Abstract
We describe the synthesis and spectral characterization of a rhenium metal–ligand complex. This complex reacts with sulfhydryl groups via an iodoacetamide side chain on the phenanthroline ligand and displays a high limiting anisotropy near 0.35 when excited at 442 nm. When covalently linked to human serum albumin, this complex displayed a mean decay time of about 1 μs. This decay time is appropriate for measuring rotational correlation times on the microsecond time scale as may occur for large macromolecular complexes.
INTRODUCTION
During the past 5 years there has been rapid development of transition metal-ligand complexes as biophysical probes and/or chemical sensors (1–5). The MLCs are typically composed of a central metal of Re, Ru, or Os and at least one diamine ligand. The photophysical properties of this class of molecules has been extensively studied (6–9). The MLCs display a number of favorable properties which facilitate their use as luminescence probes. The decay times are conveniently long, ranging from 10 ns to 10 μs. Most MLCs display polarized emission, allowing measurement of macromolecule hydrodynamics. The MLCs display large Stokes’ shifts, which eliminates the problems of self-quenching often found with fluorescein and other fluorophores with small Stokes’ shifts. And finally, most MLCs are highly stable, both photochemically and chemically.
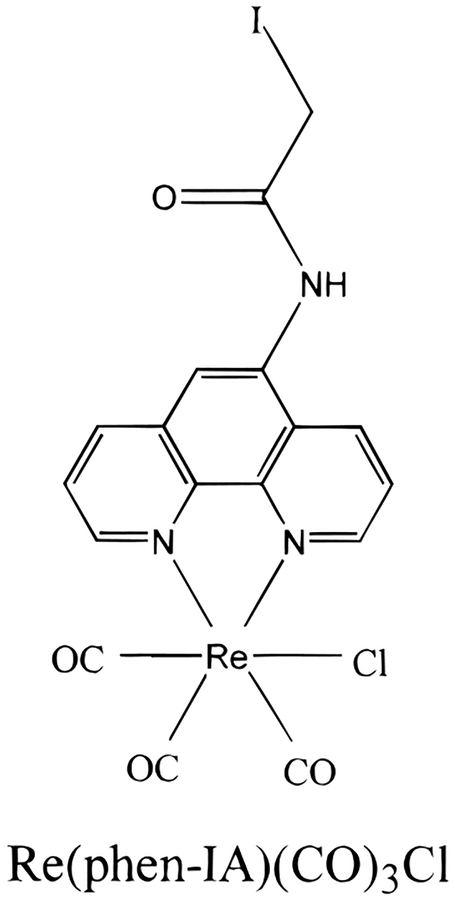
While numerous MLCs have been synthesized and characterized, relatively few MLCs have been prepared in forms suitable for conjugation with proteins. In the present report, we extend the availability of conjugatable MLCs by the synthesis of a Re MLC which is reactive with sulfhydryl residues (Figure 1). As a chloride complex, this dye was found to have a reasonable quantum yield in dichloromethane and most especially displays a high fundamental anisotropy near 0.35 and is thus useful for anisotropy measurements of large proteins.
Figure 1.
Structure of the sulfhydryl reactive Re(phen-IA)-(CO)3Cl complex.
THEORY
Time-resolved intensity and anisotropy decays were recovered from the frequency-domain data, as described previously (10–11). Intensity decays were analyzed in terms of the multiexponential model
| (1) |
where αi are the preexponential factors and τi the decay times. The fractional contributions of each decay component to the steady-state intensity are given by
| (2) |
The mean lifetime is given by
| (3) |
Anisotropy decays were analyzed in terms of the multi-correlation time (θk)
| (4) |
where r0k is the amplitude of the kth correlation time. For a fully resolved anisotropy decay, one expects the recovered total anisotropy or the time zero anisotropy r(0) = Σr0k to be equal to r0, where r0 is the fundamental anisotropy in the absence of rotational motions.
MATERIALS AND METHODS
Synthesis of Re(phen-IA)(CO)3Cl.
The synthesis of phen-IA is reported elsewhere (12). Re(CO)5Cl (76 mg, 210 mmol) (Aldrich Chemical Co., Milwaukee, WI) and phen-IA (78 mg, 215 mmol) were refluxed in toluene under argon in the dark for 6 h. The solution was cooled to room temperature and kept overnight at 4 °C. The precipitated product was collected on a medium frit. Additional product was recovered by filtering the solution into ice cold hexane. Total yield was about 77%. The recovered product was further purified using preparative thin-layer chromatography. This was done on a silica plate using a mixture of 1.0:2.7:0.05 of methanol:chloroform:water as the mobile phase. The spot containing the pure Re–Cl was scraped off the plate at the end of the experiment and extracted from the silica matrix with a mixture of 7:3 of methanol: chloroform. FAB MS: m/z = 633.5 ([M – Cl]), 668.0 ([M]).
Modification of the cysteine residue of human serum albumin (Sigma) was accomplished by addition of 50 μL of a 20 mg/mL DMSO solution of Re(phen-IA)(CO)3Cl+ to 1 mL of the protein solution (5 mg/mL, 100 mM phosphate buffer, pH 7.3). The reaction proceeded overnight at 4 °C followed by gel filtration chromatography on a Sephadex G-25 column (Sigma, St Louis, MO) equilibrated in 10 mM Tris buffer, pH 8.0. The labeled protein was eluted with the 10 mM Tris buffer, pH 8.0, and dialyzed overnight with a 10 kDa Snakeskin cutoff tubing (Pierce, Rockfield, IL) against the same buffer at 4 °C. The dye-to-protein ratio was determined by the absorption of the photoluminescent complex and protein as previously described (13) and was found to be about 0.70. This will suggest minimal stearic problems during conjugation.
INSTRUMENTATION
Absorption spectra were measured on a Hewlett-Packard 8453 diode array spectrophotometer. All steady-state fluorescence measurements were performed using a SLM 8000 spectrofluorometer. Excitation anisotropy measurements for the MLCs were done in 9:1 glycerol: dichloromethane (DCM) at −70 °C using a Neslab Endocal methanol chiller attached to the SLM 8000 spectrofluorometer. Excitation for frequency-domain lifetime and anisotropy measurements was provided by the output from a Helium-cadmium laser (442 nm, Liconix 4280N), which was amplitude modulated at single frequencies between 10 kHz and 100 MHz with an electrooptical low-frequency modulator (K2.LF, ISS) and input into an ISS K2 frequency domain fluorometer (13). Emission was collected through a Corning 2–70 510 nm cutoff filter. Rhodamine B (Aldrich Chemical Co.) in water (τ = 1.68 ns) was used as a reference for the lifetime measurements.
RESULTS AND DISCUSSION
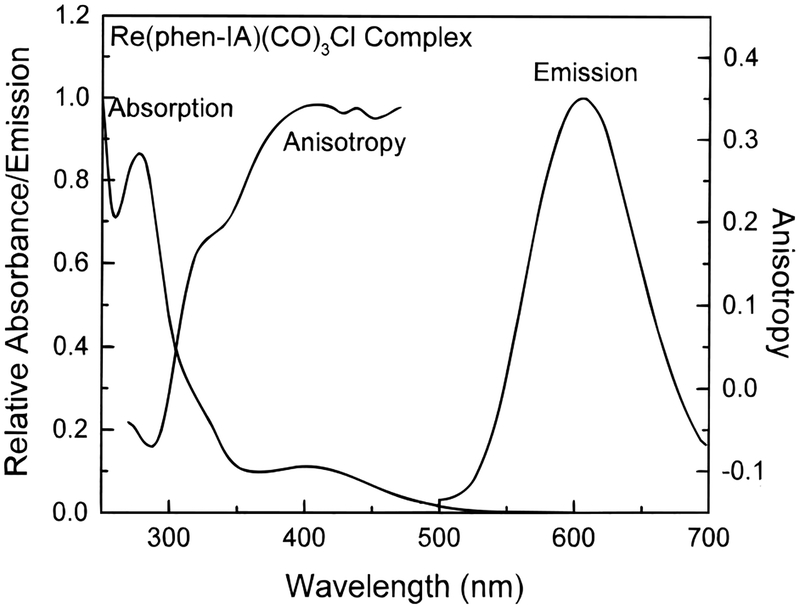
The absorption and emission spectra of Re(phen-IA)-(CO)3Cl (Re-Cl) are shown in Figure 2. Absorption maxima are seen near 410 and 280 nm, and the emission maximum near 600 nm. The large Stokes shift is typical of this class of luminophores. Its quantum yield in dichloromethane is 0.039 relative to [Ru(bpy)3]2+ in deoxygenated water (0.042). The extinction coefficients at 278, 325, and 380 nm are 23 100, 6450, and 3200, respectively.
Figure 2.
Absorption, emission and excitation anisotropy spectra of Re(phen-IA)(CO)3Cl. Absorption and emission spectra were measured at room temperature in dichloromethane. Excitation anisotropy spectra were measured in 9:1 (v/v) glycerol/dichloromethane at −70 °C with emission at 500 nm.
Figure 2 also shows the excitation anisotropy spectra for Re-Cl. It was measured in 9:1 glycerol:DCM at −70 °C to prevent rotational diffusion during the excited-state lifetime. Re–Cl displays a high fundamental anisotropy of over 0.3 when excited at wavelengths above 400 nm. Fundamental anisotropy value of 0.338 was obtained at 442 nm for this complex. This large anisotropy, over 75% of the theoretical value of 0.4, makes this complex a useful polarization probe.
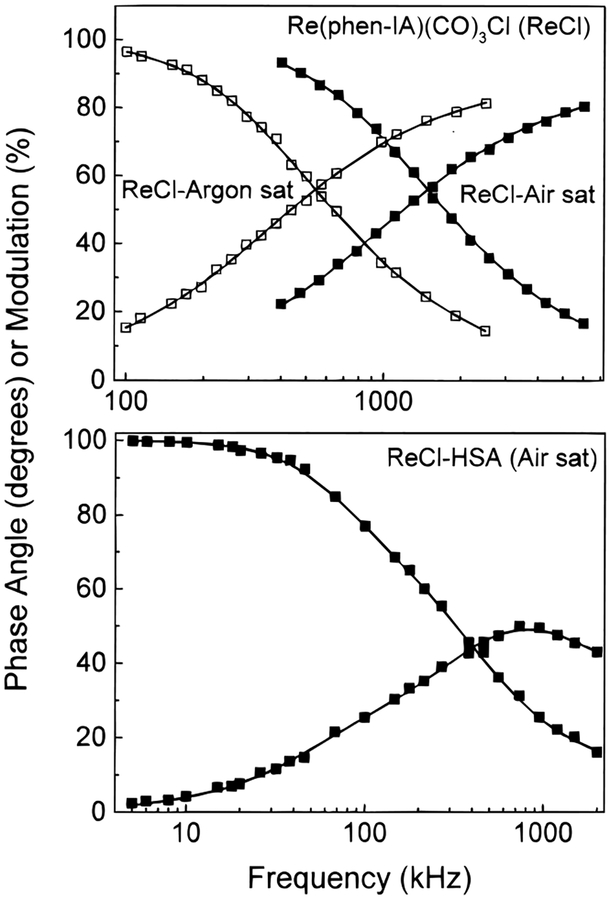
Figure 3 shows frequency-domain intensity decay of this complex when dissolved in DCM and when covalently linked to HSA. In DCM the intensity decays are single exponentials (Table 1). The decay times of the Re complex in DCM are sensitive to dissolved oxygen, which reduces the decay time from 436 ns in the absence of oxygen to 159 ns when in equilibrium with air. When bound to HSA, this complex displays a multiexponential decay (Figure 3), requiring three decay times for an acceptable fit. The mean decay time for the HSA-bound complex is 1101 ns. The much longer lifetime of the HSA-labeled complex when compared to the free probe indicates that the probe is well shielded within the protein and protected appreciably from oxygen quenching. This observed long lifetime also indicates that this probe could be used to measure long correlation times that would be expected for very large macromolecules.
Figure 3.
Representative frequency-domain intensity decays for the free Re–Cl complex and for HSA labeled with Re–Cl. Free Re–Cl measurements were made in dichloromethane while the labeled HSA was measured in aqueous solution. Excitation at 442 nm and observation above 500 nm.
Table 1.
Intensity Decay Parameters of Free and HSA-Labeled Re(phen-IA)(CO)3Cl
| metal complex | τi (ns) | αi | fi | (ns) | χR2 |
|---|---|---|---|---|---|
| Re–Cl in DCMa(air) | 159 | 1.000 | 1.00 | 159 | 2.64b |
| Re–Cl in DCM (argon) | 436 | 1.000 | 1.00 | 436 | 4.74 |
| Re–Cl–HSA (air) | 2102 | 0.026 | 0.418 | ||
| 473 | 0.128 | 0.468 | |||
| 17.4 | 0.847 | 0.114 | 1101 | 4.73 |
DCM, dichloromethane.
For calculation of χR2 the uncertainties in the phase and modulation was assumed to be 0.2 and 0.005, respectively.
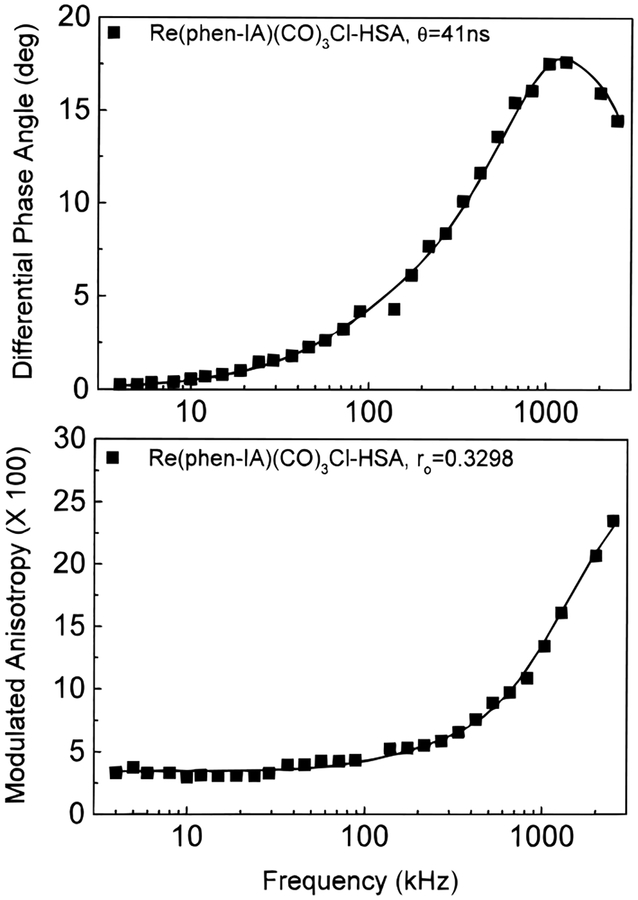
To demonstrate the utility of this Re–Cl complex for measurement of microsecond of macromolecular hydrodynamics, its anisotropy decay was measured when bound to HSA. A rotational correlation time of 41 ns was recovered when bound to HSA. This result is in good agreement with that predicted by theory and previous experimental results (13–15). In addition, the initial anisotropy of 0.32 was close to that observed in vitrified glycerol solution at 442 nm (Table 1). This result suggests that this dye is covalently bound to HSA, and hence, its anisotropy decay reflects the overall rotation of the labeled HSA. (Figure 4).
Figure 4.
Frequency domain anisotropy decays of Re(phen-IA)(CO)3Cl-HSA in aqueous solution. Excitation at 442 nm and observation above 500 nm.
Conclusion.
We have synthesized and characterized a sulfhydryl reactive Re MLC, Re(phen-IA)(CO)3Cl, that can be used to label cysteine residues in proteins. This complex is reactive with the sulfhydryl groups on proteins via an iodoacetamide side chain. This complex exhibited a long lifetime of 436 ns in an organic solvent and a high fundamental anisotropy of 0.34. The mean decay time was even longer when covalently bound to HSA, about 1101 ns. This long decay time makes this luminophore appropriate for measuring the correlation times of macromolecules of a similar time scale, as may be expected for moderate to large sized proteins. Its high anisotropy also makes it to be very useful in fluorescence polarization immunoassays of high molecular weight macromolecules (17–19).
It is also valuable to notice that this probe allows for time-resolved measurements with simple instrumentation. Wavelengths short enough to excite these complexes are available using light emitting diodes (20). Additionally, the light output of LEDs can be easily modulated to about 100 MHz (20–23), which is more than adequate for this long lifetime luminophore. Hence, the simple combination of this MLC luminophore and LED-based instrumentation can be used to study microsecond dynamics of macromolecules.
ACKNOWLEDGMENT
This work was supported by grants from the National Institutes of Health, RR-08119 and GM35154.
LITERATURE CITED
- (1).Demas JN, and DeGraff BA (1997). Applications of luminescent transition metal complexes to sensor technology and molecular probes. J. Chem. Educ 74 (6), 690–695. [Google Scholar]
- (2).Demas JN, and DeGraff BA (1991). Design and applications of highly luminescent transition metal complexes, Anal. Chem 63, 829a–837a. [Google Scholar]
- (3).Demas JN, and DeGraff BA (1994). Design and applications of highly luminescent transition metal complexes In Topics in Fluorescence Spectroscopy: Probe Design and Chemical Sensing (Lakowicz JR, Ed.) Vol. 4, pp 71–107 Plenum Press, New York. [Google Scholar]
- (4).Lakowicz JR, Terpetschnig E, Murtaza Z, and Szmacinski H (1997) Development of long-lifetime metal-ligand probes for biophysics and cellular imaging, J. Fluoresc 7 (1), 17–25. [Google Scholar]
- (5).Terpetschnig E, Szmacinski H, and Lakowicz JR (1997) Long-lifetime metal-ligand complexes as probes in biophysics and clinical chemistry, Methods in Enzymology 278, 294–321. [DOI] [PubMed] [Google Scholar]
- (6).Kalayanasundaram K (1992) Photochemistry of Polypyri-dine and Porphyrin Complexes, p 626, Academic Press, New York. [Google Scholar]
- (7).Juris A, Balzani V, Barigelletti F, Campagna S, Belser P, and Von Zelewsky A (1988) Ru(II) polypyridine complexes, photophysics, photochemistry, electrochemistry and chemiluminescence. Coord.Chem. Rev 84, 85–277. [Google Scholar]
- (8).Fredericks SM, Luong JC, and Wrighton MS (1979) Multiple emissions from rhenium (I) complexes, Intraligand and charge-transfer emission from substituted metal carbonyl cations. J. Am. Chem. Soc 101 (24), 7415–7417. [Google Scholar]
- (9).Caspar JV, and Meyer TJ (1983) Applications of the energy gap law to nonradiative, excited-state decay. J. Phys. Chem 87, 952–957. [Google Scholar]
- (10).Lakowicz JR, and Gryczynski I (1991) Frequency-domain fluorescence spectroscopy in Topics in Fluorescence Spectroscopy, Vol 1, Techniques (Lakowicz JR, Ed.) pp 293–355, Plenum Press, New York. [Google Scholar]
- (11).Beechem JM, Gratton E, Ameloot M, Knutson JR, and Brand L (1991) The global analysis of fluorescence intensity and anisotropy decay data: Second-generation theory and programs In Topics in Fluorescence Spectroscopy, Vol 2, Principles (Lakowicz JR, Ed.) Plenum Press, New York, pp 241–305. [Google Scholar]
- (12).Sigman DS, Kuwabara MD, Chen CHB, and Bruce TW (1991) Nuclease activity of 1,10-phenanthroline-copper in study of protein-DNA interactions. Methods Enzymol. 208, 414–433. [DOI] [PubMed] [Google Scholar]
- (13).Castellano FN, Dattelbaum JD, and Lakowicz JR (1998) Long-lifetime Ru(II) complexes as labeling reagents for sulfhydryl groups. Anal. Biochem 255, 165–170. [DOI] [PubMed] [Google Scholar]
- (14).Terpetschnig E, Szmacinski H, Malak H, and Lakowicz JR (1995) Metal–Ligand Complexes as a New Class of Long-Lived Fluorophores for Protein Hydrodynamics. Biophys. J 68, 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Terpetschnig E, Dattelbaum JD, Szmacinski H, and Lakowicz JR (1997) Synthesis and spectral characterization of a thiol-reactive long- lifetime Ru(II) complex. Anal. Biochem 251, 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Lakowicz JR, Cherek H, Gryczynski I, Joshi N, and Johnson ML (1987) Enhanced resolution of fluorescence anisotropy decays by simultaneous analysis of progressively quenched samples. Biophys. J 51, 755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Youn HJ, Terpetschnig E, Szmacinski H, and Lakowicz JR (1995) Fluorescence Energy Transfer Immunoassay Based on a Long-Lifetime Luminescent Metal–Ligand Complex. Anal. Biochem 232, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Terpetschnig E, Szmacinski H, and Lakowicz JR (1995). Fluorescence Polarization Immunoassay of a High Molecular Weight Antigen Based on a Long-Lifetime Ru–Ligand Complex. Anal. Biochem 227, 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Terpetschnig E, Szmacinski H, and Lakowicz JR (1996) Fluorescence polarization immunoassay of a high-molecular-weight antigen using a long wavelength-absorbing and laser diode-excitable metal-ligand complex. Anal. Biochem 240, 54–59. [DOI] [PubMed] [Google Scholar]
- (20).Sipior J, Carter GM, Lakowicz JR, and Rao G (1997) Blue light-emitting diode demonstrated as an ultraviolet excitation source for nanosecond phase-modulation fluorescence lifetime measurements Rev. Sci. Instrum 68(7), 2666–2670. [Google Scholar]
- (21).Fantini S, Franceschini MA, Fishkin JB, Barbieri B, and Gratton E (1994) Quantitative determination of the absorption spectra of chromophores in strongly scattering media, A light-emitting diode based technique. Appl. Opt 33 (22), 5204–5213. [DOI] [PubMed] [Google Scholar]
- (22).Lippitsch ME, and Wolfbeis OS (1988) Fiber-optics oxygen sensor with the fluorescence decay time as the information carrier. Anal. Chim. Acta 205, 1–6. [Google Scholar]
- (23).Sipior J, Carter GM, Lakowicz JR, and Rao G (1996) Single quantum well light emitting diodes demonstrated as excitation sources for nanosecond phase-modulation fluorescence lifetime measurements. Rev. Sci. Instrum 67 (11), 3795–3798. [Google Scholar]