Abstract
Mutations in GMPPB cause a wide spectrum of neuromuscular syndromes, including muscular dystrophies and congenital myasthenic syndrome. The mechanisms by which GMPPB mutations impair neuromuscular transmission however remain incompletely understood. We expand here upon a previous report of one such patient presenting with a myopathy-congenital myasthenic syndrome overlap phenotype. Fatigable proximal muscle weakness developed gradually between 13 and 25 years of age, with subsequent stabilization. Low-frequency repetitive nerve stimulation showed a decrement, while a muscle biopsy demonstrated the presence of a centronuclear myopathy. Genetic testing identified a homozygous c.458C>T (p.Thr153Ile) variant in GMPPB. In-vitro microelectrode recordings and ultrastructural studies showed impairment of both pre- and postsynaptic neuromuscular transmission, thus demonstrating the presence of not only postsynaptic, but also presynaptic pathology in GMPPB-related disorders.
Keywords: centronuclear myopathy, congenital myasthenic syndrome, neuromuscular junction, GMPPB, glycosylation
Introduction
Mutations in guanosine diphosphate mannose pyrophosphorylase B (GMPPB) have been found to cause a wide spectrum of neuromuscular syndromes, including congenital muscular dystrophy, limb girdle muscular dystrophy with or without cognitive impairment, recurrent rhabdomyolysis and congenital myasthenic syndrome (CMS) [1–3]. Some patients also present with myopathy-CMS overlap phenotypes [2]. Like other CMS caused by defects of glycosylation, GMPPB-CMS typically affects limb girdle muscles, without significant ocular or bulbar weakness [4]. The mechanisms by which GMPPB mutations impair neuromuscular transmission however remain incompletely understood. We expand here upon a patient we previously reported in this journal with a centronuclear myopathy and associated defect of neuromuscular transmission [5] in whom additional genetic testing recently identified a pathogenic variant in GMPPB. We thus seek to demonstrate the presence of alterations of both pre- and postsynaptic neuromuscular transmission in GMPPB-related disorders.
Case report
We summarize here the clinical and laboratory studies that were previously reported [5]. The patient, now 47 years old, is a man of Hispanic descent. His parents were fourth-degree cousins. The family history was notable for epilepsy in a sister, but there was no history of other relatives with neuromuscular disease. He was the product of an uneventful pregnancy and had normal motor development. In childhood, his athletic performance was on par with his peers.
At 13 years of age, he began developing proximal upper and lower limb weakness. His symptoms progressed over the following decade, before plateauing by age 25. Subsequently, he experienced intermittent exacerbations of the weakness that occurred several times yearly, lasting between one and 4 weeks. He also reported exertional myalgias. He did not describe fatigable weakness and denied ptosis, diplopia or bulbar weakness.
Neurological examination revealed mild to moderate proximal weakness of the upper and lower limb muscles (3/5 to 4/5 on the Medical Research Council scale), along with mild weakness of some distal muscles. Fatigability was present in proximal muscles. There was mild asymmetric enlargement of the calf muscles. There was also mild rigidity of the spine, pes cavus and contractures of the wrists and ankles. Facial weakness, ptosis and ophthalmoparesis were absent.
The creatine kinase (CK) level was 878 U/L (normal ≤336 U/L). Two Hz repetitive nerve stimulation (RNS) revealed a 19% and 35% decrement in the musculocutaneous and spinal accessory nerves, respectively. Facilitation was not observed. Edrophonium resulted in a modest reduction of the decrement in the trapezius (to 23%), while 3,4-diaminopyridine (3,4-DAP) increased the baseline compound muscle action potential amplitude by 31% and reduced the decrement to 13%. Needle examination showed early recruitment of short duration unstable motor unit potentials in proximal muscles. Tests for acetylcholine receptor and muscle-specific kinase (MuSK) antibodies were negative.
A deltoid muscle biopsy demonstrated type 1 fiber predominance and a moderate increase in internalized nuclei. Biopsies of the intercostal and serratus anterior muscles showed features of centronuclear myopathy (Figure 1A–B). In vitro electrophysiological studies indicated both pre- and postsynaptic impairment of neuromuscular transmission (Table 1) [5]. The postsynaptic defect was demonstrated by miniature endplate potentials of reduced amplitude and prolonged decay time secondary to the expression of acetylcholine receptors expressing the γ subunit (γ-AChR) at the neuromuscular junction. The presynaptic defect of neuromuscular transmission was indicated by the decreased quantal content of endplate potentials due to a reduced number of quanta available for release [5]. Endplate ultrastructural studies demonstrated simplified junctional folds and dilated vesicles in the junctional sarcoplasm, with structurally normal nerve terminals (Table 1) [5]. A molecular defect in the genes known at that time to cause CMS or centronuclear myopathy was not identified.
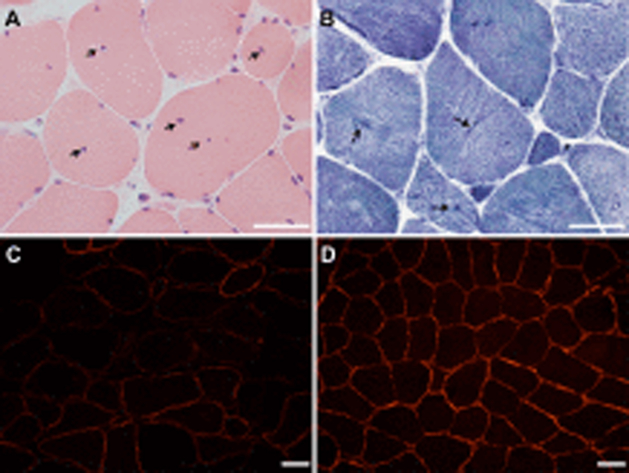
Figure 1. Intercostal muscle biopsy.
Central nucleation (arrows; A, hematoxylin and eosin stain) and radial arrangement of myofibrils (arrows; B, nicotinamide adenine dinucleotide stain), indicating the presence of a centronuclear myopathy. Patchy reduction of α-dystroglycan immunoreactivity in the patient’s muscle (C) compared to normal control muscle (D). Scale bar 50 μm in all panels.
Table 1.
Summary of in vitro electrophysiology, acetylcholine receptor (AChR) count and ultrastructure of endplate regions (EP-R) findings [5].
| Findings | |
|---|---|
| In vitro microelectrode recordings |
|
| Endplate AChR count |
|
| Endplate ultrastructure |
|
The EP-R was defined as the nerve terminal and associated postsynaptic region; one endplate may have more than one region [5]. ↑, increased; ↓, reduced; nl= normal.
The patient returned to the neuromuscular clinic almost a decade later. His neurological examination was largely unchanged. Despite the improvement in electrodiagnostic parameters, treatment with pyridostigmine and 3,4-DAP was clinically ineffective. A next generation sequencing gene panel targeting 122 known myopathy, muscular dystrophy and CMS genes was performed. This revealed a homozygous c.458C>T (p.Thr153Ile) variant in GMPPB. The frequency of this variant is 0.001% in the gnomAD database. This variant was previously reported to cause a limb-girdle muscular dystrophy in the homozygous state [6], while compound heterozygous individuals presented with recurrent rhabdomyolysis or congenital muscular dystrophy [1, 7]. In light of this finding, α-dystroglycan immunostaining was performed on the previously-obtained muscle biopsy and demonstrated patchy loss of reactivity (Figure 1C–D), as has previously been observed in GMPPB-related disorders [8].
Discussion
Our patient has evidence of a myopathy with histological features of centronuclear myopathy coexisting with a defect of neuromuscular transmission. The proximal weakness and its episodic exacerbations, the myalgias and the calf muscle enlargement we observed have also previously been noted in other patients with GMPPB-related disorders [4, 9]. However, while most GMPPB-myopathy patients show dystrophic features on muscle biopsy [2, 4], central nucleation has only rarely been observed [1].
In our patient, a neuromuscular transmission defect was clinically suggested by the presence fatigable muscle weakness and corroborated by the decremental response to low-frequency RNS. This finding is common in GMPPB-related disorders and frequently superimposed on a myopathy [2, 4]. The pathophysiology of synaptic dysfunction has not been fully elucidated. In our patient, the defect of neuromuscular transmission was further investigated by in vitro microelectrode recordings and endplate ultrastructural studies [5]. The impairment of neuromuscular transmission seen in GMPPB-related disorders had previously been attributed to impaired glycosylation of AChR subunits [4], however the finding of presynaptic dysfunction in our patient suggests the presence of additional pathophysiological mechanisms. One possible mechanism would be impaired glycosylation of agrin, a synaptic space proteoglycan causing a CMS with presynaptic impairment [10]. Indeed, decreased glycosylation of agrin was demonstrated in a mouse model of agrin-CMS [11], and could contribute to the mild facilitation observed on RNS in some patients with GMPPB-CMS [4]. Of note, simplified endplate junctional folds, as observed in our patient, have also been described in Fukuyama congenital muscular dystrophy, another secondary α-dystroglycanopathy [12], but no functional studies of the neuromuscular junction were performed in this disorder.
The p.Thr153Ile variant in GMPPB identified in our patient had not previously been associated with impaired neuromuscular transmission. While some correlations have emerged between genotype and phenotype severity in GMPPB-related disorders, the same genotype can result in a disorder with or without impaired neuromuscular transmission [9].
The combination of the newly-identified genetic defect and the previously-described morphologic and electrophysiologic findings in this patient provide insight into the pathophysiological underpinnings of GMPPB-related disorders. The mechanisms by which GMPPB mutations impair presynaptic neuromuscular transmission and cause centronuclear pathology require further investigation.
Funding:
AGE received research funding for this work from the National Institutes of Health [grant number R01NS109491], while MM received research funding from Mayo Clinic benefactors. The funding sources had no role in the study’s design, conduct or publication.
Footnotes
Declaration of interest: none
References
- [1].Cabrera-Serrano M, Ghaoui R, Ravenscroft G, Johnsen RD, Davis MR, Corbett A, et al. Expanding the phenotype of GMPPB mutations. Brain 2015;138:836–44. [DOI] [PubMed] [Google Scholar]
- [2].Belaya K, Rodriguez Cruz PM, Liu WW, Maxwell S, McGowan S, Farrugia ME, et al. Mutations in GMPPB cause congenital myasthenic syndrome and bridge myasthenic disorders with dystroglycanopathies. Brain 2015;138:2493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Carss KJ, Stevens E, Foley AR, Cirak S, Riemersma M, Torelli S, et al. Mutations in GDP-mannose pyrophosphorylase B cause congenital and limb-girdle muscular dystrophies associated with hypoglycosylation of alpha-dystroglycan. Am J Hum Genet 2013;93:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rodriguez Cruz PM, Belaya K, Basiri K, Sedghi M, Farrugia ME, Holton JL, et al. Clinical features of the myasthenic syndrome arising from mutations in GMPPB. J Neurol Neurosurg Psychiatry 2016;87:802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liewluck T, Shen XM, Milone M, Engel AG. Endplate structure and parameters of neuromuscular transmission in sporadic centronuclear myopathy associated with myasthenia. Neuromuscul Disord 2011;21:387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bergant G, Maver A, Lovrecic L, Cuturilo G, Hodzic A, Peterlin B. Comprehensive use of extended exome analysis improves diagnostic yield in rare disease: a retrospective survey in 1,059 cases. Genet Med 2018;20:303–12. [DOI] [PubMed] [Google Scholar]
- [7].Astrea G, Romano A, Angelini C, Antozzi CG, Barresi R, Battini R, et al. Broad phenotypic spectrum and genotype-phenotype correlations in GMPPB-related dystroglycanopathies: an Italian cross-sectional study. Orphanet J Rare Dis 2018;13:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Luo S, Cai S, Maxwell S, Yue D, Zhu W, Qiao K, et al. Novel mutations in the C-terminal region of GMPPB causing limb-girdle muscular dystrophy overlapping with congenital myasthenic syndrome. Neuromuscul Disord 2017;27:557–64. [DOI] [PubMed] [Google Scholar]
- [9].Montagnese F, Klupp E, Karampinos DC, Biskup S, Glaser D, Kirschke JS, et al. Two patients with GMPPB mutation: The overlapping phenotypes of limb-girdle myasthenic syndrome and limb-girdle muscular dystrophy dystroglycanopathy. Muscle Nerve 2017;56:334–40. [DOI] [PubMed] [Google Scholar]
- [10].Nicole S, Chaouch A, Torbergsen T, Bauche S, de Bruyckere E, Fontenille MJ, et al. Agrin mutations lead to a congenital myasthenic syndrome with distal muscle weakness and atrophy. Brain 2014;137:2429–43. [DOI] [PubMed] [Google Scholar]
- [11].Bogdanik LP, Burgess RW. A valid mouse model of AGRIN-associated congenital myasthenic syndrome. Hum Mol Genet 2011;20:4617–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Taniguchi M, Kurahashi H, Noguchi S, Fukudome T, Okinaga T, Tsukahara T, et al. Aberrant neuromuscular junctions and delayed terminal muscle fiber maturation in alpha-dystroglycanopathies. Hum Mol Genet 2006;15:1279–89. [DOI] [PubMed] [Google Scholar]