Abstract
Stability and reactivity have been recognized as some critical issues for gold(I) catalysts. Such issues can be well-circumvented by anchoring the gold(I) complex onto the backbones of porous organic polymer (POP) followed by coordination with a triazole ligand as illustrated in the present work via a series of gold(I)-catalyzed reactions. In this strategy, 1,2,3-triazole was used as the special “X-factor” to avoid the formation of solid AgCl involved in typical gold-activation processes. The catalyst could be readily recycled without loss of reactivity. Moreover, compared with the PPh3-modified polystyrene beads, the POP support was advantageous by providing high surface area, hierarchical porosity, and better stabilization of cations. In some cases, significantly improved reactivity was observed, even more so than using the homogeneous system, which further highlighted the great potential of this heterogeneous gold catalyst.
Keywords: heterogeneous gold catalysis, porous organic polymer, triazole-gold catalyst, recyclable gold catalyst, alkyne activation
Graphical Abstract
Gold catalysis has flourished over the past decade, enabling access to a wide range of transformations. In particular, as an efficient carbophilic Lewis acid, gold(I) catalysts have facilitated numerous interesting transformations through the activation of alkynes, allenes, and alkenes.1 However, besides the cost of gold, the utilization of gold(I) catalysts for practical applications in chemical industry has been mainly impeded by two hurdles: (A) sensitivity of cationic gold(I) catalysts and (B) catalyst activation. As shown in Scheme 1A, catalytically active [L-Au]+ cation often suffers from poor stability and may decompose into gold colloids. In homogeneous gold(I) catalysis, stable L-Au-Cl complexes are typically applied as the catalyst precursor along with activation reagents, such as Ag+ cation2 or NaBArF4 salt.3 One effective method to engender enhanced stability and catalytic activity of gold(I) catalysts is through confining the active species into heterogeneous systems.4
Scheme 1.
Challenges in Solid-Supported Gold(I) Catalyst
In 2009, our group reported 1,2,3-triazole as a special dynamic ligand to form stable gold complexes (TA-Au).5 Using this new coordination strategy, Yu and co-workers first reported a solid-supported gold using a triazole-functionalized polystyrene resin to trap the [PPh3Au]+ cation (Scheme 1B).6 Although this catalyst showed good reactivity toward some interesting transformations (such as the enyne cyclization), it suffered from gold leaching each cycle due to the dynamic coordination of triazole ligand. To avoid this problem, subsequently, they reported another heterogeneous gold(I) catalyst with polystyrene-supported phosphine ligand using Ag+ as an activation reagent (Scheme 1B).7 Although this catalyst could catalyze several alkyne and allene activation reactions, significantly reduced reactivity was observed after each cycle, likely caused by gold decomposition and a potential influence from AgCl. Thus, the avoidance of AgCl waste in catalyst activation and the improvement of the stability of [L-Au]+ are essential toward the realization of highly efficient heterogeneous gold(I) catalysts.
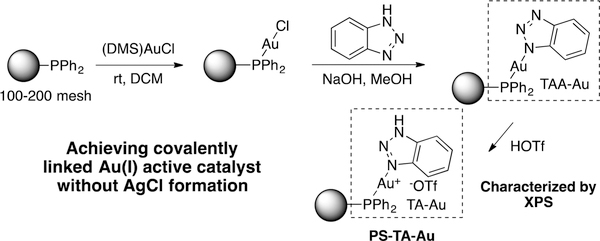
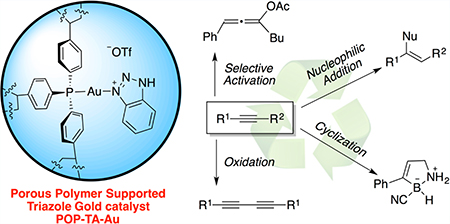
Our interest in developing a solid-supported gold(I) catalyst was initiated from the idea of using triazole as an activation strategy to access active gold(I) catalyst. As shown in Figure 1, NH-triazole can replace chloride anion under basic conditions. The resulting gold complex (triazole anion gold, TAA-Au) can be further activated through treatment with an acid, such as HOTf, forming the reactive cationic TA-Au catalyst. Using this strategy, a catalytically active solid-supported TA-Au complex can be easily prepared without the formation of solid AgCl during the process.
Figure 1.
Synthesis of PS-TA-Au.
The commercially available triphenylphosphine resin was first tested for this design and the PS-TA-Au was obtained in good yield. With this new catalyst in hand, we tested its reactivity toward phenyl acetylene hydration. Although the desired phenyl methyl ketone was formed at elevated temperature, significantly lower reactivity was observed with PS-TA-Au when comparing with the homogeneous TA-Au. More importantly, the formation of gold nanoparticles was observed with this gold complex using STEM (see SI). Though this 1,2,3-triazole activation strategy could avoid the formation of AgCl, developing a better solid support is necessary and crucial for achieving a practical heterogeneous gold(I) catalysis.
Porous materials could stabilize the corresponding metal complexes and provide a microenvironment for the substrates, which may lead to different reactivity compared with homogeneous catalysts.8 To tackle the aforementioned issues encountered for existing solid-support gold(I) catalysts, we propose that the gold(I) complex could be anchored into the backbones of porous organic polymers (POPs), followed by coordination with a triazole ligand.9
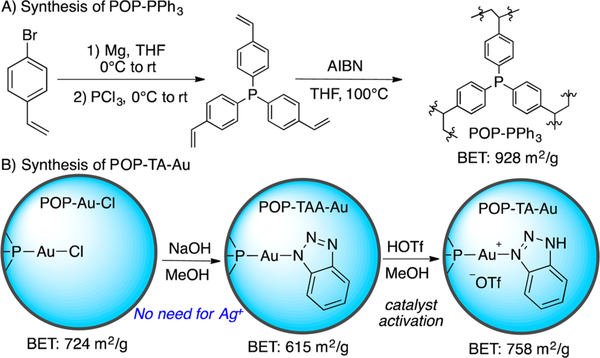
POPs represent an emerging class of porous materials possessing high surface areas, tunable pore sizes, and high water/chemical stability;10 they have been intensively investigated for potential applications in gas storage/separation, carbon capture,11 contaminant removal,12 catalysis, and so on.13 Considering the broad application of phosphine ligands in gold catalysis, we envision that the porous organic polymer (POP) synthesized from polymerization of tris(4-vinylphenyl)-phosphine (POP–PPh3), featuring excellent spatial continuity of PPh3 moieties, large surface, and hierarchical porosity, may offer a new opportunity for the development of novel heterogeneous gold catalysts with improved reactivity and catalyst stability.14 To test our hypotheses, we anchored triazole-gold(I) complex on POP–PPh3 (POP-TA-Au), as shown in Figure 2.
Figure 2.
Synthesis of heterogeneous gold(I) complex.
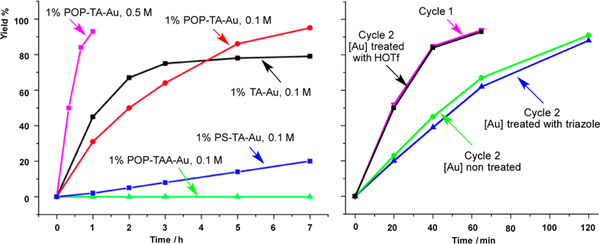
With this heterogeneous catalyst POP-TA-Au in hand, we explored its reactivity toward the Teles hydration reaction.15 Initial studies revealed that the employment of POP–PPh3 as a modification ligand has a profound effect on the catalyst durability. As shown in Figure 3, POP-TA-Au demonstrated impressive reactivity with similar reaction kinetics to the homogeneous TA-Au catalyst. Catalysts with different phosphine ligand and gold ratio (P: Au) were prepared and examined. With higher P:Au (2:1), gold decomposition was observed over time, suggesting that not all P atoms in POP are accessible. The POP-TA-Au with 5:1 P:Au ratio gave the optimal results (see SI for details). The anionic triazole complex POP-TAA-Au gave almost no reaction without acid activation. Notably, a higher yield was obtained with POP-TA-Au (95%) over homogeneous catalyst TA-Au (80%) because of improved catalyst stability. Reactions at a higher concentration (0.5 M) resulted in a much faster rate with excellent yield (>90%).
Figure 3.
POP-TA-Au-catalyzed Teles hydration.
After confirming the good reactivity of POP-TA-Au, we tested its recyclability. As shown in Figure 3, a slower reaction rate was observed after separating the heterogeneous catalyst POP-TA-Au and subsequent resubmission to another portion of starting material (cycle 2), albeit good yield (>90%) was still obtained. This may be caused by two potential reasons: (A) the loss of triazole ligand, leading to catalyst decomposition, and (B) formation of POP-TAA-Au (loss of HOTf). To test our hypothesis, we treated the gold catalyst after the first cycle with either triazole or triflic acid, followed by washing with methanol to remove extra additives. To our delight, the catalyst treated with triflic acid was activated again and catalyzed the reaction in the same efficiency as the first cycle. The catalyst treated with triazole ligand did not show any improvement of reactivity, suggesting no significant triazole ligand loss from the polymer catalyst was occurring. With this simple reactivation strategy, the POP-TA-Au catalyst has been recovered and recycled seven times without any loss of reactivity. Hydration reactions of other representative alkynes were also tested with this POP-TA-Au, and the results are summarized in Table 1.
Table 1.
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| POP-TA-Au (recycles) |
|||||||||
| TA-Au | PS-TA-Au | C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | C-7 | |
| reaction A | 85% | 25% | 93% | 88% | 88% | 87% | 90% | 85% | 87% |
| reaction B | 45% | 15% | 91% | 85% | 80% | 81% | 58% | 54% | ND |
| reaction C | 90% | 10% | 90% | 85% | 89% | 81% | 83% | 82% | 91% |
| reaction D | 89% | 8% | 91% | 85% | 86% | 83% | 82% | 88% | 90% |
General conditions: 1 mol % POP-TA-Au, MeOH:H2O = 20:1(0.5 M).
Yields determined by GC.
For all four hydration reactions that were tested, the POP-TA-Au gave better yields than both homogeneous TA-Au catalysts and the surface-modified PS-TA-Au. Hydration of propargyl alcohol and propargyl acetate gave enone in excellent yields (reactions C and D). With the less-reactive internal alkyne (reaction B), higher temperature (80 °C) and longer reaction time (12 h) are needed for 100% conversion. Excellent yields were obtained for the first four cycles. Catalyst decomposition was observed after five cycles. However, compared with homogeneous catalyst TA-Au, this porous polymer-supported gold catalyst gave significantly better reactivity and excellent recyclability. The POP-TA-Au catalyst was also successfully recycled in gram-scale reactions (see SI for details). To fully evaluate the potential of this new heterogeneous catalyst, we explored other gold-catalyzed reactions, especially the challenging examples that are difficult to achieve using common gold catalysts.
Chemoselectivity
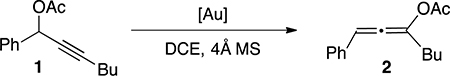
Gold(I) cation can effectively activate both alkyne and allene.16 A unique reactivity of triazole-gold catalyst (TA-Au) was the excellent chemoselectivity, activating alkyne over allene.17 Thus, treating propargyl ester with TA-Au can give the desired allene with excellent isolated yield. To test whether POP-TA-Au still maintain this chemoselectivity, we treated propargyl alcohol 1 with this heterogeneous catalyst in dry DCE (Table 2). At room temperature, no reaction proceeded (comparing with 95% yield for homogeneous TA-Au). Raising the temperature to 60 °C, allene acetate 2 was obtained in 92% yield without further activation of the allene product (formation of indene).18 This result confirmed the good chemoselectivity of TA-Au in this heterogeneous catalytic system.
Table 2.
 | |||||
|---|---|---|---|---|---|
| yield of 2 |
|||||
| conditions | time | conversion | cycle 1 | cycle 2 | cycle 3 |
| 1% TA-Au (homogeneous), rt | 2 h | 100% | 95% | ||
| 1% POP-TA-Au, rt | 24 h | <5% | ND | ||
| 1% POP-TA-Au, 60 °C | 2 h | 100% | 92% | 80%c | 55% (70%)d |
General conditions: 1 mol % gold catalyst, dry DCE (0.01 M).
Yields determined by GC.
16 h.
24 h, 70% conversion of propargyl acetate.
Hydroboration: Preventing Gold from Decomposition
One interesting transformation recently developed by our group is the TA-Au catalyzed hydroboration.19 Although cationic gold is a highly reactive π-acid, it suffers greatly from poor stability, especially with the presence of a reductant, which will cause the formation of Au(0). The discovery of TA-Au revealed a new class of gold catalyst with improved stability. However, when conducting the hydroboration reaction shown in Table 3, Ph3P-TA-Au gave only 30% yield even with 10% catalyst loading. This was clearly due to the decomposition of gold (54% conversion) under this reductive environment. To ensure high conversion, much more expensive XPhos-TA-Au has to be applied.
Table 3.
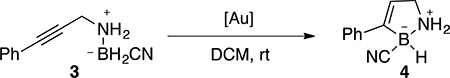 | |||||||
|---|---|---|---|---|---|---|---|
| yield (%) |
|||||||
| conditions | C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | C-7 |
| 10% [PPh3Au(TA)]OTf, 2 h | 30 | ||||||
| 10% [XPhosAu(TA)]OTf, 12 h | 75 | ||||||
| 10% POP-TA-Au, 6 h | 79 | 71 | 69 | 70 | 68 | 68 | 65 |
General conditions: 10 mol % gold catalyst, DCM (0.05 M).
Yields determined by H NMR.
To our delight, the heterogeneous POP-TA-Au catalyst could promote this transformation with good efficiency, even when only a simple PPh3 primary ligand was used. Compared with the homogeneous TA-Au catalyst (decomposed before full conversion of starting material), this newly developed heterogeneous gold(I) catalyst showed much better performance (79% yield in 6 h), likely because of the improved stabilization of the gold cation by the porous scaffold. Moreover, this POP-TA-Au could be reactivated simply by treating with triflic acid between each cycle. With this strategy, this catalyst has been successfully recycled for more than seven times, which indicated the potential for the application of this new catalyst into large-scale synthesis.
Improving TA-Au Reactivity through Lewis Acid Activation
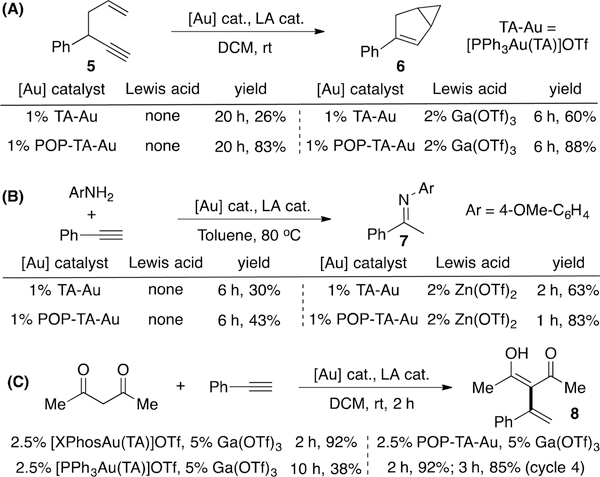
The discovery of triazole as a good ligand in gold coordination provides an opportunity to increase the stability of cationic gold catalyst. However, comparing with [L-Au]+, TA-Au has lower reactivity, which limits its application. From recent kinetic studies, we revealed that an associative ligand exchange between TA-Au and alkyne was the turnover-limiting step, which could be accelerated by Lewis acid (LA) through coordination with the TA ligand.20 On the basis of this strategy, the combination of LA and TA-Au was identified as a new catalytic system, holding both good stability and excellent reactivity. To test whether this strategy is operable in the POP-TA-Au catalysis, three representative reactions involving LA activation of TA-Au were performed: enyne cyclization/isomerization,21 alkyne hydroamination,5,22 and Nakamura reaction.23
Reaction of enyne 5 with TA-Au gave low yield (26%) without the assistance of LA. With the addition of 2% Ga(OTf)3, the desired product 6 was formed with a higher efficiency and yield (60%). Interestingly, using POP-TA-Au catalyst alone (no LA), compound 6 was obtained in 83% yield. The improved stability of the POP-TA-Au once again proved to be beneficial for challenging transformations. Furthermore, with the addition of LA, much faster reaction kinetics were observed as expected, as well as improved yield (88%, Figure 4A). Similarly, the addition of Lewis acid to POP-TA-Au could effectively facilitate alkyne hydroamination, giving improved yield with faster reaction kinetics (Figure 4B).
Figure 4.
Combination of POP-TA-Au and Lewis acid.
Recently, we reported the first successful example of gold-catalyzed intermolecular 1,3-diketone addition to alkyne (Nakamura reaction) using the combination of TA-Au and Ga(OTf)3.24 However, as shown in Figure 4C, to achieve a good yield, the expensive primary ligand XPhos was required. Significantly lower yield (38%) was obtained with PPh3 ligand. With simply polymerized PPh3 as the ligand (POP-TA-Au), excellent yield was achieved (92%). Catalyst recycling for hydroamination and Nakamura reaction were also performed (see SI).25 Even though the catalyst reactivity was lower after three cycles, better results might be achieved through further modification of the ligand, which is currently ongoing in our lab.
Gold Redox Catalysis
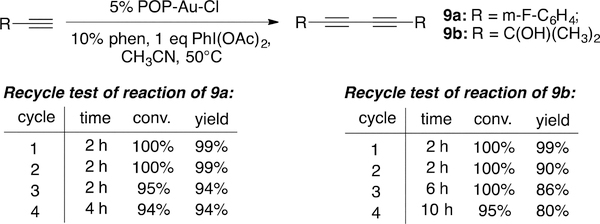
One of the most important and recent developments in gold catalysis is gold redox chemistry. Under proper conditions, Au(I) cation can be oxidized to Au(III) and give interesting new reactivity beyond the typical Au(I) π-acid activation.26 However, one major challenge is ligand oxidation with the presence of a strong oxidant. Moreover, the formation of phosphonium cation is one side reaction due to reductive elimination of substrate with the phosphine ligand from a reactive Au(III) intermediate.27 Considering all these potential problems, we were wondering whether POP-TA-Au is viable in promoting gold redox catalysis. The gold-catalyzed alkyne oxidative coupling was selected to evaluate this new heterogeneous catalyst.28 As shown in Figure 5, using POP-Au-Cl as the catalyst, the desired conjugated diynes 9a and 9b were prepared in excellent yields.29
Figure 5.
POP–Au-Cl-catalyzed oxidative alkyne coupling.
Although reduced reactivity was observed after four cycles, the fact that POP-Au could effectively promote this transformation clearly suggested that (A) the POP-Au system can survive in the presence of the strong oxidant phenyliodonium diacetate (PIDA), and (B) POP ligand is compatible with gold(III) reductive elimination process. This success certainly provides new opportunities for other challenging transformations using this POP-Au system.
In summary, we report herein an efficient porous polymer-supported triazole-gold catalyst (POP-TA-Au), which could be easily prepared from simple starting materials in high yields. In addition to the good chemoselectivity of this new heterogeneous triazole-gold catalyst, it also exhibited much better stability compared with homogeneous TA-Au. This possibly results from a microenvironment generated by the flexible porous structure of the POP–PPh3 ligand. As a recyclable gold catalyst, POP-TA-Au could be further applied into large-scale syntheses and flow reactions. The development of POPs using other phosphine ligands is currently ongoing in our lab to enrich the diversity of this type of heterogeneous gold catalyst, which will permit their extension within various applications and new reaction discovery.
Supplementary Material
ACKNOWLEDGMENTS
We thank the NIH (1R01GM120240-01), NSF (CHE-1362057), and NSFC (21629201) for financial support.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscatal.6b03211.
Experimental procedures and characterization data (PDF)
REFERENCES
- (1).For reviews of recent development of homogeneous gold catalysis:Hashmi ASK; Schwarz L; Choi JH; Frost TM Angew. Chem., Int. Ed 2000, 39, 2285–2288.Hashmi ASK; Hutchings GJ Angew. Chem., Int. Ed 2006, 45, 7896–7936.Gorin DJ; Toste FD Nature 2007, 446, 395–403.Garcia P; Malacria M; Aubert C; Gandon V; Fensterbank L ChemCatChem 2010, 2, 493–497.Hashmi ASK Angew. Chem., Int. Ed 2010, 49, 5232–5241.Sengupta S; Shi XD ChemCatChem 2010, 2, 609–619.Garayalde D; Nevado C ACS Catal. 2012, 2, 1462–1479.Wang YM; Lackner AD; Toste FD Acc. Chem. Res 2014, 47, 889–901.Dorel R; Echavarren AM Chem. Rev 2015, 115, 9028–9072.
- (2).(a) Wang D; Cai R; Sharma S; Jirak J; Thummanapelli SK; Akhmedov NG; Zhang H; Liu X; Petersen JL; Shi XJ Am. Chem. Soc 2012, 134, 9012–9019. [DOI] [PubMed] [Google Scholar]; (b) Kumar M; Hammond GB; Xu B Org. Lett 2014, 16, 3452–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Wang Z; Wang Y; Zhang LJ Am. Chem. Soc 2014, 136, 8887–8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).For selected examples of reported heterogeneous gold catalysis:Carrettin S; Blanco MC; Corma A; Hashmi ASK Adv. Synth. Catal 2006, 348, 1283–1288.García-Mota M; Cabello N; Maseras F; Echavarren AM; Perez-Ramírez J; Lopez N ChemPhysChem 2008, 9, 1624–1629.Hutchings GJ Top. Catal 2008, 48, 55–59.Neatu F; Li Z; Richards R; Toullec PY; Genêt J-P; Dumbuya K; Gottfried JM; Steinruck H-P; Pârvulescu VI; Michelet V Chem. - Eur. J 2008, 14, 9412–9418.Zhu F; Zhang F; Yang X; Huang J; Li HJ Mol. Catal. A: Chem 2011, 336, 1–7.Carriedo GA; López S; Suárez-Suárez S; Presa-Soto D; Presa-Soto A Eur. J. Inorg. Chem 2011, 2011, 1442–1447.Vaidya T; Cheng R; Carlsen PN; Frontier AJ; Eisenberg R Org. Lett 2014, 16, 800–803.Shu X.-z.; Nguyen SC; He Y; Oba F; Zhang Q; Canlas C; Somorjai GA; Alivisatos AP; Toste FD J. Am. Chem. Soc 2015, 137, 7083–7086.Rühling A; Galla H-J; Glorius F Chem. - Eur. J 2015, 21, 12291–12294.
- (5).Duan H; Sengupta S; Petersen JL; Akhmedov NG; Shi XJ Am. Chem. Soc 2009, 131, 12100–12102. [DOI] [PubMed] [Google Scholar]
- (6).Cao W; Yu B Adv. Synth. Catal 2011, 353, 1903–1907. [Google Scholar]
- (7).(a) Egi M; Azechi K; Akai S Adv. Synth. Catal 2011, 353, 287–290. [Google Scholar]; (b) Zhu Y; Laval S; Tang Y; Lian G; Yu B Asian J. Org. Chem 2015, 4, 1034–1039. [Google Scholar]
- (8).(a) Perego C; Millini R Chem. Soc. Rev 2013, 42, 3956–3976. [DOI] [PubMed] [Google Scholar]; (b) Du DY; Qin JS; Li SL; Su ZM; Lan YQ Chem. Soc. Rev 2014, 43, 4615–4632. [DOI] [PubMed] [Google Scholar]; (c) Umegaki T; Xu Q; Kojima Y Materials 2015, 8, 4512–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Qiu JH; He M; Jia MM; Yao JF Prog. Chem 2016, 28, 1016–1028. [Google Scholar]
- (9).For example of heterogeneous gold catalyst with POPs backbones, see:Wang W; Zheng A; Zhao P; Xia C; Li F ACS Catal. 2014, 4, 321–327.
- (10).(a) Slater AG; Cooper AI Science 2015, 348, aaa8075. [DOI] [PubMed] [Google Scholar]; (b) Kaur P; Hupp JT; Nguyen ST ACS Catal. 2011, 1, 819–835. [Google Scholar]; (c) Zhang YG; Riduan SN Chem. Soc. Rev 2012, 41, 2083–2094. [DOI] [PubMed] [Google Scholar]; (d) Xu Y; Jin S; Xu H; Nagai A; Jiang D Chem. Soc. Rev 2013, 42, 8012–8031. [DOI] [PubMed] [Google Scholar]; (e) Sun Q; Dai Z; Meng X; Xiao F-S Chem. Soc. Rev 2015, 44, 6018–6034. [DOI] [PubMed] [Google Scholar]
- (11).Sun L-B; Liu X-Q; Zhou H-C Chem. Soc. Rev 2015, 44, 5092–5147. [DOI] [PubMed] [Google Scholar]
- (12).(a) Li B; Zhang Y; Ma D; Shi Z; Ma S Nat. Commun 2014, 5, 5537–5545. [DOI] [PubMed] [Google Scholar]; (b) Yue Y; Mayes RT; Kim J; Fulvio PF; Sun X-G; Tsouris C; Chen J; Brown S; Dai S Angew. Chem., Int. Ed 2013, 52, 13458–13462. [DOI] [PubMed] [Google Scholar]; (c) Alsbaiee A; Smith BJ; Xiao L; Ling Y; Helbling DE; Dichtel WR Nature 2016, 529, 190–194. [DOI] [PubMed] [Google Scholar]
- (13).(a) Fischer S; Schmidt J; Strauch P; Thomas A Angew. Chem., Int. Ed 2013, 52, 12174–12178. [DOI] [PubMed] [Google Scholar]; (b) Wang F; Mielby J; Richter FH; Wang G; Prieto G; Kasama T; Weidenthaler C; Bongard H-J; Kegnæs S; Furstner A; Schuth F Angew. Chem., Int. Ed 2014, 53, 8645–8648. [DOI] [PubMed] [Google Scholar]; (c) Zhao Q; Zhang P; Antonietti M; Yuan JJ Am. Chem. Soc 2012, 134, 11852–11855. [DOI] [PubMed] [Google Scholar]; (d) Du Y; Yang H; Whiteley JM; Wan S; Jin Y; Lee S-H; Zhang W Angew. Chem., Int. Ed 2016, 55, 1737–1741. [DOI] [PubMed] [Google Scholar]; (e) Ji G; Yang Z; Zhang H; Zhao Y; Yu B; Ma Z; Liu Z Angew. Chem., Int. Ed 2016, 55, 9685–9689. [DOI] [PubMed] [Google Scholar]; (f) Hausoul PJC; Broicher C; Vegliante R; Göb C; Palkovits R Angew. Chem., Int. Ed 2016, 55, 5597–5601. [DOI] [PubMed] [Google Scholar]; (g) Ding X; Han B-H Angew. Chem., Int. Ed 2015, 54, 6536–6539. [DOI] [PubMed] [Google Scholar]; (h) Xie Y; Wang T-T; Liu X-H; Zou K; Deng W-Q Nat. Commun 2013, 4, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Wu K; Guo J; Wang C Angew. Chem., Int. Ed 2016, 55, 6013–6017. [DOI] [PubMed] [Google Scholar]; (j) Sprick RS; Bonillo B; Clowes R; Guiglion P; Brownbill NJ; Slater BJ; Blanc F; Zwijnenburg MA; Adams DJ; Cooper AI Angew. Chem., Int. Ed 2016, 55, 1792–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Sun Q; Aguila B; Verma G; Liu X; Dai Z; Deng F; Meng X; Xiao F-S; Ma S Chem. 2016, 1, 628–639. [Google Scholar]
- (14).Sun Q; Jiang M; Shen Z; Jin Y; Pan S; Wang L; Meng X; Chen W; Ding Y; Li J; Xiao F-S Chem. Commun 2014, 50, 11844–11847. [DOI] [PubMed] [Google Scholar]
- (15).Teles JH; Brode S; Chabanas M Angew. Chem., Int. Ed 1998, 37, 1415–1418. [DOI] [PubMed] [Google Scholar]
- (16).Sherry BD; Toste FDJ Am. Chem. Soc 2004, 126, 15978–15979. [DOI] [PubMed] [Google Scholar]
- (17).(a) Wang D; Ye X; Shi X Org. Lett 2010, 12, 2088–2091. [DOI] [PubMed] [Google Scholar]; (b) Motika SE; Wang Q; Ye X; Shi X Org. Lett 2015, 17, 290–293. [DOI] [PubMed] [Google Scholar]; (c) Hosseyni S; Ding S; Su Y; Akhmedov NG; Shi X Chem. Commun 2016, 52, 296–299. [DOI] [PubMed] [Google Scholar]
- (18).A molecular sieve is necessary for preventing the hydration of allene, but it may have influenced the recycle of POP-TA-Au.
- (19).(a) Wang Q; Motika SE; Akhmedov NG; Petersen JL; Shi X Angew. Chem., Int. Ed 2014, 53, 5418–5422. [DOI] [PubMed] [Google Scholar]; (b) Motika SE; Wang Q; Akhmedov NG; Wojtas L; Shi X Angew. Chem., Int. Ed 2016, 55, 11582–11586. [DOI] [PubMed] [Google Scholar]
- (20).Xi Y; Wang Q; Su Y; Li M; Shi X Chem. Commun 2014, 50, 2158–2160. [DOI] [PubMed] [Google Scholar]
- (21).Luzung MR; Markham JP; Toste FD J. Am. Chem. Soc 2004, 126, 10858–10859. [DOI] [PubMed] [Google Scholar]
- (22).Hesp KD; Stradiotto MJ Am. Chem. Soc 2010, 132, 18026–18029. [DOI] [PubMed] [Google Scholar]
- (23).Nakamura M; Endo K; Nakamura EJ Am. Chem. Soc 2003, 125, 13002–13003. [DOI] [PubMed] [Google Scholar]
- (24).Xi Y; Wang D; Ye X; Akhmedov NG; Petersen JL; Shi X Org. Lett 2014, 16, 306–309. [DOI] [PubMed] [Google Scholar]
- (25).Nakamura reaction with 10 mmol scale was performed; see SI for details.
- (26).For reviews:Wegner HA; Auzias M Angew. Chem., Int. Ed 2011, 50, 8236–8247.Hopkinson MN; Gee AD; Gouverneur V Chem. - Eur. J 2011, 17, 8248–8262.Wegner HA; Auzias M Angew. Chem., Int. Ed 2011, 50, 8236–8247. For selected examples:Ball LT; Lloyd-Jones GC; Russell CA Science 2012, 337, 1644–1648.Cai R; Lu M; Aguilera EY; Xi Y; Akhmedov NG; Petersen JL; Chen H; Shi X Angew. Chem., Int. Ed 2015, 54, 8772–8776.Huang L; Rudolph M; Rominger F; Hashmi AS K. Angew. Chem. Int. Ed 2016, 55, 4808–4813.
- (27).Kawai H; Wolf WJ; DiPasquale AG; Winston MS; Toste FD J. Am. Chem. Soc 2016, 138, 587–593. [DOI] [PubMed] [Google Scholar]
- (28).(a) Peng H; Xi Y; Ronaghi N; Dong B; Akhmedov NG; Shi XJ Am. Chem. Soc 2014, 136, 13174–13177. [DOI] [PubMed] [Google Scholar]; (b) Leyva-Pérez A; Doménech-Carbó A; Corma A Nat. Commun 2015, 6, 6703–6712. [DOI] [PubMed] [Google Scholar]
- (29).Homocoupling of 3-fluorophenylacetylene with 10 mmol scale was performed; see SI for details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.