An alternative ink design is introduced that enables fast coating of perovskite at room temperature for low-cost solar modules.
Abstract
The efficiencies of small-pixel perovskite photovoltaics have increased to above 24%, while most reported fabrication methods cannot be transferred to scalable manufacturing process. Here, we report a method of fast blading large-area perovskite films at an unprecedented speed of 99 mm/s under ambient conditions by tailoring solvent coordination capability. Combing volatile noncoordinating solvents to Pb2+ and low-volatile, coordinating solvents achieves both fast drying and large perovskite grains at room temperature. The reproducible fabrication yields a certified module efficiency of 16.4%, with an aperture area of 63.7 cm2. This method can be applied for various perovskite compositions. The perovskite modules also show a small temperature coefficient of −0.13%/°C and nearly fully recoverable efficiency after 58 cycles of shading, much better than commercial silicon and thin-film solar modules.
INTRODUCTION
Perovskite solar cells have shown rapidly improved power conversion efficiency (PCE) and stability in recent years (1–7), with a wide range of applications including tandem photovoltaics (PV) by itself or combining other solar cell technologies (8–11), flexible solar cells (12), and concentrator solar cells (13) made by various solution, vacuum, or other deposition processes (14, 15). The certified PCEs for small devices already rival those of other thin-film PV technologies (16). However, one challenge before commercialization is transferring these technologies into the marketplace using high-throughput film deposition techniques for module fabrication (15, 17, 18). A “high electrification” future in 2050 would demand an annual PV installation of 1780 GW (19), while the global installation in 2017 is only 99.1 GW (20). It requires a rapid expansion of PV manufacturing, which may be fulfilled by perovskite PV due to its low cost and rapid solution processing. One gigawatt of power needs over 6.7 million square meters of solar panels with 18% efficiency. These thin films of half a micrometer thick need to be deposited at a fast speed to be economically competitive. Therefore, fast and safe deposition of perovskite films is critically important. Deposition under ambient conditions is preferred, because it allows easy integration into mature industrial processes such as the roll-to-roll process and reduces safety issues when flammable solvents are involved. However, from a material growth kinetics point of view, rapid crystallization at low temperature generally results in perovskite films with low crystallinity, high defect density, and small grains, which reduce both efficiency and stability of perovskite solar cells. Slower and/or higher-temperature film growth have generally been applied to grow perovskite thin films with high crystallinity and large grain size, such as Ostwald ripening, thermal annealing, solvent annealing, and light sintering of perovskites (17).
Here, to reconcile the conflict between fast deposition–induced low crystallinity and the need for large grains with high crystallinity for high efficiency and stability, we propose to decouple the solid film formation and perovskite crystallization processes in blade coating by engineering new ink design (Fig. 1). We discovered that common solvents such as dimethyl sulfoxide (DMSO) and dimethylformamide (DMF) do not favor fast deposition of a compact and smooth perovskite film at room temperature, while highly volatile solvents of 2-methoxyethanol (2-ME) and acetonitrile (ACN) lead to perovskite films with low crystallinity and poor contact to the substrates. A combination of the two kinds of solvents by a certain ratio, however, avoids their respective drawback and has the merits of both. By this strategy, we realized blading of uniform perovskite films at an unprecedented speed of 99 mm/s at room temperature and produced perovskite modules with efficiency above 16% with an aperture area of over 60 cm2.
Fig. 1. Coordination tailored inks for blade coating of perovskite films.
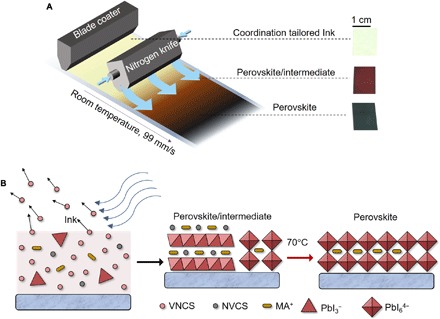
(A) Schematic illustration for N2-knife–assisted blade coating of perovskite films at 99 mm/s at room temperature using coordination tailored ink. Insets: Photograph images of as-coated ink, perovskite/intermediate film, and perovskite film. (B) Schematic illustration showing the drying of ink into a perovskite/intermediate film and full crystallization of a perovskite film. VNCS, volatile noncoordinating solvent; NVCS, nonvolatile coordinating solvent.
RESULTS
Because of the very fast blading speed and low deposition temperature (room temperature), the perovskite ink does not dry immediately. A thin solution sheet is formed right after the blade. To accelerate the drying of the liquid layer at room temperature, a nitrogen knife (N2-knife) is introduced after the blade with a fixed distance to apply a N2 flow. The solvents for the perovskite ink, in this work, are mixtures of volatile, noncoordinating solvents (VNCS) and nonvolatile, coordinating solvents (NVCS). Coordinating capability of solvents refers to the strength of the bonding between the solvents and perovskite precursors, particularly with Pb2+. Strong bonding to Pb2+ by NVCS results in solid-state intermediate phase formation. In our experiment, ~98% (volume) of the solvents for the inks are VNCS, which quickly evaporate during the blading process even at room temperature accelerated by N2 blowing. The quick evaporation of working solvent allows the formation of smooth perovskite films at a high speed and at room temperature. However, it results in small grain size and poor contact to substrate. To improve the perovskite crystallinity, we introduce ~2% NVCS into the ink, which would stay temporarily in the as-coated film in the form of intermediate phase with perovskite precursors (Fig. 1B). The following slower release of NVCS under a mild annealing process gives more time and lower energy barrier for the perovskite crystalline grains to grow into large sizes and form good contact to the substrate.
It is necessary to find out the coordinating capability of solvents so that we can choose the solvent combination for the fast blade coating of perovskites. We studied the coordinating ability of DMSO, DMF, γ-butyrolactone (GBL), 2-ME, and ACN to MAPbI3 (21, 22). DMSO and DMF can dissolve PbI2 due to their strong coordination to Pb2+ ions (23), while GBL, 2-ME, and ACN cannot dissolve PbI2 unless methylammonium iodide (MAI) is added (Fig. 2A). We speculate that MAI is first dissolved and only then is PbI2 dissolved through I− coordination to Pb2+ ions by the formation of PbI3− complexes, whose characteristic absorption peak at 390 nm is observed for GBL, 2-ME, and ACN:2-ME solutions (Fig. 2B) (23, 24). In contrast, much weaker PbI3− absorption is present in DMF- and DMSO-based solutions. ACN:2-ME mixed solvent was used here instead of pure ACN solvent, because the solubility of MAPbI3 in ACN is much lower (<0.1 M) than in the other solvents. The above experiment indicates that DMSO and DMF have strong coordination capability to Pb2+, while GBL, 2-ME, and ACN:2-ME have no or much weaker coordination capability. Since the ACN:2-ME mixed solvent exhibits “noncoordinating” behavior, we can assign ACN as a noncoordinating solvent as well. Besides, we observed that 2-ME or ACN:2-ME mixture solvent exhibits inverse temperature solubility (fig. S1), which phenomenon was also observed in GBL but not in DMF and DMSO (25, 26). It further indicates a weaker coordination ability of GBL, 2-ME, or ACN than MAI to Pb2+ so that MAPbI3 can precipitate out of the solvent at elevated temperatures (27). Donor number DN was proposed as a figure of merit to describe solvent’s coordination ability to Pb2+ (27). The DN of I− ions measured in 1,2-dichloroethane is 28.9 kcal/mol, which is comparable to that of DMF and DMSO but much larger than 2-ME, ACN, and GBL (27–29) being consistent with our results here. The DN versus vapor pressures of the five solvents are plotted in Fig. 2C, which shows that 2-ME and ACN can function as VNCS, and DMSO can be chosen for NVCS.
Fig. 2. Coordination capability of different solvents to Pb2+.
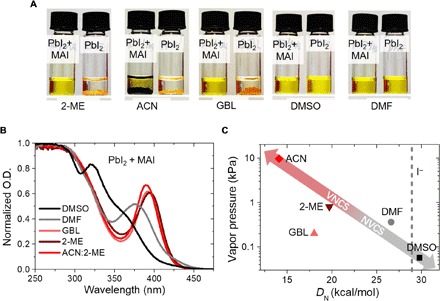
(A) Photograph images showing dissolution of PbI2:MAI = 1:1 and PbI2 alone by GBL, 2-ME, ACN, DMSO, and DMF solvents at nominal mole concentration of 1 M. (B) Ultraviolet-visible (UV-vis) absorption spectra of MAPbI3 solutions prepared from different solvents. O.D., optical density. (C) Vapor pressure and donor number (DN) of the five solvents studied and DN of iodide ion. Scanning electron microscopy (SEM) images of as-coated films from different solvents. Photo credit: Yehao Deng, University of North Carolina Chapel Hill.
We then compared using VNCS, NVCS, or a combination of the two, for room-temperature N2-assisted blade coating of perovskite films. The fast blade coating (typically >10 mm/s) of perovskites will leave a thin sheet of solution of about several micrometers thick (Fig. 1A). If the solution sheet does not dry immediately, then it will flow in certain patterns, presumably driven by surface tension or evaporation, as observed previously (30). Such solution flow results in nonuniform and discontinuous perovskite films. One way to address this problem is to reduce blade coating speed markedly so that solution dries right after the blade applicator moves away (31). However, this method limits the blade coating speed.
We observed that when DMF, DMSO, or GBL is used as solvent, the solution sheets remain wet after fast blade coating and require several to tens of minute for the solution to dry and crystallize at room temperature. The scanning electron microscopy (SEM) images in Fig. 3A and fig. S2 show that the formed films are highly discontinuous. Furthermore, x-ray diffraction (XRD) patterns of films coated from DMSO and DMF show strong XRD peaks below 10° (Fig. 3C and fig. S3), which are the intermediate phases (30). The intermediate phases are formed by the strong coordination of DMSO or DMF to perovskite precursor materials, which are stable at room temperature. In contrast, the solution sheets coated from 2-ME or ACN:2-ME (3:2 volume ratio) dry and turn into black color right after coating with pure perovskite phase formation, as evidenced by XRD in Fig. 3C and fig. S3. SEM images of the obtained perovskite films show highly compact and uniform morphology that is needed for perovskite solar modules. The results above prove that VNCS is suitable for fast and room temperature deposition of a compact and smooth perovskite film.
Fig. 3. Morphology and crystallinity of the perovskite films.
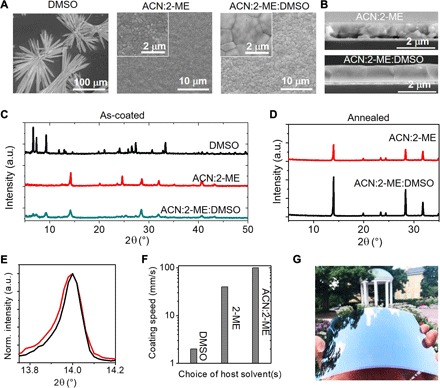
(A) SEM images of perovskite films prepared with different solvent or solvent mixtures. (B) Cross-sectional SEM images of perovskite films prepared with different solvent mixtures. (C) XRD spectra of as-coated perovskite films from different solvent or solvent mixtures. (D and E) XRD spectra of annealed perovskite films prepared with different solvent mixtures. Red, ACN:2-ME; black, ACN:2-ME:DMSO. a.u., arbitrary units. (F) Maximum coating speed for obtaining high-quality large-area perovskite films when different solvents are applied in N2-knife–assisted blade-coating process. (G) Photograph image of an as-coated perovskite film on 15 cm by 15 cm flexible substrate. Photo credit: Yehao Deng, University of North Carolina Chapel Hill.
However, pure VNCS generates perovskite films with grain size of only several hundred nanometers (Fig. 3A and fig. S2). The cross-sectional SEM images of these films (Fig. 3B) further show that these perovskite films have poor physical contact to the poly(bis(4-phenyl) (2,4,6-trimethylphenyl) amine (PTAA)–coated indium tin oxide (ITO) substrate, evidenced by the large voids in between layers. The formation of voids can be explained by earlier solidification at the top of the solution. To address this problem, we added a small amount of NCVS (DMSO) into the VNCS host solvent. Absorption spectroscopy (fig. S4) shows that the small amount of DMSO added into VNCS solutions does not notably change the coordination condition as compared to that without DMSO (Fig. 2B). However, we anticipate that the coordination ability of the solution/solid mixture with DMSO added will be increasing till DMSO leaves the as-coated films, because DMSO evaporates much slower than other solvents. We observed that the as-coated films (within several hours after blading) are composed of both intermediate phase and perovskite phase based on its brown-color appearance (Fig. 1A) and XRD spectrum (Fig. 3C). After annealing at 70°C for 1 min, the mixture films transform into the pure perovskite phase. These perovskite films have stronger and sharper XRD peaks than those formed without DMSO, with full width at half maximum of (110) peak narrowed down from 0.104° to 0.089° (Fig. 3, D and E). SEM images in Fig. 3A show that these films are still compact and uniform, but grain size increases to 1 to 2 μm in the lateral directions. The cross-sectional SEM images in Fig. 3B show that DMSO addition gives the perovskite film good physical contact to the underlying substrate. Replacing DMSO with GBL, which has similar vapor pressure (Fig. 2C), results in much smaller grain sizes and poorer contact to the substrate (fig. S2), proving that it is the coordinating ability rather than the low volatility of DMSO that improves perovskite crystallinity.
Figure 3F summarizes the allowed blade coating speed to form high-quality perovskite films with N2-knife–assisted blading method using different solvents or solvent mixtures, determined experimentally. The N2-knife was operated under pressures below 20 psi. The “high quality” refers to being uniform and compact enough for module fabrication. As discussed, pure DMSO as the solvent requires a very slow coating speed below 2 mm/s. The coating speed can increase to 40 mm/s when using 2-ME as the main solvent. With the addition of ACN at a volume ratio of 3:2 for the ACN:2-ME mixed solvent, the coating speed is further increased to 99 mm/s, which is the upper limit speed of the blade coater. Note that the viscosities of 2-ME and ACN are smaller than that of DMSO (fig. S5), so this ink formulation brings down the solution viscosity. One consequence of reduced viscosity is a thinner as-coated solution film is obtained, while we can adjust coating speed and solution concentration to increase the film thickness (32). The process of blade coating a perovskite film on flexible Corning glass with an area of ~225 cm2 at room temperature and a speed of 99 mm/s is recorded, as shown in movie S1. Photographic image of a bladed MAPbI3 film on a flexible Corning glass with an area of 225 cm2 is shown in Fig. 3G. This is 20 times faster than a recently reported N2-knife–assisted method (33).
We fabricated perovskite modules using the blade-coated perovskite films. The device structure is ITO/PTAA/MAPbI3/fullerene (C60)/bathocuproine (BCP)/metal cathode. Here, the PTAA layer was also blade-coated, and other layers were deposited by thermal evaporation. Small-area single cells could reach a high PCE of 21.3% with a VOC of 1.13 V, a JSC of 23.0 mA/cm2, and a fill factor (FF) of 81.8% (Fig. 4A). This is the best reported device performance for MAPbI3-based devices, regardless of the fabrication methods, highlighting the advantage of the blading method reported here (34). We then fabricated large-area solar modules. The J-V curves for a champion module under 1-sun illumination with an aperture area of 63.7 cm2 are shown in Fig. 4B, which have little hysteresis. The VOC, ISC, FF, and PCE values are summarized in the inserted table. The efficiency statistics of 18 modules fabricated consecutively are summarized in Fig. 4C. Approximately 90% of the modules have efficiencies of 15 to 17%, showing the highest reproducibility among perovskite module fabrication methods so far (15, 30). We investigated device uniformity along lateral direction (parallel to blade coater) and coating direction, as shown in fig. S6. The results show that the distribution of device efficiencies is rather uniform in both lateral and coating directions. We sent five modules to National Renewable Energy Laboratory (NREL) for certification. All of them have stabilized efficiencies above 15.9%, and the champion efficiency is 16.4%, which is a record of all reported perovskite modules with comparable area (fig. S7). Note that the certification was conducted by stabilizing the module around maximum power point (MPP) for 1 hour. The long-term operational stability of an encapsulated perovskite module is presented in Fig. 4D. The module was loaded at MPP, and its PCEs were measured periodically. After illumination for over 1000 hours under 1-sun equivalent light intensity [no ultraviolet (UV) filter], the module retained 87% of its peak efficiency of 15.8%. This is the best stability reported for perovskite modules under real operational conditions with PCE above 12% (18). We also investigated the performance of one module under reduced light intensity, and the measurement results are shown in fig. S8. The PCE of the module remains 16 to 17% under light intensity from 1 to 0.05 sun. Therefore, the solar module can function well under different light intensity and is promising for room light energy harvesting. As shown in fig. S8D, a ~360 cm2 submodule can charge an iPhone even in a cloudy day.
Fig. 4. Performance characterizations of perovskite solar modules.
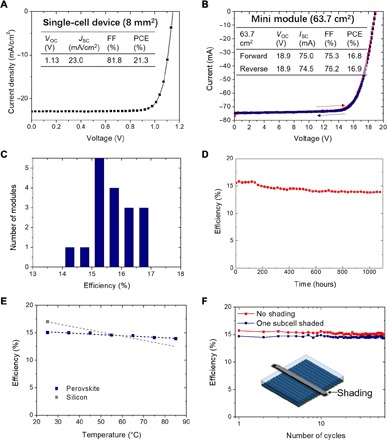
(A) J-V curve of a small-area perovskite solar cell fabricated with the N2-assisted room-temperature blade-coating method. (B) I-V curve of the champion perovskite module. (C) Distribution of efficiencies of 18 modules fabricated consecutively. (D) Long-term operational stability of an encapsulated perovskite module loaded at MPP under 1-sun equivalent illumination. (E) Averaged PCEs of perovskite modules measured at different temperatures from 25° to 85°C with a fitted temperature coefficient of −0.13%/°C. The efficiency of a typical silicon module in the market is also added for reference, which has an efficiency of 17% at 25°C and a temperature coefficient of −0.44%/°C. (F) Efficiencies of a perovskite module with one subcell going through 58 cycles of shading/de-shading. The inset shows schematically how shading is applied over one subcell.
Temperature coefficient βPCE is an important parameter that characterizes the module efficiency under real working conditions, where the temperature can easily rise to above 50°C. As temperature increases, the saturated dark current increases that lead to reduced VOC and efficiency (35). Under AM1.5G illumination, the measured temperature coefficient of the perovskite module in the temperature range of 25° to 85°C is −0.13%/°C (Fig. 4E). The efficiency loss mainly comes from VOC, which has the same coefficient (βVoc) of −0.13%/°C, while FF and ISC remain nearly unchanged (fig. S9). The efficiency of the module remains to be the same as that before testing when the temperature was reduced to 25°C, excluding degradation of the perovskite modules. This temperature coefficient is smaller than that of CdTe (−0.28%/°C), copper indium gallium selenide (CIGS) (−0.32%/°C), and crystalline silicon (c-Si) (−0.44%/°C) (36) as solar cells with larger VOC generally have smaller βVoc (35). The low temperature coefficient of perovskite modules makes them even more efficient than silicon modules under real operation temperatures above 55°C (Fig. 4E).
Shading effect is another important factor that limits PV module performances in real applications (17, 37). The shaded subcells block the photocurrent of the whole module when subcells are connected in series. The shaded subcells could be burned by the bias generated from other subcells to resume photocurrent output. Silicon solar modules have large breakdown voltages over 15 V (38, 39), and CdTe and CIGS solar modules have lower breakdown voltage below 10 V. More than 50% of power for those solar modules is lost after breakdown even with a shading area of only ~10% (38, 39). Besides, the breakdown results in permanent damage to CdTe and CIGS modules and PCE loss of 4 to 14% after 20 s of shading (40, 41). Here, we mimicked the extreme case that one subcell in the module was entirely shaded, while all other subcells were exposed to 1-sun illumination (Fig. 4F, inset). The breakdown of the shaded subcell was observed during MPP tracking over 2 to 4 min (fig. S10). After breakdown, the module resumes its power generation with a small power loss of relatively 6.0%, which is proportional to the nominal area reduction (6.25%). This means that the shaded subcell does not negatively affect the remaining subcells in the perovskite module. To evaluate the damage, one module was shaded for 4 min. The module recovered almost 100% of its original power output when shading was removed, indicating no permanent damage. We performed >50 cycles of shading/de-shading on the same subcell of a module. A slight reduction of PCE from 15.7 to 15.1% is observed after the first 20 cycles, and then, the module PCE stabilizes in the following cycles (Fig. 4F and fig. S10). A recent study on reverse bias behavior of a single perovskite solar cell pointed out that ion migration would induce tunneling breakdown (37). The lack of permanent damage after recovery and a low breakdown voltage of ~0.4 V support this mechanism (fig. S10E). Ion migration is a unique property in halide perovskites, which explains why perovskite solar modules have such superior shading tolerance relative to other commercial PV modules. Confining ions within perovskite layer may avoid undesired degradation process such as perovskite decomposition and degradation of adjacent layers (42) but still preserve good shading tolerance, which requires ions to migrate just within the perovskite layer.
DISCUSSION
In summary, we have demonstrated fast blade coating of large-area perovskite films at room temperature. Using volatile host solvents allows for quick formation of compact perovskite films with the assistance of N2-knife. The added coordinating solvents slowly release from the as-coated solid film, giving enough time for the perovskite grains to grow into large sizes with high crystallinity and good contact to substrate. A coating speed as high as 99 mm/s has been realized, yielding perovskite modules with a certified stabilized PCE of 16.4% with an aperture area of 63.7 cm2. The as-fabricated modules also show superior temperature and shading effect tolerance compared to commercialized PV technologies including c-Si, CdTe, and CIGS, which increase the competitiveness of perovskite PV in the future PV market.
MATERIALS AND METHODS
Materials
All chemicals were purchased from Sigma-Aldrich unless otherwise specified and used without further purification. Methylammonium iodide was purchased from GreatCell Solar. Methylammonium hypophosphite was synthesized according to our previous publication (43).
Device fabrication
Prepatterned ITO/glass substrates were washed with detergent, deionized water, isopropanol, and acetone sequentially and dried in an oven at 60°C overnight. PTAA/toluene solution was blade-coated on UV-ozone–treated ITO/glass substrate at 20 mm/s with 200-μm coating gap. Then, perovskite layer was blade-coated with air knife blowing at room temperature. The solution composition was ~1.0 M MAPbI3 in a mixture solvent composed of ACN (60%, v/v)/2-ME (40%, v/v) for coating at 99 mm/s for best-performing devices. The molar ratio of DMSO to MAPbI3 is ~20%. l-α-Phosphatidylcholine, methylammonium chloride, and methylammonium hypophosphite were added into the solution as additives at molar percentages of ~0.025, ~0.8, and ~1.0% to MAPbI3, respectively. Note that those additives are not required for high-speed room-temperature perovskite film coating here but can improve device efficiency (44, 45). The blade coater gap was 200 to 300 μm. The air knife worked below 20 psi. The as-coated solid film was annealed at 70°C for several minutes and then at 100°C for 5 to 20 min. Then, the perovskite film was thermally evaporated with C60 (30 nm) and BCP (6 nm). Laser scribing was then performed twice before and after electrode deposition to complete the module fabrication. For the modules sent for certification, polydimethylsiloxane antireflection coatings were applied.
Device characterizations
The J-V measurement of perovskite modules were performed with the Keithley 2400 Source Meter under simulated AM1.5G irradiation produced by a xenon lamp–based solar simulator (Oriel Sol3A, Class AAA Solar Simulator). The light intensity was calibrated by a silicon reference cell (Newport 91150V-KG5). The scan rate was 1 V/s for modules and there was no preconditioning before measurement. To measure the long-term operational stability of perovskite module, the module was encapsulated, illuminated by 1-sun equivalent metal halide lamp, and loaded at MPP. To measure the module efficiency at elevated temperatures, the encapsulated module was placed on a large hotplate, and the temperatures of the module were measured with an infrared thermometer. The temperature variation over the module’s aperture area is less than 5°C. The SEM images were taken by a Quanta 200 FEG environmental scanning electron microscope. The XRD pattern was obtained with a Rigaku sixth generation MiniFlex X-ray diffractometer.
Supplementary Material
Acknowledgments
Funding: This work was supported by Office of Naval Research under award N00014-17-1-2619 and University of North Carolina’s Research Opportunities Initiative (UNC ROI) through Center of Hybrid Materials Enabled Electronic Technology. This work was performed, in part, at the Chapel Hill Analytical and Nanofabrication Laboratory, CHANL, a member of the North Carolina Research Triangle Nanotechnology Network, RTNN, which is supported by the National Science Foundation, grant ECCS-1542015, as part of the National Nanotechnology Coordinated Infrastructure, NNCI. Author contributions: J.H. and Y.D. conceived the idea. Y.D. designed the experiments and conducted most of the device fabrication and measurement. C.H.V.B. and B.C. assisted device fabrication and certification. X.D. helped with solution preparation. J.Z. performed XRD measurements. Y.D. and J.H. wrote the paper, and all authors reviewed the paper. Competing interests: J.H. and Y.D. are inventors on a patent application related to this work filed by University of North Carolina Chapel Hill (PCT/US2019/025237, filed 01 April 2019). The other authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from J.H. (jhuang@unc.edu).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/12/eaax7537/DC1
Fig. S1. Photographic images of MAPbI3 solutions prepared by dissolving in 2-ME or ACN/2-ME solvent at room temperature and then heated to 80°C.
Fig. S2. SEM images of N2 knife–assisted blade-coated perovskite films using DMF, GBL, 2-ME, ACN:2-ME, and ACN:2-ME:GBL as solvents.
Fig. S3. XRD spectra of as-coated films deposited from DMF-, GBL-, or 2-ME–based solutions.
Fig. S4. UV-vis absorption spectra of perovskite precursor solution based on VNCS with a little DMSO added.
Fig. S5. Viscosity of ACN, 2-ME, and DMSO solvents.
Fig. S6. The efficiency uniformity over a perovskite module.
Fig. S7. NREL certification of a perovskite submodule with an aperture area of 63.7 cm2 and a stabilized efficiency of 16.4%.
Fig. S8. The performance of a perovskite module under low intensity sun light illumination.
Fig. S9. The performance of a perovskite module at elevated temperatures.
Fig. S10. The performance of a perovskite module experiencing shading effect.
Movie S1. Room-temperature blade coating of perovskite film at 99 mm/s.
REFERENCES AND NOTES
- 1.Kojima A., Teshima K., Shirai Y., Miyasaka T., Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Christians J. A., Schulz P., Tinkham J. S., Schloemer T. H., Harvey S. P., de Villers B. J. T., Sellinger A., Berry J. J., Luther J. M., Tailored interfaces of unencapsulated perovskite solar cells for >1,000 hour operational stability. Nat. Energy 3, 68–74 (2018). [Google Scholar]
- 3.Hou Y., Du X., Scheiner S., McMeekin D. P., Wang Z., Li N., Killian M. S., Chen H., Richter M., Levchuk I., Schrenker N., Spiecker E., Stubhan T., Luechinger N. A., Hirsch A., Schmuki P., Steinrück H.-P., Fink R. H., Halik M., Snaith H. J., Brabec C. J., A generic interface to reduce the efficiency-stability-cost gap of perovskite solar cells. Science 358, 1192–1197 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Tan H., Jain A., Voznyy O., Lan X., García de Arquer F. P., Fan J. Z., Quintero-Bermudez R., Yuan M., Zhang B., Zhao Y., Fan F., Li P., Quan L. N., Zhao Y., Lu Z.-H., Yang Z., Hoogland S., Sargent E. H., Efficient and stable solution-processed planar perovskite solar cells via contact passivation. Science 355, 722–726 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Kim H.-S., Lee C.-R., Im J.-H., Lee K.-B., Moehl T., Marchioro A., Moon S.-J., Humphry-Baker R., Yum J.-H., Moser J. E., Grätzel M., Park N.-G., Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2, 591 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arora N., Dar M. I., Hinderhofer A., Pellet N., Schreiber F., Zakeeruddin S. M., Grätzel M., Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20%. Science 358, 768–771 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Turren-Cruz S.-H., Hagfeldt A., Saliba M., Methylammonium-free, high-performance, and stable perovskite solar cells on a planar architecture. Science 362, 449–453 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Han Q., Hsieh Y.-T., Meng L., Wu J.-L., Sun P., Yao E.-P., Chang S.-Y., Bae S.-H., Kato T., Bermudez V., Yang Y., High-performance perovskite/Cu (In, Ga) Se2 monolithic tandem solar cells. Science 361, 904–908 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Zhao D., Chen C., Wang C., Junda M. M., Song Z., Grice C. R., Yu Y., Li C., Subedi B., Podraza N. J., Zhao X., Fang G., Xiong R.-G., Zhu K., Yan Y., Efficient two-terminal all-perovskite tandem solar cells enabled by high-quality low-bandgap absorber layers. Nat. Energy 3, 1093–1100 (2018). [Google Scholar]
- 10.Sahli F., Werner J., Kamino B. A., Bräuninger M., Monnard R., Paviet-Salomon B., Barraud L., Ding L., Leon J. J. D., Sacchetto D., Cattaneo G., Despeisse M., Boccard M., Nicolay S., Jeangros Q., Niesen B., Ballif C., Fully textured monolithic perovskite/silicon tandem solar cells with 25.2% power conversion efficiency. Nat. Mater. 17, 820–826 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Bush K. A., Palmstrom A. F., Zhengshan J. Y., Boccard M., Cheacharoen R., Mailoa J. P., McMeekin D. P., Hoye R. L., Bailie C. D., Leijtens T., Peters I. M., Minichetti M. C., Rolston N., Prasanna R., Sofia S., Harwood D., Ma W., Moghadam F., Snaith H. J., Buonassisi T., Holman Z. C., Bent S. F., McGehee M. D., 23.6%-efficient monolithic perovskite/silicon tandem solar cells with improved stability. Nat. Energy 2, 17009 (2017). [Google Scholar]
- 12.Bu T., Li J., Zheng F., Chen W., Wen X., Ku Z., Peng Y., Zhong J., Cheng Y.-B., Huang F., Universal passivation strategy to slot-die printed SnO2 for hysteresis-free efficient flexible perovskite solar module. Nat. Commun. 9, 4609 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z., Lin Q., Wenger B., Christoforo M. G., Lin Y.-H., Klug M. T., Johnston M. B., Herz L. M., Snaith H. J., High irradiance performance of metal halide perovskites for concentrator photovoltaics. Nat. Energy 3, 855–861 (2018). [Google Scholar]
- 14.Ávila J., Momblona C., Boix P. P., Sessolo M., Bolink H. J., Vapor-deposited perovskites: The route to high-performance solar cell production? Joule 1, 431–442 (2017). [Google Scholar]
- 15.Chen H., Ye F., Tang W., He J., Yin M., Wang Y., Xie F., Bi E., Yang X., Grätzel M., Han L., A solvent-and vacuum-free route to large-area perovskite films for efficient solar modules. Nature 550, 92–95 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Green M. A., Hishikawa Y., Warta W., Dunlop E. D., Levi D. H., Hohl-Ebinger J., Ho-Baillie A. W. H., Solar cell efficiency tables (version 50). Prog. Photovolt. Res. Appl. 25, 668–676 (2017). [Google Scholar]
- 17.Li Z., Klein T. R., Kim D. H., Yang M., Berry J. J., van Hest M. F. A. M., Zhu K., Scalable fabrication of perovskite solar cells. Nat. Rev. Mater. 3, 18017 (2018). [Google Scholar]
- 18.Rong Y., Hu Y., Mei A., Tan H., Saidaminov M. I., Seok S. I., McGehee M. D., Sargent E. H., Han H., Challenges for commercializing perovskite solar cells. Science 361, eaat8235 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Mayer J. N., Philipps S., Hussein N. S., Schlegl T., Senkpiel C., Current and future cost of photovoltaics. Long-term scenarios for market development, system prices and LCOE of utility-scale PV systems. Fraunhofer ISE , 1–82 (2015). [Google Scholar]
- 20.Schmela M., Global Market Outlook for Solar Power 2018–2022. SolarPower Europe , 1–81 (2018). [Google Scholar]
- 21.Hendriks K. H., van Franeker J. J., Bruijnaers B. J., Anta J. A., Wienk M. M., Janssen R. A., 2-Methoxyethanol as a new solvent for processing methylammonium lead halide perovskite solar cells. J. Mater. Chem. A 5, 2346–2354 (2017). [Google Scholar]
- 22.Noel N. K., Habisreutinger S. N., Wenger B., Klug M. T., Hörantner M. T., Johnston M. B., Nicholas R. J., Moore D. T., Snaith H. J., A low viscosity, low boiling point, clean solvent system for the rapid crystallisation of highly specular perovskite films. Energ. Environ. Sci. 10, 145–152 (2017). [Google Scholar]
- 23.Sharenko A., Mackeen C., Jewell L., Bridges F., Toney M. F., Evolution of iodoplumbate complexes in methylammonium lead iodide perovskite precursor solutions. Chem. Mater. 29, 1315–1320 (2017). [Google Scholar]
- 24.Stamplecoskie K. G., Manser J. S., Kamat P. V., Dual nature of the excited state in organic–inorganic lead halide perovskites. Energ. Environ. Sci. 8, 208–215 (2015). [Google Scholar]
- 25.Saidaminov M. I., Abdelhady A. L., Murali B., Alarousu E., Burlakov V. M., Peng W., Dursun I., Wang L., He Y., Maculan G., Goriely A., Wu T., Mohammed O. F., Bakr O. M., High-quality bulk hybrid perovskite single crystals within minutes by inverse temperature crystallization. Nat. Commun. 6, 7586 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadro J. M., Nonomura K., Gachet D., Grätzel M., Hagfeldt A., Facile route to freestanding CH3NH3PbI3 crystals using inverse solubility. Sci. Rep. 5, 11654 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamill J. C. Jr., Schwartz J., Loo Y.-L., Influence of solvent coordination on hybrid organic–inorganic perovskite formation. ACS Energy Letters 3, 92–97 (2017). [Google Scholar]
- 28.Lau K. W., Aron M.-H. H., Yen M. H.-J., Fung E. Y., Grzybicki S., Matamoros R., Curtis J. C., Solvent, electrolyte and solute shape effects on optical electron transfer in mixed-valence ruthenium ammine dimers. Inorg. Chim. Acta 226, 137–143 (1994). [Google Scholar]
- 29.Linert W., Jameson R. F., Taha A., Donor numbers of anions in solution: The use of solvatochromic Lewis acid–base indicators. J. Chem. Soc. Dalton Trans. , 3181–3186 (1993). [Google Scholar]
- 30.Deng Y., Zheng X., Bai Y., Wang Q., Zhao J., Huang J., Surfactant-controlled ink drying enables high-speed deposition of perovskite films for efficient photovoltaic modules. Nat. Energy 3, 560–566 (2018). [Google Scholar]
- 31.He M., Li B., Cui X., Jiang B., He Y., Chen Y., O’Neil D., Szymanski P., Ei-Sayed M. A., Huang J., Lin Z., Meniscus-assisted solution printing of large-grained perovskite films for high-efficiency solar cells. Nat. Commun. 8, 16045 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.T. Schneller, R. Waser, M. Kosec, D. Payne, Chemical Solution Deposition of Functional Oxide Thin Films (Springer, 2013). [Google Scholar]
- 33.Ding J., Han Q., Ge Q.-Q., Xue D.-J., Ma J.-Y., Zhao B.-Y., Chen Y.-X., Liu J., Mitzi D. B., Hu J.-S., Fully air-bladed high-efficiency perovskite photovoltaics. Joule 3, 402–406 (2019). [Google Scholar]
- 34.Shin S. S., Yeom E. J., Yang W. S., Hur S., Kim M. G., Im J., Seo J., Noh J. H., Seok S. I., Colloidally prepared La-doped BaSnO3 electrodes for efficient, photostable perovskite solar cells. Science 356, 167–171 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Dupré O., Vaillon R., Green M. A., Physics of the temperature coefficients of solar cells. Sol. Energy Mater. Sol. Cells 140, 92–100 (2015). [Google Scholar]
- 36.Fu F., Feurer T., Weiss T. P., Pisoni S., Avancini E., Andres C., Buecheler S., Tiwari A. N., High-efficiency inverted semi-transparent planar perovskite solar cells in substrate configuration. Nat. Energy 2, 16190 (2017). [Google Scholar]
- 37.Bowring A. R., Bertoluzzi L., O'Regan B. C., McGehee M. D., Reverse bias behavior of halide perovskite solar cells. Adv. Energy Mater. 8, 1702365 (2018). [Google Scholar]
- 38.Khaing H. H., Liang Y. J., Htay N. N. M., Fan J., Characteristics of different solar PV modules under partial shading. Int. J. Electr. Comput. Electron Commun. Eng. 8, 1328–1332 (2014). [Google Scholar]
- 39.C. Tzikas, G. Gómez, M. van den Donker, K. Bakker, A. H. M. Smets, W. Folkerts, Do thin film PV modules offer an advantage under partial shading conditions? 33rd European Photovoltaic Solar Energy Conference and Exhibition (2017). [Google Scholar]
- 40.Silverman T. J., Deceglie M. G., Deline C., Kurtz S., Partial shade stress test for thin-film photovoltaic modules. Reliability of Photovoltaic Cells, Modules, Components, and Systems VIII 9563, 95630F (2015). [Google Scholar]
- 41.Silverman T. J., Mansfield L., Repins I., Kurtz S., Damage in monolithic thin-film photovoltaic modules due to partial shade. IEEE J. Photovoltaics 6, 1333–1338 (2016). [Google Scholar]
- 42.Bai Y., Lin Y., Ren L., Shi X., Strounina E., Deng Y., Wang Q., Fang Y., Zheng X., Lin Y., Chen Z.-G., Du Y., Wang L., Huang J., Oligomeric silica-wrapped perovskites enable synchronous defect passivation and grain stabilization for efficient and stable perovskite photovoltaics. ACS Energy Letters 4, 1231–1240 (2019). [Google Scholar]
- 43.Xiao Z., Wang D., Dong Q., Wang Q., Wei W., Dai J., Zeng X., Huang J., Unraveling the hidden function of a stabilizer in a precursor in improving hybrid perovskite film morphology for high efficiency solar cells. Energ. Environ. Sci. 9, 867–872 (2016). [Google Scholar]
- 44.Chen B., Yu Z., Liu K., Zheng X., Liu Y., Shi J., Spronk D., Rudd P. N., Holman Z., Huang J., Grain engineering for perovskite/silicon monolithic tandem solar cells with efficiency of 25.4%. Joule 3, 177–190 (2019). [Google Scholar]
- 45.Zheng X., Chen B., Dai J., Fang Y., Bai Y., Lin Y., Wei H., Zeng X. C., Huang J., Defect passivation in hybrid perovskite solar cells using quaternary ammonium halide anions and cations. Nat. Energy 2, 17102 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/12/eaax7537/DC1
Fig. S1. Photographic images of MAPbI3 solutions prepared by dissolving in 2-ME or ACN/2-ME solvent at room temperature and then heated to 80°C.
Fig. S2. SEM images of N2 knife–assisted blade-coated perovskite films using DMF, GBL, 2-ME, ACN:2-ME, and ACN:2-ME:GBL as solvents.
Fig. S3. XRD spectra of as-coated films deposited from DMF-, GBL-, or 2-ME–based solutions.
Fig. S4. UV-vis absorption spectra of perovskite precursor solution based on VNCS with a little DMSO added.
Fig. S5. Viscosity of ACN, 2-ME, and DMSO solvents.
Fig. S6. The efficiency uniformity over a perovskite module.
Fig. S7. NREL certification of a perovskite submodule with an aperture area of 63.7 cm2 and a stabilized efficiency of 16.4%.
Fig. S8. The performance of a perovskite module under low intensity sun light illumination.
Fig. S9. The performance of a perovskite module at elevated temperatures.
Fig. S10. The performance of a perovskite module experiencing shading effect.
Movie S1. Room-temperature blade coating of perovskite film at 99 mm/s.


