Abstract
Aims
The role of long non-coding RNA’s (lncRNA) in the biology of ulcerative colitis (UC) is not well understood. We have previously detected changes in lncRNA’s associated with UC. This study aims to characterize one specific lncRNA, CDKN2B-AS1 whose expression was downregulated in UC patients.
Main methods
UC biopsies were used to determine the levels of linear and circular CDKN2B-AS1 relative to healthy controls. In situ hybridization was used to determine the localization of CKDN2B-AS1 in the colon. The intestinal epithelial cell line, Caco-2, was used to study the effects of shRNA mediated loss of CDKN2B-AS1. Transepithelial electrical resistance was used to measure barrier function. An RT-PCR array, immunoblots and immunohistochemistry were used to determine tight junction proteins that CDKN2B-AS1 regulates.
Key findings
CDKN2B-AS1 is transcribed into not only linear transcripts but also as circular RNA through back-splicing and both forms are decreased in IBD. CDKN2B-AS1 is expressed mainly in colonic epithelial cells. Cells with down-regulated CDKN2B-AS1 exhibited increased proliferation and no alterations in apoptosis. Targeting both the linear and circular transcripts of CDKN2B-AS1 with short hairpin RNAs enhanced barrier function. We subsequently determined that Claudin-2, a “leaky Claudin” known to decrease barrier function, was decreased in CDKN2B-AS1 knockdown cells.
Significance
This study identifies a novel lncRNA with both linear and circular transcripts affecting UC biology.
Keywords: Inflammatory bowel disease, Non-coding RNA, Intestinal barrier
1. Introduction
Inflammatory bowel disease (IBD) is a set of gastrointestinal disorders comprised of ulcerative colitis (UC) and Crohn’s disease (CD) affecting roughly 1.6 million Americans [1]. The gastrointestinal system is a complicated web of interactions between environmental factors, commensal bacteria, epithelial cells, stromal components, resident immune cells, and recruited bone-marrow derived cells. The delicate balance that exists during homeostasis is perturbed in IBD patients as a result of many disparate reasons [2]. Factors such as toxin exposures, changes in microbial composition, genetic and epigenetic alterations have been implicated in the manifestation of this debilitating disease. While much progress has been made in the last twenty years in the management of IBD, there still exists a lack of understanding as to how this inflammatory disease arises and how to control and cure it. Therapies directed against key mediators of this inflammatory process such as tumor necrosis factor-alpha, IL-23, sphingosine-1-phosphate receptor, and integrins have been relatively successful in treating certain aspects of IBD [3]. However, approximately 40% of patients do not initially respond to anti-TNF therapy and an additional 30% of patients lose response over time [4]. Additionally, powerful immunomodulators used in long-term management of these diseases increase the risk of infections and malignancies [5]. Given the high refractory response rates, the potential side effects of these medications and the expanding clinical need, new therapeutic targets and prognostic tools are needed to combat IBD and expand the potential pipeline of tools.
Much of the previous work on IBD pathophysiology has focused on protein-coding genes, however, these only represent 2% of the total RNA expressed in cells [6]. The majority of the expressed RNAs are in the form of non-coding transcripts that lack open reading frames to produce proteins [6]. These transcripts function as components of the spliceosome, telomerase machinery, ribosomal RNA, and regulators of gene expression such as microRNAs and long non-coding RNAs (lncRNAs). Long non-coding RNAs are a relatively new field of study for gene regulation. They are transcripts measuring over 200 base-pairs in length and lacking an open reading frame [6]. Decades ago these transcripts were thought to be transcriptional noise, however, recent research is uncovering a wealth of information regarding their potential mechanisms of action. Working in either cis- or trans- functions, these lncRNAs can bring an array of chromatin remodeling complexes to local or distant genes to fine-tune their gene expression and affect processes such as tumor progression and inflammation. Genes such as lincRNA-EPS, lincRNA-Cox2, and IFNG-AS1/NeST have all been implicated in regulating the immune system through its regulation of broad chromatin remodeling complexes that regulate an expanse of the genome [7–9]. In addition to the linear transcripts, a relatively new concept regarding RNA biology is showing that RNAs can form circular variants through a process of back-splicing [10]. These circular variants have been implicated in microRNA sponging, protein scaffolding, and chromatin regulation [11]. The breadth of potential regulatory mechanisms attainable via the myriad of RNA products are expanding and pose interesting targets for new therapeutic and prognostic targets.
In order to understand the role of lncRNA’s in inflammatory bowel disease pathophysiology, our group and others have uncovered a several lncRNAs that are differentially expressed between IBD patient colonic samples versus controls [7,12,13]. For only a few of these lncRNAs have functions been ascribed as it relates to the pathophysiology of IBD. In addition, for IFNG-AS1 to regulating interferon gamma production [7,14], BC012900 has been shown to regulate intestinal epithelial cell apoptosis [13]. By profiling over 30,000 lncRNAs, we previously identified over 700 differentially expressed lncRNAs in patients with IBD [7]. Of the multiple differentially expressed lncRNAs, one lncRNA was commonly found to be downregulated in the IBD patients when compared to controls, CDKN2B-AS1. Interestingly, two previous investigations of lncRNA expression in IBD patients also found CDKN2B-AS1 to be downregulated in IBD patients [12,13]. Given the prevalence of CDKN2B-AS1 alterations in multiple independent datasets, we chose to pursue this lncRNA for further mechanistic study. CDKN2B-AS1 is transcribed as a 1605 basepair lncRNA in the antisense direction from the INK4b-ARFCDKN2B INK4a gene cluster. The INK4a/ARF/INK4b locus is on located on chromosome 9p21. This locus is deleted in a wide range of human cancers with an estimated frequency of 30–40%, making it one of the most frequent cytogenic events in human cancer [15]. The products of the locus include p15INK4b, p16INK4a, and ARF, which play important roles in tumor suppression [16,17]. The INK4b/ARF/INK4b locus which includes CDKN2B-AS1 is a well-defined genetic risk locus associated with multiple human diseases including coronary artery disease (CAD) [18]. In addition, CDKN2B-AS1 has been found to be associated with periodontitis, diabetes, open angle glaucoma and various cancers [19–21]. Studies in prostate tissue and fibroblast cells revealed that CDKN2B-AS1 epigenetically represses its neighboring CDKN2A/B genes [22,23]. CDKN2B-AS1 has also been shown to increase cell proliferation, increase cell adhesion, and decrease apoptosis [24,25]. Interestingly, CDKN2B-AS1 is transcribed as a circular RNA with specific functions [24,26]. Recently, Holdt et al. showed that the circular form of CDKN2B-AS1 RNA confers atheroprotection by controlling ribosomal RNA (rRNA) maturation and modulating pathways of atherogenesis [24].
Through the studies described in this manuscript, we have shown that CDKN2B-AS1 to be significantly downregulated in UC patient samples. Interestingly, both linear and circular forms of this gene appear to have decreased expression in the setting of colitis representing the first example of altered circular RNA expression in UC patient samples. Mechanistically, linear and circular CDKN2B-AS1 affects the proliferative capacity of colonic epithelial cells. Reductions in expression of CDKN2B-AS1 enhance the barrier formation of colonic epithelial monolayers through disruptions in Claudin-2 expression.
2. Methods
2.1. Clinical samples and microarray
Normal controls and patients with a clinical and histological diagnosis of ulcerative colitis with active endoscopic inflammation were obtained by colonic biopsy from the left colon from the University of Chicago after obtaining informed consent in accordance with University policies under IRB 15573A. Anonymized samples analyzed at UCLA under approved experimental protocols. Control patients included patients who had no colonic inflammation or adenomas at the time of colonoscopy. The biobank excluded patients under the age of 18 and who used anti-coagulants. An Qiagen miRNeasy kit was used to extract RNA from de-identified samples per manufacturers recommendations. Arraystar performed the lncRNA microarray. For the circular form of CDKN2B-AS1 19 controls and 17 UC samples were used. For the linear form of CDKN2B-AS1 10 controls and 10 UC samples were used. For the microarray 7 controls and 8 UC samples were used.
In situ assay - Freshly cut 10 um sections from formalin fixed paraffin embedded sections were deparrafinized in xylene for 5 min, then dehydrated in 100% ethanol for 5 min. Slides were then air dried overnight. Slides were baked and an RNA-scope 2.5 brown kit (Advanced Cell Diagnostics) was used for subsequent steps according the manufacturer. The CDKN2B-AS1 probe used contained the antisense sequence to base-pairs 9–1252. All probes were from Advanced Cell Diagnostics.
2.2. Cell culture
Caco2 BBE cells were purchased from the American Type Culture Collection. Caco2 BBE cells were grown in FBS (Sigma Aldrich) that was screened for tetracycline activity. Trypsin and PBS were from Corning. To induce down-regulation of CDKN2B-AS1, cells were treated for 5 days with 1 μg/ml doxycycline hyclate (Fisher Scientific).
2.3. Cell line production
SMARTvector inducible lentiviral shRNA glycerol stocks were purchased from Dharmacon. The sequences for the shRNAs are as follows: Target 1, 5′- TTCCAAATAGATCTCCCCG −3′; Target 2, 5′- TTGAATC AGAATGAGGCTT −3′. The control vector contained a non-targeting sequence (#VSC11651). Viruses were produced using a generation 2 lentiviral production system in HEK293T cells. Resulting viruses were the titered on Caco2 BBE cells before subsequent infection at an MOI < 1. After transduction, 2 μg/ml Puromycin (Calbiochem) was used to select to transduced cells.
2.4. RNA extraction, cDNA synthesis and qPCR
An Aurum RNA mini (Biorad) was used to extract, DNase, and purify total RNA from cells according to the manufacturer. For cDNA synthesis, an iScript cDNA kit (Biorad) was used to reverse transcribe 1 μg RNA into cDNA according the manufacturer. A CFX 384 well Real-Time system (Biorad) was used to amplify and detect SYBR green (Biorad) mediated qPCR reactions. To analyze tight junction mRNA levels, the PrimePCR tight junction array (Biorad) was used. The following primers were used: linear CDKN2B-AS1 (F-AGCTCAGAGCAATTCCAGTG CAAG, R-GCTGACCAGTGTGCTGCAAAGC), circular CDKN2B-AS1 (F-GCTGGGATTACAGGTGTGAGACACC), R-GAATCAGAATGAGGCTTATT CTTCTCATC), p15 (F-GTTTACGGCCAACGGTGGAT, R- GCCCATCATC ATGACCTGGA), HPRT1 (F-GACCAGTCAACAGGGGACAT, R- GGCTGG CCTATAGGCTCATAGTGC), GAPDH (F-GGGTGTGAACCATGAGAAGT, R- CCTTCCACGATACCAAAGTT), 36 s pre-rRNA (F-GGTCCCGTTTGCT GTCTC, R- TAGCTGCGTTCTTCATCGAC). All primers were purchased from Integrated DNA Technologies. The comparative Ct method [27], relative to HPRT1/Hprt1, was used to calculate fold change.
2.5. Western blots
Confluent monolayers were washed in PBS and harvested in laemmli buffer. To shear genomic DNA, lysates were slowly passed through 20 ga and 25 ga needles. Lysates were boiled for 5 min before loading 20 μl of each sample on 4–20% SDS containing polyacrylamide gels (Biorad). A TransBlot turbo kit was used to transfer gels onto PVDF (Biorad). Membranes were then washed in PBS and then blocked in western blot blocking reagent (Biorad). Blots were then washed in PBS before primary antibody incubation overnight. Primary antibodies were diluted in 5% BSA in PBS. Streptavidin conjugated secondary antibodies were from Jackson Immunolabs. A clarity ECL kit (BioRad) and ChemiDox Touch imager (BioRad) were from were used according to manufacturer’s instructions. When probing for Claudin-4, Claudin-2 blots were stripped using western blot stripping reagent (ThermoFisher) according to manufacturer’s instructions. Both Claudin-2 (#PA5–13335) and Claudin-4 (#32–9400) antibodies were from ThermoFisher. The actin antibody (#A2066) was from Sigma Aldrich.
2.6. Immunofluorescence and immunohistochemistry
For immunofluorescence, qPCR validated CDKN2B-AS1 downregulated cells and control shRNA cells were fixed with 1% formaldehyde (Fisher Scientific). After 0.3% Triton X100 mediated permeablization and bovine serum albumin blocking, cells were incubated with an anti-PES1 (Abcam, ab72539) antibody at the concentration of 1:100. Secondary antibodies were from Invitrogen. Nuclei were stained with DAPI and prolong gold (Invitrogen) was used as the mount media. Slides were images on a confocal microscope (Zeiss). Double blinded images were quantified by three independent reviewers. For immunohistochemistry, cells were washed 3 times in PBS and then incubated in a non-enzymatic cell dissociation for 3 min. Cells were then pelleted and fixed in 4% formaldehyde overnight. Cell pellets were then embedded in histogel and paraffinized. A pH 6.0 sodium citrate solution was used for antigen retrieval after re-hydration. Cells were blocked in bovine serum albumin and incubated with primary antibodies overnight. Claudin-2 (#PA5–13335) and Occludin (#71–1500) antibodies were from ThermoFisher.
2.7. Barrier assay
One-hundred and fifty thousand cells were plated on 6.5 mm inserts containing 0.4 μm polyester membranes. STX2 electrodes and an EVOM from World Precision Instruments were used to measure transepithelial electrical resistance.
2.8. Proliferation assays
For cell counting assays, 50,000 cells were plated in 24-well plates in quadruplicate. Each day post seeding cells were trypsinized, counterstained with trypan blue and counted under a light microscope. For xcelligence mediated proliferation assays (ACEA Biosciences) 5000 cells were plated in 96-well gold plated plates and normalized to the cell index at the 6-h time-point.
2.9. RNase R treatment
RNase R was purchased from Epicentre. 1 μg RNA was treated with buffer alone or RNase R according to manufacturer’s instructions.
2.10. Nuclear/Cytoplasmic fractionation
A PARIS kit (Invitrogen) was used according to the manufacturer’s instructions.
2.11. Apoptosis assay
Twenty-thousand cells were plated in 96 well plates. Three to four days after plating cells were treated with 2 μM staurosporine or DMSO (1:1000). Eight hours after staurosporine treatment 50 μl of CaspaseGlo caspase 3/7 assay reagent (Promega) was added to the wells and luminescence was measured by a IVIS imaging system (Perkin Elmer).
2.12. Statistics
A students t-test was used to determine significance between control and experimental samples.
3. Results
3.1. CDKN2B-AS1 is decreased in whole colon biopsies from actively inflamed ulcerative colitis patients
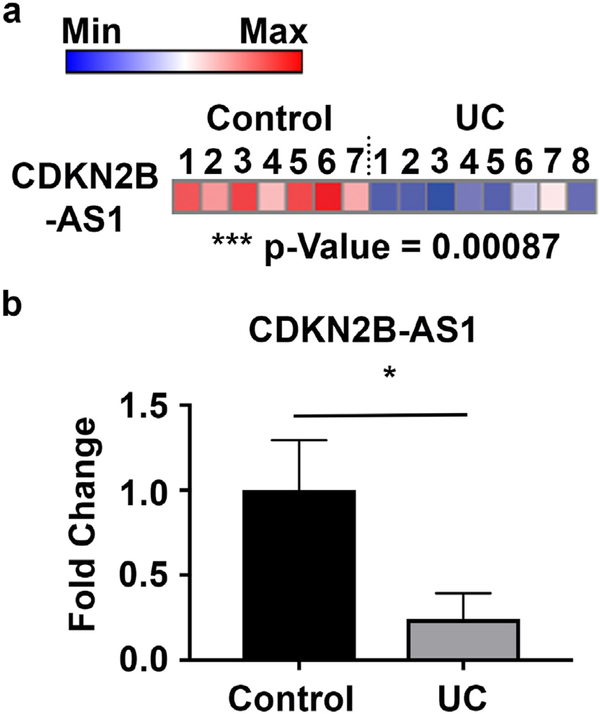
To better understand the role for long non-coding RNAs in the pathogenesis of IBD we performed a lncRNA microarray of colonic biopsies from healthy controls and actively inflamed IBD patients. One of the most significantly decreased genes on the array was CDKN2B-AS1 with a 12.2-fold downregulation and p = 0.00087 (Fig. 1a). This result is similar to that reported by others that have shown that CDKN2B-AS1 is significantly down-regulated in both ulcerative colitis and Crohn’s disease patients [7,12,13]. Given the reproducibility and large decrease of CDKN2B-AS1 in IBD patients, we sought to validate and better understand the role for CDKN2B-AS1 in colitis. We first attempted to detect the possible murine lncRNA ortholog to CDKN2B-AS1, Gm12610. However, a variety of PCR-based methods on both purified epithelial and immune cell populations in wild type and colitic mice failed to detect the presence of Gm12610 (data not shown), similar to findings that Holdt et al. also reported [24]. Therefore, to better understand the mechanistic role of CDKN2B-AS1 in IBD, we focused on studies involving human tissue and cell lines.
Fig. 1.
CDKN2B-AS1 is decreased in whole colon biopsies from actively inflamed ulcerative colitis patients. (a) Heat map representation of microarray results of CDKN2B-AS1 levels in IBD patients or controls. (b) qPCR validation of the alterations in CDKN2B-AS1 RNA levels in ulcerative colitis patients compared to controls. Mean ± SEM. For the validation n = 10 samples per group. For the microarray n = 7–8 samples per group. * p < 0.05.
3.2. CDKN2B-AS1 forms circular splice forms that are altered in ulcerative colitis patients with active inflammation
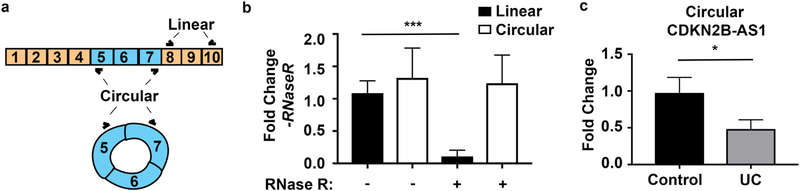
To validate the microarray results, we analyzed the expression of CDKN2B-AS1 in an independent cohort by qPCR in colonic biopsies obtained from healthy controls or ulcerative colitis patients. Similar to microarray results, we observed a 5-fold decrease in CDKN2B-AS1 in UC-active patients compared to controls p = 0.0365 (Fig. 1b). Interestingly, CDKN2B-AS1 has been reported to form back-spliced circular transcripts [24,26]. Therefore, to test the hypothesis that CDKN2B-AS1 also forms circular transcripts, we performed RNase R experiments. RNase R is an enzyme that degrades linear but not circular RNA. We observed that while linear CDKN2B-AS1 was almost entirely degraded by RNase R, outward facing primers to detect circular CDKN2B-AS1 still produced similar amounts of signal as compared to buffer controls (Fig. 2a). Previously, microarray technology measuring transcript expression was not equipped to measure circular RNAs. Additionally, RNA-sequencing techniques have not analyzed for circular RNA expression in IBD samples. For these reasons, circular RNA expression in CDKN2B-AS1 has not previously been investigated. We hypothesized that circular CDKN2B-AS1 might also be downregulated in IBD patients and may be important in IBD. Using whole colon biopsies and primers directed at the circular form of CDKN2B-AS1, we observed a 2-fold decrease in circular CDKNB-AS1 in UC-active patients compared to controls (Fig. 2b).
Fig. 2.
CDKN2B-AS1 forms circular splice forms that are altered in ulcerative colitis patients with active inflammation. (a) A graphical representation of both linear and circular CDKN2B-AS1 and primer sets used to detect them. (b) RNA was purified from the colonic epithelial cell line, Caco2 BBE and was subjected to either RNase R or RNase R buffer treatment. Subsequent RNA was reverse transcribed and qPCR was performed against the linear form of CDKN2B-AS1 or the circular form, exons 7–5. Mean ± SEM. 3 independent experiments. *** p < 0.001. (c) Whole colon biopsy RNA from healthy controls or inflamed Ulcerative colitis patients was reverse transcribed and outward facing primers against exons 7 and 5 were amplified. Mean ± SEM. n = 17 controls and 19 UC samples. * p < 0.05.
3.3. CDKN2B-AS1 primarily localizes to the colonic epithelium
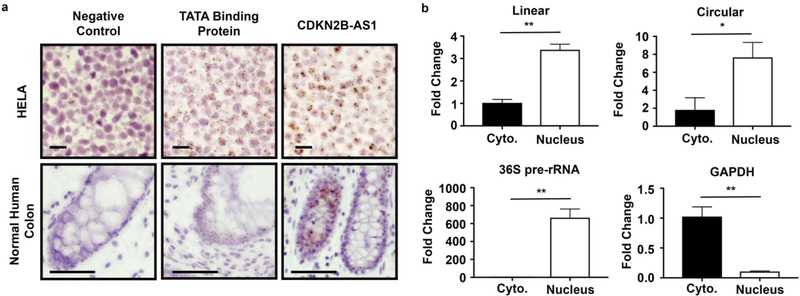
To identify the cell specificity of CDKN2B-AS1, in situ probes were first tested on HELA cell pellets and then on normal human colon biopsies (Fig. 3a). A probe directed at all annotated transcripts of CDKN2B-AS1 localized from the epithelial crypt base to the villus in the human colon. Additionally, in both HELA and colonic epithelial cells, CDKNB-AS1 displayed a nuclear localization. To ensure both epithelial and immune cells contained viable RNA, sections were analyzed for TATA binding protein mRNA signal. Indeed, both immune cells and epithelial cells were positive for TATA protein mRNA (Fig. 3a). Lastly, a negative control probe against the bacterial gene dapB did not produce a signal in either HELA or colonic epithelial cells (Fig. 3a). To support the in situ results, we fractionated the nucleus from the cytoplasm in a colonic epithelial cell line, purified RNA and performed qPCR. Similar to in situ results, both linear and circular forms of CDKN2B-AS1 primarily fractionated to the nuclear fraction (Fig. 3b). To ensure proper extraction, the non-spliced form of a pre-rRNA, which is restricted to the nucleus, was measured in both cytoplasmic and nuclear fractions and was seen to be 500-fold elevated in the nuclear compartment as compared to cytoplasmic fractions (Fig. 3b). Additionally, the positive control for a cytoplasmic localized mRNA, GAPDH, was 10-fold elevated in the cytoplasmic compartment compared to nuclear in these samples, confirming the interpretation of that CDKN2B-AS1 is localized to the nucleus (Fig. 3b).
Fig. 3.
CDKN2B-AS1 primarily localizes to the colonic epithelium. (a) In situ images from HELA or normal human colon samples. The negative control was against dapB, or a gene expressed in bacteria not present in humans. The positive control was against the TATA binding protein mRNA that is the ubiquitously expressed general transcription factor. The probe for CDKN2B-AS1 contained the antisense sequence against the longest transcript expressed, which would indicate the localization of both linear and circular forms. Top scale bar = 20 μm, Bottom scale bar = 100 μm. (b) After separating nuclear and cytoplasmic fractions of Caco2 BBE cells RNA was purified and qPCR performed against CDKN2B-AS1. GAPDH was used a cytoplasmic localized RNA control and 36S pre-rRNA was used a nuclear localized RNA control. Mean ± SEM. 3 independent experiments. * p < 0.05, ** p < 0.01.
3.4. CDKN2B-AS1 inhibits colonic epithelial cell proliferation
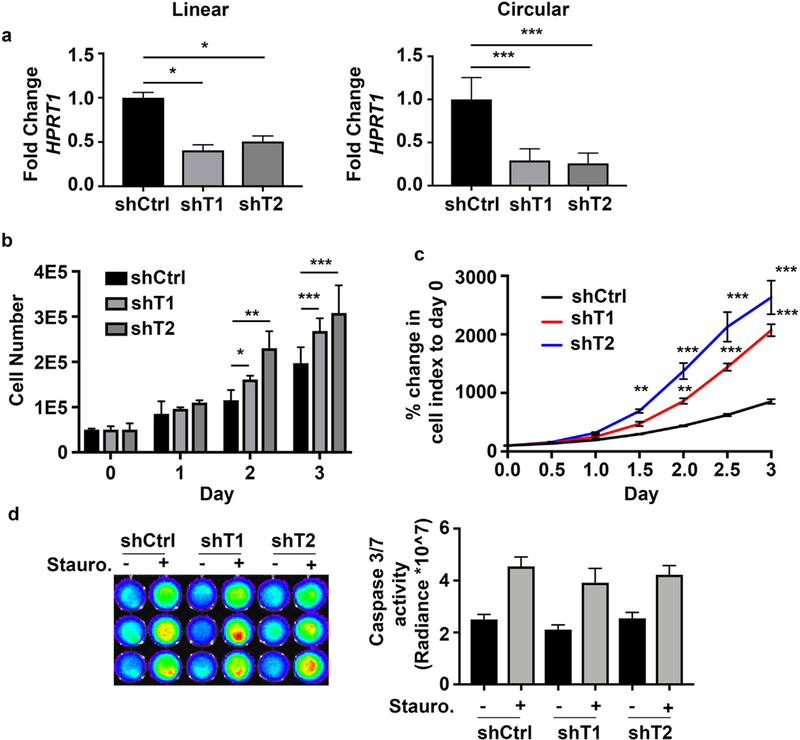
Given that CDKN2B-AS1 is only expressed in human cells, we elected to study a human colonic epithelial system with the capacity to measure barrier function, Caco2 BBE cells. To test if CDKN2B-AS1 regulates basic cellular functions of colonic epithelial cells we first measured the ability of CDKN2B-AS1 depleted Caco2 BBE to proliferate. As IBD patients displayed reduced linear and circular levels of CDKN2B-AS1, shRNAs were designed against exons 5–7 to downregulate both linear and circular CDKN2B-AS1. After doxycycline induction, two distinct shRNAs against CDKN2B-AS1 were able to downregulate the linear form to 50–60% and the circular form to near 80% compared to non-targeting control shRNA cells (Fig. 4a). Cell proliferation was first measured by cell count using a hemocytometer after down-regulation of CDKN2B-AS1. Both cell lines with down-regulated CDKN2B-AS1 displayed significantly elevated cell numbers after induction of the shRNAs (Fig. 4b). We next performed another assay to measure cell proliferation which creates a cell index based on impedance of cells on a gold matrix (xCelligence Real Time Cell Analysis, Acea Biosystems, Inc). Similar to hemocytometer results both cell lines with down-regulated CDKN2B-AS1 had an elevated cell index over time (Fig. 4c). Another aspect of epithelial barrier function is apoptosis. Therefore, cells with down-regulated CDKN2B-AS1 were treated with and without the apoptosis inducing chemical, staurosporine, and caspase 3/7 activity was assayed as a measure of apoptosis. At baseline there were no differences in apoptosis, nor were there any alterations after the induction of apoptosis (Fig. 4d).
Fig. 4.
CDKN2B-AS1 inhibits colonic epithelial cell proliferation. (a) Control shRNA (shCtrl) Caco2 BBE cells contained a non-targeting shRNA sequence. Two different shRNAs (shT1, shT2) directed at exons 5–7 were used to down-regulate both linear and circular CDKN2B-AS1 in Caco2 BBE cells. (b) Parallel sets of cells were counted with a hemocytometer over time until cells were no longer sub-confluent. Mean ± SEM. 3 independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001. (c) An xcelligence assay was used to determine cell proliferation over time. Mean ± SEM. 3 independent experiments * p < 0.05, *** p < 0.001. (d) Eight hours after staurosporine or DMSO (control) treatment caspase 3/4 activity was measured.
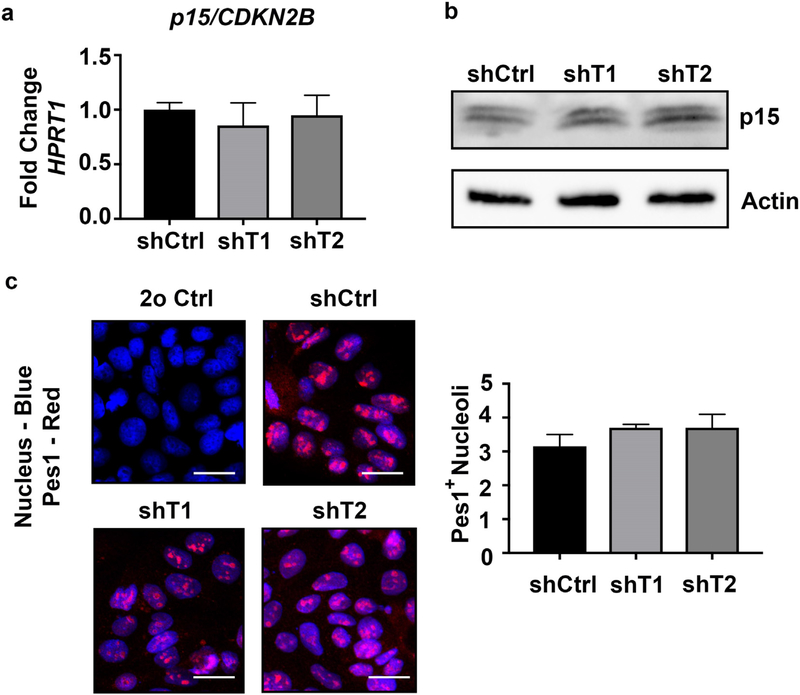
3.5. Down-regulation of CDKN2B-AS1 in colonic epithelial cells does not affect p15 levels or PES1 localization
We next wanted to determine how CDKN2B-AS1 is involved in cell proliferation. CDKN2B-AS1 has been shown to regulate the cis-related cell-cycle inhibitory gene p15/CDKN2B [22], however qPCR analyses revealed that in colonic epithelial cells, p15/CDKN2B mRNA and protein levels were not altered in cells with CDKN2B-AS1 knockdown (Fig. 5a and b). Additionally, previous publications have suggested that the circular, but not linear form, of CDKN2B-AS1 can negatively regulate proliferation thorough an inhibition of ribosome maturation [24]. The binding of CDKN2B-AS1 to PES1, an rRNA splicing complex protein, was seen to regulate the number of PES1-positive nucleoli. Therefore, we immuno-stained PES1 in control and CDKN2B-AS1 downregulated cells and counter stained with a nuclear stain to quantify the number PES1 positive nucleoli. Both the downregulated and control cell lines displayed, on average, 3 PES1 positive nucleoli (Fig. 5c).
Fig. 5.
Down-regulation of CDKN2B-AS1 in colonic epithelial cells does not affect p15 levels or PES1 localization. (a) RNA was harvested from control shRNA (shCtrl) Caco2 BBE cells and Caco2 BBE cells containing two different shRNA sequences (shT1, shT2) against CDKN2B-AS1. qPCR was performed against p15 and HPRT1 to normalize samples. Mean ± SEM. 3 independent experiments. (b) After harvesting protein from sub-confluent cells western blots were performed against p15 and actin. (c) Pes1 immunostaining in shCtrl, shT1 and shT2 cells. Scale Bar = 50 μm. Double blinded images were quantified by three separate reviewers and the experiment was replicated 3 times. Mean ± SEM.
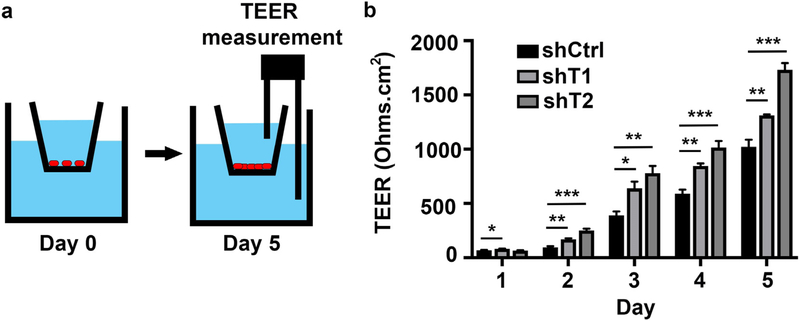
3.6. CDKN2B-AS1 antagonizes colonic epithelial barrier function
We next investigated the effects of CDKN2B-AS1 on barrier function, as this is a critical function of epithelial cells and an intrinsic component in the pathogenesis of IBD. To assess barrier function, we measured transepithelial electrical resistance (TEER), which is quantifies ability of ions to cross an epithelial monolayer (Fig. 6a). We plated control and CDKN2B-AS1-deficient cells on transwell inserts and measured their TEER over 5 days. Two days after plating both cell lines with down-regulated CDKN2B-AS1 had an increased TEER compared to control cells that persisted throughout the experimental time course (Fig. 6b). Four-kilodalton dextran did not flux through the junctions in any of the cell lines at the endpoint (data not shown). Importantly, the effects of proliferation were minimized as cells were plated at a near excess on day zero.
Fig. 6.
CDKN2B-AS1 antagonizes colonic epithelial barrier function. (a) On day 0 cells are plated on semi-permeable transwell inserts. By day 5 trans-epithelial resistance reaches maximal levels. (b) The day after plating (Day 1) trans-epithelial electrical resistance was measured and repeated daily. Mean ± SD. Representative results of 3 independent experiments. * p < 0.05, *** p < 0.001.
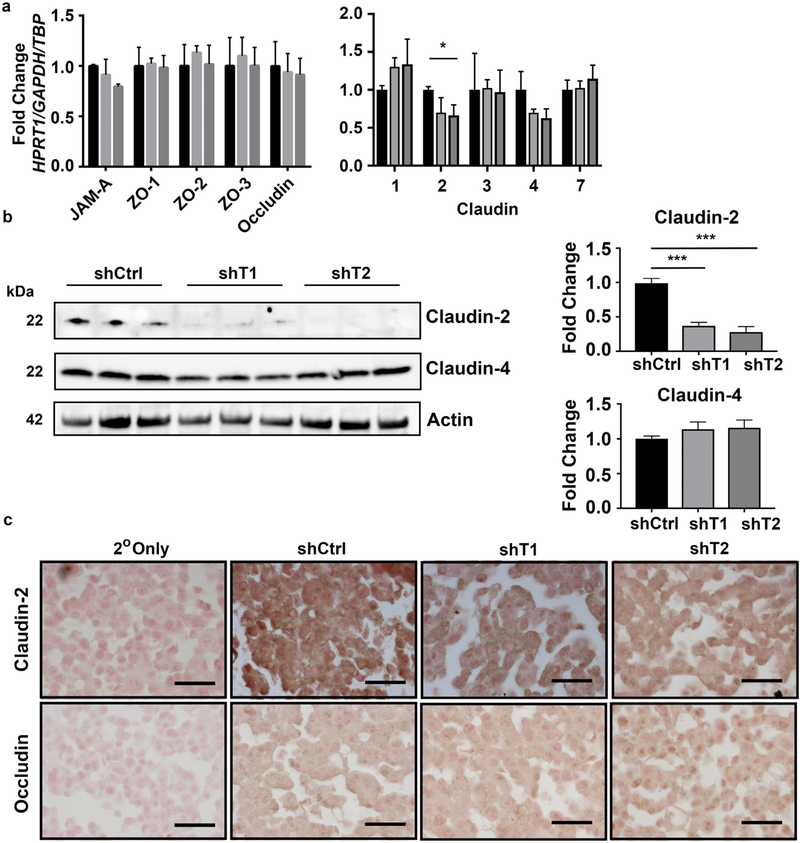
3.7. CDKN2B-AS1 regulates Claudin-2
As Claudin family members are the major molecules that regulate barrier by forming either tight or leaky pores between cells [28], we next performed an mRNA based array against tight junction proteins, including the Claudin family of proteins. While some were highly variable between replicates, of the 5 with the most consistent measurements only the leaky Claudin, Claudin-2, was observed to be significantly altered (Fig. 7a) [29]. Lastly, we measured Claudin-2 protein levels by western blot in control and CDKN2B-AS1 downregulated cells and observed an almost entire loss in Claudin-2 in CDKN2B-AS1 downregulated cells as compared to controls (Fig. 7b). We did not observe any differences in the tight Claudin, Claudin-4, in cells with downregulated CDKN2B-AS1 (Fig. 7b). As an alternative method to determine the relative levels of Claudin-2 immunohistochemistry was performed on cells that were lightly dissociated and pelleted as immunofluorescence staining for Claudin-2 was unsuccessful in Caco-2 BBE cells (data not shown). In control cells Claudin-2 staining was diffuse and dark brown whereas in both cell lines with down-regulated CDKN2B-AS1 a lighter brown was observed suggesting lower levels of Claudin-2 (Fig. 7c). The signal intensity for Occludin on serial sections was similar between all three conditions (Fig. 7c).
Fig. 7.
CDKN2B-AS1 regulates Claudin-2. (a) A tight junction PCR array was used to determine the difference in mRNA levels of major tight junction proteins. Mean ± SD. Samples were assayed in triplicate. * p < 0.05, ** p < 0.01, *** p < 0.001. (b) Whole cell protein was harvested on cells a day after confluence and western blots were performed against Claudin-2, Claudin-4 or Actin. Western blots were cut at below the 37 kD marker and the top section was probed for Actin while the bottom section were probed for Claudin-2 and Claudin-4. Mean ± SEM. 2 independent experiments. ** p < 0.01, *** p < 0.001. (c) Paraffin embedded cell pellets were stained for either Claudin-2 or Occludin. Scale bars = 50 μm.
4. Discussion
Many studies have profiled protein coding-genes in IBD patients compared to healthy individuals and have shown significant changes in key mediators of IBD pathophysiology. These studies led to the identification of treatments or biomarkers for IBD such as TNF therapies or calprotectin assays. In 2015 and 2016, three manuscripts were published that the characterized an understudied class of RNA molecules named long non-coding RNAs that are altered in IBD patients [7,12,13]. However, the function of the majority of lncRNAs which are dysregulated in IBD patients is not known [13]. We chose to study one of the most significantly decreased lncRNAs in IBD patients, CDKN2B-AS1. CDKN2B-AS1 has a complicated gene structure with over 20 splice variants including back-spliced circular RNA molecules. We determined that longest linear splice variant and major circular form of CDKN2B-AS1 were decreased in IBD biopsies. Finding that the possible mouse ortholog was undetectable in purified cell populations we decided to study CDKN2B-AS1 in human colonic cells as in situ results identified the majority of all CDKN2B-AS1 was expressed in those cells exclusively.
We observed in histology from normal colons, a probe recognizing all forms of CDKN2B-AS1 localized to the nucleus of epithelial cells. Another study has performed in situ against CDKN2B-AS1 in atheroslectoric plaques and they observed a similar nuclear localization that co-stained with smooth muscle and macrophage cells. In the colon, we were only able to detect CDKN2B-AS1 in epithelial cells and did not observe smooth muscle staining (data not shown). We chose to downregulate both the majority of the linear forms and the majority of the circular forms, as to mimic the alterations observed in IBD patients. This was accomplished with shRNAs directed against exons 5 and 6. However, a caveat of this study as compared to others studying CDKN2B-AS1 is the dual loss of both linear and circular forms in this study. We observed that loss of both linear and circular forms of CDKN2B-AS1 increased cell proliferation and enhanced barrier function.
As CDKN2B-AS1 is predominantly nuclear it is unlikely to bind to tight junction localized proteins to alter their function. Therefore, we tested if CDKN2B-AS1 alters the mRNA levels of barrier conferring proteins and eventually determined that Claudin-2 protein levels were dramatically decreased in cells lacking CDKN2B-AS1. To better understand why the cells lacking CDKN2B-AS1 proliferation we analyzed the mRNA and protein levels of cis-related cell cycle inhibitor gene Cyclin Dependent Kinase Inhibitor 2B (CDKN2B, p15) however this gene was not altered in intestinal epithelial cells with down-regulated CDKN2B-AS1. Circular CDKN2B-AS1 has been implicated in altering the localization of the ribosomal assembly protein PES1, however we did not observe alterations in PES1 localization. These differences between our study and others could be due to different splice variants of CDKN2B-AS1 expressed different cell types, inherent differences in cell type specific functions of CDKN2B-AS1, or co-downregulation of the linear and circular forms of CDKN2B-AS1. To answer these questions future studies should analyze the effects of down-regulating specific splice variants on intestinal epithelial cell physiology.
Our results represent the first investigation of circular RNA biology in IBD pathophysiology. While continued research is needed to understand this area of biology, this study highlights the importance of identifying new arenas in which to investigate potential mechanistic insights. Our finding that down-regulation of CDKN2B-AS1 enhances barrier function suggests that the loss in patients could be a protective mechanism to enhance barrier after insult. It was clear that down-regulation of CDKN2B-AS1 resulted in a loss of Claudin-2, which has been shown to form a leaky epithelial barrier [28]. Previous clinical studies have shown that Claudin-2 is increased in IBD samples [30,31]. The seeming discrepancy between this clinical data and our experimental results highlight the complexity of signaling and gene regulation within clinical IBD samples. It is possible that multiple pathways other than CDKN2B-AS1 are regulating Claudin-2. Our results demonstrate CDKN2B-AS1 important role in colonic epithelial biology and warrant further investigation into additional mechanisms and potential uses in IBD.
Dysregulation of circular RNAs has been seen in a few areas of biology such as oncology and cardiac research. While the significance of circular RNA biology is still being investigated, these novel area of investigation have the potential to enhance our understanding of the disease process. To date, there have been no unbiased high-throughput analyses of circular RNAs in IBD pathophysiology. Microarray technology or RNA-seq analysis could be performed that recognize circular RNA splice junctions and profile the differentially expressed circular RNA variants critical for IBD pathophysiology. As circular RNAs have been seen to have potent cellular functions, this represents a next step in IBD transcriptomics with the aim of developing the next generation of prognostic, diagnostic, and therapeutic tools.
5. Conclusions
Long non-coding RNA biology is a relatively new field of interest in IBD research. Our study focuses on a key lncRNA that is commonly dysregulated in ulcerative colitis patients. Interestingly, the lncRNA, CDKN2B-AS1 forms both linear and circularized RNA transcripts. Mechanistically, CKDN2B-AS1 down-regulation results in increased colonic barrier integrity possibly through decreases in Claudin-2 expression. By investigating lncRNA biology in IBD, new and exciting areas of biology are opened up for understanding this debilitating disease.
Acknowledgments
Grant support
J.P. is supported by U of C DDRCC: NIDDK P30DK42086. C.P. is supported by RO1 DK60729 and P30 DK 41301-26. D.P. is supported by CCFA career development award, CURE DDRC DK41301, and UCLA CTSI UL1TR0001881. The UCLA Integrated Molecular Technologies core was supported by CURE/P30 grant DK041301.
Footnotes
Competing interests
The authors declare no competing interests.
References
- [1].Molodecky NA, Soon IS, Rabi DM, et al. , Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review, Gastroenterology 142 (2012) 46–54 e42 quiz e30. [DOI] [PubMed] [Google Scholar]
- [2].Ananthakrishnan AN, Epidemiology and risk factors for IBD, Nat Rev Gastroenterol Hepatol 12 (2015) 205–217. [DOI] [PubMed] [Google Scholar]
- [3].Rutgeerts P, Vermeire S, Van Assche G, Biological therapies for inflammatory bowel diseases, Gastroenterology 136 (2009) 1182–1197. [DOI] [PubMed] [Google Scholar]
- [4].Papamichael K, Cheifetz AS, Therapeutic drug monitoring in IBD: the new standard-of-care for Anti-TNF therapy, Am. J. Gastroenterol 112 (2017) 673–676. [DOI] [PubMed] [Google Scholar]
- [5].Targownik LE, Bernstein CN, Infectious and malignant complications of TNF inhibitor therapy in IBD, Am. J. Gastroenterol 108 (2013) 1835–1842 quiz 1843. [DOI] [PubMed] [Google Scholar]
- [6].Iyer MK, Niknafs YS, Malik R, et al. , The landscape of long noncoding RNAs in the human transcriptome, Nat. Genet 47 (2015) 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Padua D, Mahurkar-Joshi S, Law IK, et al. , A long noncoding RNA signature for ulcerative colitis identifies IFNG-AS1 as an enhancer of inflammation, Am. J. Physiol. Gastrointest. Liver Physiol 311 (2016) G446–G457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Atianand MK, Hu W, Satpathy AT, et al. , A long noncoding RNA lincRNA-EPS acts as a transcriptional brake to restrain inflammation, Cell 165 (2016) 1672–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carpenter S, Aiello D, Atianand MK, et al. , A long noncoding RNA mediates both activation and repression of immune response genes, Science 341 (2013) 789–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Salzman J, Gawad C, Wang PL, et al. , Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types, PLoS One 7 (2012) e30733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen LL, The biogenesis and emerging roles of circular RNAs, Nat Rev Mol Cell Biol 17 (2016) 205–211. [DOI] [PubMed] [Google Scholar]
- [12].Mirza AH, Berthelsen CH, Seemann SE, et al. , Transcriptomic landscape of lncRNAs in inflammatory bowel disease, Genome Med 7 (2015) 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wu F, Huang Y, Dong F, et al. , Ulcerative colitis-associated long noncoding RNA, BC012900, regulates intestinal epithelial cell apoptosis, Inflamm. Bowel Dis. 22 (2016) 782–795. [DOI] [PubMed] [Google Scholar]
- [14].Gomez JA, Wapinski OL, Yang YW, et al. , The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-gamma locus, Cell 152 (2013) 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nobori T, Miura K, Wu DJ, et al. , Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers, Nature 368 (1994) 753–756. [DOI] [PubMed] [Google Scholar]
- [16].Wrensch M, Jenkins RB, Chang JS, et al. , Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility, Nat. Genet 41 (2009) 905–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hamada K, Kohno T, Kawanishi M, et al. , Association of CDKN2A(p16)/CDKN2B (p15) alterations and homozygous chromosome arm 9p deletions in human lung carcinoma, Genes Chromosomes Cancer 22 (1998) 232–240. [DOI] [PubMed] [Google Scholar]
- [18].Holdt LM, Beutner F, Scholz M, et al. , ANRIL expression is associated with atherosclerosis risk at chromosome 9p21, Arterioscler. Thromb. Vasc. Biol 30 (2010) 620–627. [DOI] [PubMed] [Google Scholar]
- [19].Burdon KP, Macgregor S, Hewitt AW, et al. , Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1, Nat. Genet 43 (2011) 574–578. [DOI] [PubMed] [Google Scholar]
- [20].Shea J, Agarwala V, Philippakis AA, et al. , Comparing strategies to fine-map the association of common SNPs at chromosome 9p21 with type 2 diabetes and myocardial infarction, Nat. Genet 43 (2011) 801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li WQ, Pfeiffer RM, Hyland PL, et al. , Genetic polymorphisms in the 9p21 region associated with risk of multiple cancers, Carcinogenesis 35 (2014) 2698–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yu W, Gius D, Onyango P, et al. , Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA, Nature 451 (2008) 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kotake Y, Nakagawa T, Kitagawa K, et al. , Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene, Oncogene 30 (2011) 1956–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Holdt LM, Stahringer A, Sass K, et al. , Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans, Nat. Commun 7 (2016) 12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Holdt LM, Hoffmann S, Sass K, et al. , Alu elements in ANRIL non-coding RNA at chromosome 9p21 modulate atherogenic cell functions through trans-regulation of gene networks, PLoS Genet. 9 (2013) e1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Burd CE, Jeck WR, Liu Y, et al. , Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk, PLoS Genet. 6 (2010) e1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schmittgen TD, Livak KJ, Analyzing real-time PCR data by the comparative C(T) method, Nat. Protoc 3 (2008) 1101–1108. [DOI] [PubMed] [Google Scholar]
- [28].Van Itallie CM, Anderson JM, Claudins and epithelial paracellular transport, Annu. Rev. Physiol 68 (2006) 403–429. [DOI] [PubMed] [Google Scholar]
- [29].Amasheh S, Meiri N, Gitter AH, et al. , Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells, J. Cell Sci 115 (2002) 4969–4976. [DOI] [PubMed] [Google Scholar]
- [30].Zeissig S, Burgel N, Gunzel D, et al. , Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease, Gut 56 (2007) 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Weber CR, Nalle SC, Tretiakova M, et al. , Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation, Lab. Investig 88 (2008) 1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]