Abstract
Objective
Currently, the correlation between preoperative bilirubin level and overall survival (OS) remains poorly defined in respectable perihilar cholangiocarcinoma (CC). The objectives of the current study were to evaluate the outcomes of perihilar CC after resection and then to analyze factors influencing curative resection, tumor recurrence and OS.
Methods
115 patients with perihilar CC underwent surgical resection were retrospectively analyzed based on clinic characteristics, operative details, tumor recurrence and long-term survival data.
Results
The 1-, 3-, and 5-year OS rates after resection were 75.9%, 36.5%, 21.7%, whereas the corresponding tumor recurrence rates were 29.6%, 70.8%, 85.3%, respectively. Preoperative bilirubin level combined with liver resection, resection margin, vascular invasion and perineural invasion, lymph node metastasis and TNM stage were found to be correlated with OS and tumor recurrence. Multivariate analysis showed that preoperative bilirubin level together with resection margin, perineural invasion, and TNM stage were independent predictors of OS and tumor recurrence. Furthermore, preoperative bilirubin level was related with R0 resection, lymph node metastasis, TNM stage and postoperative liver function recovery.
Conclusion
Preoperative bilirubin level may effectively reflect the severity of perihilar CC and predict the OS and tumor recurrence after resection for perihilar CC patients.
Keywords: perihilar cholangiocarcinoma, surgical outcomes, predicting factors, bilirubin level, R0 resection
Background
Cholangiocarcinoma (CC), described as malignancy that arising from the biliary tract epithelia, is the second most common primary liver malignancy.1 CC can be divided into intrahepatic, perihilar, or distal CC according to the tumor location in the biliary tree. Perihilar CC is the most common CC, involving the hepatic duct bifurcation between the second-order bile ducts and the origin of cystic duct.2,3 Complete surgical resection remains the only hope for long-term survival in patients with perihilar CC.4 In the past 20 years, surgical management of perihilar CC has progressed due to improvement in surgical techniques and perioperative assessment, which offer possible chance of cure within acceptable morbidity and mortality rates.5 However, the rates of resectability and 5-year survival from previous reports vary widely, ranging from 28% to 94% and from 11% to 45%, respectively.4 Currently, there are still controversies in surgical procedure for perihilar CC, especially for types I and II perihilar CC. Furthermore, the necessity and method of preoperative biliary drainage to decompress biliary obstruction are still debated.6,7
The objectives of the current study were to primarily evaluate the outcomes of patients with perihilar CC who underwent surgical resection in our center and then analyze risk factors influencing curative resection, tumor recurrence and overall survival (OS).
Methods
Patients Population and Data Collection
From September 2006 to January 2015, 115 patients underwent surgical resection and pathologically proven perihilar CC were retrospectively reviewed in the First Affiliated Hospital of Nanjing Medical University, China (Supplementary Figure 1). The patients with mixed CC/hepatocellular carcinoma were excluded. Patients who died within post-operation 30 days were excluded (n=2). This study was approved by the institutional review boards of the hospital, and consent was obtained from every patient. This study was conducted in accordance with the Declaration of Helsinki. Patients’ records were anonymized and de-identified prior to analysis.
Demographic information including sex, age and clinical presentation were collected for each patient. Preoperative parameters including carbohydrate antigen19-9 (CA19-9), carcinoembryonic antigen (CEA) and preoperative bilirubin level (prior to any biliary intervention) were also recorded. Pathologic data included histologic grade, tumor size, TNM stage, resection margin, lymph node metastasis, vascular and perineural invasion were ascertained based on final pathologic assessment. Pathologic tumor staging was based on the TNM classification of American Joint Committee on Cancer (7th edition). Extent of bile duct involvement was typed according to the Bismuth-Corlette classification.8 Date of last follow-up, OS and tumor recurrence were collected for all patients.
Preoperative Evaluation and Surgical Procedures
The location and extent of the tumor, lymph nodes and distant metastasis, peritoneal seeding, Bismutch-Corlette type, remnant liver volume and resectability were evaluated according to computed tomography, or magnetic resonance. Patients with serum bilirubin above 12 mg/dl and need to combine liver resection routinely considered preoperative biliary drainage (PBD). Surgical procedures were decided according to preoperative Bismutch-Corlette type and intraoperative exploration. Overt distant metastasis, intrahepatic metastasis, or peritoneal seeding were defined as non-resectability.
Statistical Analysis
Continuous variables were expressed as mean SD or median (range). Categorical variables were expressed as number (percentage). The Student’s t-test and the χ2 test were used to analyze continuous or categorical variables, respectively. OS was defined as the interval between the date of liver resection and the date of death or last follow-up evaluation. Tumor recurrence is determined by pathological diagnosis or imaging examination (CT or MRI). OS and tumor recurrence rates were calculated using the log-rank test. Univariate and multivariate analysis of prognostic factors for survival and tumor recurrence were performed using Cox regression hazard model. Statistical calculations were performed using SPSS version 19.0 (Chicago, IL, USA). Statistical significance was defined as p value of <0.05.
Results
Demographic and Clinic-Pathologic Characteristics
A total of 115 patients who underwent surgical resection for perihilar CC and met the inclusion criteria were identified. Baseline characteristics of the population were presented in the Supplementary Table 1. The median age of this cohort was 60.4 years (range, 26–81 years) and male gender predominated (n=74, 64.3%). The average preoperative total bilirubin was 8.2±7.9 mg/dl whereas the number of bilirubin levels >3 mg/dL and >12 mg/dL were 56 (48.7%) and 28 (24.3%) respectively. The number of patients with CA19-9>39U/mL and carcinoembryonic antigen (CEA) >4.7 ng/mL were 56 and 24, respectively. The two main clinical symptoms were jaundice (48.7%) and epigastric pain (40%).
The clinicopathologic characteristics of patients were showed in Supplementary Table 2. Among them, there were about 82 (71.3%) patients with negative tumor margin. Vascular invasion was found in 7 patients (6.1%), while patients with lymph node metastasis and perineural invasion were 47.0% and 42.6%, respectively. Most tumors were TNM stage 2 or 3 (n = 37, 32.2% and n = 58, 50.4%, respectively). Among them, 47 patients were diagnosed as type IV, 17 as type IIIa and 27 as type IIIb while only 21 patients were diagnosed as type II and 3 as type I of perihilar CC.
Operative Procedure and Curability
The rates of R0 resection for the different surgical procedures in various types of perihilar CC were showed in Supplementary Table 3. 115 patients underwent resection and the rate of R0 resection was 71.3%. The R0 resection rate in types I and II was 100% and 71.4%, respectively. There was no statistical difference in R0 resection rate between type IIIa (82.4%) and type IIIb (74.1%) (P = 0.716). There was lower R0 resection rate in type IV (63.8%) than other types of tumor. In terms of the surgical procedures, the types I and II patients were more likely to undergo bile duct resection, whereas combined major hepatectomy were more common in types III and IV patients. The rate of R0 resection was higher in patients underwent combined liver resection than those underwent segmental bile duct resection in types III and IV patients (72% vs 55.6%).
Overall Survival and Prognostic Factors Analysis
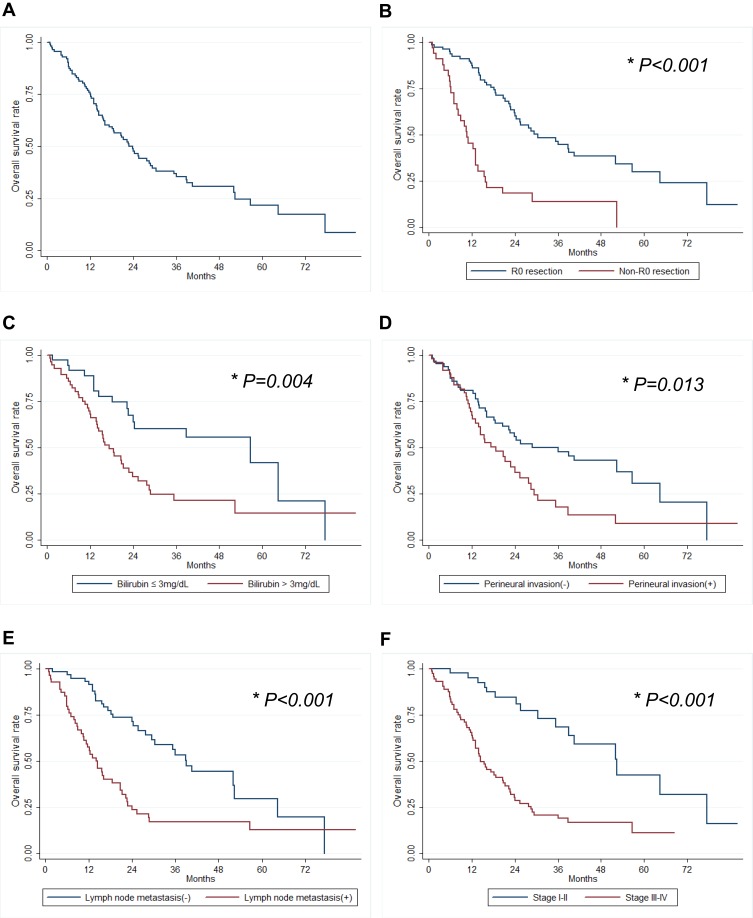
The 1-, 3- and 5-year OS rates were 75.9%, 36.5%, 21.7%, respectively (Figure 1A). Preoperative total bilirubin level combines liver resection, resection margin, vascular invasion and perineural invasion, lymph node metastasis, and TNM stage were found to be correlated with OS upon univariate analysis (Table 1). The 1-, 3- and 5-year OS rates of patients with R0 resection were 88.6%, 46.4%, 29.7%, while non-R0 resection was 45.5%, 13.4%, 0%, respectively (Figure 1B). Patients with low preoperative bilirubin level were found to have significantly better OS rate than patients with high preoperative bilirubin level (Figure 1C). Furthermore, the OS rate was significantly better for patients without vascular invasion (P=0.032) and perineural invasion (P=0.015), absence of lymph node metastasis (P < 0.001), and early TNM stage (P <0.001) (Figure 1D–F). The OS rates according to the Bismuth-Corlette classification were not found to show significant statistical difference. Patients who underwent combined liver resection had significantly better OS than patients without combined liver resection in types III and IV tumors (P=0.049). However, patients with combined caudate lobectomy during hepatectomy have similar OS compared to those without caudate lobectomy. Otherwise, there was no difference in OS observed in terms of CA19-9 level, tumor size and differentiation. In our multivariate analysis, preoperative high bilirubin level (HR 2.34, P=0.012), positive resection margin (HR 2.79, P= 0.003), perineural invasion (HR 2.42, P=0.008), and TNM stage (HR 4.67, P=0.013) were found to be independent prognostic factors of OS (Table 1).
Figure 1.
The overall survival rates in patients with perihilar CC. (A) Overall survival, (B) the status of resection margin (R0), (C) preoperative bilirubin level, (D) perineural invasion, (E) lymph node metastasis and (F) tumor stage (N= 115). *P<0.05.
Table 1.
Univariate and Multivariate Analysis for OS and Tumor Recurrence
| Characteristic | OS | Tumor Recurrence | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | |
| Age ≥60 | 0.163 | 1.39 (0.88–2.20) | 0.326 | 1.26 (0.79–.02) | ||||
| Sex (female) | 0.458 | 0.84 (0.52–1.34) | 0.385 | 0.81 (0.50–.31) | ||||
| PBD | 0.195 | 1.44 (0.83–2.48) | 0.089 | 1.60 (0.93–2.75) | ||||
| Bismuth type (III-IV vs I-II) |
0.185 | 1.50 (0.82–2.74) | 0.253 | 1.44 (0.77–2.68) | ||||
| Bilirubin (>3mg/dL) | 0.005 | 2.26 (1.28–4.01) | 0.002 | 2.55 (1.41–4.63) | ||||
| Bilirubin (>6mg/dL) | 0.005 | 2.13 (1.26–3.61) | 0.011 | 2.00 (1.17–3.41) | ||||
| Bilirubin (>12mg/dL) | <0.001 | 3.55 (2.04–~6.18) | 0.012 | 2.34 (1.21–4.54) | <0.001 | 3.29 (1.89–5.71) | 0.029 | 2.07 (1.08–3.97) |
| CEA ≥4.7 U/mL | 0.354 | 1.35 (0.71–2.57) | 0.686 | 1.14 (0.59–2.20) | ||||
| CA19-9≥39U/mL | 0.261 | 1.60 (0.70–3.65) | 0.333 | 1.54 (0.64–3.71) | ||||
| CL (types III and IV) | 0.709 | 1.15 (0.55–2.38) | 0.646 | 1.19 (0.57–2.46) | ||||
| Liver resection (types III and IV) |
0.049 | 0.45 (0.20–1.00) | 0.024 | 0.40 (0.18–0.88) | ||||
| Positive tumor margin | <0.001 | 3.57 (2.20–5.80) | 0.003 | 2.79 (1.42–5.49) | <0.001 | 3.29 (2.01–5.40) | <0.001 | 3.52 (1.74–7.14) |
| Tumor size ≥2 cm | 0.330 | 1.36 (0.73–2.55) | 0.249 | 1.45 (0.77–2.71) | ||||
| Differentiation | ||||||||
| Moderate vs well | 0.121 | 1.63 (0.88–3.02) | 0.206 | 1.49 (0.80–2.75) | ||||
| Poor vs well | 0.143 | 2.03 (0.79–5.26) | 0.488 | 1.45 (0.51–4.12) | ||||
| Vascular invasion | 0.032 | 2.35 (1.08–5.16) | 0.955 | 1.03 (0.32–3.29) | ||||
| Perineural invasion | 0.015 | 1.78 (1.12–2.83) | 0.008 | 2.42 (1.26–4.65) | 0.017 | 1.78 (1.11–2.87) | 0.001 | 3.31 (1.68–6.55) |
| Lymph node metastasis | <0.001 | 2.64 (1.65~–4.22) | <0.001 | 2.55 (1.57–4.12) | ||||
| TNM stage (III–IV vs I–II) | <0.001 | 3.85 (2.17–6.85) | 0.013 | 4.67 (1.38–15.87) | <0.001 | 3.49 (1.94–6.31) | 0.017 | 4.37 (1.30–14.71) |
Risk Factors Analysis for Perihilar CC Recurrence
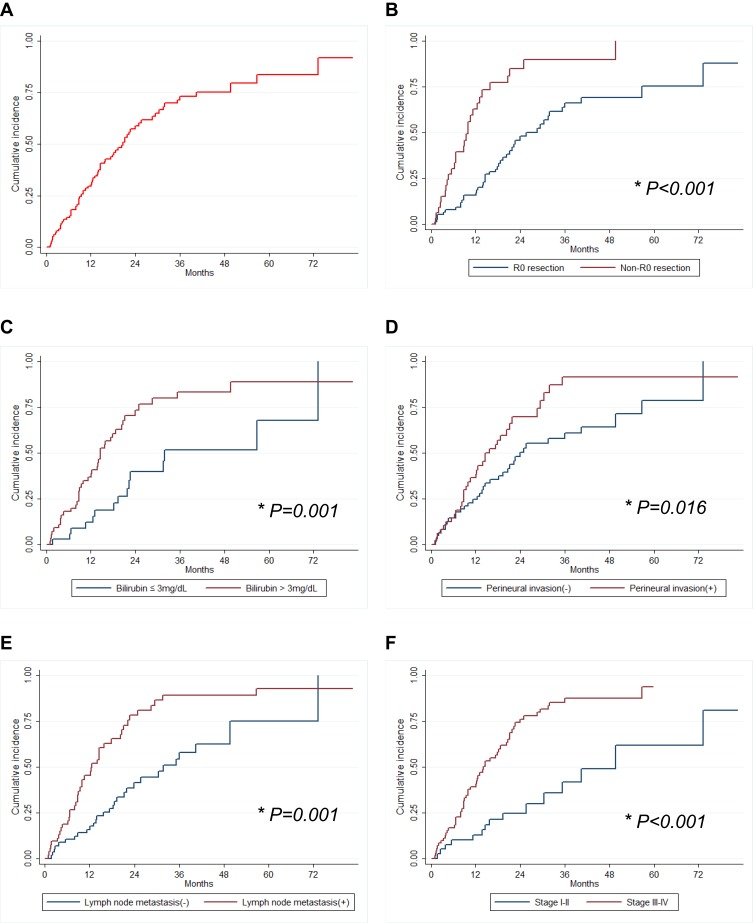
The 1-, 3- and 5-year tumor recurrence rates were 29.6%, 70.8%, 85.3%, respectively (Figure 2). The most common recurrence site was intrahepatic local recurrence (91.4%). From our univariate analysis, total bilirubin level combined with liver resection, resection margin, perineural invasion, lymph node metastasis, and TNM stage were associated with perihilar CC recurrence (Table 1). Preoperative high bilirubin level (HR 2.07, P=0.029) along with positive resection margin (HR 3.52, P <0.001), perineural invasion (HR 3.31, P=0.001), and late TNM stage (HR 4.37, P=0.017) were independent predictors of tumor recurrence (Table 1). Analysis was also performed focused on patients who developed early (within 1 year) recurrence (n=32). In the multivariate analysis, preoperative high bilirubin level (HR 3.42, P=0.007) and lymph node metastasis (HR 7.47, P=0.008) were independent predictors of early tumor recurrence after surgery (Table 2).
Figure 2.
Tumor recurrence rates in patients with perihilar CC. (A) Overall survival, (B) the status of resection margin (R0); (C) preoperative bilirubin level; (D) perineural invasion; (E) lymph node metastasis; and (F) tumor stage (N= 115). *P<0.05.
Table 2.
Risk Factor for Early Recurrence of Hilar CC (Within 1 Year)
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| P Value | HR (95% CI) | P Value | HR (95% CI) | |
| Bismuth types (III-IV vs I-II) | 0.432 | 1.47 (0.56–3.81) | ||
| Liver resection (type III or IV) | 0.141 | 0.45 (0.15–1.30) | ||
| Serum bilirubin (>3mg/dL) | 0.019 | 3.61 (1.23–10.57) | ||
| Serum bilirubin (>6mg/dL) | 0.008 | 3.50 (1.39–8.83) | ||
| Serum bilirubin (>12mg/dL) | <0.001 | 4.78 (2.08–10.91) | 0.007 | 3.42 (1.40–8.37) |
| Positive tumor margin | <0.001 | 5.12 (2.49–10.52) | ||
| Vascular invasion | 0.245 | 2.03 (0.62~–6.66) | ||
| Perineural invasion | 0.229 | 1.53 (0.76–3.07) | ||
| Lymph node metastasis | 0.002 | 3.34 (1.54–7.24) | 0.008 | 7.47 (1.71–32.63) |
| TNM stage (III–IV vs I–II) | 0.010 | 3.51 (1.35–9.13) | ||
Preoperative Bilirubin Level Was an Effectively Predictor for Prognostic Outcomes of Perihilar CC
On Univariate analysis, our results showed that preoperative bilirubin level was significantly associated with OS and tumor recurrence. Preoperative high bilirubin level (>12 mg/dl) was an independent predictors of OS and tumor recurrence after surgery (Table 1). The 1-, 3- and 5-year OS rates of patients with low preoperative bilirubin level were 87.5%, 46.9%, 37.5%, while high preoperative bilirubin level was 53.6%, 13.9%, 0%, respectively. Patients with high preoperative bilirubin level were found to have significantly higher early tumor recurrence than patients with lower preoperative bilirubin level. Our further analysis showed that high bilirubin level was related with lower R0 resection, more lymph node metastasis and advanced TNM stage. In turn, higher preoperative bilirubin level was found in those patients with R1 resection or lymph node metastasis or high TNM stage (Table 3). Furthermore, preoperative level of bilirubin was associated with postoperative liver function recovery (Supplementary Figure 2). However, the PBD did not affect OS and tumor recurrence after resection for perihilar CC patients.
Table 3.
Perioperative Bilirubin Level Was Related with Lymph Node Metastasis and R0 Resection
| Characteristic | Bilirubin Level (mg/dL) | Bilirubin Level (mg/dL) | ||||
|---|---|---|---|---|---|---|
| Mean±sd | P | ≤12 | >12 | P | ||
| R0 resection | Yes | 7.07±7.62 | 0.029 | 51 | 16 | 0.046 |
| No | 11.04±8.05 | 14 | 12 | |||
| Perineural invasion | No | 7.52±7.78 | 0.379 | 37 | 14 | 0.651 |
| Yes | 8.98±8.08 | 28 | 14 | |||
| Lymph node metastasis | No | 6.37±7.91 | 0.032 | 36 | 9 | 0.045 |
| Yes | 9.87±7.60 | 29 | 19 | |||
| TNM | I–II | 5.75±7.03 | 0.031 | 27 | 5 | 0.033 |
| III–IV | 9.46±8.09 | 38 | 23 | |||
Discussion
In this project, we analyzed risk factors influencing curative resection, tumor recurrence and OS of perihilar CC after liver surgery. Our results showed that lymph node metastasis, perineural invasion, high preoperative bilirubin level, combine liver resection and positive resection margin were important prognostic factors for perihilar CC after resection. In our cohort, 47.0% (54 of 115) of patients had lymph node metastasis, which had higher tumor recurrence rate and lower overall survival rate than those patients without lymph node metastasis. These results were similar with those reported in the literature.9,10,11 Aoba T et al research showed that the number of lymph node metastasis was an important prognostic factor in perihilar CC Italian multicenter analysis showed that an LNR (the number of positive lymph nodes divided by the total number of harvested nodes) exceeding 0.20 was the independent prognostic factor for OS in N1 perihilar CC patients.12 Furthermore, perineural invasion was also reported to be independent prognostic factor.13,14 In our study, 48 (42.3%) of patients have perineural invasion, which was confirmed as an independent prognostic factor of OS and tumor recurrence after surgery for perihilar CC patients.
Recently, more evidence showed that surgical procedure affected the outcomes of perihilar CC. Surgical radical excision (R0 resection) was still the only chance of a potential cure for perihilar CC.15,16 Our result also confirmed that positive resection margin was independent prognostic factor on tumor recurrence and OS after resection for perihilar CC. The 1-, 3- and 5-year OS rates of patients with R0 resection were 88.6%, 46.4%, 29.7%, while non-R0 resection were 45.5%, 13.4%, 0%, respectively. R0 and R1 resection resulted in early tumor recurrence rates of 15.0% and 60.6%, respectively. Furthermore, bile duct resection combined with hepatic resection has been widely accepted for the treatment of perihilar CC and can effectively increase the rate of R0 resection and long-term OS.16–18 In our study, we also demonstrated that combined with hepatectomy was found to be an important prognostic factor of OS and tumor recurrence for types III and IV perihilar CC. The above results suggested that to types III and IV tumors, combine with liver resection should be performed to achieve higher R0 resection and increase long-term OS. However, for types I and II perihilar CC, there were still controversies regarding whether major hepatic resection can improve survival. Some paper reported that single bile duct resection was enough for types I and II tumors and there was no significant difference in survival between combine with hepatectomy and bile duct resection alone.19 However, other data suggested that bile duct resection combined with hepatectomy in types I and II perihilar CC might contribute to the improvement of R0 resection and survival rate.20,21 In our study, all of 3 type I patients underwent to bile duct resection and the R0 resection rate was 100%. However, the rate of R0 resection in type II patients who underwent bile duct resection was only 68.4%, which significantly lower than the type II patients who underwent bile duct resection combined with hepatectomy. It was also even lower than types III and IV tumors. These results suggested that segmental bile duct resection should be offered only to type I patient, whereas bile duct resection-combined hepatectomy might contribute to the improvement of R0 resection and survival rate in type II perihilar CC. Due to the contiguity and close anatomical relationship between the caudate lobe and the perihilar bile duct, some centers suggested combined resection of the caudate lobe for the treatment of perihilar CC. Several studies showed that combined caudate lobectomy was an independent prognostic factor and increased the rate of R0 resection and OS21–24. However, our results showed that combined caudate lobectomy did not increase the rate of R0 resection and OS in the cohort. The possible reason was that most of types III and IV tumors underwent hepatectomy including caudate lobectomy.
Currently, the role and necessity of preoperative biliary drainage for types III and IV perihilar CC and the optimal preoperative bilirubin level is still a matter of debate.6,7,25 The morbidity and mortality after hepatectomy were higher in patients with obstructive jaundice than in patients with normal bilirubin levels.25 Multicenter European study also showed that high preoperative total bilirubin level (≥3mg/dL) was significantly associated with increased complications after major hepatectomy for perihilar CC.7 Therefore, PBD has been widely performed to reverse the cholestasis-associated risk of liver and renal failure after resection.26–28 There are still controversies regarding the necessity of PBD since it can be associated with an increase in procedure-related adverse events.29 A recently published meta-analysis revealed that PBD seems to be associated with higher postoperative morbidity and increases the risk of wound infections.30 Another multicenter retrospective study showed that PBD did not decrease postoperative morbidity and mortality in perihilar CC.7 Furthermore, PBD did not impact the long-term prognosis of perihilar CC after surgical resection. There was no significant difference in OS and tumor recurrence rates between the patients with or without underwent PBD.31 Currently, the correlation between preoperative bilirubin level and OS remains poorly defined. In this study, our results showed that patients with high preoperative bilirubin level was found to have lower OS and higher tumor recurrence rates than the patients with lower preoperative bilirubin level. Our further analysis also demonstrated that high bilirubin level was related with lower R0 resection, more lymph node metastasis and advanced TNM stage. The above results suggested that preoperative bilirubin level may effectively reflect the severity of perihilar CC and as an important prognostic factor after surgery for perihilar CC patients in some extent. Furthermore, the PBD could not rescue the negative effect of high preoperative bilirubin levels to long-term survival and tumor recurrence. The possible reason may be PBD do not change the existed fact of preoperative high bilirubin level although the bilirubin levels can be decreased after PBD.
In conclusion, complete surgical resection, including hepatic resection, can result in long-term survival in the patients with perihilar CC.Pathological factors such as advanced TNM stage, lymph node and perineural invasion were independent predictors of OS and tumor recurrence. Preoperative bilirubin level may effectively reflect the severity of perihilar CC and as an independent prognostic factors on OS and tumor recurrence after resection for perihilar CC patients.
Acknowledgments
This study was supported by National Science Foundation of China (NSFC) (81700572, 81670570), Natural Science Foundation of Jiangsu Province, China (BK20171077) and Key Research and Development Program of Jiangsu Province (BE2016789).
Abbreviations
CC, cholangiocarcinoma; OS, overall survival; CA19-9, antigen19-9; PBD, biliary drainage.
Ethics Approval and Consent to Participate
This study was approved by the institutional review boards of the First Affiliated Hospital, Nanjing Medical University and consent was obtained from every patient. This study was conducted in accordance with the Declaration of Helsinki. Patients’ records were anonymized and de-identified prior to analysis.
Data Sharing Statement
The datasets supporting the conclusions of this article are included within the article.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
There is no conflict of interest in this work.
References
- 1.El-Serag HB, Engels EA, Landgren O, et al. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: a population-based study of U.S. veterans. Hepatology. 2009;49(1):116–123. doi: 10.1002/hep.22606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–1229. doi: 10.1053/j.gastro.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245(5):755–762. doi: 10.1097/01.sla.0000251366.62632.d3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito F, Cho CS, Rikkers LF, Weber SM. Hilar cholangiocarcinoma: current management. Ann Surg. 2009;250(2):210–218. doi: 10.1097/SLA.0b013e3181afe0ab [DOI] [PubMed] [Google Scholar]
- 5.Igami T, Nishio H, Ebata T, et al. Surgical treatment of hilar cholangiocarcinoma in the “new era”: the Nagoya University experience. J Hepatobiliary Pancreat Sci. 2010;17(4):449–454. doi: 10.1007/s00534-009-0209-0 [DOI] [PubMed] [Google Scholar]
- 6.Kawakami H, Kuwatani M, Onodera M, et al. Endoscopic nasobiliary drainage is the most suitable preoperative biliary drainage method in the management of patients with hilar cholangiocarcinoma. J Gastroenterol. 2011;46(2):242–248. doi: 10.1007/s00535-010-0298-1 [DOI] [PubMed] [Google Scholar]
- 7.Farges O, Regimbeau JM, Fuks D, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg. 2013;100(2):274–283. doi: 10.1002/bjs.8950 [DOI] [PubMed] [Google Scholar]
- 8.Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140(2):170–178. [PubMed] [Google Scholar]
- 9.Nuzzo G, Giuliante F, Ardito F, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012;147(1):26–34. doi: 10.1001/archsurg.2011.771 [DOI] [PubMed] [Google Scholar]
- 10.Aoba T, Ebata T, Yokoyama Y, et al. Assessment of nodal status for perihilar cholangiocarcinoma: location, number, or ratio of involved nodes. Ann Surg. 2013;257(4):718–725. doi: 10.1097/SLA.0b013e3182822277 [DOI] [PubMed] [Google Scholar]
- 11.Bird NTE, McKenna A, Dodd J, Poston G, Jones R, Malik H. Meta-analysis of prognostic factors for overall survival in patients with resected hilar cholangiocarcinoma. Br J Surg. 2018;105(11):1408–1416. doi: 10.1002/bjs.2018.105.issue-11 [DOI] [PubMed] [Google Scholar]
- 12.Giuliante F, Ardito F, Guglielmi A, et al. Association of lymph node status with survival in patients after liver resection for hilar cholangiocarcinoma in an italian multicenter analysis. JAMA Surg. 2016;151(10):916–922. doi: 10.1001/jamasurg.2016.1769 [DOI] [PubMed] [Google Scholar]
- 13.Ercolani G, Zanello M, Grazi GL, et al. Changes in the surgical approach to hilar cholangiocarcinoma during an 18-year period in a Western single center. J Hepatobiliary Pancreat Sci. 2010;17(3):329–337. doi: 10.1007/s00534-009-0249-5 [DOI] [PubMed] [Google Scholar]
- 14.Cho MS, Kim SH, Park SW, et al. Surgical outcomes and predicting factors of curative resection in patients with hilar cholangiocarcinoma: 10-year single-institution experience. J Gastrointest Surg. 2012;16(9):1672–1679. doi: 10.1007/s11605-012-1960-0 [DOI] [PubMed] [Google Scholar]
- 15.Kondo S, Takada T, Miyazaki M, et al. Guidelines for the management of biliary tract and ampullary carcinomas: surgical treatment. J Hepatobiliary Pancreat Surg. 2008;15(1):41–54. doi: 10.1007/s00534-007-1279-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234(4):507–517; discussion 517–509. doi: 10.1097/00000658-200110000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nimura Y, Hayakawa N, Kamiya J, Kondo S, Shionoya S. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14(4):535–543; discussion 544. doi: 10.1007/BF01658686 [DOI] [PubMed] [Google Scholar]
- 18.Ito F, Agni R, Rettammel RJ, et al. Resection of hilar cholangiocarcinoma: concomitant liver resection decreases hepatic recurrence. Ann Surg. 2008;248(2):273–279. doi: 10.1097/SLA.0b013e31817f2bfd [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki M, Kimura F, Shimizu H, et al. Extensive hilar bile duct resection using a transhepatic approach for patients with hepatic hilar bile duct diseases. Am J Surg. 2008;196(1):125–129. doi: 10.1016/j.amjsurg.2007.04.020 [DOI] [PubMed] [Google Scholar]
- 20.Ikeyama T, Nagino M, Oda K, Ebata T, Nishio H, Nimura Y. Surgical approach to bismuth type I and II hilar cholangiocarcinomas: audit of 54 consecutive cases. Ann Surg. 2007;246(6):1052–1057. doi: 10.1097/SLA.0b013e318142d97e [DOI] [PubMed] [Google Scholar]
- 21.Song SC, Choi DW, Kow AW, et al. Surgical outcomes of 230 resected hilar cholangiocarcinoma in a single centre. ANZ J Surg. 2013;83(4):268–274. doi: 10.1111/j.1445-2197.2012.06195.x [DOI] [PubMed] [Google Scholar]
- 22.Liu CL, Fan ST, Lo CM, Tso WK, Lam CM, Wong J. Improved operative and survival outcomes of surgical treatment for hilar cholangiocarcinoma. Br J Surg. 2006;93(12):1488–1494. doi: 10.1002/(ISSN)1365-2168 [DOI] [PubMed] [Google Scholar]
- 23.Dinant S, Gerhards MF, Busch OR, Obertop H, Gouma DJ, Van Gulik TM. The importance of complete excision of the caudate lobe in resection of hilar cholangiocarcinoma. HPB (Oxford). 2005;7(4):263–267. doi: 10.1080/13651820500372376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsao JI, Nimura Y, Kamiya J, et al. Management of hilar cholangiocarcinoma: comparison of an American and a Japanese experience. Ann Surg. 2000;232(2):166–174. doi: 10.1097/00000658-200008000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paik WH, Loganathan N, Hwang JH. Preoperative biliary drainage in hilar cholangiocarcinoma: when and how? World J Gastrointest Endosc. 2014;6(3):68–73. doi: 10.4253/wjge.v6.i3.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SG, Lee YJ, Park KM, Hwang S, Min PC. One hundred and eleven liver resections for hilar bile duct cancer. J Hepatobiliary Pancreat Surg. 2000;7(2):135–141. doi: 10.1007/s005340050167 [DOI] [PubMed] [Google Scholar]
- 27.Nagino M, Takada T, Miyazaki M, et al. Preoperative biliary drainage for biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg. 2008;15(1):25–30. doi: 10.1007/s00534-007-1277-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Gaag NA, Kloek JJ, de Castro SM, Busch OR, van Gulik TM, Gouma DJ. Preoperative biliary drainage in patients with obstructive jaundice: history and current status. J Gastrointest Surg. 2009;13(4):814–820. doi: 10.1007/s11605-008-0618-4 [DOI] [PubMed] [Google Scholar]
- 29.Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T, et al. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2013;28(4):593–607. doi: 10.1111/jgh.2013.28.issue-4 [DOI] [PubMed] [Google Scholar]
- 30.Celotti A, Solaini L, Montori G, Coccolini F, Tognali D, Baiocchi G. Preoperative biliary drainage in hilar cholangiocarcinoma: systematic review and meta-analysis. Eur J Surg Oncol. 2017;43(9):1628–1635. doi: 10.1016/j.ejso.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 31.Zhang XF, Beal EW, Merath K, et al. Oncologic effects of preoperative biliary drainage in resectable hilar cholangiocarcinoma: percutaneous biliary drainage has no adverse effects on survival. J Surg Oncol. 2018;117(6):1267–1277. doi: 10.1002/jso.24945 [DOI] [PubMed] [Google Scholar]