Abstract
While carbon dots (C-dots) have been extensively investigated pertaining to their fluorescent, phosphorescent, electrochemiluminescent, optoelectronic, and catalytic features, their inherent chemical exchange saturation transfer magnetic resonance imaging (CEST MRI) properties are unknown. By virtue of their hydrophilicity and abundant exchangeable protons of hydroxyl, amine, and amide anchored on the surface, we report here that C-dots can be adapted as effective diamagnetic CEST (diaCEST) MRI contrast agents. As a proof-of-concept demonstration, human glioma cells were labeled with liposomes with or without encapsulated C-dots and implanted in mouse brain. In vivo CEST MRI was able to clearly differentiate labeled cells from non-labeled cells. The present findings may encourage new applications of C-dots for in vivo imaging in deep tissues, which is currently not possible using conventional fluorescent (near-infrared) C-dots.
Keywords: carbon dots, cell labeling, CEST MRI, contrast agent, intracranial implantation
Carbon dots (C-dots),[1] defined as discrete quasi-spherical carbogenic nanoparticles of several nm in size,[2] are a new type of organic materials composed primarily of carbon, oxygen, and hydrogen. C-dots are considered to be more biocompatible than the more widely used heavy metal-based quantum dots,[3] with both displaying favorable distinctive properties pertaining to electrochemiluminescence,[4] optoelectronics,[5] catalysis,[6] and size-dependent fluorescence behavior.[7] Consequently, numerous nanosystems integrating C-dots with functional groups have been designed for pH imaging,[8] sensing of metal ions and molecules,[9] drug delivery,[10] and monitoring of hydrogel degradation,[11] all of which used fluorescence intensity as readout. However, noninvasive in vivo fluorescence imaging using C-dots or quantum dots is hampered by the limited light penetration depth (approximately 1–2 cm) even in the near-infrared spectrum.[12]
Unlike fluorescence imaging, magnetic resonance imaging (MRI) is able to acquire images of deeply seated organs. Two strategies have been pursued to adapt carbon dots for MRI contrast enhancement: 1) to incorporate GdIII or MnII in Cdots for T1 contrast enhancing,[13] and 2) to hybridize with iron oxide nanoparticles for .T2/T2* contrast enhancing.[14] Doping with paramagnetic metals, however, raises toxicity concerns,[15] while the C-dots/iron oxide hybrid produces an unfavorable negative contrast. Herein we propose to take advantage of the natural chemical exchange saturation transfer (CEST) MRI contrast properties of metal-free C-dots to generate contrast without the need for (super)paramagnetic labels.
CESTis a relatively new powerful MRI technique that can detect diamagnetic agents by their water exchangeable protons.[16] In a CEST study, exchangeable protons are magnetically tagged using a radiofrequency saturation pulse irradiated at their specific resonance frequencies, followed by exchange of the tagged protons with surrounding water to decrease the water MRI signal. As proton exchange occurs many times when a long saturation pulse is applied, the reduction of MRI signal is amplified over time, and as a result, low concentrations of agents (mm range) can be detected.[16] Many common functional groups, for instance, hydroxyl, amine, amide, guanidinium, and imine, contain exchangeable protons, making CEST MRI applicable to a broad spectrum of diamagnetic compounds such as sugars,[17] peptides and proteins,[18] amino acids,[19] nucleic acids,[20] and drugs.[21] Since hydrophilic C-dots have a surface with abundant exchangeable protons, we hypothesized that they are inherently CEST MRI detectable.
In a typical synthesis, arginine-modified C-dots (AC-dots) were prepared by simple microwave irradiation (Figure 1A, see the Supporting Information for details). Matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy (MALDI-TOF MS) was used to assess the purity of AC-dots (Supporting Information, Figure S1). Arginine was used as a dopant to improve the production yield of carbon dots and as a contributor to the diaCEST contrast.[19a,22] The AC-dots were single and spherical in shape, with a narrow size distribution with a mean size of 4.7 nm, as characterized by transmission electron microscopy (TEM), atomic force microscopy (AFM), and dynamic light scattering (DLS; Figure 1B and Supporting Information, Figures S2 and S3). ξ potential measurements of the AC-dots in PBS produced a value of −8.0 mV, suggesting the presence of carboxyl groups on surfaces; however, positive charges induced by the guanidinium group of arginine can also be expected. This was confirmed by X-ray photoelectron spectroscopy (XPS). In the full-scan XPS spectrum, peaks related to C1s, N1s, and O1s were identified, with an atomic ratio of 63%:12%:25% (Figure 1C). A high-resolution N1s spectrum consisted of two individual spectra with peaks centered at 399.4 and 400.0 eV, corresponding to −NH2 and C–NH–C, respectively,[23] is shown in Figure 1E. Similarly, the C1s spectrum (Figure 1D) was deconvoluted into four peaks centered at 284.8, 286.1, 287.8, and 289.0 eV, corresponding to C=C, C–O/C–N, C=O/C=N, and COOH, respectively. The O1s spectrum was fitted with two peaks centered at 530.8 and 532.2 eV, corresponding to CO-C/C-OH and C=O, respectively (Figure 1F).[24] Fourier transform infrared (FTIR) spectroscopy (Supporting Information, Figure S4) further supports the co-existence of carboxyl and guanidinium with the observation of an intense peak at 1600 cm−1 with respect to stretching vibration of C=O and a broad peak at 3065 cm−1 relative to N–H absorption. The absorption band at 1666 cm−1 was attributed to stretching vibration of C=N in the guanidinium group.[25] The broad band around 3500 cm−1 furthermore indicates the presence of −OH. The UV/Vis and fluorescent emission spectra of the AC-dots solution are shown in Figure 1G. In the optical spectrum, there was a broad absorption band around 295 nm with increasing absorption towards 500 nm, which is typical of carbon dots.[26] The solution demonstrated multiple emissions from 430 to 550 nm when excited from 300 to 500 nm, with the highest efficiency at 470 nm upon excitation at 400 nm. This multi-color fluorescence is still characteristic of carbon dots, presumably caused by particles of different sizes and/or the existence of multiple emissive domains on each single particle.[27]
Figure 1.
Synthesis and characterization of AC-dots. A) Synthesis of AC-dots and surface modification with guanidinium and carboxyl groups. Note that the positive charge of the guanidinium cation is delocalized. B) TEM images of AC-dots. A picture of diluted AC-dots in solution is shown. C–F) Full-scan XPS profiles of AC-dots and highresolution spectra for C1s, N1s, and O1s with peak deconvolution. G) Optical spectrum and excitation wavelength-dependent fluorescence emission spectra of AC-dots in solution.
Owing to the location of exchangeable protons on the surface of AC-dots, we next studied its CEST MRI properties (Figures 2A–C). At pH 7.4, the Z-spectra show a more attenuated signal at the positive frequency offset when increasing the concentration of AC-dots from 0.5 to 10 mgmL−1, while the MTRasym plots indicate an increase in CEST signal for all concentrations. At 10 mgmL−1 of AC-dots, the maximum MTRasym intensity was observed at 2 ppm, consistent with the diaCEST contrast of the guanidinium protons of arginine (Supporting Information, Figure S5).[28] This observation also confirms the preservation of guanidinium groups from arginine on the carbon dots. By correlating the MTRasym intensity at 2 ppm with the concentration of AC-dots up to 5 mgmL−1, we obtained a linear relationship, that is, MTRasym(%, 2 ppm)=4.0 × [AC-dots], with a detection limit for the AC-dots less than 0.5 mgmL−1 (Figure 2D). The MTRasym signal at 2 ppm is also a function of saturation field strength (B1) and saturation time (tsat), as shown in Figure S6 in the Supporting Information. The pH-dependency of CEST contrast is shown in Figure 2E, with a MTRasym signal maximum at 1 ppm at pH 6.1 and 6.5. This is the result of the presence of surface hydroxyl groups, which is overtaken by the signal of guanidinium protons at 2 ppm at pH≥7.0. We also measured the effects of AC-dots on T1 and T2 relaxation (Figure 2F). They exhibited no enhanced T1 relaxation but did enhance T2 relaxation, which can be explained by the overall proton exchange shortening the water T2 relaxation times.[29] To demonstrate that the CEST effect is indeed an inherent feature of hydrophilic carbon dots, we prepared and characterized two other nitrogen-doped C-dots (See the Experimental Section and Figures S7 and S8 in the Supporting Information). Concentration-dependent diaCEST contrast were clearly observed for the two C-dots (Supporting Information, Figure S9), with a strong signal peak occurring at 1 ppm resulting from the large number of hydroxyl groups on the particle surfaces.
Figure 2.
The diaCEST MR properties of AC-dots. A) Illustration of AC-dots and surface modification of exchangeable protons in the guanidinium group (red-labeled) and the hydroxyl group (blue-labeled). B,C) Mass concentration-dependence of Z-spectra (B, relative water signal with and without saturation) and MTRasym spectra (C, asymmetry calculation of Z-spectra relative to water frequency at 0 ppm) for 0.5–10 mgmL−1 AC-dots at pH 7.4. D) MTRasym intensity at 2 ppm (CEST effect) as a function of AC-dots mass concentration. E) pH-dependence of MTRasym spectra of AC-dots (10 mgmL−1) in 10 mm PBS. F) Relaxation rates as a function of AC-dots mass concentration in 10 mM PBS (pH 7.4, 37°C) and calculated relaxivities. Data points are an average of three measurements. MR conditions: B1 =3.6 μT, tsat =3 s, and B0 =11.7 T.
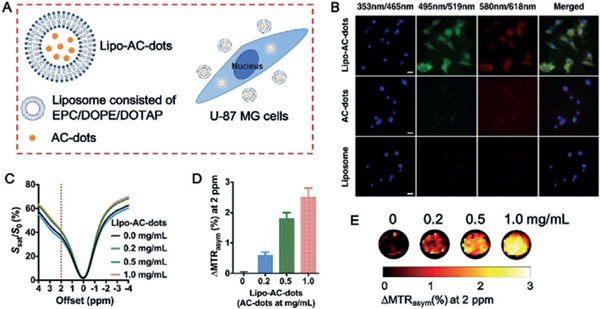
The inherent fluorescence of AC-dots allows the microscopic evaluation of cellular internalization as part of in vivo CEST MRI transplantation studies. No significant uptake of particles was observed after incubation with nonencapsulated AC-dots (Figure 3B), which can be expected given the negative surface charges of both the AC-dots and the cell membrane, leading to repulsion instead of binding and internalization. Several methods exist to increase the cellular uptake of nanoparticles,[30] including electroporation,[31] surface coating with peptides,[32] and liposome encapsulation.[33] We decided to use the third approach and prepared AC-dots encapsulated within liposomes (Lipo–AC-dots, see the Supporting Information for synthetic details). ξ potential measurements of the Lipo–AC-dots showed a positive value of +17 mV, which is beneficial for effective cell membrane binding followed by internalization (Figure 3A). Indeed, the presence of green and red fluorescence signal originating from the AC-dots inside cells could be clearly appreciated in cells labeled with Lipo–AC-dots, in contrast to cells labeled with AC-dots alone (Figure 3B). 3D cross-section and xy-section fluorescent images of the Lipo–AC-dots-labeled cells further confirmed the location of particles inside cells rather than on the cell membrane surface (Supporting Information, Figures S10 and S11). An approximately linear increase of fluorescence intensity was observed when the incubation time increased from 3 to 12 h (Supporting Information, Figure S12). After incubation for 12 h, the uptake of Lipo–AC-dots was 10, 22, and 36 pg AC-dots/cell relative to the addition of particles at 0.2, 0.5, and 1.0 mgmL−1, respectively (Supporting Information, Figure S13 and Table S1). In contrast, for the same concentration of direct AC-dots (1.0 mgmL−1), the cellular uptake was only 4 pg AC-dots/cell. Cytotoxicity experiments for both Lipo–AC-dots and AC-dots showed greater than 90% cell viability for all concentrations even up to 1.0 mgmL−1 after incubation for 24 h (Supporting Information, Figure S14), suggesting excellent biocompatibility of AC-dots regardless of liposome encapsulation.
Figure 3.
Liposome encapsulation of AC-dots and cellular uptake by human U-87 MG glioma cells. A) Schematic illustration of the cellular uptake of AC-dots following liposome encapsulation. B) Fluorescence imaging of cells after 6 h incubation with Lipo–AC-dots, AC-dots, or empty liposome. Nuclei are counterstained with DAPI. Scale bar=20 μm. C–E) CEST MRI measurements of cells labeled with Lipo–AC-dots at different concentrations (37°C, B0=11.7 T). Data in D: mean±SD (n=3).
We next evaluated the detectability of the Lipo–AC-dots-labeled cells by CEST MRI. Two million U-87 MG cells were incubated with different concentrations of Lipo–AC-dots for 12 h. Cells incubated with increasing concentrations of Lipo–AC-dots exhibited appreciably higher CEST contrast at 2 ppm (Figure 3C–E). It is important to note that the effect of liposome encapsulation on the CEST signal of AC-dots was negligible (Supporting Information, Figure S15).
Finally, we investigated the feasibility to detect Lipo–AC-dots-labeled cells transplanted in mouse brain in vivo using CEST MRI (Figure 4). Cells labeled with Lipo–AC-dots were injected into the left striatum, and cells labeled with empty liposomes were injected into the right side as a control. CEST MRI was performed at 24 hours after injection. The locations of implanted cells can be visualized on T2-weighted (T2w) images. As expected, the cells labeled with Lipo–AC-dots showed an overall higher but rather inhomogeneous CEST contrast enhancement at 2 ppm compared to the control cells (Figure 4B,C and Supporting Information, Figure S16). For all the five mice tested, the region-of-interest (ROI) analysis (Figure 4D) showed a significant increase of MTRasym intensity at 2 ppm by 28% for Lipo–AC-dots-labeled cells compared to liposome-labeled cells (mean MTRasym=6.4±0.3% and 5.0±0.6%, respectively; P=0.0019, two-tailed, paired Student’s t test). The CEST MRI findings were validated using ex vivo fluorescence imaging, which clearly shows the presence of AC-dots in the injected sites as evidenced by the green fluorescence emitted by the AC-dots. It should be noted that the CEST signal at 2 ppm is not specific enough to separate the CEST signal of AC-dots from the endogenous CEST background.[34]
Figure 4.
In vivo CEST MRI of mouse brain 1 day after implanting AC-dots-labeled U-87 MG cells in the striatum. A) Illustration of the preparation and implantation of Lipo–AC-dots-labeled cells, with empty-liposome-labeled cells as the control. B) T2-weighted image and the corresponding CEST image at 2 ppm of a mouse brain at 24 hours after the implantation. C) MTRasym plots of C-dots-labeled cells and control cells using manually drawn ROIs based on the T2w image. D) Comparison of CEST contrast at 2 ppm between Lipo–AC-dots-labeled cells and liposome-labeled cells. E) Ex vivo fluorescence imaging of the brain slice. Excitation wavelength=495 nm; emission wavelength=519 nm.
Considering their favorable size and biocompatibility, Cdots are promising diaCEST contrast agents for a wide array of biomedical applications, for example, tumor screening or even theranostics by cancer cell[35]- or tumor vasculature[36]specific markers. However, there are still a few technical considerations towards potential future studies. Firstly, given the presence of abundant in vivo endogenous proteins and metabolites whose CEST contrasts fall in the frequency range of 0–4 ppm,[16b] specificity of AC-dots (approximately 2 ppm) is relatively inefficient. Thus, it is imperative to design other C-dots with exchangeable protons that resonate downfield (larger frequency offsets). A post-synthetic surface modification would be a viable option, which is currently under investigation. Compared to the previously reported C-dot/ metal-hybrid nanoparticles,[13,14] our first generation AC-dots have a relatively low CEST detectability, attributed to the inherently inferior sensitivity of CEST MRI (mm range) than those of T1 or T2/T2* methods (mm range) and low doping rate of arginine per each particle. We will improve the sensitivity of C-dots in future studies by modification with more sensitive CEST probes (those with highly shifted chemical shifts and faster exchange rates) and optimizing the incorporation rates. Secondly, the in vivo pharmacokinetic properties, biodistribution, and toxicity of these CEST C-dots need to be investigated thoroughly in animal models, and the injection dose needs to be optimized accordingly. Thirdly, although this study was feasible at our preclinical MRI scanner operating at high magnetic field (11.7 T), it will be important to evaluate the CEST and relaxation properties of these C-dots on 3.0 T clinical scanners. Finally, it is still a challenge to reproducibly perform large-scale synthesis of C-dots in terms of particle size, because even tiny atomic variation could cause significantly distinct clearance pathways of nanoparticles.[37] Taken together, we foresee a vast potential for the C-dots to be used as a new group of CEST MRI contrast agents.
In summary, we have demonstrated that hydrophilic Cdots show appreciable CEST MRI contrast around 1–2 ppm offset from the water proton frequency, attributed to exchangeable hydroxyl and guanidinium protons on the particle surfaces. Using a liposome-mediated encapsulation method, human glioma cells were labeled with AC-dots, making them CEST-MRI-detectable both in vitro and in vivo after the intracranial implantation. Overall, this work may be further extended to the design and synthesis of CESTtraceable C-dots with new structures and functions, and we believe that this new exploitation of C-dots as CEST MRI contrast agents will revitalize them for in vivo imaging applications.
Supplementary Material
Acknowledgements
Financial support from the NIH grants R03 EB021573, R01 CA211087, R21 CA215860, R01 EB015032, R01 EB023647, R01 DK106972, R56 NS098520, and P41 EB024495 is acknowledged. We thank Dr. Patricia McGuiggan for generous help in performing AFM, Dr. Joel Tang for assistance in conducting FTIR, Dr. Katherine Tripp for help with the in vitro fluorescence measurements, Dr. Scott Kuo for invaluable suggestions, and Dr. Jiadi Xu for help with the mouse MRI experiment.
Footnotes
Supporting information and the ORCID identification number(s) for the author(s) of this article can be found under: https://doi.org/10.1002/anie.201904722.
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Jia Zhang, The Russell H. Morgan Department of Radiology and Radiological Science, Division of MR Research, The Johns Hopkins University School of Medicine, Baltimore, MD (USA).
Yue Yuan, The Russell H. Morgan Department of Radiology and Radiological Science, Division of MR Research, The Johns Hopkins University School of Medicine, Baltimore, MD (USA); Cellular Imaging Section and Vascular Biology Program, Institute for Cell Engineering, The Johns Hopkins University School of Medicine, Baltimore, MD (USA).
Minling Gao, Department of Neurology, The Johns Hopkins University School of Medicine, Baltimore, MD (USA).
Zheng Han, The Russell H. Morgan Department of Radiology and Radiological Science, Division of MR Research, The Johns Hopkins University School of Medicine, Baltimore, MD (USA).
Chengyan Chu, The Russell H. Morgan Department of Radiology and Radiological Science, Division of MR Research, The Johns Hopkins University School of Medicine, Baltimore, MD (USA); Cellular Imaging Section and Vascular Biology Program, Institute for Cell Engineering, The Johns Hopkins University School of Medicine, Baltimore, MD (USA).
Yuguo Li, The Russell H. Morgan Department of Radiology and Radiological Science, Division of MR Research, The Johns Hopkins University School of Medicine, Baltimore, MD (USA).
Peter C. M. van Zijl, The Russell H. Morgan Department of Radiology and Radiological Science, Division of MR Research, The Johns Hopkins University School of Medicine, Baltimore, MD (USA) F.M Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD (USA).
Mingyao Ying, Department of Neurology, The Johns Hopkins University School of Medicine, Baltimore, MD (USA)
Jeff W. M. Bulte, The Russell H. Morgan Department of Radiology and Radiological Science, Division of MR Research, The Johns Hopkins University School of Medicine, Baltimore, MD (USA) F.M Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD (USA); Cellular Imaging Section and Vascular Biology Program, Institute for Cell Engineering, The Johns Hopkins University School of Medicine, Baltimore, MD (USA).
Guanshu Liu, The Russell H. Morgan Department of Radiology and Radiological Science, Division of MR Research, The Johns Hopkins University School of Medicine, Baltimore, MD (USA); F.M Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD (USA).
References
- [1].Sun YP, Zhou B, Lin Y, Wang W, Fernando KA, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang H, Luo PG, Yang H, Kose ME, Chen B, Veca LM, Xie SY, J. Am. Chem. Soc 2006, 128, 7756–7757. [DOI] [PubMed] [Google Scholar]
- [2].Zheng XT, Ananthanarayanan A, Luo KQ, Chen P, Small 2015, 11, 1620–1636. [DOI] [PubMed] [Google Scholar]
- [3].Luo PG, Sahu S, Yang S-T, Sonkar SK, Wang J, Wang H, LeCroy GE, Cao L, Sun Y-P, J. Mater. Chem. B 2013, 1, 2116–2127. [DOI] [PubMed] [Google Scholar]
- [4].a) Zheng L, Chi Y, Dong Y, Lin J, Wang B, J. Am. Chem. Soc 2009, 131, 4564–4565; [DOI] [PubMed] [Google Scholar]; b) Zhao WW, Wang J, Zhu YC, Xu JJ, Chen HY, Anal. Chem 2015, 87, 9520–9531. [DOI] [PubMed] [Google Scholar]
- [5].a) Kwon W, Do S, Lee J, Hwang S, Kim JK, Rhee S-W, Chem. Mater 2013, 25, 1893–1899; [Google Scholar]; b) Chistyakov AA, Zvaigzne MA, Nikitenko VR, Tameev AR, Martynov IL, Prezhdo OV, J. Phys. Chem. Lett 2017, 8, 4129–4139. [DOI] [PubMed] [Google Scholar]
- [6].a) Martindale BC, Hutton GA, Caputo CA, Reisner E, J. Am. Chem. Soc 2015, 137, 6018–6025; [DOI] [PubMed] [Google Scholar]; b) Li X-B, Tung C-H, Wu L-Z, Nat. Rev. Chem 2018, 2, 160–173. [Google Scholar]
- [7].Li H, He X, Kang Z, Huang H, Liu Y, Liu J, Lian S, Tsang CH, Yang X, Lee ST, Angew. Chem. Int. Ed 2010, 49, 4430–4434; Angew. Chem. 2010, 122, 4532–4536. [DOI] [PubMed] [Google Scholar]
- [8].Shangguan J, He D, He X, Wang K, Xu F, Liu J, Tang J, Yang X, Huang J, Anal. Chem 2016, 88, 7837–7843. [DOI] [PubMed] [Google Scholar]
- [9].Zhang J, Yu S-H, Mater. Today 2016, 19, 382–393. [Google Scholar]
- [10].Fowley C, McHale AP, McCaughan B, Fraix A, Sortino S, Callan JF, Chem. Commun 2015, 51, 81–84. [DOI] [PubMed] [Google Scholar]
- [11].Wang L, Li B, Xu F, Li Y, Xu Z, Wei D, Feng Y, Wang Y, Jia D, Zhou Y, Biomaterials 2017, 145, 192–206. [DOI] [PubMed] [Google Scholar]
- [12].a) Antaris AL, Chen H, Cheng K, Sun Y, Hong G, Qu C, Diao S, Deng Z, Hu X, Zhang B, Zhang X, Yaghi OK, Alamparambil ZR, Hong X, Cheng Z, Dai H, Nat. Mater 2016, 15, 235–242; [DOI] [PubMed] [Google Scholar]; b) Chen Y, Montana DM, Wei H, Cordero JM, Schneider M, Le Guevel X, Chen O, Bruns OT, Bawendi MG, Nano Lett. 2017, 17, 6330–6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].a) Xu Y, Jia XH, Yin XB, He XW, Zhang YK, Anal. Chem 2014, 86, 12122–12129; [DOI] [PubMed] [Google Scholar]; b) Yao YY, Gedda G, Girma WM, Yen CL, Ling YC, Chang JY, ACS Appl. Mater. Interfaces 2017, 9, 13887–13899. [DOI] [PubMed] [Google Scholar]
- [14].a) Wang H, Shen J, Li Y, Wei Z, Cao G, Gai Z, Hong K, Banerjee P, Zhou S, Biomater. Sci 2014, 2, 915–923; [DOI] [PubMed] [Google Scholar]; b) Liu X, Jiang H, Ye J, Zhao C, Gao S, Wu C, Li C, Li J, Wang X, Adv. Funct. Mater 2016, 26, 8694–8706. [Google Scholar]
- [15].Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB, Lancet Neurol. 2017, 16, 564–570. [DOI] [PubMed] [Google Scholar]
- [16].a) Ward KM, Aletras AH, Balaban RS, J. Magn. Reson 2000, 143, 79–87; [DOI] [PubMed] [Google Scholar]; b) van Zijl PCM, Yadav NN, Magn. Reson. Med 2011, 65, 927–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].a) van Zijl PCM, Jones CK, Ren J, Malloy CR, Sherry AD, Proc. Natl. Acad. Sci. USA 2007, 104, 4359–4364; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Walker-Samuel S, Ramasawmy R, Torrealdea F, Rega M, Rajkumar V, Johnson SP, Richardson S, Goncalves M, Parkes HG, Arstad E, Thomas DL, Pedley RB, Lythgoe MF, Golay X, Nat. Med 2013, 19, 1067–1072; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Liu G, Banerjee SR, Yang X, Yadav N, Lisok A, Jablonska A, Xu J, Li Y, Pomper MG, van Zijl P, Nat. Biomed. Eng 2017, 1, 977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].a) Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC, Nat. Med 2003, 9, 1085–1090; [DOI] [PubMed] [Google Scholar]; b) Bar-Shir A, Liang Y, Chan KW, Gilad AA, Bulte JW, Chem. Commun 2015, 51, 4869–4871; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Song X, Airan RD, Arifin DR, Bar-Shir A, Kadayakkara DK, Liu G, Gilad AA, van Zijl PC, McMahon MT, Bulte JW, Nat. Commun 2015, 6, 6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].a) McMahon MT, Gilad AA, DeLiso MA, Berman SM, Bulte JW, van Zijl PC, Magn. Reson. Med 2008, 60, 803–812; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Cai K, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H, Detre JA, Reddy R, Nat. Med 2012, 18, 302–306; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Farrar CT, Buhrman JS, Liu G, Kleijn A, Lamfers ML, McMahon MT, Gilad AA, Fulci G, Radiology 2015, 275, 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].a) Snoussi K, Bulte JW, Gueron M, van Zijl PC, Magn. Reson. Med 2003, 49, 998–1005; [DOI] [PubMed] [Google Scholar]; b) Bar-Shir A, Liu G, Greenberg MM, Bulte JW, Gilad AA, Nat. Protoc 2013, 8, 2380– 2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].a) Li Y, Chen H, Xu J, Yadav NN, Chan KW, Luo L, McMahon MT, Vogelstein B, van Zijl PC, Zhou S, Liu G, Oncotarget 2016, 7, 6369–6378; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu H, Jablonska A, Li Y, Cao S, Liu D, Chen H, van Zijl PC, Bulte JW, Janowski M, Walczak P, Liu G, Theranostics 2016, 6, 1588–1600; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lock LL, Li Y, Mao X, Chen H, Staedtke V, Bai R, Ma W, Lin R, Li Y, Liu G, Cui H, ACS Nano 2017, 11, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Airan RD, Bar-Shir A, Liu G, Pelled G, McMahon MT, van Zijl PC, Bulte JW, Gilad AA, Magn. Reson. Med 2012, 68, 1919–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stevens JS, de Luca AC, Pelendritis M, Terenghi G, Downes S, Schroeder SLM, Surf. Interface Anal 2013, 45, 1238–1246. [Google Scholar]
- [24].Sun S, Zhang L, Jiang K, Wu A, Lin H, Chem. Mater 2016, 28, 8659–8668. [Google Scholar]
- [25].Guodong F, Mingming G, Qi L, Hongyu M, Guanghua L, Qiang M, Qiang F, Yanfu H, Zhiguang S, New J Chem. 2016, 40, 8444–8450. [Google Scholar]
- [26].Zhang J, Yuan Y, Liang G, Yu SH, Adv. Sci. 2015, 2, 1500002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Das SK, Liu Y, Yeom S, Kim DY, Richards CI, Nano Lett. 2014, 14, 620–625. [DOI] [PubMed] [Google Scholar]
- [28].Chan KW, Liu G, Song X, Kim H, Yu T, Arifin DR, Gilad AA, Hanes J, Walczak P, van Zijl PC, Bulte JW, McMahon MT, Nat. Mater. 2013, 12, 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].a) Zhang J, Li Y, Slania S, Yadav NN, Liu J, Wang R, Zhang J, Pomper MG, van Zijl PC, Yang X, Liu G, Chem. Eur. J. 2018, 24, 1259–1263; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yadav NN, Xu J, Bar-Shir A, Qin Q, Chan KW, Grgac K, Li W, McMahon MT, van Zijl PC, Magn. Reson. Med. 2014, 72, 823–828; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Gore JC, Brown MS, Mizumoto CT, Armitage IM, Magn. Reson. Med. 1986, 3, 463–466. [DOI] [PubMed] [Google Scholar]
- [30].Stewart MP, Sharei A, Ding X, Sahay G, Langer R, Jensen KF, Nature 2016, 538, 183–192. [DOI] [PubMed] [Google Scholar]
- [31].Walczak P, Kedziorek DA, Gilad AA, Lin S, Bulte JW, Magn. Reson. Med. 2005, 54, 769–774. [DOI] [PubMed] [Google Scholar]
- [32].Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, Weissleder R, Nat. Biotechnol. 2000, 18, 410–414. [DOI] [PubMed] [Google Scholar]
- [33].Bulte JW, Ma LD, Magin RL, Kamman RL, Hulstaert CE, Go KG, The TH, de Leij L, Magn. Reson. Med. 1993, 29, 32–37. [DOI] [PubMed] [Google Scholar]
- [34].Jin T, Wang P, Zong X, Kim SG, Neuroimage 2012, 59, 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhao M, Beauregard DA, Loizou L, Davletov B, Brindle KM, Nat. Med. 2001, 7, 1241–1244. [DOI] [PubMed] [Google Scholar]
- [36].Hatakeyama S, Sugihara K, Shibata TK, Nakayama J, Akama TO, Tamura N, Wong SM, Bobkov AA, Takano Y, Ohyama C, Fukuda M, Fukuda MN, Proc. Natl. Acad. Sci. USA 2011, 108, 19587–19592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Du B, Jiang X, Das A, Zhou Q, Yu M, Jin R, Zheng J, Nat. Nanotechnol. 2017, 12, 1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.