Abstract
The present clinical-experimental study aims to examine the effect of pure experimental fluoride solutions and stannous chloride on the initial oral bioadhesion under in situ conditions. After 1 min of pellicle formation on bovine enamel slabs, 12 subjects rinsed with 8 ml of the fluoride test solutions (NaF, Na2PO3F, AmF, SnF2,) with 500 ppm fluoride concentration each for 1 min. Additionally, rinsing without a solution (control) and rinsing with 1563 ppm SnCl2 solution took place for 1 min. Afterwards, fluorescence microscopy took place to visualize bacterial adhesion and glucan formation (8 h oral exposition) with DAPI and ConA and the BacLight method. TEM was performed to visualize the pellicle ultrastructure together with EDX to detect stannous ions. The rinsing solutions with pure SnF2 and SnCl2 reduced significantly the initial bacterial colonization (DAPI). While, NaF and Na2PO3F showed no significant effect compared to the control. There was no significant difference between AmF, SnF2 and SnCl2. All tested experimental solutions showed no reducing effect on the glucan formation. Considerable alterations of the pellicle ultrastructure resulted from rinsing with the Sn-containing solutions. SnF2 appears to be the most effective type of fluoride to reduce initial bacterial colonization in situ. The observed effects primarily have to be attributed to the stannous ions’ content.
Subject terms: Dental biofilms, Fluoridation
Introduction
According to the WHO oral health report of october 2017, dental caries is still a major public health problem and the most common non transmittable disease worldwide, especially in industrialized countries affecting 60–90% of the school children and a vast majority of adults. It is expensive to treat; hence consuming 5–10% of healthcare budgets and is the main reason for hospitalization of children in some high-income countries. In this context, the use of fluorides is recommended worldwide. In order to cite the WHO: “It is important that population-wide prevention interventions are universally available and accessible. Such interventions include the use of fluoride, and comprehensive patient-centred essential oral health care”. This quote emphasizes the use and application of fluorides in daily dental health care. It is even more important that the mechanisms, effects and actions of fluorides are fully understood, especially when the use is recommended worldwide on a daily basis. Nevertheless, there are different explanations how fluorides work. Current explanatory models are based on studies with combinations of fluorides or fluoride solutions with different amounts of constituents. Thereby, many studies on the anticariogenic effect of fluorides focused on their role in reducing enamel and dentin demineralization and enhancing remineralization of early caries lesions1,2. Multiple clinical studies demonstrated the cariostatic effect of fluorides in various multifaceted application forms.
Initial biofilm formation starts with the adhesion of oral microorganisms to the pellicle layer, a physiological proeinaceous layer with protective and antibacterial properties3,4. The adhesion of singular bacterial cells is followed by coaggregation and bacterial cell multiplication as well as glucan synthesis5,6. Despite the well known efficacy of fluorides, little is known about the interactions of pure fluoride substances with the pellicle layer and the effect of these substances on initial bioadhesion as the initial point of biofilm formation under in situ conditions7. In contrast, fluoride compounds in already existing mouth rinsing solutions or tooth pastes are the objective of multiple clinical and experimental trials7. These mouth rinses have a high variety of constituents and combinations of fluoride components7,8. Thereby, chemical interactions of the constituents are possible and the antiadherent effect might be influenced by specific components of the mouthrinses.
In this context, in vitro research showed, that fluoride concentrations above 300 ppm can significantly reduce acidogenicity, acidurity and the development of three dimensional bacterial aggregates of streprococcus mutrans communities9. In addition, it is known that fluorides can inhibit the bacterial enzymes9.
Previous studies and reports have shown that especially the metal cations of fluorides play an important role in prevention and treatment of enamel erosions10,11. Thereby, significant differences between the cations were revealed for their anti-erosive effect10,11 So far, there is little evidence of their influence in initial oral bioadhesion processes. An earlier in vitro study has shown, that sodium fluoride was superior to amine fluoride concerning its anti-erosive capacity10. Stannous ions in stannous fluorides can inhibit the bacterial enzymes aldolase and glycerinaldehyde dehydrogenase by oxidation of thiol-groups. As a consequence, the bacterial metabolism is negatively influenced. In addition, the bacterial cell-cell-cohesion and adhesion to enamel surfaces are inhibited by stannous ions. This can lead to an antiadherent effect induced by these metal ions12. These results are very important findings supporting an increase of knowledge of the actions and mechanisms of fluorides. Nevertheless, to our best knowledge there is no in situ study that has examined the effect of pure fluorides (sodium fluorophosphates, sodium fluoride, amine fluoride, stannous fluoride) on initial oral bioadhesion processes and the modifying action on the ultrastructure.
Results
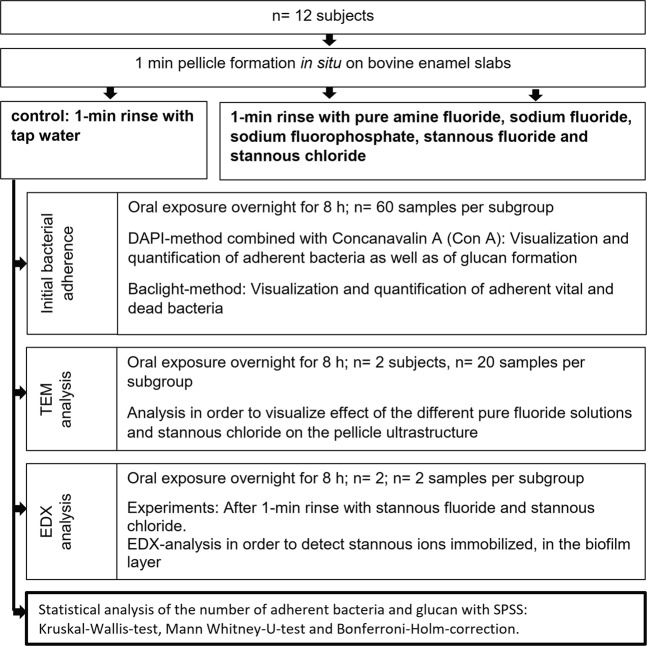
The bovine specimens were analysed with DAPI, BacLight, SEM and EDX-analysis (Fig. 1).
Figure 1.
Flow chart of the in situ experiments. Thereby, every volunteer (n = 12) rinsed with the different fluoride solutions and the stannous chloride solution at different days (2-day wash out period between the different experiments). After oral overnight exposure for 8 h, the specimens were removed and used for the experiments (DAPI, BacLight) irrespective of their position on the splint. The experiments for TEM and EDX analysis took place with selected subjects (n = 2). The statistical analysis was performed for the number of adherent bacteria and glucans with the Kruskal-Wallis-test, the Mann Whitney-U-test and the Bonferroni-Holm-correction.
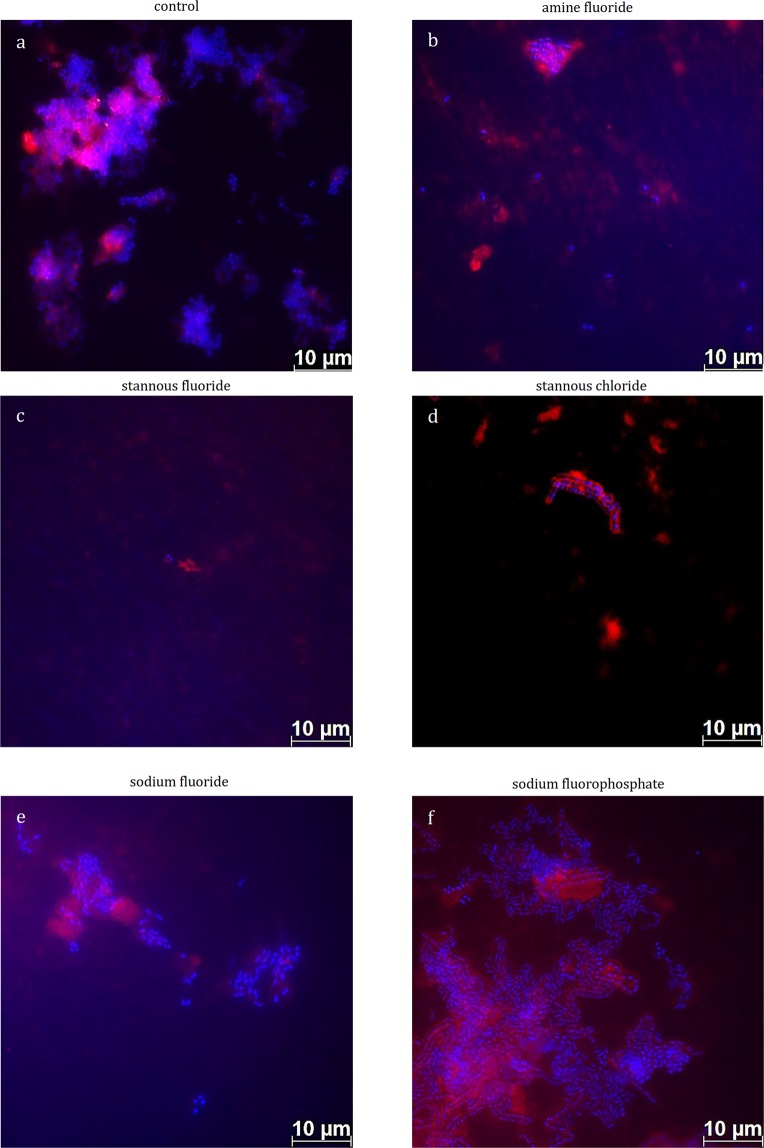
Initial bacterial colonization was thereby visualized by DAPI (Fig. 2) and BacLight staining (Fig. 3). On the enamel slabs of the control group, monolayers of adherent bacteria were visible covering more than 50% of the enamel surface (Fig. 2a). The rinsing solutions with pure stannous fluoride (Fig. 2c) and stannous chloride (Fig. 2d) significantly reduced the initial bacterial colonization in situ. Thereby single bacterial cells, and small aggregates were detectable on enamel (Fig. 2c,d). In addition, the visualization of the glucan formation took place with the fluorescence dye Concanavalin A (Fig. 2). In general, all tested experimental solutions showed no reducing effect on the glucan formation (Fig. 2a–f). Thereby, glucan formation was detectable on all enamel samples, in particular, around adhering bacterial cells (Fig. 2d).
Figure 2.
Fluorescence microscopic images displaying a combination of simultaneous DAPI (blue) and glucan (red) staining. Thereby DAPI-staining indicates adherent bacteria at the pellicle layer as well as bacteria-glucan-agglomerates in combination with the glucan staining (Con A – red). The highest number of adhering bacteria is detected after 1-min-rinsing with tap water (a, control), sodiumfluoride (e) and sodium fluorophosphate (f). In contrast, less adherent bacteria are stained after 1-min-rinsing with amine fluoride (b), stannous fluoride (c) and stannous chloride (d). In situ pellicle formation time: 8 h.
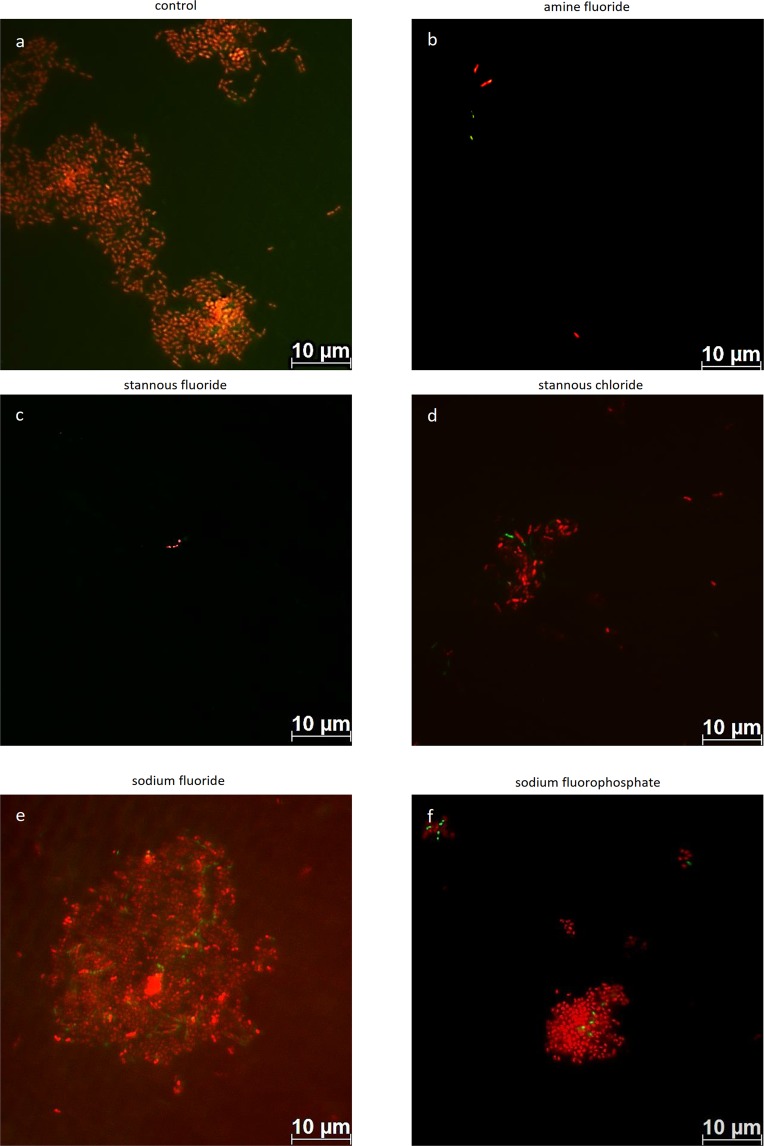
Figure 3.
Fluorescence microscopic images after staining with the Live/dead staining BacLight kit. Thereby, vital bacteria (green) and dead bacteria (red) are detectable at the pellicle layer. The highest number of vital and dead bacteria is detected after 1-min-rinsing with tap water (a, control), sodiumfluoride (e) and sodium fluorophosphate (f). In contrast, less adherent bacteria are stained after 1-min-rinsing with amine fluoride (b), stannous fluoride (c) and stannous chloride (d). In situ pellicle formation time: 8 h.
With the BacLight live/dead staining a clear differentiation of vital (green) and dead (red) bacteria was possible (Fig. 3). Thereby, less vital bacteria were detectable after rinsing with amine fluoride (Fig. 3b) and stannous fluoride (Fig. 3c) compared to the control (Fig. 3a) and the sodium fluoride group (Fig. 3e). In addition less adherent dead bacteria were visible on the enamel surface after rinsing with amine fluoride (Fig. 3b) and stannous fluoride (Fig. 3c) compared to the control (Fig. 3a) and sodium fluoride group (Fig. 3e). Further, significantly fewer dead bacteria were detectable after the rinse with stannous fluoride (Fig. 3b) and amine fluoride (Fig. 3b) compared to sodium fluorophosphate (Fig. 3f).
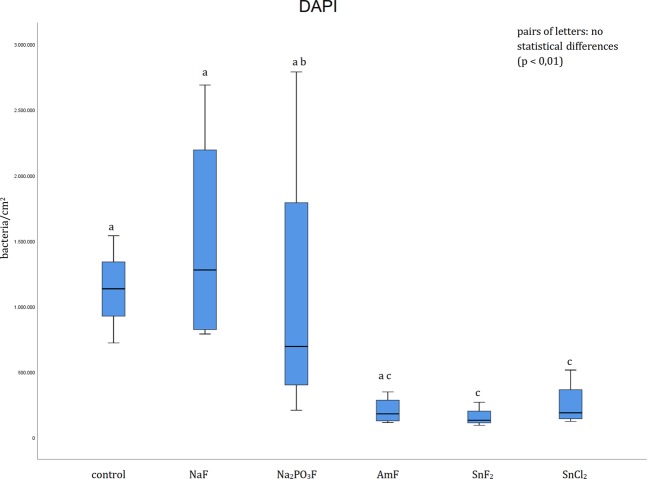
A quantification of the adherent bacteria was performed after visualization of the bacterial cells with DAPI (Fig. 4). In the control group, the participants rinsed with tap water and significantly higher numbers were detected on enamel compared to stannous fluoride and stannous chloride. In contrast, amine fluoride, sodium fluoride and sodium fluorophosphate showed no significant effect compared to the control. After rinsing with sodium fluoride significantly more adherent bacteria were detected compared to stannous fluoride and stannous chloride after visualization with DAPI. In addition, significantly fewer bacteria were visible after the rinse with stannous fluoride compared to sodium fluorophosphate. Besides, no significant difference was revealed by the DAPI method between the rinsing solutions amine fluoride, stannous fluoride and stannous chloride (Fig. 4).
Figure 4.
Boxplot diagram. Influence of pure fluoride solutions on initial bacterial adhesion to enamel bovine slabs in situ. Evaluation of the bacterial colonization with the DAPI method. Columns sharing the same letter (enamel: lower case letters) are not significantly different (Mann-Whitney U test and Bonferroni-Holm correction, p < 0.01).
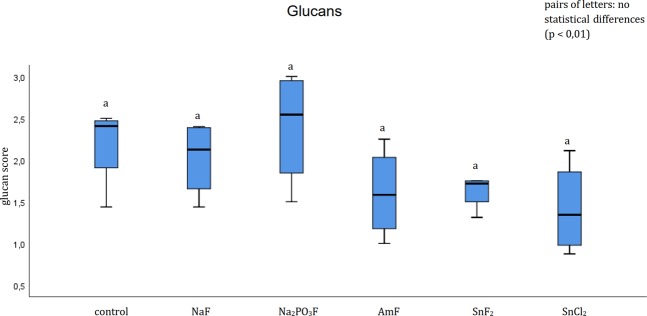
The evaluation of the glucan formation showed no significant effect of all pure fluoride solutions and stannous chloride (Fig. 5).
Figure 5.
Boxplot diagram. Influence of pure fluoride solutions on glucan formation on enamel bovine slabs in situ, staining dye Concanavalin A. Columns sharing the same letter (lower case letters) are not significantly different (Mann-Whitney U test and Bonferroni- Holm correction, p < 0.01), in summary no statistical differences were shown.
Evaluating the number of adherent bacteria, the number of vital bacteria was significantly decreased after rinsing with amine fluoride and stannous fluoride compared to the control and the sodium fluoride group (Fig. 6). Besides, the number of dead bacteria was significantly decreased after rinsing with amine fluoride and stannous fluoride compared to the control and sodium fluoride group. Further, significantly fewer dead bacteria were detected after the rinse with amine fluoride and stannous fluoride compared to sodium fluorophosphate. Nevertheless, no significant difference was revealed by the BacLight method between the rinsing solutions amine fluoride, stannous fluoride and stannous chloride (Fig. 6).
Figure 6.
Boxplot diagram. Influence of pure fluoride solutions on initial bacterial adhesion to enamel bovine slabs in situ. Evaluation of vital (green) and dead (red) bacteria with the BacLight method. Columns sharing the same letter (enamel: lower case letters) are not significantly different (Mann-Whitney U test, and Bonferroni-Holm correction, p < 0.01). Significantly lower numbers of bacteria are detectable after rinsing with amine fluoride (vital and dead bacteria) and stannous fluoride (vital and dead bacteria) compared to the control (rinsed with tap water), sodium fluoride and sodium fluorophosphate.
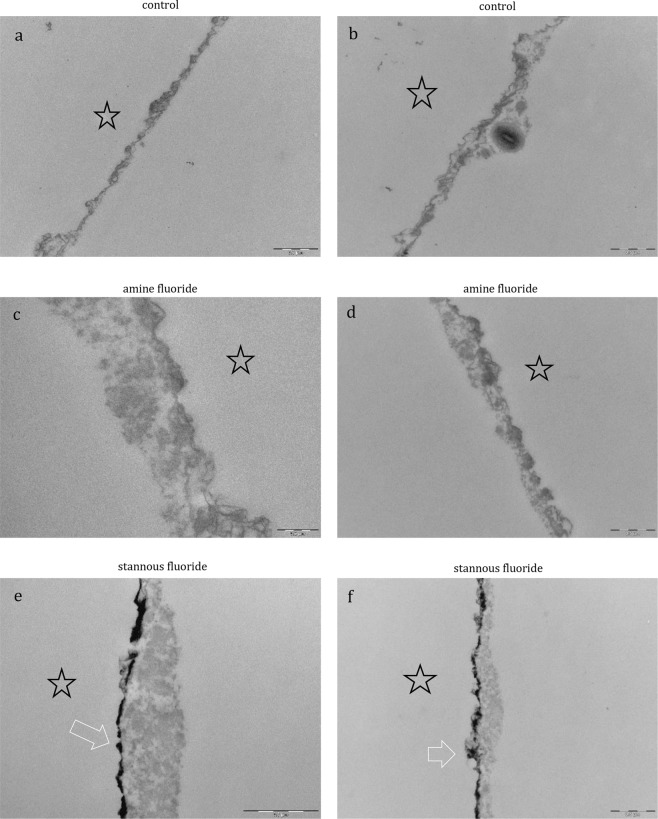
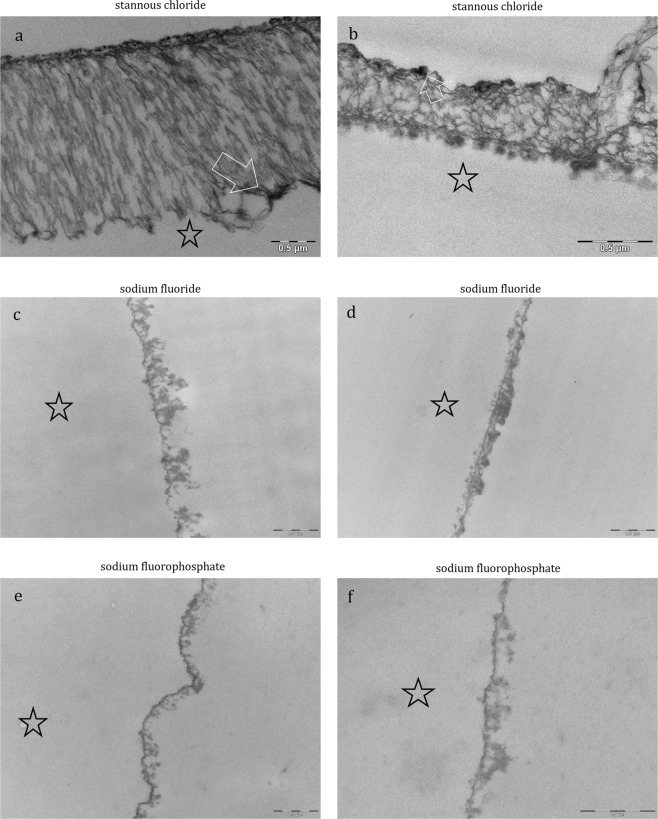
The physiological accumulation of an electron-dense basal layer and a more inhomogenous second granular layer with less electron dense granular and globular structures has been confirmed by SEM as expected (Fig. 7a,b). After rinsing with sodium fluoride (Fig. 8c,d) and sodium fluorophosphate (Fig. 8e,f), the characteristic ultrastructure was visualized and showed no significant differences compared to the control. In contrast, distinct differences in the ultrastructure of the pellicle were visualized after rinsing with the pure amine fluoride, pure stannous fluoride and stannous chloride solutions (Figs. 7 and 8). Thereby, the pellicles’ thickness varied between the different pure fluoride rinsing solutions (Figs. 7c–f and 8c–f). Besides, after rinsing with amine fluoride (Fig. 7c,d) the pellicle showed an increased thickness of the less electron-dense granular and globular second layer and was denser compared to the control, sodium fluoride (Fig. 8c,d) and sodium fluorophosphate (Fig. 8e,f). But most interestingly, rinsing with stannous ions containing solutions (Figs. 7e,f and 8a,b) appeared to notably affect the characteristic ultrastructural appearance of the pellicle. After the application of stannous fluoride, the basal layer of the pellicle showed a higher electron density. In contrast, rinsing with stannous chloride (Fig. 8a,b) lead to demineralization of the enamel surface. As a consequence, infiltrated areas with stannous ions were visible (Fig. 8a,b) and stannous precipitates were detectable (Fig. 8a,b). Further, the pellicle ultrastructure showed an increased thickness compared to all other tested solutions after rinsing with amine fluoride and stannous fluoride (Fig. 7b–d).
Figure 7.
Representative TEM images visualize the pellicle´s ultrastructure. Intraoral exposure time: 8 h. All pellicles show an electron-dense basal layer. This basal layer is covered by a varying less electron-dense granular and globular layers (a, b, control). There are distinct ultrastructural differences detectable after rinsing with pure fluoride solutions (c–f) compared to the control (a,b). (c,d) Amine fluoride: An increased thickness of the less electron-dense granular and globular layer is detectable. (e,f) Stannous fluoride: A higher electron density of the basal layer with stannous precipitates (arrow) can be observed. The enamel was removed during processing of the specimens, the former enamel side is marked with an asterisk.
Figure 8.
Representative TEM images visualize the pellicle´s ultrastructure. Intraoral exposure time: 8 h. All pellicles show an electron-dense basal layer. This basal layer is covered by a varying less electron-dense granular and globular layers. There are distinct ultrastructural differences detectable after rinsing with pure fluoride solutions (c–f) or stannous chloride (a,b). (a,b) Stannous chloride: A demineralization of the enamel surface took place. Infiltrated areas on the enamel surface are visible and stannous precipitates are detectable in infiltrated zones in the enamel (arrow). (c,d) Sodium fluoride: The accumulation of an electron-dense basal layer and a more inhomogenous granular layer is visible after rinsing with sodium fluoride. (e,f) Sodium fluorophosphate: A thin, electron-dense basal layer with a less electron-dense granular and globular layer with varying thickness is detectable. The enamel was removed during processing of the specimens, the former enamel side is marked with an asterisk.
EDX analysis was performed after the 1-min rinse of stannous fluoride and stannous chloride and the oral exposure time of 8 h. Thereby, stannous ions were detected in the pellicle layer.
Discussion
A decline of severity and prevalence of carious lesions has been attributed to the application of fluorides13. Nevertheless, caries is still a global problem for the oral health systems of industrialized countries. The inhibitory effect of customary available fluoride mouthrinses against oral biofilms and their antibacterial effect has thereby been the focus of many previous studies14,15. However, to the authors’ best knowledge, pure fluoride substances have not been tested concerning initial bioadhesion and the modification of the pellicle´s ultrastructure under in situ conditions.
Screening the literature, there were suggestions that the pellicle retaines fluorides at the tooth surface16. Amine fluoride and stannous fluoride are assumed to have a high affinity towards hydroxyapatite surfaces and the pellicle layer, respectively. In this context, it was shown that stannous ions (Sn2+) in stannous fluoride (SnF2) have antibacterial properties17. These properties are the result of the inhibition of bacterial enzymes responsible for the glucose transport and bacterial metabolism18. The antimicrobial properties are not transient19 due to the very good substantivity of dental hard tissues, especially in dental biofilms20. In addition, Sn2+ ions inhibit MMPs (matrix metalloproteinases)21. The stannous ion as a compound of stannous fluoride or stannous chloride forms a superficial layer on the dental hard tissues20. Thereby, the precipitation of a calcium fluoride like product, and a stannous phosphate can bee shown22. Thereby, proteins from the saliva adhere to these resultant products22. This creates a good foundation for pellicle formation23. These findings are in good accordance with the results of this study. The accumulated stannous ions in the pellicle were confirmed by EDX analysis after rinsing with stannous fluoride and stannous chloride in the present study. This important finding was approved by TEM. After rinsing with stannous fluoride and stannous chloride, the incorporation of stannous ions was visible as an electron dense structure inside the pellicle layers and after rinsing with stannous chloride in the enamel surface as well. Rykke et al.22 have reported that they collected about twice as much pellicle material from the buccal surfaces of 24 stannous fluoride-treated-teeth compared to native surfaces in vivo22. Moreover, Tinanoff et al.12 detected an increase of pellicle thickness after the treatment with stannous fluoride by SEM12. Even this finding was affirmed in the present study. After visualization with SEM, a thicker electron dense morphology of the pellicle was detected after rinsing with pure stannous fluoride and stannous chloride (Figs. 7c,d and 8b–d). The different composition and thickness of the pellicle formed after the application of stannous fluoride seem to have an influence on the adherence of bacterial species and biofilm formation. The In vitro-study of Algarni confirms this assumption24. They examined the effect of stannous and fluoride ions on the pellicle proteome. Thereby, a higher incidence of proteins was detected after the treatment with stannous fluorides. They concluded that pellicle proteins have an impact on the precipitation of CaF2 reaction products on the tooth surface after the treatment with fluorides. Further, they increase the efficacy of a fluoride treatment. Overall, after the stannous fluoride treatment a higher abundance of mucins (MUC7, MUC5B), albumin and carbonic anhydrase was detected. These mucins can improve the synergy to other proteins like amylase24,25. This enhances the efficacy of the modified pellicle against initial bioadhesion on the tooth surface. In addition, the transient antimicrobial properties were proven by the DAPI and BacLight method after 8 h oral exposition to the oral cavity in situ. These results reassure the assumption that rinsing with stannous ions containing solutions leads to a retention of the stannous ions inside the pellicle layers. Hence, the pellicle can serve as a reservoir for the antibacterial stannous ions and the antibacterial, antiadherent properties of the pellicle are increased. Most interestingly, rinsing with stannous fluoride and stannous chloride reduced significantly the adherence of bacteria to the enamel surface without distinct differences between these two solutions (DAPI).
The available literature12,19,21 devoted to this topic gives no information about this effect. Instead, examining the antibacterial effects of stannous chloride, the accessable studies evaluate commercially availabe products that contain stannous chloride. Thereby, the most frequent combination is stannous chloride with sodium fluoride12,15. Accordingly, antibacterial effects were not completely attributable to stannous chloride or sodium fluoride. Nevertheless, it can be pointed out clearly, that pure stannous fluoride and stannous chloride have antiadherent effects and the stannous ions provide this effect irrespective of the combination of the tin ion with fluoride or chloride. In addition, a modified pellicle ultrastructure with thicker basal layer and thicker less electron dense granular and globular layer was detected after rinsing with the stannous ions containing solutions (Fig. 8). This observation leads to the assumption that protective properties of the pellicle are improved by stannous fluoride and chloride. Thereby, the stannous ions play an important role. The cariostatic effect of Sn2+ can mainly be attributed to two of its characteristics. These include antimicrobial properties and a high affinity to apatite surfaces. Oppermann and Johansen18 assumed that the antimicrobial activity is caused by an inhibition of microbial enzymes, which are involved in the transport, and the metabolism of glucose in bacterial cells. In addition, the acid production of biofilms is inhibited too. These antimicrobial properties are not temporary due to the high substantivity of Sn2+ to the tooth surface and dental biofilms20. Previous studies on dental erosion have already shown, that rinsing with stannous chloride and stannous fluoride leads to a precipitation of stannous ions at the tooth surface11. This layer maintains even after embedding in citric acid (pH 2.3). Some previous publications assume a potential molecular interaction between stannous ions and components of the pellicle layer17. In contrast to monovalent sodium ions, the influence of bivalent stannous ions leads to a cross-linking and an increase in adsorption of specific proteins to the pellicle layer. As a result, an increase in pellicle thickness can be detected in TEM. This includes salivary proteins like mucins and albumin. It is well known, that an increase in pellicle thickness can be associated with a decreased number of adherent bacteria to the tooth surface. In addition, the ratio of dead and vital bacteria can also be influenced. The present study has demonstrated these correlations. It could therefore be concluded that rinsing with stannous fluoride and stannous chloride leads to an increase of the pellicle thickness, resulting in a decrease of bacterial adhesion and bacterial viability.
Amine fluoride is a positively charged, antimicrobial substance with anti-plaque capacity26. In a previous In-situ-study with an amine fluoride containing mouthrinse, the potential accumulation of the amine fluorides could not be approved (EDX)7 and no antiadherent effect could be confirmed7. Adopting the same experimental set-up as Hannig et al.7 described in their in situ study, the present study detected as well no significant reducing effect of bacterial adherence in situ with DAPI. Nevertheless, as confirmed in the present study with the BacLight viability kit, biofilm viability decreased significantly after amine fluoride treatment26. This reduction of vital bacterial cells is mostly attributable to the electrostatic charges of the positively charged amine fluoride and the anionic bacterial cell surfaces26. These results underline the fact that pure amine fluoride solutions can have a significant effect on bacterial viability.
In order to obtain similar preconditions in the present study, the fluoride concentration was always adjusted to 500 ppm. As a consequence, the sample weight and measured pH-value differed among the used experimental fluoride solutions. Thereby, the most effective pure mouthrinse solutions were amine fluoride, stannous fluoride and stannous chloride, respectively. However, they displayed the lowest pH-values among the tested solutions (Fig. 1).
Interestingly, after rinsing with stannous chloride, zones of infiltration were identified in the enamel (TEM, Fig. 8). Thereby, a demineralization of the enamel surface took place. Infiltrated, etched areas on the enamel surface are visible and stannous precipitates are detectable in the infiltrated zones of the superficial enamel (Fig. 8, arrow). They can be interpreted as demineralization of the tooth surface due to the low pH of the stannous chloride rinsing solution (Fig. 1). These zones of infiltration show a demineralized, etched enamel surface filled with proteinaceous structures. In the literature, these areas are also known as subsurface pellicle and it is assumed that they have a barrier function, avoiding a further demineralization of the enamel surface27. Wei and Forbes already showed such an effect in their study28. They examined the effect of 10% stannous fluoride to the enamel surface (3 molars). Thereby, the penetration of the stannous ions was around 20 µm after 30 seconds embedding time. After these 30 seconds a demineralization of the enamel was observed and the authors of this publication described this area as an irregular zone II enamel interface configuration. Recapitulating the images from this study, Wei and Forbes have already observed the formation of a subsurface pellicle and the precipitation of stannous ions at the enamel surface after rinsing with a stannous fluoride solution in 197228.
In the present study, these areas of infiltration were not detectable after rinsing with amine fluoride although both solutions had similar pH-values. Consequently, the fluoride component in stannous fluoride or the higher pH-value might be the reason that prevented the enamel surface from the demineralization event. It would certainly be very interesting to examine the antiadherent properties of stannous chloride at a higher pH-value. Thereby, demineralization could be prevented and the anticariogenic effect could still maintain.
The fluoridation of acidic agents like amine fluoride (pH 3.6) and stannous fluoride (pH 4.5) lead to changes on the hydroxyapatite surface. Thereby, acidic conditions induce complex chemical changes in the structure and compounds like Ca(OH)2, fluoride apatite and CaF2 are formed29–31. It was reported stannous solutions can precipitate at pH levels of 4.5 form precipitates and they can lead to dull feelings on the tooth surfaces and astringency32. The twelve participants of the present study did not express such feelings but stannous precipitates were detected by TEM in the ultrastructure of the pellicle after rinsing with stannous fluoride and stannous chloride.
In addition, it was shown that a higher pH of fluoride solutions is associated with lower efficiency32. This could be an explanation for the lower efficacy of the sodium fluoride and sodium fluorophosphate solutions in the present study. Further experiments are necessary to detect if the pH value has an influence on the bacteria-reducing effect of sodium fluoride and sodium fluorophosphate.
The present experiments on initial bacterial adherence were accomplished on bovine enamel slabs. An experimental set-up maintained in the majority of earlier studies on in situ7 and in vitro8 pellicle formation and initial bioadhesion. Thereby, the application of the fluorides was performed with a solution to ensure that mechanical brushing with a toothbrush had no additional effect. A recent study showed that an prolonged application time doesn’t lead to a thicker surface layer or an increased number of pellicle bound fluoride ions33. Therefore, the used application time of the fluorides was 1 min in the present study.
The use of human teeth is limited in in situ studies. They are difficult to obtain in higher quantities due to the degree of destruction caused by extended carious lesions leading to the extraction of the specific teeth. Thereby, it is difficult to yield a specific sample size and homogeneity of the sample surface34. Accordingly, alternative substrates came into focus. Different analysing methods have shown that bovine enamel mostly resembles human enamel compared to other animal species (human versus bovine, porcine and ovine)34. A recent study of Pelá et al.35 compared the pellicle proteome based on human and bovine enamel slabs under in situ conditions. Thereby, the majority of proteins were similar. The authors assume that a reason might be the similar structure of the inorganic components35. Therefore, human enamel can be substituted by bovine enamel when examining pellicle formation and bioadhesion under in situ conditions35. Comparing the studies on pellicle formation and investigations regarding the effect of different agents on initial bioadhesion in situ, all available comparative studies were conducted on bovine enamel slabs7. Accordingly, bovine enamel slabs were selected for the present in situ study.
In summary, the null hypothesis has been rejected as in the present analysis significant antiadherent effects of stannous containing solutions on the initial bacterial colonization were found. After rinsing with pure SnF2 and pure SnCl2 considerable antiadherent effects and alterations of the pellicle ultrastructure were detected. Further studies investigating the effects of pure fluorides and stannous chloride on in vivo biofilm formation are necessary to give more information.
Material and Methods
Subjects
A number of 12 caries-inactive subjects participated in the present study (Fig. 1). They were all non-smokers and showed no signs of caries or periodontitis in the last two years. Prior to the start of the study, these volunteers had given informed, written consent. An experienced dentist carried out visual oral examination. The study was conducted at the department of operative dentistry at the Dresden university hospital, Germany. The ethics committees of the Dresden (Vote: EK 275092012) University checked and approved the study design. Thereby, all research was performed in accordance with relevant guidelines/regulations. Informed written consent was obtained from all participants.
The aim of this experimental clinical in situ study was to compare the anti-adhesive effect of pure concentration-adjusted fluoride solutions, and one stannous-containing solution, regarding initial bacterial colonization, glucan formation and modification of the ultrastructure of the pellicle.
Specimens
Cylindrical enamel slabs (diameter 5 mm, 19.63 mm surface area, 1.5 mm height) were gained from the facial surfaces of bovine incisors of two-year old cattle (BSE-negative animals). Slabs with structural alterations of the enamel were excluded from the study. The surfaces of the enamel slabs were polished with wet grinding abrasive paper (400–4000 grit) and the smear layer was removed by ultrasonication (us) with NaOCl (3%) for 3 min36. After double washing in distilled water for 5 min (us), the slabs were disinfected in ethanol (70%) for 30 min. Afterwards, they were washed in distilled water again37,38. Before oral exposition, the enamel slabs were stored in 4 °C aqua dest. for 24 h to form a hydration layer37–39.
Tested pure solutions
Concentration-adjusted (to 500 ppm) pure experimental fluoride solutions were produced. Thereby, the the solution volume was 100 ml. Sodium fluoride (Ferdinand Kreutzer Sabamühle GmbH, Nürnberg, Germany), sodium fluorophosphates (Omya Schweiz AG, Oftringen, Switzerland), amine fluoride (Permafluor) 33% (Permcos GmbH, Arisdorf, Switzerland), stannous fluoride (Honeywell Specialty Chemicals GmbH, Seelze, Germany), and stannous chloride dihydrate (Honeywell Specialty Chemicals GmbH, Seelze, Germany) were tested in the present study. Thereby, the natural pH of the solutions was maintained during the experiments (Fig. 1): amine fluoride (pH 3.6), stannous fluoride (pH 4.5), stannous chloride (pH 3.5), sodium fluoride (pH 7.1) and sodium fluorophosphate (pH 6.6). Nonfluoridated tap water served as a reference. Between the different examination days, a two-day wash out period was performed.
Pellicle formation
For in situ pellicle formation individual upper jaw splints were customized from stainless-steel clamps and polymethylmethacrylate40,41. Cavities for the insertion of the enamel slabs were prepared on the buccal sites of the premolars and 1st molar on both sites (n = 8/splint). The fixation of the enamel specimens was performed with polyvinyl siloxane impression material (Aquasil, Denstply De-Trey, Konstanz, Germany). One hour before wearing the splints, the subjects brushed their teeth without toothpaste, flossing was optional, and eating was not allowed. To allow pellicle formation and initial bacterial colonization on the specimens‘ surfaces, the splints were carried intraorally for 1 min followed by a 1-min rinse with the specific test solution (tap water, sodium fluoride, sodium fluorophosphate, amine fluoride, stannous fluoride and stannous chloride), and 8 h of intraoral exposure (Fig. 1).
Total bacterial count (DAPI) and glucan formation (Con A)
DAPI staining was conducted as described previously36. DAPI (4′,6-diamidino-2-phenylindole) stains DNA unspecifically by binding to the AT-rich regions of double stranded DNA42. Upon binding to DNA, the DAPI molecule fluoresces intensely. First, the samples were rinsed with sodium chloride. For staining, the samples were covered with 1 ml DAPI solution (Merck, Darmstadt, Germany) in a dark chamber. After 15 min the DAPI solution was removed, and the samples were covered with methanol for 4 min. In the following, the specimens were dried at room temperature and coated with Citifluor (Citifluor Ltd., London, UK) and analysed by epifluorescence microscopy (Axioskop II, Zeiss, Oberkochen, Germany). The initial biofilms were analysed at 1000-fold magnification using the light filter for DAPI (BP 365, FT 395, LP 397). The number of cells observed in ten randomized microscopic ocular grid fields per sample was counted. The area of ocular grid (0.0156 mm2) allowed calculating the numbers of bacteria per square centimeter. DAPI staining was combined with concanavalin A (Invitrogen, Molecular probes, Darmstadt, Germany) for visualization of glucan formation. The stock solution was 5 mg/ml Alexa Fluor 594 conjugate in 0.1 M NaH2PO4 buffer, pH 8.3. The stock solution was stored at −20 °C. The working solution was a 10-μl stock solution in 490 μl PBS (1 mM CaCl2, 1 mM MnCl2, 1 mM MgCl2).
BacLightTM viability assay
The LIVE/DEAD® BacLight™ Bacterial Viability Kit (Invitrogen, Molecular probes, Darmstadt, Germany) adopts two nucleic acid stains: green-fluorescent SYTO® 9 stain and red-fluorescent propidium iodide stain43. The BacLight kit was used for staining of enamel samples exposed to the oral fluids for visualization of vital and dead bacteria in the adherent state. Similar amounts of component A (Syto9 1.67 mM/propidium iodide 1.67 mM, 300 μl DMSO) and B (Syto9 dye 1.67 mM/propidium iodide 18.3 mM, 300 μl DMSO) were mixed; 2 μl were added to 1 ml of saline solution. The samples were rinsed with sodium chloride. Afterwards the enamel slabs were incubated with this solution in a dark chamber for 10 min. Finally, the samples were rinsed with saline solution and evaluated immediately with a fluorescence microscope using the FDA filter and the ethidium bromide filter7.
Ultrastructural analysis of the in situ formed pellicle (TEM)
The pellicle-covered enamel slabs were fixed in 2.5% glutaraldehyde/1.5% formaldehyde for 2 h. Postfixation took place in 1% osmium tetroxide for 2 h. The specimens were dehydrated in an ascending series of alcohol and embedded in Araldite CY 212 (Plano, Wetzlar, Germany). After decalcification in 1 M HCl re-embedding was accomplished with Araldite. Ultrathin sections of the pellicle layer were cut with a diamond knife, mounted on pioloform-coated copper grids and contrasted with uranyl acetate and lead citrate. TEM analysis of the pellicle´s ultrastructure was performed at 30,000–100,000 fold magnification in a TEM TECNAI 12 Biotwin (FEI Eindhoven, The Netherlands).
Energy-dispersive X-Ray spectroscopy (EDX)
EDX-analysis was performed after application of stannous fluoride and stannous chloride in situ (Fig. 1). The analysis was carried out on these block-face specimens with a XL 30 ESEM (philips, Eindhoven, Netherlands) with an EDX system (Phoenix, EDAX INC., Mahwah, USA) in order to detect stannous ions immobilized in the pellicle layer.
Statistics
Statistical evaluation of the number of adherent bacteria (DAPI and BacLight) as well as the number of glucans was carried out using the Kruskal-Wallis and the Mann–Whitney U-test (p < 0.05)44. Afterwards, a Bonferroni-Holm correction took place. The used Software was SPSS 21.0 (IBM, Ehningen, Germany). Regarding the statistical sample size analysis (Fig. 1) the power of the study is 80%. In the present study the following null hypothesis is tested with DAPI, ConA, BacLight and TEM: No significant effects will be found between the applied pure fluoride rinsing solutions and the control group.
Acknowledgements
This work was not supported by a research foundation. We thank our lab staff for substantial input regarding the experimental part of the study.
Author contributions
J.K. main author of the manuscript, interpretation of the data and statistical data analysis; P.W. responsible for the clinical/experimental part of the study, acquisition of volunteers, preparation of specimens and splints; S.B. clinical/experimental part of the study; B.L. preparation of TEM-samples and performance of transmission electron microscopy; N.P. performance of TEM-coupled EDX-analysis; A.K. assistance with the interpretation of the data; C.H. and M.H. Project leadership and initiators of the study, gave critical and innovative impulses and advices for the manuscript.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smits MT, Arends J. Influence of xylitol- and/or fluoride-containing toothpastes on the remineralization of surface softened enamel defects in vivo. Caries research. 1985;19:528–535. doi: 10.1159/000260893. [DOI] [PubMed] [Google Scholar]
- 2.Hellwig E, Lussi A. What is the optimum fluoride concentration needed for the remineralization process? Caries research. 2001;35(Suppl 1):57–59. doi: 10.1159/000049112. [DOI] [PubMed] [Google Scholar]
- 3.Lendenmann U, Grogan J, Oppenheim FG. Saliva and dental pellicle–a review. Advances in dental research. 2000;14:22–28. doi: 10.1177/08959374000140010301. [DOI] [PubMed] [Google Scholar]
- 4.Hannig M, Joiner A. The structure, function and properties of the acquired pellicle. Monographs in oral science. 2006;19:29–64. doi: 10.1159/000090585. [DOI] [PubMed] [Google Scholar]
- 5.Hannig C, Hannig M. The oral cavity–a key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clinical oral investigations. 2009;13:123–139. doi: 10.1007/s00784-008-0243-3. [DOI] [PubMed] [Google Scholar]
- 6.Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries research. 2011;45:69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannig C, et al. Effect of conventional mouthrinses on initial bioadhesion to enamel and dentin in situ. Caries research. 2013;47:150–161. doi: 10.1159/000345083. [DOI] [PubMed] [Google Scholar]
- 8.Veeregowda DH, van der Mei HC, Busscher HJ, Sharma PK. Influence of fluoride-detergent combinations on the visco-elasticity of adsorbed salivary protein films. European journal of oral sciences. 2011;119:21–26. doi: 10.1111/j.1600-0722.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- 9.Pandit S, Cai JN, Jung JE, Jeon JG. Effect of 1-Minute Fluoride Treatment on Potential Virulence and Viability of a Cariogenic Biofilm. Caries research. 2015;49:449–457. doi: 10.1159/000434731. [DOI] [PubMed] [Google Scholar]
- 10.Ganss C, Schlueter N, Hardt M, Schattenberg P, Klimek J. Effect of fluoride compounds on enamel erosion in vitro: a comparison of amine, sodium and stannous fluoride. Caries research. 2008;42:2–7. doi: 10.1159/000111743. [DOI] [PubMed] [Google Scholar]
- 11.Wiegand A, Bichsel D, Magalhaes AC, Becker K, Attin T. Effect of sodium, amine and stannous fluoride at the same concentration and different pH on in vitro erosion. Journal of dentistry. 2009;37:591–595. doi: 10.1016/j.jdent.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Tinanoff N, Brady JM, Gross A. The effect of NaF and SnF2 mouthrinses on bacterial colonization of tooth enamel: TEM and SEM studies. Caries research. 1976;10:415–426. doi: 10.1159/000260234. [DOI] [PubMed] [Google Scholar]
- 13.Ten Cate JM. Fluorides in caries prevention and control: empiricism or science. Caries research. 2004;38:254–257. doi: 10.1159/000077763. [DOI] [PubMed] [Google Scholar]
- 14.Addy M, Jenkins S, Newcombe R. The effect of triclosan, stannous fluoride and chlorhexidine products on: (I) Plaque regrowth over a 4-day period. Journal of clinical periodontology. 1990;17:693–697. doi: 10.1111/j.1600-051X.1990.tb01055.x. [DOI] [PubMed] [Google Scholar]
- 15.Brecx M, Netuschil L, Reichert B, Schreil G. Efficacy of Listerine, Meridol and chlorhexidine mouthrinses on plaque, gingivitis and plaque bacteria vitality. Journal of clinical periodontology. 1990;17:292–297. doi: 10.1111/j.1600-051X.1990.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 16.Arends J, Schuthof J. Effect of fluoridation on lesion depth and microhardness indentations of artificial white spot lesions. Caries research. 1981;15:176–178. doi: 10.1159/000260515. [DOI] [PubMed] [Google Scholar]
- 17.Kensche A, et al. Effect of fluoride mouthrinses and stannous ions on the erosion protective properties of the in situ pellicle. Scientific reports. 2019;9:5336. doi: 10.1038/s41598-019-41736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oppermann RV, Johansen JR. Effect of fluoride and non-fluoride salts of copper, silver and tin on the acidogenicity of dental plaque in vivo. Scandinavian journal of dental research. 1980;88:476–480. doi: 10.1111/j.1600-0722.1980.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 19.Kasturi, R. et al. Effects of nine weeks’ use of a new stabilized stannous fluoride dentifrice on intrinsic plaque virulence expressed as acidogenicity and regrowth: a modified PGRM study. The Journal of clinical dentistry6 Spec No, 71–79 (1995). [PubMed]
- 20.Scott DC, Coggan JW, Cruze CA, He T, Johnson RD. Topical oral cavity pharmacokinetic modeling of a stannous fluoride dentifrice: an unusual two compartment model. Journal of pharmaceutical sciences. 2009;98:3862–3870. doi: 10.1002/jps.21691. [DOI] [PubMed] [Google Scholar]
- 21.Cvikl B, Lussi A, Carvalho TS, Moritz A, Gruber R. Stannous chloride and stannous fluoride are inhibitors of matrix metalloproteinases. Journal of dentistry. 2018;78:51–58. doi: 10.1016/j.jdent.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Rykke M, Ellingsen JE, Sonju T. Chemical analysis and scanning electron microscopy of acquired pellicle formed in vivo on stannous fluoride treated enamel. Scandinavian journal of dental research. 1991;99:205–211. doi: 10.1111/j.1600-0722.1991.tb01886.x. [DOI] [PubMed] [Google Scholar]
- 23.Ellingsen JE, Rolla G. Treatment of dentin with stannous fluoride–SEM and electron microprobe study. Scandinavian journal of dental research. 1987;95:281–286. doi: 10.1111/j.1600-0722.1987.tb01843.x. [DOI] [PubMed] [Google Scholar]
- 24.Algarni AA, et al. The impact of stannous, fluoride ions and its combination on enamel pellicle proteome and dental erosion prevention. PloS one. 2015;10:e0128196. doi: 10.1371/journal.pone.0128196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine MJ, et al. Structural aspects of salivary glycoproteins. Journal of dental research. 1987;66:436–441. doi: 10.1177/00220345870660020901. [DOI] [PubMed] [Google Scholar]
- 26.van der Mei HC, Engels E, de Vries J, Busscher HJ. Effects of amine fluoride on biofilm growth and salivary pellicles. Caries research. 2008;42:19–27. doi: 10.1159/000111746. [DOI] [PubMed] [Google Scholar]
- 27.Meckel AH. The formation and properties of organic films on teeth. Archives of oral biology. 1965;10:585–598. doi: 10.1016/0003-9969(65)90004-x. [DOI] [PubMed] [Google Scholar]
- 28.Wei SH, Forbes WC. Effect of particle size on reactions of powdered sound human enamel and dentine with stannous fluoride solution. Archives of oral biology. 1972;17:1467–1472. doi: 10.1016/0003-9969(72)90106-9. [DOI] [PubMed] [Google Scholar]
- 29.Petzold M. The influence of different fluoride compounds and treatment conditions on dental enamel: a descriptive in vitro study of the CaF(2) precipitation and microstructure. Caries research. 2001;35(Suppl 1):45–51. doi: 10.1159/000049110. [DOI] [PubMed] [Google Scholar]
- 30.Gerth HU, Dammaschke T, Schafer E, Zuchner H. A three layer structure model of fluoridated enamel containing CaF2, Ca(OH)2 and FAp. Dental materials: official publication of the Academy of Dental Materials. 2007;23:1521–1528. doi: 10.1016/j.dental.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Muller F, et al. Elemental depth profiling of fluoridated hydroxyapatite: saving your dentition by the skin of your teeth? Langmuir: the ACS journal of surfaces and colloids. 2010;26:18750–18759. doi: 10.1021/la102325e. [DOI] [PubMed] [Google Scholar]
- 32.Wiegand A, Waldheim E, Sener B, Magalhaes AC, Attin T. Comparison of the effects of TiF4 and NaF solutions at pH 1.2 and 3.5 on enamel erosion in vitro. Caries research. 2009;43:269–277. doi: 10.1159/000217859. [DOI] [PubMed] [Google Scholar]
- 33.Faidt T, et al. Time Dependence of Fluoride Uptake in Hydroxyapatite. ACS Biomaterials Science & Engineering. 2017;3:1822–1826. doi: 10.1021/acsbiomaterials.6b00782. [DOI] [PubMed] [Google Scholar]
- 34.Teruel Jde D, Alcolea A, Hernandez A, Ruiz AJ. Comparison of chemical composition of enamel and dentine in human, bovine, porcine and ovine teeth. Archives of oral biology. 2015;60:768–775. doi: 10.1016/j.archoralbio.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Pela VT, et al. Proteomic analysis of the acquired enamel pellicle formed on human and bovine tooth: a study using the Bauru in situ pellicle model (BISPM) Journal of applied oral science: revista FOB. 2018;27:e20180113. doi: 10.1590/1678-7757-2018-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hannig C, et al. Fluorescence microscopic visualization and quantification of initial bacterial colonization on enamel in situ. Archives of oral biology. 2007;52:1048–1056. doi: 10.1016/j.archoralbio.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Hannig C, Spitzmuller B, Hannig M. Characterisation of lysozyme activity in the in situ pellicle using a fluorimetric assay. Clinical oral investigations. 2009;13:15–21. doi: 10.1007/s00784-008-0213-9. [DOI] [PubMed] [Google Scholar]
- 38.Hannig C, et al. Effect of safflower oil on the protective properties of the in situ formed salivary pellicle. Caries research. 2012;46:496–506. doi: 10.1159/000339924. [DOI] [PubMed] [Google Scholar]
- 39.Fu B, et al. Evidence of chemisorption of maleic acid to enamel and hydroxyapatite. European journal of oral sciences. 2004;112:362–367. doi: 10.1111/j.1600-0722.2004.00149.x. [DOI] [PubMed] [Google Scholar]
- 40.Weber MT, Hannig M, Potschke S, Hohne F, Hannig C. Application of Plant Extracts for the Prevention of Dental Erosion: An in situ/in vitro Study. Caries research. 2015;49:477–487. doi: 10.1159/000431294. [DOI] [PubMed] [Google Scholar]
- 41.Kensche A, Basche S, Bowen WH, Hannig M, Hannig C. Fluorescence microscopic visualization of non cellular components during initial bioadhesion in situ. Archives of oral biology. 2013;58:1271–1281. doi: 10.1016/j.archoralbio.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz T, Hoffmann S, Obst U. Formation of natural biofilms during chlorine dioxide and u.v. disinfection in a public drinking water distribution system. Journal of applied microbiology. 2003;95:591–601. doi: 10.1046/j.1365-2672.2003.02019.x. [DOI] [PubMed] [Google Scholar]
- 43.Hannig C, Follo M, Hellwig E, Al-Ahmad A. Visualization of adherent micro-organisms using different techniques. Journal of medical microbiology. 2010;59:1–7. doi: 10.1099/jmm.0.015420-0. [DOI] [PubMed] [Google Scholar]
- 44.Kruskal WH. A nonparametric test for the several sample problem. Volume 23, 525–540. Ann. Math. Statist. 1952;23:15. doi: 10.1214/aoms/1177729332. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.