Abstract
Background
Aurora A kinase (AAK) plays an integral role in mitotic entry, DNA damage checkpoint recovery, and centrosome and spindle maturation. Alisertib (MLN8237) is a potent and selective AAK inhibitor. In pediatric preclinical models, antitumor activity was observed in neuroblastoma, acute lymphoblastic leukemia, and sarcoma xenografts. We conducted a phase 2 trial of alisertib in pediatric patients with refractory or recurrent solid tumors or acute leukemias ().
Procedures
Alisertib (80 mg/m2/dose) was administered orally, daily for 7 days every 21 days. Pharmacogenomic (PG) evaluation for polymorphisms in the AURK gene and drug metabolizing enzymes (UGT1A1*28), and plasma pharmacokinetic studies (PK) were performed. Using a two-stage design, patients were enrolled to 12 disease strata (10 solid tumor and 2 acute leukemia). Response was assessed after cycle 1, then every other cycle.
Results
A total of 139 children and adolescents (median age 10 years) were enrolled, 137 were evaluable for response. Five objective responses were observed (2 complete responses and 3 partial responses). The most frequent toxicity was myelosuppression. The median alisertib trough concentration on day 4 was 1.3 μM, exceeding the 1 μM target trough concentration in 67% of patients. No correlations between PG or PK and toxicity were observed.
Conclusion
Despite alisertib activity in pediatric xenograft models and cogent pharmacokinetic-pharmacodynamic relationships in preclinical models and adults, the objective response rate in children and adolescents receiving single agent alisertib was less than 5%.
Keywords: Aurora A Kinase, Clinical Trial, Childhood Cancer, Pharmacokinetics, Pharmacogenomics
Introduction
The Aurora kinase family is essential in the regulation of chromosome segregation and cytokinesis during mitosis1. Aurora A kinase (AAK) plays an integral role in mitotic entry, DNA damage checkpoint recovery, and centrosome and spindle maturation1. Aurora A is overexpressed in many adult tumors including bladder, breast, lung and head and neck cancers; 1–4 as well as pediatric malignancies.5–8 The Aurora A kinase gene (AURK) has 2 two common polymorphisms; the phe31Ile polymorphism, which alters the kinase function and is associated with tumorigenesis or advanced cancers;9,10 and the Va571Ile polymorphism that in combination with phe31IIe may be associated with an increased risk of cancer or treatment related adverse events.11–14
Alisertib (MLN8237) is a potent and selective AAK inhibitor previously investigated alone and in combination with chemotherapy as a potential treatment for patients with relapsed/refractory peripheral T-cell lymphoma as well as advanced solid tumors15–18. In preclinical models, antitumor activity and maximum pharmacodynamic effect were associated with alisertib concentrations exceeding 1 μM.19,20 In adults, the recommended dose of alisertib is 50 mg twice daily for 7 days with associated dose limiting toxicities (DLT) of neutropenia and stomatitis.21 The maximum concentration (Cmax) and area under concentration time curve (AUC) following administration of 50 mg enteric coated tablets were 2.9 μM and 20.9 μM•h, respectively; and the steady state trough concentration (Cmin) exceeded 1 μM.22 Pharmacokinetic parameters derived from a population pharmacokinetic (PK) model based on data from 363 adults enrolled on 7 alisertib single agent trials showed a terminal half-life of 19.3 h and an apparent clearance (CL/F) of 4.25 L/h23. Although the major metabolic pathway of alisertib is glucuronidation via the UDP-glucuronosyltransferase, UGT1A1, alisertib CL/F was not altered in adult subjects with UGT1A1*28 polymorphisms. Single agent phase 2 trials of alisertib demonstrated modest activity in adults with ovarian cancer24, acute myeloid leukemia (AML),25 and T- or B- cell lymphoma.26
In the Pediatric Preclinical Testing Program (PPTP) alisertib was active in neuroblastoma (NBL) and acute lymphoblastic leukemia (ALL) xenografts with maintained complete responses observed in 3 of 7 NBL xenografts and 6 out of 6 ALL xenografts; sustained concentrations ≥ 1μM were associated with response.27 Statistically significant improvement in event free survival in Wilms tumor (WT), rhabdomyosarcoma (RMS), and osteosarcoma (OS) xenograft models was also observed.28 In these preclinical studies, alisertib was administered 5 days per week for 3 and 6 consecutive weeks for ALL and solid tumor models, respectively. In addition, alisertib was active in p53-wildtype, therapy-refractory NBL cell lines,29 as a result of disruption of the Aurora-A/N-Myc complex resulting in inhibition of N-Myc dependent transcription.30 Alisertib has also been shown to induce cell death and augment radiation sensitivity in atypical teratoid rhabdoid (ATRT) cell lines that overexpress AAK and have mutations in SMARCB1 (SNF5/INI1), a tumor suppressor and component of chromatin remodeling.7
Based on the preclinical anti-tumor activity in pediatric cell lines and xenograft models and the clinical anti-tumor activity in adult trials, a Children’s Oncology Group phase 1 trial of alisertib in children and adolescents with relapsed or refractory solid tumors evaluated both once daily and twice daily schedules31. The recommended phase 2 dose and schedule was 80 mg/m2 orally, once daily for 7 days. DLTs included myelosuppression, mood alterations, somnolence, mucositis, fatigue, alopecia, elevated hepatic transaminases, agitation and euphoria. In contrast to adults, the twice daily schedule in children resulted in a higher frequency of neutropenia and palmar-plantar erythrodysesthesia. There was marked inter-patient variability in the alisertib PK parameters in children. At the recommended dose, the Cmax and AUC were 7.5 ± 0.1 μM and 75 ± 13.5 μM•h, respectively. The alisertib trough 24 hours after the first dose at the 80 mg/m2 dose level was 1.1 μM. Of 33 response evaluable children in the phase 1 trial, one with hepatoblastoma (HBL) had a PR and 8 [NBL (n=4) and sarcoma (n=4)] had stable disease for 5–35 cycles.31
Based on the mechanism of action and preclinical activity of alisertib, a phase 2 trial was conducted to evaluate the objective response rate of alisertib in children and adolescents with relapsed/refractory solid tumors or acute leukemia. In parallel, and subsequent to the establishment of a dose and schedule for alisertib in a pediatric population, we selected two ALL xenografts against which we have previously reported single-agent alisertib efficacy at a dose resulting in drug plasma levels that are achievable in humans for additional testing to compare dosing schedules.
Methods
Patient Population
Patients with relapsed or refractory cancer were enrolled in one of 12 strata based on histology, including two strata for neuroblastoma (NBL); one for those with measurable disease by computed tomography (CT) or magnetic resonance imaging (MRI) and another for those with disease evaluable by MIBG scintigraphy, but no measurable disease. Patients with NBL limited to the bone marrow were not eligible. Patients with other solid tumors were required to have measurable disease as defined by Response Evaluation Criteria in Solid Tumors (RECIST 1.1). 32 Strata for patients with sarcoma included rhabdomyosarcoma (RMS), non-RMS soft tissue sarcoma (NRSTS), osteosarcoma (OS), or Ewing sarcoma/ peripheral PNET (EWS). Additional solid tumor strata included enrollment of patients with Wilms tumor (WT), hepatoblastoma (HBL), malignant germ cell tumors (GCT), and rhabdoid tumors (central nervous system atypical teratoid rhaboid or other malignant rhabdoid tumors) with loss of INI1 by immunohistochemistry or molecular analysis. Patients with hematological malignancies without CNS involvement who were refractory or recurrent after at least 2 prior induction chemotherapy regimens, including those with acute myeloid leukemia (AML) and at least 5% myelobasts in the bone marrow or those with acute lymphoblastic leukemia (ALL) and greater than 25% blasts (M3) in bone marrow, were also eligible. Subjects enrolled on the COG phase 1 trial (ADVL0812) who received alisertib at the recommended phase 2 dose and who met criteria for inclusion in one of the 12 disease stratum defined in this trial were included in this study population by prospective design.31
Patients were required to swallow alisertib tablets intact. Other inclusion criteria included age > 12 months and < 22 years; performance status of ≥ 50 by the Karnofsky scale for patients > 16 years or by the Lansky scale if ≤ 16 years; adequate renal function (normal serum creatinine for age and gender); and hepatic function (total bilirubin ≤ 1.5-fold greater than the upper limit of normal, alanine aminotransferase (ALT) less than 225 U/L, and serum albumin of at least 2 g/dL) was required. In patients with solid tumors, bone marrow function for patients without known tumor infiltration of bone marrow included an absolute neutrophil count (ANC) ≥ 1000/μL, platelet count ≥ 100,000/μL, and hemoglobin ≥ 8 gm/dL; for patients with solid tumors and known bone marrow metastatic disease an ANC ≥ 750/μL, platelet count ≥ 50,000/μL, and hemoglobin ≥ 8 gm/dL were required. Patients with leukemia could enroll if they were not refractory to red blood cell or platelet transfusions.
Patients were required to have recovered from the acute toxic effects of all prior treatment. Requirements for the interval of time from prior therapy were standard.31 Exclusion criteria included uncontrolled infection; pregnancy; lactation; concurrent administration of selected P-glycoprotein substrates (digoxin, cyclosporine, tacrolimus or sirolimus); or use of daily benzodiazepines, because of the potential benzodiazepine-like effects of alisertib.
This study was conducted in compliance with the Declaration of Helsinki, the International Conference on Harmonization, Guideline for Good Clinical Practice, and applicable national and local regulatory requirements. Institutional Review Boards at participating institutions approved the study. Informed consent was obtained from patients, ages 18 years or older, or from parents/legal guardians of children aged less than 18 years, with child assent when appropriate, according to institutional policies.
Treatment Program
Alisertib (Millennium Pharmaceuticals, Inc. Cambridge, MA) was distributed by the NCI Cancer Therapy Evaluation Program as 10 mg enteric-coated tablets. Alisertib (80 mg/m2) was administered orally once daily for seven consecutive days. Cycle duration was 21 days. The dose was reduced to 60 mg/m2 for reversible toxicity as outlined in the protocol. The maximum daily dose of alisertib was 160 mg. Adherence was monitored using daily dosing diaries completed by the patient or parent/guardian. In the absence of progressive disease or unacceptable toxicity, the maximum total duration of protocol therapy was 35 cycles, approximately 2 years.
Toxicity Monitoring and Dose Modifications
The Common Terminology and Adverse Events (CTCAE v 4.0) criteria were used to grade toxicity. Prior to each cycle, physical examination, complete blood count (CBC), serum electrolytes, creatinine, and liver function tests were performed. During cycle 1, physical examinations and serum chemistries were performed weekly; CBCs were performed twice weekly. In subsequent cycles, CBCs were performed weekly or more frequently if hematological toxicity occurred.
Response
Disease evaluations were performed at baseline, the end of cycle 1 and after completion of every other cycle of protocol therapy. In patients with non-CNS solid tumors, response was assessed using RECIST version 1.1 criteria.32 Response in NBL subjects with non-measurable but MIBG evaluable disease was assessed using the Curie Score criteria.33 Response for subjects with AML was assessed using the International Working Group Criteria 34 and for those with ALL response was defined by morphology. Response in patients with central nervous system ATRT was assessed based on the sum of the products of the longest diameter × perpendicular diameter. All objective responses were confirmed by central review.
Any eligible patient who received at least one dose of alisertib was considered evaluable for response provided: (1) the patient was observed on protocol therapy for at least one cycle and the tumor was not removed surgically prior to the time an objective response was confirmed; or (2) the patient demonstrated a complete or partial response as confirmed by central review; or (3) the patient demonstrated progressive disease or died while on protocol therapy. All other evaluable patients with solid tumors were considered to be non-responders. The maximum evaluation period for determination of the overall best response was six treatment cycles for ADVL0921; the evaluation period for determination of overall best response for ADVL0812 was the time from enrollment to termination of protocol therapy.
Pharmacokinetics and Pharmacogenomics
To characterize the PKs of alisertib in children and adolescents, blood samples (3 mL, EDTA) were required in all participants during cycle 1 prior to alisertib administration on days 1, 4±1, and 7±1. If consent was provided, optional sampling was performed at 1–2, 3–4, and 6–8 hours after the first dose. Plasma was stored at −80°C until analysis. Alisertib concentrations were measured as previously described and PK parameters were calculated using non-compartmental analyses.31,35
Consenting patients provided whole blood in EDTA tubes prior to day 7 of the first cycle for genotyping of patients for germline polymorphisms in UGT1A1 or aurora AAK gene (AURK, Phe31Ile and Val57Ile). DNA was extracted by QIAamp DNA Blood Mini Kit as per manufacturer’s instructions. Methods were validated with a panel of 60 Caucasian DNA samples from the Coriell Institute. Positive and negative controls were included for each analysis. For UGT1A1 *28 (rs8175347), the number of TA repeats in the promoter region were detected and quantified by a modification of the method described by Akaba et al.36 UGT1A1 polymorphisms rs4124874 and rs10929302 were evaluated with PCR amplification and dye-terminator sequencing. Specific primers were designed and validated to amplify the region for both SNPs. Forward and reverse primers are AGTTCTCTTCACCTCCTCCT and AATAAA CCCCACCTCACCAC, respectively. For AURKA, genotyping for the G>A polymorphism (rs1047972 in codon 57) and T>A polymorphism (rs2273535 in codon 31) was performed by amplification and detected on a Bio-Rad CFX384 Real-Time PCR detection system (Hercules CA). The real time PCR methods were validated against a standard PCR reaction with sequence detection of the polymorphisms. Primer and probe sequences were provided by Millennium Pharmaceuticals, Inc. (Cambridge, MA). The forward and reverse primer sequences for rs227353 were CTGGCCACTATTTACAGGTAATGGA and TGGAGGTCCAAAACGTGTTCTC, respectively with probe/reporter 1 (VIC-labeled) sequence ACTCAGCAATTTCCTT and probe/reporter 2 (FAM-labeled) sequence CTCAGCAAATTCCTT. The forward and reverse primer sequences for rs1047972 were CGGCTTGTGACTGGAGACA and GGGTCTTGTGTCCTTCAAATTCTTC, respectively with probe/reporter 1 (VIC-labeled) sequence CAGCGCGTTCCTT and probe/reporter 2 (FAM-labeled) sequence CAGCGCATTCCTT. The AURKA haplotypes were determined using the Phe31Ile and val57Ile SNPs as described by Ishikawa et al.14
Statistical Analysis Plan
A two-stage design was used to evaluate alisertib anti-tumor activity in seven primary strata: NBL with RECIST measurable disease, NBL with MIBG only evaluable disease, OS, EWS, RMS, ALL, and AML. For each of the two NBL strata, 14 patients were enrolled at the first stage. If no patients experienced a complete or partial response, alisertib was considered inactive in that stratum, and further enrollment to that stratum was terminated. If one or more patients achieved an objective response, 10 additional patients were enrolled to the stratum. Alisertib was active if ≥ 4 of 24 patients in an expanded stratum experienced an objective response. With this design, alisertib was identified as inactive with probability 0.96 if the true response rate was 5% and as active with probability 0.91 if the true response rate was 25%. For the other five secondary strata (NRSTS, HBL, GCT, WT and rhabdoid tumors), at the first stage for each stratum, 10 patients were enrolled. If no patients experienced an objective response, alisertib was considered inactive in that stratum and enrollment was terminated. If one or more patients experienced an objective response, 10 additional patients were enrolled to that stratum. Alisertib was considered active if ≥ 3 of 20 patients in an expanded stratum experienced an objective response. With this design, alisertib would be identified as inactive with probability 0.93 if the true response rate was 5%, and was identified as active with probability 0.88 if the true response rate was 25%. Because of the rarity of tumors in the secondary strata, enrollment to the study was designed to be closed, irrespective of enrollment numbers, when the evaluation of the seven primary strata was completed. If sufficient enrollment was obtained, the two-stage design used for the non-NBL stratum was applied to the secondary stratum.
Xenograft studies
Subsequent to the prior preclinical evaluation in which alisertib was administered on a twice-daily schedule for 5 days and repeated for 3-weeks,27 the maximum tolerated dose in pediatric patients was determined to be once daily for 7 days.31 An experiment was designed to compare the preclinical and clinical schedules. ALL xenografts were generated as described previously.28,37 ALL-8 and ALL-19 xenograft cells, generated from 2 patients with relapsed ALL, were inoculated into NOD/SCID mice and engraftment monitored by weekly flow cytometric enumeration of the percentage of human CD45 (%huCD45) cells in murine peripheral blood (PB). When the %huCD45 cells reached a median of >1% for the entire cohort, mice were randomized to receive treatment with alisertib or vehicle control. Alisertib was administered using two alternative schedules: Schedule A) twice daily for 7 days; or Schedule B) twice daily for 5 days repeated for 3 weeks. In both cases the dose used was 10.4 mg/kg, administered by oral gavage as a suspension in 10% cyclodextrin. Groups of 4–6 mice were euthanized at Days 0, 7 and 21 post treatment initiation and at the end of the evaluation period (Day 42) to assess leukemic infiltration of PB, bone marrow and spleens. An additional experimental endpoint for each mouse was when the %huCD45 cells in PB reached 25% (deemed an event). Mice were euthanized if morbid or if they experienced weight loss ≥20%. Event free survival (EFS), Treated-Control (T-C), T/C and overall response measure (ORM) estimations were carried out according to established methodology.37 Individual mice were assigned an ORM depending on the leukemic growth characteristics observed in the 42 days following treatment according to the established criteria used for evaluating single agents, and the median ORM was used to obtain the group score.
Results
Patients
Characteristics for all patients are presented in Table I. All patients (n=139) were eligible. Two patients, one with RECIST-measurable NBL and one with AML, were not evaluable for response due to rapid progression of disease prior to the start of protocol therapy. The median number of treatment cycles for 137 response-evaluable patients was 2 (range 1–35). A total of 500 cycles of alisertib were delivered. Three patients completed 35 cycles (24 months) of protocol therapy.
Table I.
Patient Characteristics at Enrollment
| Total | NBL Measurable | NBL Evaluable | AML | ALL | EWS | RMS | NR-STS | OS | WT | HBL | GCT | MRT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eligible (N) | 139 | 25 | 24 | 11 | 10 | 10 | 10 | 10 | 10 | 10 | 8 | 7 | 4 |
| Age (y)* | 10 (2–21) | 9 (3–20) | 8 (4–19) | 10 (3–15) | 13 (7–19) | 14 (6–20) | 12 (4–21) | 15 (9–20) | 17 (12–21) | 10 (3–18) | 6 (3–14) | 11 (3–19) | 5 (2–7) |
| Female (%) | 45% | 48% | 29% | 55% | 30% | 30% | 70% | 50% | 30% | 70% | 38% | 57% | 50% |
| Race n(%) | |||||||||||||
| Caucasian | 80 (58%) | 19 (76%) | 15 (63%) | 2 (18%) | 5 (50%) | 8 (80%) | 7 (70%) | 4 (40%) | 7 (70%) | 5 (50%) | 4 (50%) | 4 (57%) | - |
| Black | 21 (15%) | 2 (8%) | 4 (17%) | 2 (18%) | 2 (20%) | - | 1 (10%) | 1 (10%) | 2 (20% | 2 (20%) | 2 (20%) | - | 3 (75%) |
| Other | 6 (4%) | 0 (0%) | 2 (8%) | 1 (9%) | - | - | - | - | 1 (10%) | - | 1 (10%) | 1 (14%) | - |
| Not Specified | 32 (23%) | 4 (16%) | 3 (12%) | 6 (55%) | 3 (30%) | 2 (20%) | 2 (20%) | 5 (50%) | 3 (30%) | 1 (10%) | 2 (29%) | 1 (25%) | |
| Ethnicity N (%) | |||||||||||||
| Hispanic | 30 (22%) | 3 (12%) | 1 (4%) | 4 (36%) | 2 (20%) | 1 (10%) | 1 (10%) | 3 (30%) | 3 (30%) | 4 (40%) | 3 (38%) | 4 (57%) | 1 (25%) |
| Non-Hispanic | 97 (70%) | 19 (76%) | 22 (92%) | 5 (46%) | 8 (80%) | 8 (80%) | 7 (70%) | 6 (60%) | 7 (70%) | 5 (50%) | 5 (62%) | 2 (29%) | 3 (75%) |
| Not Reported | 12 (8%) | 3 (12%) | 1 (4%) | 2 (18%) | - | 1 (10%) | 2 (20%) | 1 (10%) | - | 1 (10%) | - | 1 (14%) | - |
Median (range)
NBL –neuroblastoma; AML- Acute Myeloid Leukemia; ALL- Acute Lymphoblastic Leukemia; EWS-Ewing Sarcoma; RMS- Rhabdomyosarcoma; NR-STS- Non-Rhabdo Soft Tissue Sarcoma; OS- Osteosarcoma; WT- Wilms Tumor; HBL- Hepatoblastoma; GCT- Malignant Germ Cell Tumor; MRT- Malignant Rhaboid Tumor (2 patients with CNS atypical teratoid rhabdoid tumors and 2 patients with extracranial malignant rhabdoid tumors).
Toxicity
During cycle 1, 18 patients (13%) experienced dose limiting toxicity including myelosuppression, mucositis, febrile neutropenia, enterocolitis, diarrhea, depression, hypersomnia, photophobia, tumor lysis syndrome, hyperbilirubinemia and/or electrolyte abnormalities. The frequency of alisertib related grade 3 and 4 toxicities is shown in Table 2. Alisertib-related grade 3 or 4 toxicities that occurred in ≥10% of delivered cycles (n=500) were anemia (13.6%), lymphopenia (12.2%), neutropenia (51.8%), thrombocytopenia (20.8%) and leukopenia (33%). During cycle 1, two patients had fatal adverse events possibly related to alisertib: a patient with pelvic soft tissue sarcoma experienced grade 5 respiratory failure and a patient with hepatoblastoma experienced a fatal hepatic hemorrhage.
Table 2:
Frequency of Alisertib Related Grade 3 or 4 Toxicity in All Cycles (n=500)
| CTCAE Class | Toxicity | Grade 3 n (%) | Grade 4 n (%) | Grade 5 n (%) |
|---|---|---|---|---|
| Hematologic | Anemia | 63 (12.6%) | 5 (1%) | |
| Febrile neutropenia | 18 (3.6%) | |||
| Lymphopenia | 47 (9.4%) | 14 (2.8%) | ||
| Neutropenia | 124 (24.8%) | 137 (27.4%) | ||
| Thrombocytopenia | 54 (10.8%) | 50 (10%) | ||
| Serum amylase increased | 1 (0.2%) | |||
| Leukopenia | 117 (23.4%) | 48 (9.6%) | ||
| Eye Disorders | Photophobia | 1 (0.2%) | ||
| Gastrointestinal | Diarrhea | 2 (0.4%) | ||
| Enterocolitis | 1 (0.2%) | |||
| Oral mucositis | 19 (3.8%) | |||
| Oral pain | 5 (1%) | |||
| Nausea | 2 (0.4%) | |||
| Vomiting | 2 (0.4%) | |||
| Investigations (Laboratory) | ALT increased | 17 (3.4%) | ||
| AST increased | 10 (2%) | |||
| Hyperbilirubinemia | 3 (0.6%) | |||
| GGT increased | 1 (0.2%) | |||
| INR increased | 1 (0.2%) | |||
| Infection | Infection | 1 (0.2%) | ||
| Pneumonia | 1 (0.2%) | |||
| Urinary tract infection | 1 (0.2%) | |||
| Metabolism/Nutrition | Anorexia | 1 (0.2%) | ||
| Dehydration | 6 (1.2%) | |||
| Hyperuricemia | 1 (0.2%) | |||
| Hypoalbuminemia | 1 (0.2%) | |||
| Hypocalcemia | 1 (0.2%) | |||
| Hypokalemia | 4 (0.8%) | 1 (0.2%) | ||
| Hyponatremia | 3 (0.6%) | |||
| Hypophosphatemia | 1 (0.2%) | |||
| Tumor lysis syndrome | 1 (0.2%) | |||
| Psychiatric | Depression | 1 (0.2%) | ||
| Neurological | Dizziness | 14 (2.8%) | ||
| Hypersomnia | 1 (0.2%) | |||
| Hepatobiliary | Hepatic hemorrhage | 1 (0.2%) | ||
| Respiratory | Respiratory failure | 1 (0.2%) | ||
| Skin/Dermatological | Palmar-plantar erythrodysesthesia | 2 (0.4%) |
Response
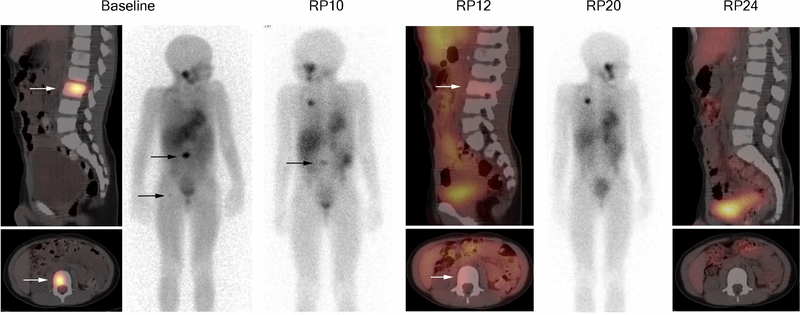
Five objective responses were observed. Two patients had complete responses, one patient with MIBG-only evaluable NBL (Figure 1) and one patient with Wilms tumor. Three patients had partial responses, one each with RECIST-measurable NBL, MIGB evaluable NBL and HBL. The patient with HBL and partial response was previously reported in the phase 1 trial.31 Unlike the phase 1 study (ADVL0812), prolonged stable disease was not considered as a response in this trial and was not centrally reviewed. No responses were achieved in the other primary disease strata (OS, EWS, RMS, ALL, AML). The objective responses are summarized in Table 3 and includes the number of cycles of alisertib administered for patients with a best response of stable disease. Accrual to secondary strata (HBL, WT, GCT, NRSTS) was discontinued due to insufficient response in the primary strata. At the time the study was closed, accrual to the first stage was not completed for HBL, GCT or rhabdoid tumors.
Figure 1.
Sagittal and axial SPECT/CT images from 123I-MIBG examination performed at baseline, reporting periods (RP) 12 and 24; 123I-MIBG anterior projection planar images at baseline, RP10 and RP 20. Each reporting period is one cycle. Arrows indicate MIBG-avid neuroblastoma in the L3 vertebral body and proximal right femur. The other areas of MIBG positivity on planar imagines include physiologic uptake in liver, salivary glands, renal collecting system, GI tract, and excretion in bladder. Uptake projecting of the thorax at RP 10 and 10 is residual tracer at the port injection site.
Table 3.
Responses to Alisertib
| Stratum | Response Evaluable | Responder | Non-responders | |||
|---|---|---|---|---|---|---|
| Complete Response | Partial Response | Stable Disease n patients, median (range) cycles administered | Non-responders | Progressive Disease | ||
| Neuroblastoma (Measurable) | 24 | 1 | 2 (6, 13 cycles) | 5 | 16 | |
| Neuroblastoma (MIBG Evaluable) | 24 | 1 | 1 | 9 13 (5–35) cycles | 4 | 9 |
| Acute Lymphoblastic Leukemia | 10 | 3 (1,2,2 cycles) | 1 | 6 | ||
| Acute Myeloid Leukemia | 10 | 10 | ||||
| Ewing Sarcoma | 10 | 3 (4, 5, 5 cycles) | 2 | 5 | ||
| Rhabdomyosarcoma | 10 | 1 15 cycles | 2 | 7 | ||
| Non-Rhabdo Soft Tissue Sarcoma | 10 | 1 5 cycles | 2 | 7 | ||
| Osteosarcoma | 10 | 2 | 8 | |||
| Wilms tumor | 10 | 1 | 1 31 cycles | 1 | 7 | |
| Hepatoblastoma | 8 | 1* | 1 5 cycles | 2 | 4 | |
| Germ Cell Tumor | 7 | 2 4 and 5 cycles | 1 | 4 | ||
| Rhabdoid Tumors | 4 | 1 | 3 | |||
| Total | 137 | 2 | 3 | 23 | 33 | 76 |
Reported previously in ADVL0812 publication.31
Pharmacokinetics and Pharmacogenomics
Forty-five patients provided consent for optional PK samples on ADVL0912 and 2 patients from ADVL0812 had trough levels obtained on day 4. The alisertib PK parameters were highly variable (Supplemental Table 1). There was no correlation between age or gender and alisertib Cmin or AUC. The alisertib Cmax exceeded 1μM in 98% (44/45) of patients participating in detailed PK studies on day 1. The median Cmin on day 4 was 1.6 μM, exceeding 1μM target trough concentration in 67% (26/39) of patients. The median Cmin on day 7 was 0.9 μM, exceeding 1μM in 41% of patients (11/27).
A total of 87 patients underwent genotyping for AURKA and UGT1A1 (Table 4A). There was no relationship between cycle 1 toxicity and either the Phe31Ile or Val57Ile single nucleotide polymorphisms (SNP) in the AURKA genotype or in the AURKA haplotypes (Table 4B). There was no relationship between ≥ grade 2 toxicities and AURKA genotype for the Ile31Phe SNP or the AURKA haplotypes. However, patients that were heterozygous (WV) for the Val57Ile SNP appeared to have fewer ≥ grade 2 toxicities (Table 4B). Given the small patient cohort, it is not possible to determine if this is clinically significant. In addition, there were no relationships between treatment response and AURKA genotype for either SNP.
Table 4A:
AURKA, UGT1A1*28 and UGT1A1 PBREM genotype distribution.
| Gene | RsSNP ID | Description | Genotypea (WW/WV/VV) |
|---|---|---|---|
| AURKA | rs2273535 | 91 A>T, Ile31Phe | 51/27/8 b |
| rs1047972 | 169 G>A, Val57Ile | 63/21/2 b | |
| UGT1A1*28 | rs8175347 | TA repeat (5,6,7,8) | 39/32/11 |
| UGT1A1 PBREM | rs4124874 | −3279 T>G | 23/42/22 |
| rs10929302 | −3156 G>A | 46/33/8 | |
W = Wild Type Allele, V = Variant allele; For UGT1A1*28- WW = 66 or 57, WV = 67 or 68, and VV = 77 or 78
One sample could not be genotyped with the available DNA
Five samples could not be genotyped with the available DNA
Table 4B.
Toxicity versus AURKA genotype
| Cycle 1 DLT | Grade 2+ Toxicity | ||||
|---|---|---|---|---|---|
| AURKA Genotype | Yes | No | Yes | No | |
| 91 A>T | Total | 13 | 73 | 72 | 14 |
| WW | 8 | 43 | 42 | 9 | |
| WV | 5 | 22 | 22 | 5 | |
| VV | 0 | 8 | 8 | 0 | |
| 169G>A | Total | 13 | 73 | 72 | 14 |
| WW | 10 | 53 | 54 | 9 | |
| WV | 3 | 18 | 17 | 4 | |
| VV | 0 | 2 | 1 | 1 | |
| Haplotype | Total | 13 | 73 | 72 | 14 |
| H1 | 5 | 25 | 26 | 4 | |
| H2 | 8 | 48 | 46 | 10 | |
Paired data for UGT1A1 phenotype and day 4 Cmin was obtained in 32 patients. There did not appear to be a difference in Day 4 Cmin between the intermediate (IM) and poor metabolizer (PM) phenotypes, therefore, the data for these patients were pooled (Table 4C). The mean ± SE alisertib trough concentration for the extensive metabolizer group (EM, n=16) was 1.01 ± 0.23 μM (95% C.I. of 0.53 – 1.49 μM) and for IM/PM (n=16) was 2.06 ± 0.30 μM (95% C.I. of 1.42 – 2.70 μM). The difference in the population means, 1.05 μM (95% C.I. of 0.28 – 1.82 μM), was statistically significant (p-value of pooled t-test, 0.0091). The mean alisertib trough concentration for patients with and without ≥ grade 2 toxicities were 1.50 ± 0.25 μM (95% C.I. of 0.96 – 2.04 μM) and 1.58 ± 0.35 μM (95% C.I. of 0.83 – 2.32 μM), respectively. The difference in the population means, 0.08 μM (95% C.I. of −0.79 – 0.94 μM), was not statistically significant. While alisertib trough concentrations were statistically significantly higher for IM/PM patients, there did not appear to be a relationship with the occurrence of ≥ grade 2 toxicity (p-value = 0.86). Furthermore, we found no significant interaction between EM (yes/no) and ≥ grade 2 adverse events (yes/no) (p-value = 1.0) (Table 4C).
Table 4C:
Toxicity versus UGT1A1 metabolizer phenotype and alisertib trough concentration (Cmin) on day 4.
| EM | I/E, I, P | |||
|---|---|---|---|---|
| Grade ≥2: No (n=7) | Grade ≥ 2: Yes (n=9) | Grade ≥ 2; No (n=8) | Grade ≥ 2: Yes (n=8) | |
| Alisertib Cmin (μM) Mean ± SD |
0.81± 0.43 | 1.17± 1.15 | 2.25± 1.53 | 1.87±0.82 |
| Alisertib Cmin (μM) Median (range) |
0.99 (0.2–1.24) | 0.53 (0.08–3.51) | 2.22 (0.6–5.65) | 1.80 (0.97–3.10) |
Xenograft studies
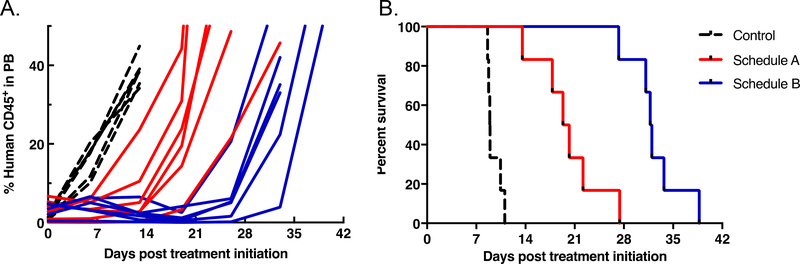
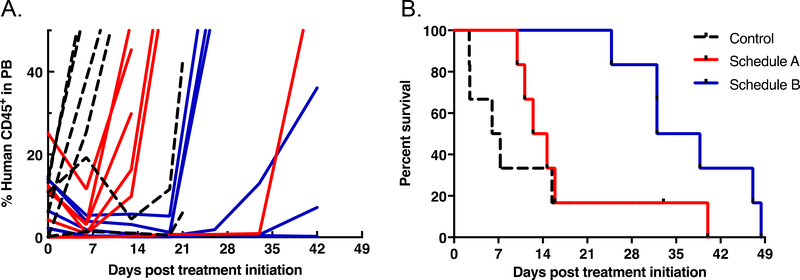
For the T-lineage ALL-8, based on serial peripheral blood parameters, leukemia progression was significantly delayed compared to vehicle control for both of the treatment schedules, resulting in increased EFS (Figure 2 and Table 5). Leukemia progression was delayed by an additional 12.1 days in mice treated with Schedule B compared with Schedule A (p=0.004), with T/C values 3.6 and 2.2, respectively. However, neither of the treatment schedules induced an objective response. Data are summarized in Table 5 and Supplementary Table 2. Engraftment levels for ALL-8 engrafted mice detected in the three compartments analyzed at autopsy (blood, bone marrow and spleen) are shown in Supplementary Figure 1, Figure 2 and Figure 3, respectively. Treatment with both alisertib schedules limited leukemia progression to a similar extent by Day 7 in all organs analyzed. These effects were not complete, with 5–10 % human cells in the spleen and approximately 20% human cells in the bone marrow as the lowest levels achieved. Schedule B was more effective than Schedule A in reducing leukemia levels measured at Day 21 in the three compartments analyzed, but the differences between the treatments were transient and after drug treatments ceased there was a rapid progression of the disease (Day 42).
Figure 2.
Percentage of huCD45 cells in peripheral blood over time (A), and event-free survival curves (B) for ALL-8 engrafted NOD/SCID mice treated with alisertib at 10.4 mg/kg twice daily for 7 days (Schedule A, red), or twice daily for 5 days repeated for 3 weeks (Schedule B, blue) in relation to vehicle-treated controls (dotted line).
Table 5.
Leukemic growth delay summary and clinical scoring for the two treatment schedules for xenografts ALL-8 and ALL-19.
| Treatment Schedule* | Median EFS (days) | EFS T-C (days) | EFS T/C | p value** | Median Response Score | ||
|---|---|---|---|---|---|---|---|
| Control | Treated | ||||||
| ALL-8 | Schedule A | 8.9 | 19.8 | 10.9 | 2.2 | 0.002 | PD2 |
| Schedule B | 8.9 | 31.9 | 23.0 | 3.6 | 0.002 | SD | |
| ALL-19 | Schedule A | 6.6 | 13.6 | 7.0 | 2.0 | 0.584 | PD2 |
| Schedule B | 6.6 | 31.2 | 24.6 | 4.7 | 0.002 | CR | |
Schedule A, twice daily × 7 days; Schedule B, twice daily × 5 days × 3 weeks
Statistically significant results are in bold.
For the B-lineage ALL-19, based on serial peripheral blood parameters, leukemia progression was not significantly different from that of controls for Schedule A (Figure 3 and Table 5), However, Schedule B significantly delayed ALL-19 progression by 24.6 days relative to controls, which was 17.6 days greater than Schedule A (p=0.048) with T/C values 4.7 and 2.0, respectively. Furthermore, while treatment on Schedule A resulted in progressive disease (PD), Schedule B induced a complete response (CR). Data are summarized in Table 5 and Supplementary Table S2. Engraftment levels for ALL-19 engrafted mice detected in the three compartments analyzed at autopsy (blood, bone marrow and spleen) are shown in Supplementary Figure 1, Figure 2 and Figure 3, respectively. They followed a similar pattern to those of ALL-8, with the exception of being higher at Day 7, particularly in the bone marrow.
Figure 3.
Percentage of huCD45+ cells in peripheral blood over time (A), and event-free survival curves (B) for ALL-19 engrafted NOD/SCID mice treated with alisertib at 10.4 mg/kg twice daily for 7 days (Schedule A, red), or twice daily for 5 days repeated for 3 weeks (Schedule B, blue) in relation to vehicle-treated controls (dotted line).
Discussion
In this phase 2 study, anti-tumor activity of alisertib was evaluated in 137 children and adolescents in 7 primary and 5 secondary disease strata. We demonstrated that children achieved target concentrations established in adults and preclinical models. The higher alisertib trough concentration in patients with intermediate and poor metabolizer UGT1A1 phenotypes compared to the extensive metabolizer phenotype was statistically significant. However, we did not find a difference in frequency of toxicity among these groups. Evaluation of AURK somatic mutation status and AAK expression in archival tumor specimens from children enrolled on this study was not performed, therefore, the impact of enrollment stratification by somatic mutation or expression in tumors of children enrolled on this trial is unknown. Pharmacogenomic profiling of germline AURKA in adults has focused on cancer susceptibility and early adverse reactions.14 In this study, we evaluated germline AURKA SNPs and did not find correlation with toxicity during cycle 1 or response.
Despite striking efficacy in pediatric xenograft models in which objective responses were reported in 80% of solid tumor pediatric solid tumor models and all leukemia models28, the objective response rate in children and adolescents receiving single agent alisertib on this trial was less than 5%. In patients receiving 50 mg BID, the Cmax and AUC0–24 h were 1.3 and 40μM h, respectively27. At the recommended phase 2 dose of 50 mg BID for 7 days, average trough concentrations exceeded 1μM, the efficacious concentration estimated in previous preclinical work. In mice receiving alisertib at 10 mg/kg, the Cmax and AUC0–24 h were 16 and 39μM h, respectively, with the 12 h level being 1.2 μM 27. These data suggest that continuous drug exposure above 1μM throughout each 24-hour dosing period which can only be achieved with twice-daily dosing in mice, is crucial for anti-tumor activity. The initial preclinical studies evaluating alisertib in pediatric patient-derived xenograft models used a dose and schedule that was employed in preclinical assessment of adult cancer models in an attempt to mirror pharmacokinetic-pharmacodynamic relationships expected to be tolerated in humans. However, the continuous treatment schedule was too myelosuppressive in adult phase 1 studies and a 7-day on, 14-day off regimen was adopted to minimize toxicity. Thus, we hypothesized that the dose and schedule used in our trial might account for the discordance between the preclinical and clinical activity observed.
Concurrent with this trial, we tested alisertib in ALL xenograft models utilizing an intermittent dose and schedule, and found significantly less efficacy compared with the previously tested schedule. This demonstrates an important point in the clinical translation of new agents. While careful consideration is made to maximize the clinical relevance and translatability of pre-clinical oncology studies, numerous variables can influence the extent to which a drug against a given target will cause toxicity. This study highlights the critical importance of performing reverse translational studies to rigorously reproduce results when dose or schedule significantly change as a result of early phase human clinical trials. The continuous treatment schedule of alisertib, which was shown to be more effective in preclinical models than the 1-week administration schedule, was not feasible due to toxicity to pursue in the clinical setting, providing a potential explanation for the differential observed between preclinical and clinical anti-tumor activity. To increase our confidence in the translation of results from preclinical studies, there needs to be continued efforts to redesign preclinical experiments as we learn from the corresponding human experience.
Identifying applicable preclinical cancer models remains a major challenge in augmenting the effectiveness of drug development and predicting success in the clinic. All models are limited and interrogating the complexity of human cancers in the laboratory remains a challenge that contributes appreciably to attrition in drug development. In recent years, patient-derived xenografts obtained by direct implants of human tumors in immunodeficient mice and then passaged directly from mouse to mouse have emerged as an important platform for translational oncology research38. The ability of these models to predict clinical outcomes is being optimized through murine humanization strategies to improve the reach of these models as reliable tools for exploring tumor intrinsic and extrinsic heterogeneity, clonal evolution under the selective pressure of our therapies, discovery of integral biomarkers and predictability of drug response in the clinic38. Numerous challenges and limitations remain, including the lack of a proper anatomical and metastatic niche, engraftment failure of certain tumor subtypes, access to imaging technologies for robust tumor visualization, and hurdles to achieve complete human immune system reconstitution38.
To improve the efficiency of this two-stage phase 2 trial, this trial was prospectively designed to include data from 21 patients on the phase 1 single agent alisertib trial (ADVL0812), who were treated at the recommended dose and met eligibility criteria for this trial, including 5 patients with NBL- measurable disease, 12 patients with NBL-MIGB evaluable disease, 2 patients with non-RMS soft tissue sarcoma and 2 patients with hepatoblastoma. Given the rarity of relapsed pediatric cancers, trial designs that improve efficiency are essential.
Clinical trials evaluating alisertib in combination with cytotoxic agents have shown anti-tumor activity in children and adolescents with relapsed solid tumors including neuroblastoma 39,40. Given the lack of objective response observed in this comprehensive single agent clinical trial of alisertib as well as dose limiting myelosuppression, alternative strategies, including novel:novel combinations simultaneously targeting other oncogenic signaling pathways or exploiting the pro-apoptotic machinery41, should be explored to harness this pathway and simultaneously minimize toxicity.
Supplementary Material
Statement of Translational Relevance.
The Aurora kinases are a family of serine-threonine kinases that play an essential role in regulating chromosome assembly and segregation during mitosis and are critical for cell proliferation. Aurora A dysregulation has been implicated in cancer, and as such, is a rational therapeutic target. This phase 2 study of alisertib (MLN2837), an oral small molecular inhibitor of Aurora A kinase, was evaluated in 137 pediatric patients with relapsed/refractory solid tumors or acute leukemia. The lack of robust objective responses suggests that alisertib, as a single agent, has limited anti-tumor activity and alternative strategies, including novel combinations simultaneously targeting other oncogenic signaling pathways, should be explored to harness this pathway and simultaneously minimize toxicity. The continuous treatment schedule of alisertib, which was shown to be more effective in preclinical models than the 1-week administration schedule, was not feasible in the clinical setting, providing a potential explanation for the differential observed between preclinical and clinical anti-tumor activity.
Acknowledgments
Financial Support: UM1CA097452 Phase 1 and Pilot Consortium Grant; National Institutes of Health National Cancer Institute NOI-CM-42216 and NOI-CM-91001-03; St Baldrick’s Foundation; and Cookies for Kids Foundation
Abbreviations Key Table
- AAK
Aurora A Kinase
- ALL
Acute Lymphoblastic Leukemia
- ALT
Alanine Aminotransferase
- ANC
Absolute Neutrophil Count
- AML
Acute Myeloid Leukemia
- ATRT
Atypical Teratoid Rhabdoid Tumor
- AUC
Area Under the Concentration × Time Curve
- AURK
Aurora A Kinas Gene
- CBC
Complete Blood Count with Differential
- CL/F
Apparent Clearance
- Cmax
Maximum Concentration, peak
- Cmin
Minimum Concentration, trough
- CR
Complete Remission, Complete Response
- CT
Computed Tomography Imaging
- CTCAE
Common Terminology Criteria for Adverse Events
- dL
Deciliter
- DLT
Dose Limiting Toxicity
- EDTA
Ethylenediaminetetraacetic acid
- EWS
Ewing Sarcoma Family of Tumors
- GCT
Germ Cell Tumor (Malignant)
- GGT
Gamma-Glutamyl Transferase
- g
Gram
- h
Hour
- HBL
Hepatoblastoma
- INR
International Normalized Ratio
- L
Liter
- NBL
Neuroblastoma
- NRSTS
Non- Rhabdo Soft Tissue Sarcoma
- m2
Square meters of body surface area
- mg
Milligram
- MIBG
Metaiodobenzylguanidine
- mm3
Cubic millimeters
- MRI
Magnetic Resonance Imaging
- OS
Osteosarcoma
- PB
Peripheral blood
- PD
Progressive Disease
- PK
Pharmacokinetics
- PG
Pharmacogenomics
- PPTP
Pediatric Preclinical Testing Program
- PR
Partial Response, Partial Remission
- RECIST
Response Criteria in Solid Tumors
- RMS
Rhabdomyosarcoma
- SD
Stable Disease
- UGT1A1
UDP-glucuronosyltransferase 1A1
- ULN
Upper Limit of Normal
- WBC
White Blood Cell Count
- WT
Wilms Tumor
- μL
Microliter
- μM
micromolar
Footnotes
Conflict of Interest Disclosure Statement: The authors declare no potential conflict of interest.
References
- 1.Gautschi O, Heighway J, Mack PC, et al. : Aurora kinases as anticancer drug targets. Clinical cancer research : an official journal of the American Association for Cancer Research 14:1639–48, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Nigg EA: Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol 2:21–32, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Lapenna S, Giordano A: Cell cycle kinases as therapeutic targets for cancer. Nature reviews. Drug discovery 8:547–66, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Sen S, Zhou H, Zhang RD, et al. : Amplification/overexpression of a mitotic kinase gene in human bladder cancer. Journal of the National Cancer Institute 94:1320–9, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Borges KS, Moreno DA, Martinelli CE Jr., et al. : Spindle assembly checkpoint gene expression in childhood adrenocortical tumors (ACT): Overexpression of Aurora kinases A and B is associated with a poor prognosis. Pediatr Blood Cancer 60:1809–16, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Hartsink-Segers SA, Zwaan CM, Exalto C, et al. : Aurora kinases in childhood acute leukemia: the promise of aurora B as therapeutic target. Leukemia 27:560–8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venkataraman S, Alimova I, Tello T, et al. : Targeting Aurora Kinase A enhances radiation sensitivity of atypical teratoid rhabdoid tumor cells. Journal of neuro-oncology 107:517–26, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romain C, Paul P, Kim KW, et al. : Targeting Aurora kinase-A downregulates cell proliferation and angiogenesis in neuroblastoma. J Pediatr Surg 49:159–65, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewart-Toland A, Briassouli P, de Koning JP, et al. : Identification of Stk6/STK15 as a candidate low-penetrance tumor-susceptibility gene in mouse and human. Nature genetics 34:403–12, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Dicioccio RA, Song H, Waterfall C, et al. : STK15 polymorphisms and association with risk of invasive ovarian cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 13:1589–94, 2004 [PubMed] [Google Scholar]
- 11.Egan KM, Newcomb PA, Ambrosone CB, et al. : STK15 polymorphism and breast cancer risk in a population-based study. Carcinogenesis 25:2149–53, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Tang W, Qiu H, Jiang H, et al. : Aurora-A V57I (rs1047972) polymorphism and cancer susceptibility: a meta-analysis involving 27,269 subjects. PloS one 9:e90328, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu L, Zhou X, Jiang F, et al. : STK15 rs2273535 polymorphism and cancer risk: a meta-analysis of 74,896 subjects. Cancer epidemiology 38:111–7, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa A, Suga T, Shoji Y, et al. : Genetic variants of NPAT-ATM and AURKA are associated with an early adverse reaction in the gastrointestinal tract of patients with cervical cancer treated with pelvic radiation therapy. Int J Radiat Oncol Biol Phys 81:1144–52, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Barr PM, Li H, Spier C, et al. : Phase II Intergroup Trial of Alisertib in Relapsed and Refractory Peripheral T-Cell Lymphoma and Transformed Mycosis Fungoides: SWOG 1108. J Clin Oncol 33:2399–404, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melichar B, Adenis A, Lockhart AC, et al. : Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: a five-arm phase 2 study. Lancet Oncol 16:395–405, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Friedberg JW, Mahadevan D, Cebula E, et al. : Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas. J Clin Oncol 32:44–50, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manfredi MG, Ecsedy JA, Chakravarty A, et al. : Characterization of Alisertib (MLN8237), an investigational small-molecule inhibitor of aurora A kinase using novel in vivo pharmacodynamic assays. Clin Cancer Res 17:7614–24, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Manfredi MG, Ecsedy JA, Chakravarty A, et al. : Characterization of Alisertib (MLN8237), an investigational small-molecule inhibitor of aurora A kinase using novel in vivo pharmacodynamic assays. Clinical cancer research : an official journal of the American Association for Cancer Research 17:7614–24, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Yang JJ, Li Y, Chakravarty A, et al. : Preclinical drug metabolism and pharmacokinetics, and prediction of human pharmacokinetics and efficacious dose of the investigational Aurora A kinase inhibitor alisertib (MLN8237). Drug metabolism letters 7:96–104, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Cervantes A, Elez E, Roda D, et al. : Phase I pharmacokinetic/pharmacodynamic study of MLN8237, an investigational, oral, selective aurora a kinase inhibitor, in patients with advanced solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research 18:4764–74, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Falchook G, Kurzrock R, Gouw L, et al. : Investigational Aurora A kinase inhibitor alisertib (MLN8237) as an enteric-coated tablet formulation in non-hematologic malignancies: phase 1 dose-escalation study. Investigational new drugs 32:1181–7, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkatakrishnan K, Zhou X, Ecsedy J, et al. : Dose selection for the investigational anticancer agent alisertib (MLN8237): Pharmacokinetics, pharmacodynamics, and exposure-safety relationships. J Clin Pharmacol 55:336–47, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Matulonis UA, Sharma S, Ghamande S, et al. : Phase II study of MLN8237 (alisertib), an investigational Aurora A kinase inhibitor, in patients with platinum-resistant or -refractory epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. Gynecologic oncology 127:63–9, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Goldberg SL, Fenaux P, Craig MD, et al. : An exploratory phase 2 study of investigational Aurora A kinase inhibitor alisertib (MLN8237) in acute myelogenous leukemia and myelodysplastic syndromes. Leukemia research reports 3:58–61, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedberg JW, Mahadevan D, Cebula E, et al. : Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 32:44–50, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carol H, Boehm I, Reynolds CP, et al. : Efficacy and pharmacokinetic/pharmacodynamic evaluation of the Aurora kinase A inhibitor MLN8237 against preclinical models of pediatric cancer. Cancer chemotherapy and pharmacology 68:1291–304, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maris JM, Morton CL, Gorlick R, et al. : Initial testing of the aurora kinase a inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP). Pediatr Blood Cancer, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michaelis M, Selt F, Rothweiler F, et al. : Aurora kinases as targets in drug-resistant neuroblastoma cells. PloS one 9:e108758, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brockmann M, Poon E, Berry T, et al. : Small molecule inhibitors of aurora-a induce proteasomal degradation of N-myc in childhood neuroblastoma. Cancer cell 24:75–89, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosse YP, Lipsitz E, Fox E, et al. : Pediatric phase I trial and pharmacokinetic study of MLN8237, an investigational oral selective small-molecule inhibitor of Aurora kinase A: a Children’s Oncology Group Phase I Consortium study. Clinical cancer research : an official journal of the American Association for Cancer Research 18:6058–64, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–47, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Ady N, Zucker JM, Asselain B, et al. : A new 123I-MIBG whole body scan scoring method--application to the prediction of the response of metastases to induction chemotherapy in stage IV neuroblastoma. European journal of cancer 31A:256–61, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Cheson BD, Bennett JM, Kopecky KJ, et al. : Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 21:4642–9, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Lipsitz E, Moorthy G, Mosse Y, et al. : A sensitive and selective liquid chromatography/tandem mass spectrometry method for determination of MLN8237 in human plasma. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 878:2369–73, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akaba K, Kimura T, Sasaki A, et al. : Neonatal hyperbilirubinemia and mutation of the bilirubin uridine diphosphate-glucuronosyltransferase gene: a common missense mutation among Japanese, Koreans and Chinese. Biochem Mol Biol Int 46:21–6, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Houghton PJ, Morton CL, Tucker C, et al. : The pediatric preclinical testing program: description of models and early testing results. Pediatr Blood Cancer 49:928–40, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Byrne AT, Alferez DG, Amant F, et al. : Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer 17:254–268, 2017 [DOI] [PubMed] [Google Scholar]
- 39.DuBois SG, Marachelian A, Fox E, et al. : Phase I Study of the Aurora A Kinase Inhibitor Alisertib in Combination With Irinotecan and Temozolomide for Patients With Relapsed or Refractory Neuroblastoma: A NANT (New Approaches to Neuroblastoma Therapy) Trial. J Clin Oncol 34:1368–75, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubois SG, Mosse YP, Fox E, et al. : Phase 2 Trial of Alisertib in Combination with Irinotecan and Temozolomide for Patients with Relapsed or Refractory Neuroblastoma. Clin Cancer Res, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ham J, Costa C, Sano R, et al. : Exploitation of the Apoptosis-Primed State of MYCN-Amplified Neuroblastoma to Develop a Potent and Specific Targeted Therapy Combination. Cancer Cell 29:159–72, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.