Abstract
Tumor patients are at a high risk of venous thromboembolism (VTE), and the mechanism by which this occurs may involve tumor-derived microvesicles (MVs). Previously, it has been shown that tumor MVs become attached to endothelial cells in static conditions. To investigate whether this process occurs under physiologically relevant flow rates, tumor MVs were perfused across a microfluidic device coated with growing human umbilical vein endothelial cells (HUVECs). Cell lines were screened for their ability to form tumor spheroids, and two cell lines, ES-2 and U87, were selected; spheroids formed were transferred to a microfluidic chip, and a second endothelial cell biochip was coated with HUVECs and the two chips were linked. Media flowed through the spheroid chip to the endothelial chip, and procoagulant activity (PCA) of the tumor media was determined by a one-stage prothrombin time assay. Tumor MVs were also quantified by flow cytometry before and after interaction with HUVECs. Confocal images showed that HUVECs acquired fluorescence from MV attachment. Labeled MVs were proportionally lost from MV rich media with time when flowed over HUVECs and were not observed on a control chip. The loss of MV was accompanied by a proportional reduction in PCA. Flow cytometry, confocal microscopy, and live flow imagery captured under pulsatile flow confirmed an association between tumor MVs and HUVECs. Tumor MVs attached to endothelial cells under physiological flow rates, which may be relevant to the VTE pathways in cancer patients.
INTRODUCTION
Microfluidic technology is already showing potential in areas related to medicine and medical diagnostics through the manipulation of biological samples and the use of miniaturized devices.1 Microfluidic culture systems are able to function, assess, and provide data for nanoenvironments through their ability to mimic in vivo biological systems onto closely resembling in vitro microfluidic environments.2 Moreover, microfluidics has the ability to handle microliter volumes in microchannels of 1 μm–1000 μm and where fluid flow is strictly laminar and concentrations of molecules can be well-controlled. Since the early 1990s, this technology has been used for biological research methods for specific analyses such as polymerase chain reaction and DNA microarrays and also appears to be an ideal tool for the study of cancer.3 As such, microfluidics has been utilized to study tumor biopsies.
Tumors are known to release subcellular extracellular vesicles (EVs) composed of both larger (100–1000 nm) microvesicles (MVs) and nanosized exosomes (<100 nm) into the bloodstream.4,5 MVs are shed from cells via a number of pathways such as apoptosis and membrane remodeling.6,7 MVs are released into the circulation where they can then be detected in blood samples using a standardized flow cytometry technique.8 MVs were originally thought to be simply inert cellular debris, but they have been found to play a number of roles depending on the antigens they retain from the parent cells.9,10 Target cells are modulated by MV through their capacity to facilitate cell-to-cell interactions, where proteins and mRNA are transferred to neighboring cells, raising the expression of protein on the target cell membrane and inducing cell signaling.11,12 MVs have been implicated in the prothrombotic state associated with cancer, and Tissue Factor (TF)-bearing MVs (TFMVs), in particular, are found in cancer patients' plasma and have been suggested as a possible risk factor for the occurrence of venous thromboembolism (VTE).13
Many tumor cells express TF, especially cancers that originate in the epithelium, and TFMVs are spontaneously released into the circulation by these tumors.14,15 TF is a transmembrane, 47-KDa-glycoprotein16 and the key activator for hemostasis, serving as the protein component of tissue thromboplastin.17 TF also plays a vital role in a number of cellular processes including intracellular signaling, cell proliferation, and blood vessel development.18
TFMVs are found in the blood of healthy individuals19 as well as those with cancer, but levels tend to be higher in cancer patients; this has been recorded in a number of malignancies including breast cancer,20 colorectal cancer,21 and pancreatic cancer.22,23 Together, these results suggest the potential of TFMVs as a biomarker identifying those who are a high thrombosis risk among cancer patients.24,25 The procoagulant potential of TFMVs mostly depends on the presence of TF, which can drive coagulation26 and also anionic phospholipid expression, particularly phosphatidylserine (PS). Lacroix and Dignat-George26 describe MVs that contain both PS and TF as particularly procoagulant,27 and a significant number of prothrombotic conditions have been reported to have elevated MV numbers in plasma.28
A number of studies have also found links between TFMV and thrombosis using in vivo mice models. One such experiment by Thomas et al.29 involved infusion of MVs derived from cancer cells that showed an accumulation at the injury site as well as a reduction in the tail bleeding time and the time of arteriole and venule occlusions. The study showed that MV derived from cancer cells and carrying TF and P-selectin glycoprotein ligand 1 (PSGL-1) were active in forming an in vivo thrombus.29 The shedding by a tumor of TF-bearing MVs through leaking blood vessels in the tumor mass, tumor-induced upregulation of TF expression in monocytes and endothelial cells, and upregulation of endothelial cell TF expression by chemotherapeutic agents together led to elevated circulating TF levels.30 Tumors that are sensitive to chemotherapy would be more likely to cause VTE, given that such tumors are more likely to shed greater numbers of MV via apoptosis.31 The involvement of TF in tumor progression has also been demonstrated via hematogenous metastasis.32,33 In vitro data have also confirmed a role for TFMV in coagulation and thrombin-generation34,35 and have been shown to promote metastasis through angiogenesis, immune suppression, cancer cell survival, and invasion.13 All of these processes require the ability to interact with the endothelium.
A microfluidic device has been shown previously to be capable of extracting antigen-specific MVs from biologically complex samples, such as serum and conditioned medium from cultured cells. The majority of MVs isolated via this method retained their native morphology.36 Wu et al. developed a microfluidic platform that first filters red blood cells out from blood and then further analyses the remaining vesicles based on their smaller size; this way, over 99% of RBCs can be removed from the initial sample, and the exosomes with the desired size were further purified with an efficiency of over 98%.37 Therefore, microfluidic devices are ideal candidates to study the translation of biomarkers such as tumor MVs to study clinically relevant questions.
The purpose of this present work is to investigate how tumor MVs are able to interact with endothelial cells in vitro utilizing a microfluidic platform. Understanding more fully the mechanisms of endothelial involvement in thrombotic events may help in the development of better therapeutic solutions in cancer management.
MATERIALS AND METHODS
Cell lines and culture
The ovarian carcinoma cell line ES2 (ATCC, UK) and glioblastoma cell line U87 (ATCC, UK) were seeded at 1 × 106/ml cells into a 25 cm² tissue culture flask (Sarstedt, UK) and left to adhere overnight at 37 °C in a 5% CO2 incubator and maintained in McCoy's 5A media or DMEM, respectively, supplemented with 10% (v/v) Fetal Bovine Serum (FBS) and 1% (v/v) Penicillin/Streptomycin (all Lonza, UK). Spheroids were formed using ultralow adherence 96-well plates (ThermoFisher, UK) seeded with 2 × 105 cells and cultivated over 5–7 days prior to use. Primary human umbilical vein endothelial cells (HUVECs; PromoCell, Heidelberg, Germany) were cultured in complete endothelial cell growth media (ECGM, PromoCell). HUVECs were seeded at 1 × 106/ml cells into 25 cm² cell+ tissue culture flasks (Sarstedt) and cultured at 37 °C in a 5% CO2 incubator. HUVECs were utilized at passages 3–6.
Procoagulant activity
The procoagulant potential of cell-free supernatant and cells was measured using the semiautomated Thrombotrack SOLO coagulometer. This machine works by mechanical detection of the clotting end point method. Samples (100 μl) were placed into a cuvette containing a steel ball and 25 mM CaCl2 (100 μl) were added; finally, 100 μl of control plasma (NormTrol, Helena Biosciences, UK) were added, and the time taken for clot formation (prothrombin time, PT) was automatically determined.
CFSE staining protocol
MV released from ES-2 and U87 tumor cells were labeled via 5(6)-carboxyfluorescein diacetate N-hydroxysuccinimidyl ester (CFSE) staining of the parent cells. Harvested cells (1 × 106 cells/ml) from ES-2 and U87 cancer cells were suspended in 1 ml phosphate buffered saline (PBS) and incubated with a CellTrace™ CFSE dye (Invitrogen, UK) at 5 μM as a final working concentration and incubated for 20 min at room temperature or 37 °C in the dark. Stained cells were washed twice with PBS and seeded into a series of 25 cm2 cell culture flasks in 10 ml of the appropriate medium and incubated for 24 h at 37 °C and 5% CO2. Unlabeled cells were used as the negative control.
Microfluidic chips
Two chips were used for the HUVEC experiments, either a μ-Slide I Luer (Ibidi, Germany) or a Vena8 endothelial cell biochip (Cellix, Ireland). Slides were treated with UV irradiation for 20 min and coated by dispensing approximately 12 μl of type B 2% v/v gelatin (Sigma Aldrich, UK) into the channel. Control chips were also treated in the same way to account for nonspecific binding. Then, the biochips were then incubated for 24 h at 4 °C. Cultured HUVECs (2000 cells) were added into each channel, and the reservoirs were filled with 60 μl of the media. The biochips were incubated in the CO2 incubator for 24 h at 37 °C. Then, labeled/unlabeled MVs were perfused over the HUVECs for 6 h and PCA, MV quantification, and microscopy images were assessed to evaluate MV interaction with HUVECs. Control chips without HUVECs were done in parallel to the experimental setup.
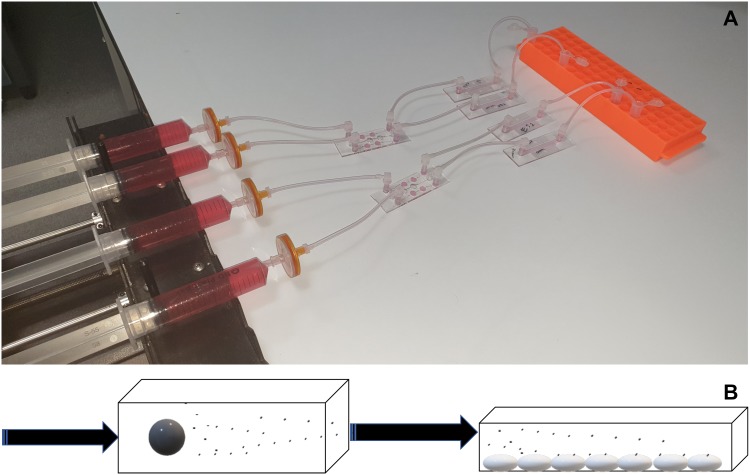
A μ-slide III 3D perfusion was used to hold the spheroids and then flow was applied via a syringe pump (4 μl/min). A μ-Slide I Luer was precoated with HUVECs and was attached to the output of the μ-slide III 3D chip as shown in Figs. 1(a) and 1(b). Samples were then collected via the output of the μ-Slide I Luer into sterile 1.5 ml polypropylene tubes.
FIG. 1.
(a). Basic experimental setup showing tumor fresh media contained in syringes linked to a multiwell μ-slide III 3D chip containing either ES-2 or U87 spheroids linked through to a μ-Slide I Luer containing HUVECs and finally sample collection tubes. The experiments were carried out in a 37 °C incubator. (b). Schematic of the experimental setup. Media were flowed via either a syringe pump (constant flow) or a Kima pump (pulsatile flow) through a microfluidic chip containing tumor spheroids. MVs are released from the spheroids into the media, which is then connected to a second chip coated with HUVECs to study their interaction.
Ultrafiltration
CFSE-labeled MV cell-free media were harvested from ES2 and U87 cells and centrifuged at 300g at 4 °C for 4 min to remove detached cells. Supernatant (6 ml) was collected and filtered through Vivaspin® 6 ml concentrators (Sartorius, UK). MVs were recovered from the media concentrate and PCA was assessed. The molecular weight cutoff was 100 kDa; MVs were presumed not to pass through as filtrate due to their relatively large size in comparison to the cutoff value.
Flow cytometry
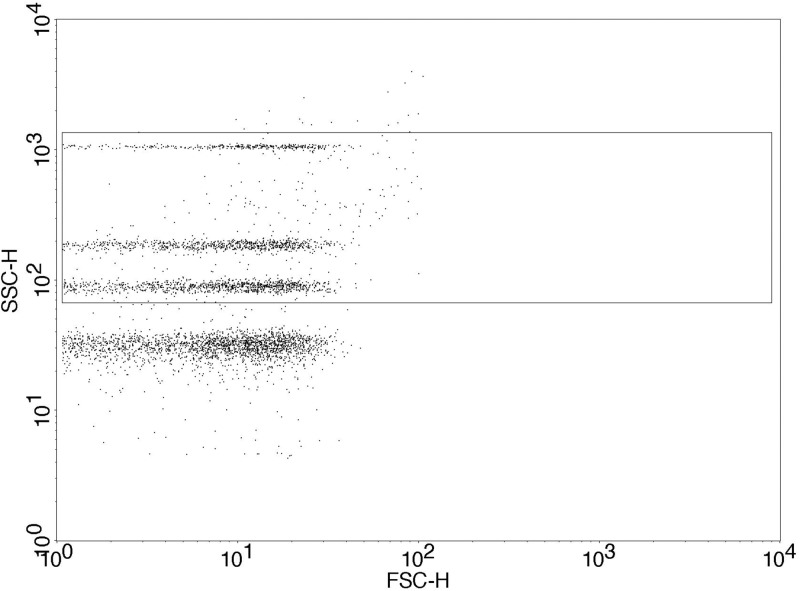
CFSE-labeled MVs released from ES2 and U87 tumor cells were quantified by flow cytometry before and after being passed through the biochip. 50 μl of labeled [either CFSE or anti-TF: FITC (Bio-rad)] and unlabeled samples were immediately analyzed by flow cytometry by adding an equal volume of Accucheck beads (Invitrogen, UK) and 150 μl of 0.2 μm-filtered sterile PBS. Unlabeled MV samples were used as negative control. A flow cytometer (BD FACSCalibur) was setup with Megamix SSC beads (Biocytex, France) that are used to define a MV gate according to side scatter characteristics of the beads (Fig. 2) following the manufacturer's protocol.
FIG. 2.
Defined MV gate based on Megamix SSc beads’ manufacturer protocol. The box around the 3 differentially sized Megamix SSc beads on side scatter represents the MV gate set at approximately 0.2–0.5 μm. The lowest SSc beads are 0.16 μm and do not form part of the MV gate.
Microscopy
The μ-Slide I Luer microfluidics biochips were coated with HUVECs cells and washed twice with PBS to investigate the immobilization of (CFSE)-labeled MVs. Labeled/unlabeled MVs of ES2 and U87 cell-free medium were perfused over the HUVECs for 6 h. Confocal microscopy was performed using a Zeiss LSM710 Laser Scanning Confocal Microscope, and images were acquired using ZEN software (Zeiss Group, Oberkochen, Germany).
The interaction of MV on HUVECs was further studied using an automated microfluidic platform (VenaFlux and Vena8 Endothelial+ biochips; Cellix, Dublin, Ireland) in order to mimic the physiological flow status. The Vena8 chip was coated with HUVECs (same conditions as previously described) and connected to a Kima pump (Cellix), which delivers pulsatile flow with shear stress at 450 μl/min for 6 min followed by 5 min of absence of flow. The flow chamber was then connected to the Mirus Evo Nanopump (Cellix), the channels were rinsed three times with 25 μl of media prior to each experiment, and MV adhesion was initiated by the addition of CFSE-labeled MV supernatant (ES-2 and U87) and unlabeled MV as well. Interaction of MV was recorded every second under a shear stress of 1 dyne/cm2 in phase contrast, and the settings were equal in all conditions (exposure time 344 ms, magnification 32×) for 5 min.
RESULTS
Procoagulant activity
PCA of ES-2 and U87 cells and media were assessed via the one-stage PT assay. For the same concentration of cells within the assay (3 × 105), the PT was similar between ES2 (33.0 s) and U87 (32.6 s). The cell-free media harvested at the same time was shown in both cells line to be procoagulant with ES-2 media supporting a PT of 76.9 ± 3.4 s (n = 4) and U87 media was less procoagulant with a PT of 137.1 ± 4.3 s (n = 4). Ultrafiltration using a Vivaspin (100 kDa MWCO) was shown to remove all associated PCA of the filtrated media confirming that the PCA was MV associated whereas the concentrate diluted with fresh media to the original volume was shown to retain and slightly increase PCA (ES2: 41.4 ± 9.2 s; U87: 112.8 ± 13.3 s).
TF labeling of MV and interaction with HUVECs
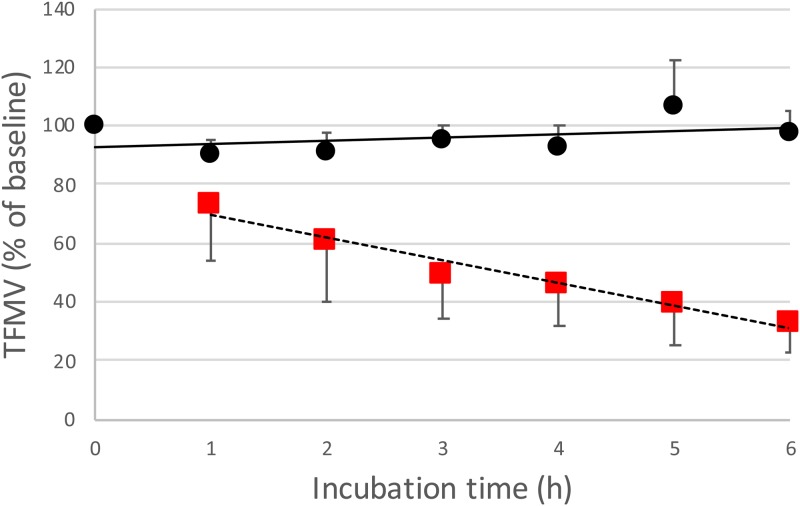
Initially, TF labeling (anti-human TF: FITC) of MV from media of ES-2 and U87 spheroids cultured on a μ-slide 3D chip was used to quantify the interaction with HUVECs under flow and the relationship between TFMV with PCA. Over a time course of 6 h, TFMV linearly decreased (through 1–6 h) from the media collected after perfusion across HUVECs on a μ-slide Luer chip when compared to a coated control chip containing no HUVECs (Fig. 3).
FIG. 3.
TFMVs of ES-2 and U87 as a percentage of baseline values (n = 8) when perfused across a μ-Slide I Luer containing cultured HUVECs (red square) or a gelatin control chip (black circle) with no HUVECs present (n = 4) for 6 h. Error bars are SD.
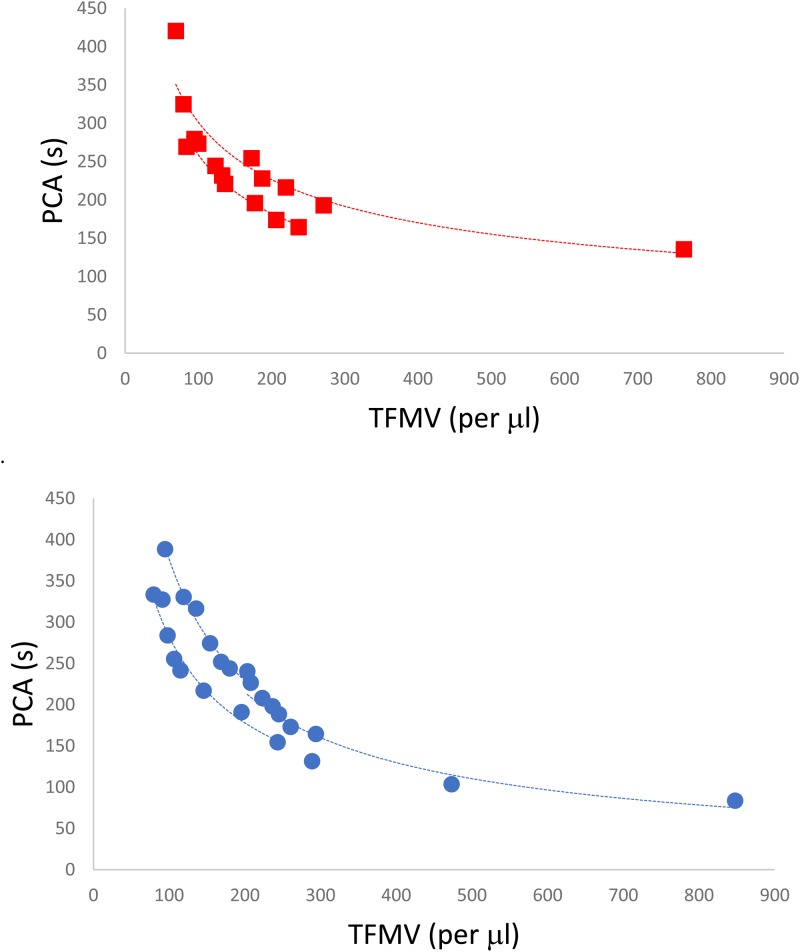
The loss of TFMV after perfusion across HUVEC coated slides was further investigated through analysis of the PCA associated with the media following perfusion across the 6 h time frame. A clear power relationship was observed between TFMV and PCA for both cell lines, and the subsequent loss of detected TFMV over time with HUVECs perfusion resulted in a slower PCA (Fig. 4). Control samples of MV rich tumor media passed through gelatin coated μ-slide Luer chips showed no change in PCA across the 6 h experimental window (ES-2: 224.7 ± 4.8 s; U87: 190.2 ± 7.4 s). HUVECs were analyzed by flow cytometry for TF expression postperfusion and showed a mean fluorescent ratio (fluorescent intensityTF/fluorescent intensitynegative control) increase relative to control HUVECs (ratio of 1) to 2.41 ± 0.13 for HUVECs perfused with ES-2 MV and 2.16 ± 0.26 for HUVECs perused with U87 MV (n = 4).
FIG. 4.
Relationship between TFMV and PCA of U87(red square, n = 16) and ES-2 (blue circle, n = 24) media when perfused over HUVECs for 6 h. Lines of best fit are for a power relationship and R2 values range from 0.904 to 0.985.
CFSE labeling and detection of tumor MV
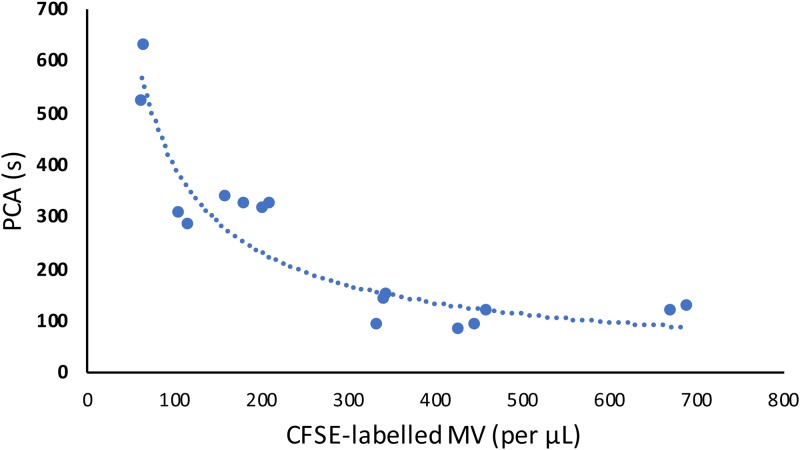
To visualize the observed interaction between MV and HUVECs under flowing conditions, ES2 and U87 (1 × 106 cells/ml) were fluorescently labeled with CFSE and incubated for 24 h. Cell-free media from each tumor cell line was then harvested and perfused over HUVECs adhered to a microfluidic chip (μ-Slide I Luer) for 6 h (equating to approximately 6.7 × 105 total MVs). PCA and MV quantifications were determined before and after being perfused over HUVECs. The MV gate was defined using Megamix SSc beads, and CFSE-labeled MV could be clearly identified on a fluorescence plot in comparison to unlabeled MV (Fig. 5). Independent measurements showed a relationship between PCA and the quantity of CFSE-labeled MV (Fig. 6).
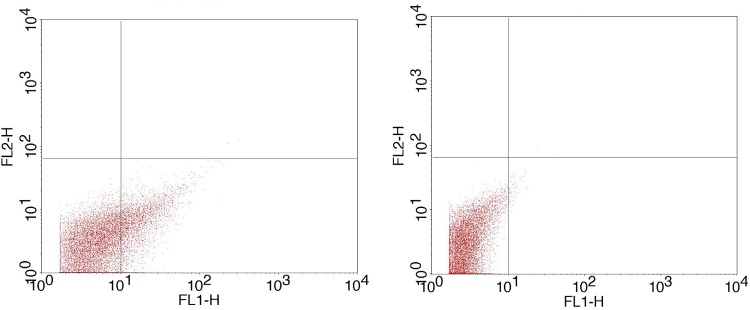
FIG. 5.
CFSE fluorescently labeled (left panel) and unlabeled (right) MV populations. The individual MV events in the lower right quadrant correspond to CFSE-labeled MVs (increased FL-1 signal), which were then quantified using counting beads.
FIG. 6.
The correlation between CFSE- labeled MV with PCA of tumor media (n = 16, independent measurements).
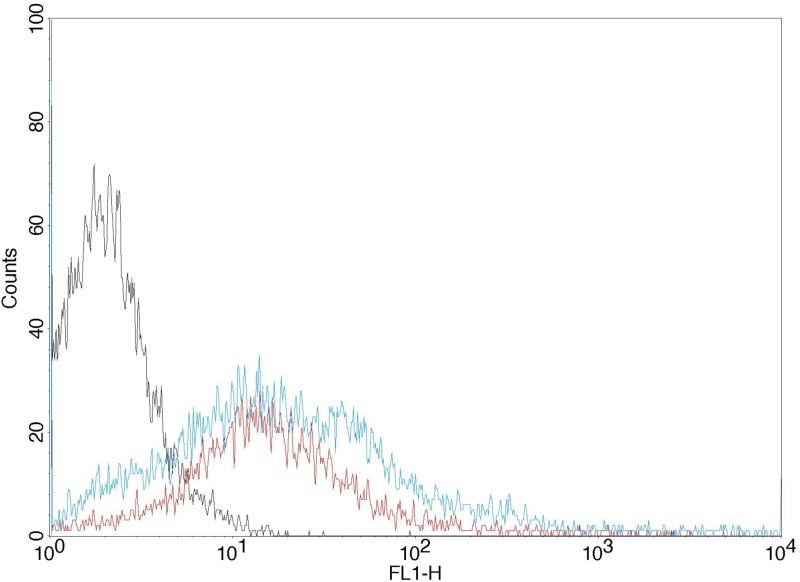
HUVECs were detached from the μ-Slide I Luer chip at the end of the 6 h period and analyzed for PCA and the acquisition of fluorescent properties from the CFSE-labeled MVs. The PCA of HUVECs (3 × 105 per assay) incubated with ES2 tumor media was 149 ± 0.5 s and 127 ± 1.2 s for HUVECs incubated with U87 tumor media. The PCA of HUVECs with fresh (no TFMV) media was 370 ± 17.5 s. The cells were further characterized by flow cytometry, and the results showed an increased fluorescence (gained from labeled tumor MV) for HUVECs compared to the control cells (Fig. 7). The mean fluorescence ratio of HUVECs relative to control increased to 10.52 ± 1.77 for HUVECs perfused with ES-2 CFSE-labeled MV and 7.53 ± 0.64 for HUVECs perused with U87 MV (n = 3).
FIG. 7.
Representative (n = 3) histogram plot of fluorescence of HUVECs perfused with U87 or ES-2 MV rich media for 6 h, compared to HUVECs perfused with control media (left peak). MVs were labeled with CFSE from the parent cell.
Confocal microscopy
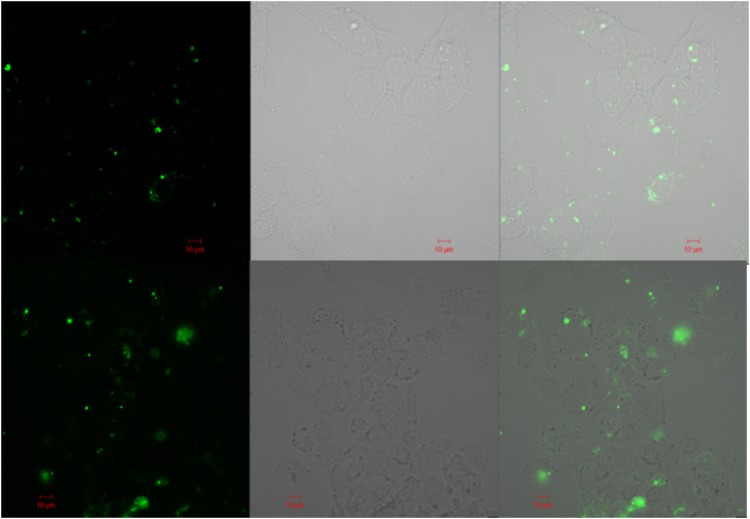
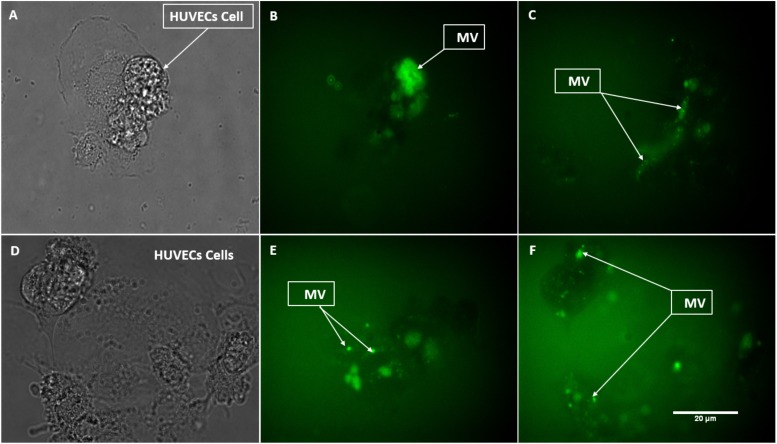
To further define the MV interaction with HUVECs, CFSE-labeled MVs were perfused over HUVECs on a μ-Slide I Luer channel for 6 h and then washed and analyzed by confocal microscopy (Fig. 8). Images obtained showed fluorescence localized at the surface of HUVECs.
FIG. 8.
Confocal microscopy of HUVECs incubated on a μ-Slide I Luer perfused with tumor media (ES-2 top panels, U87 bottom panels) with CFSE-labeled MVs. The left panels correspond to the fluorescent detection channel, middle panels are brightfield detection channel, and the right panels are the combined images.
Automated image capture under flow
The Cellix system allows for live image capture under physiological flow conditions and was utilized to further confirm that the association of tumor MV with HUVECs, observed by confocal microscopy, was not due to any period of static flow between the experiment and analysis. CFSE-MVs were constantly passed over a Vena8 microfluidic chip precoated with HUVECs, and images were captured “as live” under flowing conditions. MVs were again observed to be associated with HUVECs (Fig. 9).
FIG. 9.
Still images captured from a live recording showing CFSE-labeled MV aggregated on HUVECs under flow. The top panel [(a)–(c)] shows labeled ES-2 MV deposition. (a) shows HUVEC cells with unlabeled ES2-MV. (b) and (c) show two different MV depositions with HUVECs. (d) shows U87 unlabeled MV with HUVECs. (e) and (f) represent the labeled U87 MV deposition on HUVECs.
DISCUSSION
In this study, we demonstrate for the first time that MVs formed in vitro from tumor spheroids interact with endothelial cells under flow conditions in a dual microfluidic chip assembly. After initially adhering to the HUVEC surfaces in a static condition, TFMVs were shown to associate with HUVECs under dynamic flow conditions in a time dependent way. The correlation of loss of PCA and reduction in detected TFMV when tumor media was passed over HUVECs under flow suggests direct evidence for PCA being determined by TFMV concentration and also that TFMVs are lost due to their association with the endothelial cells. Control chips coated with gelatin but without HUVECs showed no loss of MV or PCA through the experiments; therefore, the observed loss can be attributed to the interaction with HUVECs. We have previously shown that PCA is linked to tumor spent media concentration23 in a power relationship, as also observed here for the MV concentration (Fig. 4). The observation that the concentration of spent tumor media concentration and quantified MV both determine PCA suggests that the MVs in tumor media are responsible for the associated PCA. Fluorescent and confocal microscopy (Figs. 8 and 9) clearly showed fluorescence attributed to the presence of CFSE-labeled MV on HUVECs within the microfluidic chip after tumor MVs were perfused over the cells. From both TF and CFSE analysis of HUVECs post perfusion, the ES-2 MV conferred an increased PCA and showed a greater fluorescence when compared to U87 MV suggesting that more MVs were associated with HUVECs. ES-2 MV rich media were also found to possess a greater PCA prior to incubation with HUVECs, which may be indicative of a greater MV concentration. After removal of MV from the media via ultrafiltration (Vivaspin, 100 kDa), the filtrate no longer supported coagulation. The pore size of these filtration units would allow soluble TF to pass through (47 kDa), if present in a monomeric or dimeric form, and so the data suggest that the PCA associated with tumor conditioned media is MV dependent as discussed above.
A possible limitation of the study would be whether the interaction with endothelial cells seen here is ubiquitous and occurs for MVs derived from any cell exposed to the circulation. However, the acquisition of a more procoagulant HUVEC phenotype via the acquisition of TF expressing tumor MV as shown here would be more specific to tumor-derived MV. Furthermore, it was shown that although flow cytometry is currently the only standardized method8 across laboratories for enumeration of MV, the method has a size limitation where smaller MV cannot be detected.38 The methodology described here, using Megamix beads, creates an MV size window of detection of 0.2–0.5 μm (Fig. 2).
The binding of procoagulant tumor MVs to endothelial cells could have relevance to the in vivo mechanism of VTE formation in cancer patients where TFMVs have been proposed to be associated with a high thrombosis risk.24,25 If tumor MVs are able to bind to endothelial cells within the circulation, then this could be a basis for increased procoagulant potential. Future work should focus on the exact mechanism of tumor MV binding to endothelial cells and the response of endothelial cells to the stimuli in terms of activation, apoptosis, or altered cell surface marker expression.
It has been proposed that MV endocytosis by endothelial cells occurs through interaction between anionic phospholipids at the MV surface and endothelial cell surface expressed αvβ3 integrin.39 This process was shown to be inhibited in the presence of annexin V and the internalization of MV, and subsequent protein digestion at the MV surface by trypsin additionally provides evidence of a phospholipid role in the binding of MVs to endothelial cells.39 The engulfment and recycling of MV through the Rab family of the Golgi-endosomal transport network has also been demonstrated.40 Furthermore, there is evidence of TF–VIIa–protease-activated receptor (PAR) 2 signaling in thrombin generation (and activation of other transmembrane G protein-coupled receptors) leading to transcription of prothrombotic genes, signal transduction amplification cascades, and also the establishment of tumors.41,42 PAR1 can also transactivate PAR2, which can promote an extra thrombin generation response in the endothelium and tumor environment.43
When cells are exposed to inflammatory cytokines, leukocytes are more likely to undergo microvesiculation and are, therefore, capable of the production of TFMV, which may become associated with developing thrombus via P-selectin glycoprotein ligand 1–P-selectin interactions and also may stabilize the thrombus by fibrin formation induction.44,45 Neutrophils can also recruit TFMV,46 and the extracellular traps that they project have been demonstrated in vitro to serve as an adherence site for tumor-derived TFMV.47 This may be a significant process for localizing TFMV and concentrating additional TF into the developing thrombus. There is also evidence to suggest that MVs are able to transfer their procoagulant potential to other cell types and in doing so can exacerbate endothelial activation48 as suggested here. While TF expression can be induced in cultured endothelial cells in response to inflammatory, in vivo it is probable that the TF associated with endothelial cells is derived from TFMV released by monocytes or tumor cells.49,50 Moreover, the expression of TF on the endothelium in response to both monocyte-derived MVs and inflammatory mediators accompanies the concomitant translocation of phospholipids, such as PS, that could enhance the binding of coagulation factors.51 The induction of endothelial cell apoptosis is related to MV generation and downregulation of TF pathway inhibitor thrombomodulin and glycosaminoglycans such as heparan sulfate on the endothelial surface.52 The resultant impairment of activation of the protein C anticoagulant pathway and reduced antithrombin III activity may be attributed to the disrupted integrity of the endothelium. In addition, activated endothelial cells express cell surface adhesion molecules that increase platelet adhesion and attract monocytes and neutrophils, all of which might further contribute to coagulation initiation or amplification.53,54
In summary, we report a microfluidic two-chip setup which showed that tumor MV released from spheroids bind to endothelial cells under dynamic, physiologically relevant flow conditions.
CONCLUSION
Tumor-derived procoagulant MVs were shown to become associated with endothelial cells under flow conditions within a dual microfluidic setup. Tumor MVs were shown to be the cause of procoagulant activity in vitro.
ACKNOWLEDGMENTS
This research was funded through a Ph.D. scholarship from the Northern Border University (Saudi Arabia) to A.A.
The authors declare no conflict of interest.
REFERENCES
- 1.Chen C., Skog J., Hsu C.-H., Lessard R. T., Balaj L., Wurdinger T., Carter B. S., Breakefield X. O., Toner M., and Irimia D., “Microfluidic isolation and transcriptome analysis of serum microvesicles,” Lab Chip 10(4), 505–511 (2010). 10.1039/B916199F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choudhury D., Ramsay W. T., Kiss R., Willoughby N. A., Paterson L., and Kar A. K., “A 3D mammalian cell separator biochip,” Lab Chip 12(5), 948–953 (2012). 10.1039/c2lc20939j [DOI] [PubMed] [Google Scholar]
- 3.Zhang Z. and Nagrath S., “Microfluidics and cancer: Are we there yet?,” Biomed. Microdevices 15(4), 595–609 (2013). 10.1007/s10544-012-9734-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freyssinet J. M., “Cellular microparticles: What are they bad or good for?,” J. Thromb. Haemost. 1(7), 1655–1662 (2003). 10.1046/j.1538-7836.2003.00309.x [DOI] [PubMed] [Google Scholar]
- 5.Minciacchi V. R., Freeman M. R., and Di Vizio D., “Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes,” Semin. Cell Dev. Biol. 40, 41–51 (2015). 10.1016/j.semcdb.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aupeix K., Hugel B., Martin T., Bischoff P., Lill H., Pasquali J.-L., and Freyssinet J.-M., “The significance of shed membrane particles during programmed cell death in vitro, and in vivo, in HIV-1 infection,” J. Clin. Invest. 99(7), 1546–1554 (1997). 10.1172/JCI119317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnouf T., Chou M.-L., Goubran H., Cognasse F., Garraud O., and Seghatchian J., “An overview of the role of microparticles/microvesicles in blood components: Are they clinically beneficial or harmful?,” Transfus. Apher. Sci. 53(2), 137–145 (2015). 10.1016/j.transci.2015.10.010 [DOI] [PubMed] [Google Scholar]
- 8.Lacroix R., Dubois C., Leroyer A., Sabatier F., and Dignat-George F., “Revisited role of microparticles in arterial and venous thrombosis,” J. Thromb. Haemost. 11, 24–35 (2013). 10.1111/jth.12268 [DOI] [PubMed] [Google Scholar]
- 9.Nomura S. and Shimizu M., “Clinical significance of procoagulant microparticles,” J. Intensive Care 3(1), 2 (2015). 10.1186/s40560-014-0066-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shai E. and Varon D., “Development, cell differentiation, angiogenesis—Microparticles and their roles in angiogenesis,” Arterioscler. Thromb. Vasc. Biol. 31(1), 10–14 (2011). 10.1161/ATVBAHA.109.200980 [DOI] [PubMed] [Google Scholar]
- 11.Essayagh S., Xuereb J.-M., Terrisse A.-D., Tellier-Cirioni L., Pipy B., and Sié P., “Microparticles from apoptotic monocytes induce transient platelet recruitment and tissue factor expression by cultured human vascular endothelial cells via a redox-sensitive mechanism,” Thromb. Haemost. 98(10), 831–837 (2007). 10.1160/TH07-02-0082 [DOI] [PubMed] [Google Scholar]
- 12.Simak J. and Gelderman M. P., “Cell membrane microparticles in blood and blood products: Potentially pathogenic agents and diagnostic markers,” Transfus. Med. Rev. 20(1), 1–26 (2006). 10.1016/j.tmrv.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 13.Hisada Y., Alexander W., Kasthuri R., Voorhees P., Mobarrez F., Taylor A., McNamara C., Wallen H., Witkowski M., and Key N. S., “Measurement of microparticle tissue factor activity in clinical samples: A summary of two tissue factor-dependent FXa generation assays,” Thromb. Res. 139, 90–97 (2016). 10.1016/j.thromres.2016.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Nedawi K., Meehan B., Micallef J., Lhotak V., May L., Guha A., and Rak J., “Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells,” Nat. Cell Biol. 10(5), 619 (2008). 10.1038/ncb1725 [DOI] [PubMed] [Google Scholar]
- 15.Khorana A. A., Ahrendt S. A., Ryan C. K., Francis C. W., Hruban R. H., Hu Y. C., Hostetter G., Harvey J., and Taubman M. B., “Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer,” Clin. Cancer Res. 13(10), 2870–2875 (2007). 10.1158/1078-0432.CCR-06-2351 [DOI] [PubMed] [Google Scholar]
- 16.Han X., Guo B., Li Y., and Zhu B., “Tissue factor in tumor microenvironment: A systematic review,” J. Hematol. Oncol. 7(1), 54 (2014). 10.1186/s13045-014-0054-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bach R. R., “Tissue factor encryption,” Arterioscler. Thromb. Vasc. Biol. 26(3), 456–461 (2006). 10.1161/01.ATV.0000202656.53964.04 [DOI] [PubMed] [Google Scholar]
- 18.Abdulkadir S. A., Carvalhal G. F., Kaleem Z., Kisiel W., Humphrey P. A., Catalona W. J., and Milbrandt J., “Tissue factor expression and angiogenesisin human prostate carcinoma,” Hum. Pathol. 31(4), 443–447 (2000). 10.1053/hp.2000.6547 [DOI] [PubMed] [Google Scholar]
- 19.Madden L. A., Vince R. V., Sandström M. E., Taylor L., McNaughton L., and Laden G., “Microparticle-associated vascular adhesion molecule-1 and tissue factor follow a circadian rhythm in healthy human subjects,” Thromb. Haemost. 99(11), 909–915 (2008). 10.1160/TH08-01-0030 [DOI] [PubMed] [Google Scholar]
- 20.Trappenburg M. C., van Schilfgaarde M., Bredewold E. O., van Aalderen M. C., Spronk H. M., ten Cate H., Leyte A., and Terpstra W. E., “Elevated numbers and altered subsets of procoagulant microparticles in breast cancer patients using endocrine therapy,” Thromb. Res. 127(4), 363–369 (2011). 10.1016/j.thromres.2010.12.015 [DOI] [PubMed] [Google Scholar]
- 21.Hron G., Kollars M., Weber H., Sagaster V., Quehenberger P., Eichinger S., Kyrle P. A., and Weltermann A., “Tissue factor-positive microparticles: Cellular origin and association with coagulation activation in patients with colorectal cancer,” Thromb. Haemost. 97(01), 119–123 (2007). 10.1160/TH06-03-0141 [DOI] [PubMed] [Google Scholar]
- 22.Thaler J., Ay C., Mackman N., Metz-Schimmerl S., Stift J., Kaider A., Müllauer L., Gnant M., Scheithauer W., and Pabinger I., “Microparticle-associated tissue factor activity in patients with pancreatic cancer: Correlation with clinicopathological features,” Eur. J. Clin. Invest. 43(3), 277–285 (2013). 10.1111/eci.12042 [DOI] [PubMed] [Google Scholar]
- 23.Yates K. R., Welsh J., Echrish H. H., Greenman J., Maraveyas A., and Madden L. A., “Pancreatic cancer cell and microparticle procoagulant surface characterization: Involvement of membrane-expressed tissue factor, phosphatidylserine and phosphatidylethanolamine,” Blood Coagulation Fibrinolysis 22(8), 680–687 (2011). 10.1097/MBC.0b013e32834ad7bc [DOI] [PubMed] [Google Scholar]
- 24.Dvorak H., Quay S., Orenstein N., Dvorak A., Hahn P., Bitzer A., and Carvalho A., “Tumor shedding and coagulation,” Science 212(4497), 923–924 (1981). 10.1126/science.7195067 [DOI] [PubMed] [Google Scholar]
- 25.Falanga A., Marchetti M., and Vignoli A., “Coagulation and cancer biological and clinical aspects,” J. Thromb. Haemost. 11(2), 223–233 (2013). 10.1111/jth.12075 [DOI] [PubMed] [Google Scholar]
- 26.Lacroix R. and Dignat-George F., “Microparticles as a circulating source of procoagulant and fibrinolytic activities in the circulation,” Thromb. Res. 129, S27–S29 (2012). 10.1016/j.thromres.2012.02.025 [DOI] [PubMed] [Google Scholar]
- 27.Key N. S., Chantrathammachart P., Moody P. W., and Chang J.-Y., “Membrane microparticles in VTE and cancer,” Thromb. Res. 125, S80–S83 (2010). 10.1016/S0049-3848(10)70020-7 [DOI] [PubMed] [Google Scholar]
- 28.Falanga A., Marchetti M., and Russo L., “The mechanisms of cancer-associated thrombosis,” Thromb. Res. 135, S8–S11 (2015). 10.1016/S0049-3848(15)50432-5 [DOI] [PubMed] [Google Scholar]
- 29.Thomas G. M., Panicot-Dubois L., Lacroix R., Dignat-George F., Lombardo D., and Dubois C., “Cancer cell–derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo,” J. Exp. Med. 206(9), 1913–1927 (2009). 10.1084/jem.20082297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasthuri R. S., Taubman M. B., and Mackman N., “Role of tissue factor in cancer,” J. Clin. Oncol. 27(29), 4834 (2009). 10.1200/JCO.2009.22.6324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boles J. C., Williams J. C., Hollingsworth R. M., Wang J.-G., Glover S. L., Owens A. P. III, Barcel D. A., Kasthuri R. S., Key N. S., and Mackman N., “Anthracycline treatment of the human monocytic leukemia cell line THP-1 increases phosphatidylserine exposure and tissue factor activity,” Thromb. Res. 129(2), 197–203 (2012). 10.1016/j.thromres.2011.06.022 [DOI] [PubMed] [Google Scholar]
- 32.Bromberg M. E., Konigsberg W. H., Madison J. F., Pawashe A., and Garen A., “Tissue factor promotes melanoma metastasis by a pathway independent of blood coagulation,” Proc. Natl. Acad. Sci. U.S.A. 92(18), 8205–8209 (1995). 10.1073/pnas.92.18.8205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole M. and Bromberg M., “Tissue factor as a novel target for treatment of breast cancer,” Oncologist 18(1), 14–18 (2013). 10.1634/theoncologist.2012-0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diamant M., Tushuizen M. E., Sturk A., and Nieuwland R., “Cellular microparticles: New players in the field of vascular disease?,” Eur. J. Clin. Invest. 34(6), 392–401 (2004). 10.1111/j.1365-2362.2004.01355.x [DOI] [PubMed] [Google Scholar]
- 35.Zwicker J. I., Furie B. C., and Furie B., “Cancer-associated thrombosis,” Crit. Rev. Oncol. Hematol. 62(2), 126–136 (2007). 10.1016/j.critrevonc.2007.01.001 [DOI] [PubMed] [Google Scholar]
- 36.Iliescu F. S., Poenar D. P., Yu F., Ni M., Chan K. H., Cima I., Taylor H. K., Cima I., and Iliescu C., “Recent advances in microfluidic methods in cancer liquid biopsy,” Biomicrofluidics 13(4), 041503 (2019). 10.1063/1.5087690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu M., Ouyang Y., Wang Z., Zhang R., Huang P.-H., Chen C., Li H., Li P., Quinn D., and Dao M., “Isolation of exosomes from whole blood by integrating acoustics and microfluidics,” Proc. Natl. Acad. Sci. U.S.A. 114(40), 10584–10589 (2017). 10.1073/pnas.1709210114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obeid S., Ceroi A., Mourey G., Saas P., Elie-Caille C., and Boireau W., “Development of a NanoBioAnalytical platform for “on-chip” qualification and quantification of platelet-derived microparticles,” Biosens. Bioelectron. 93, 250–259 (2017). 10.1016/j.bios.2016.08.100 [DOI] [PubMed] [Google Scholar]
- 39.Terrisse A., Puech N., Allart S., Gourdy P., Xuereb J., Payrastre B., and Sie P., “Internalization of microparticles by endothelial cells promotes platelet/endothelial cell interaction under flow,” J. Thromb. Haemost. 8(12), 2810–2819 (2010). 10.1111/j.1538-7836.2010.04088.x [DOI] [PubMed] [Google Scholar]
- 40.Collier M. E., Mah P.-M., Xiao Y., Maraveyas A., and Ettelaie C., “Microparticle-associated tissue factor is recycled by endothelial cells resulting in enhanced surface tissue factor activity,” Thromb. Haemost. 110(11), 966–976 (2013). 10.1160/TH13-01-0055 [DOI] [PubMed] [Google Scholar]
- 41.Rickles F. R., Patierno S., and Fernandez P. M., “Tissue factor, thrombin, and cancer,” Chest 124(3), 58S–68S (2003). 10.1378/chest.124.3_suppl.58S [DOI] [PubMed] [Google Scholar]
- 42.Ruf W., Disse J., Carneiro-Lobo T. C., Yokota N., and Schaffner F., “Tissue factor and cell signalling in cancer progression and thrombosis,” J. Thromb. Haemost. 9, 306–315 (2011). 10.1111/j.1538-7836.2011.04318.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McEachron T. A., Pawlinski R., Richards K. L., Church F. C., and Mackman N., “Protease-activated receptors mediate crosstalk between coagulation and fibrinolysis,” Blood 116(23), 5037–5044 (2010). 10.1182/blood-2010-06-293126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Østerud B. and Bjørklid E., “Sources of tissue factor,” Semin. Thromb. Hemost. 32, 11–23 (2006). 10.1055/s-2006-933336 [DOI] [PubMed] [Google Scholar]
- 45.Ruf W. and Ruggeri Z. M., “Neutrophils release brakes of coagulation,” Nat. Med. 16(8), 851 (2010). 10.1038/nm0810-851 [DOI] [PubMed] [Google Scholar]
- 46.Martinod K. and Wagner D. D., “Thrombosis: Tangled up in NETs,” Blood 123(18), 2768–2776 (2014). 10.1182/blood-2013-10-463646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas G., Brill A., Mezouar S., Crescence L., Gallant M., Dubois C., and Wagner D., “Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases the incidence of deep vein thrombosis in mice,” J. Thromb. Haemost. 13(7), 1310–1319 (2015). 10.1111/jth.13002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morel O., Toti F., Hugel B. N. D., Bakouboula B., Camoin-Jau L., Dignat-George F. O., and Freyssinet J.-M., “Procoagulant microparticles: Disrupting the vascular homeostasis equation?” Arterioscler. Thromb. Vasc. Biol. 26(12), 2594–2604 (2006). 10.1161/01.ATV.0000246775.14471.26 [DOI] [PubMed] [Google Scholar]
- 49.Yu J., May L., Milsom C., Anderson G. M., Weitz J. I., Luyendyk J. P., Broze G., Mackman N., and Rak J., “Contribution of host-derived tissue factor to tumor neovascularization,” Arterioscler. Thromb. Vasc. Biol. 28(11), 1975–1981 (2008). 10.1161/ATVBAHA.108.175083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osterud B. and Bjorklid E., “Tissue factor in blood cells and endothelial cells,” Front. Biosci. E4(1), 289–299 (2012). 10.2741/e376 [DOI] [PubMed] [Google Scholar]
- 51.Aird W. C., “The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome,” Blood 101(10), 3765–3777 (2003). 10.1182/blood-2002-06-1887 [DOI] [PubMed] [Google Scholar]
- 52.Pradier A. and Ettelaie C., “The influence of exogenous tissue factor on the regulators of proliferation and apoptosis in endothelial cells,” J. Vasc. Res. 45(1), 19–32 (2008). 10.1159/000109074 [DOI] [PubMed] [Google Scholar]
- 53.Bombeli T., Karsan A., Tait J. F., and Harlan J. M., “Apoptotic vascular endothelial cells become procoagulant,” Blood 89(7), 2429–2442 (1997). 10.1182/blood.V89.7.2429 [DOI] [PubMed] [Google Scholar]
- 54.Schouten M., Wiersinga W. J., Levi M., and Van Der Poll T., “Inflammation, endothelium, and coagulation in sepsis,” J. Leukocyte Biol. 83(3), 536–545 (2008). 10.1189/jlb.0607373 [DOI] [PubMed] [Google Scholar]