Abstract
From Kant to current perspectives in neuroaesthetics, the experience of beauty has been described as disinterested, i.e. focusing on the stimulus perceptual features while neglecting self-referred concerns. At a neurophysiological level, some indirect evidence suggests that disinterested aesthetic appreciation might be associated with attentional enhancement and inhibition of motor behaviour. To test this hypothesis, we performed three auditory-evoked potential experiments, employing consonant and dissonant two-note musical intervals. Twenty-two volunteers judged the beauty of intervals (Aesthetic Judgement task) or responded to them as fast as possible (Detection task). In a third Go-NoGo task, a different group of twenty-two participants had to refrain from responding when hearing intervals. Individual aesthetic judgements positively correlated with response times in the Detection task, with slower motor responses for more appreciated intervals. Electrophysiological indexes of attentional engagement (N1/P2) and motor inhibition (N2/P3) were enhanced for more appreciated intervals. These findings represent the first experimental evidence confirming the disinterested interest hypothesis and may have important applications in research areas studying the effects of stimulus features on learning and motor behaviour.
Subject terms: Emotion, Learning and memory, Human behaviour
Introduction
Most current theories of aesthetics describe aesthetic appreciation as a mental state focusing on the stimulus perceptual features, while neglecting self-referred concerns1–8. This idea of aesthetic pleasure as disinterested, originated in the western positivist philosophical tradition. Kant, in the ‘Critique of Judgement’, defined taste as “the faculty of judging an object […] by an entirely disinterested satisfaction or dissatisfaction”9. This notion was further adapted into a psychological theory of aesthetics by the philosopher Schopenhauer10, according to whom aesthetic experiences free the observer from “will”, allowing him or her to achieve a transitory will-less [willenlos] perception of the world.” Therefore, aesthetic appreciation is defined as independent from any material or social reward or loss (i.e., disinterested; for a recent discussion see e.g., Kreitman11) and at the same time prompted by a special attitude of attention (i.e., focused on the stimulus features; see e.g., Stolnitz12). For the philosopher Dewey, aesthetic experiences involve an intense engagement in the ever-changing present moment and stand out from more mechanical and routine interactions with the environment13. The temporary suspension of prototypical responses that results from psychological distance (i.e. absence of personal goals or threats) makes room for a higher intensity of the felt sensations and emotions elicited by beautiful objects (Distancing-Embracing model8). This enables observers to fully embrace the “here and now” of perception for its own sake, and the subjectively felt intensity of sensations being rewarding in its own right14.
Interestingly, recent neuroaesthetic research has proposed neurofunctional models of aesthetic appreciation that refer to the same theoretical framework described above. Aesthetic pleasure is considered as a peculiar reward, directed to promote contemplation (i.e., “sensing and learning pleasures”5,15–18), while preventing the craving for objects by inhibiting motor activation3. We will refer to this link between aesthetic appreciation, attention to stimulus features and inhibition of motor behaviour, as the disinterested interest hypothesis.
Some neuroimaging results support this hypothesis. Through an electrophysiological study, de Tommaso et al.19 found increased motor inhibition in response to beautiful images as compared to ugly ones. More specifically, the amplitude of the event-related potential (ERP) P3 component, known to be modulated by motor-inhibition, was greater for visual stimuli perceived as more beautiful than for neutral or ugly images. Kawabata and Zeki20 found significantly greater fMRI activations in bilateral motor cortices during the observation of paintings judged as ugly, as compared to paintings rated as beautiful. Interestingly, motor activations were linearly increasing with the subjectively perceived stimulus ugliness. Similarly, Di Dio and colleagues found increased activations peaking in the left motor cortex after the presentation of images of statues rated as ugly compared to beautiful images21. Moreover, in the auditory domain, the existence of a relation between motor responses to sounds and their pleasantness has also been described18. Roy and colleagues22 found that the startle eye blink reaction amplitude was larger during unpleasant compared with pleasant consonant intervals. Moreover, a number of neuroimaging studies16,23–27 revealed the presence of enhanced sensory processing for more appreciated visual and auditory stimuli, which might be attributed to the effect of increased attentional engagement28–30. Additionally, fMRI studies investigating “disinterested” aesthetic judgements showed partially functionally dissociable networks underlying judgements of beauty and more pragmatic (e.g. symmetry) judgments25. During aesthetic judgements only, more appreciated visual stimuli caused a “beauty-induced” signal boost in higher visual processing areas25 that mimics the effect of increased attention. However, to the best of our knowledge, there is no direct evidence of a link between beauty-related motor inhibition and attention engagement towards beautiful stimuli, as postulated by the disinterested interest hypothesis.
In the present study, we aim at testing this hypothesis, both at a behavioural and at an electrophysiological level, with auditory stimuli. Sounds, as well as images, can induce an aesthetic response, and, at the same time, it is possible to control for sounds’ basic features (e.g., frequency, duration, complexity, volume) in a very precise way. Previous studies demonstrated that ERPs may provide neurophysiological indexes of both attentional engagement/selection31,32 and motor inhibition33–35, thus making EEG a suitable technique to investigate the possible correlates of disinterested aesthetic appreciation. We will present content-free auditory stimuli, consisting in more or less consonant two-note intervals (i.e., two synthetic tones displayed simultaneously), because consonance is known to influence aesthetic appreciation36 and to modulate cortical responses measured with EEG37–41. Even though some studies found peference for mild dissonance over consonance42, more consonant musical intervals are normally (especially in non musicians43) more appreciated than dissonant ones36,44–48. Importantly, it is possible to produce consonant and dissonant intervals sharing comparable physical features by just varying the ratio between the frequency (Hz) of single tones36 (§ Stimuli). This was crucial in the present experiment to control for potential confounding effects, due to tones’ basic physical features, known to affect EEG responses49.
To test our hypothesis, we performed three EEG experiments. In Experiment 1, participants were asked to evaluate the beauty of single intervals (aesthetic judgement task, from now on AJ task). In Experiment 2 participants listened to the same intervals intermixed with white noise (intervals were presented in 50% of trials) and had to respond as fast as possible by pressing a button whenever they heard an interval (Detection task). This task aimed at investigating the relationship between aesthetic experience and motor behaviour. In Experiment 3, participants had to perform a Go-NoGo task, which is usually employed to investigate motor-inhibition mechanisms and their electrophysiological correlates50,51. In this task subjects had to respond to frequent Go stimuli, while avoiding to respond to infrequent NoGo stimuli.
If the disinterest interest hypothesis is correct, we expect the following results: 1) Slower response times in the detection task for more appreciated intervals (as a behavioural index of related motor inhibition); 2) The enhancement of ERP components related to motor inhibition (the N2/P3 complex) for more appreciated intervals; 3) The enhancement of attention-related ERP components (such as the N1/P2 complex) for more appreciated intervals.
Results
Experiment 1 (AJ task)
Behavioural results
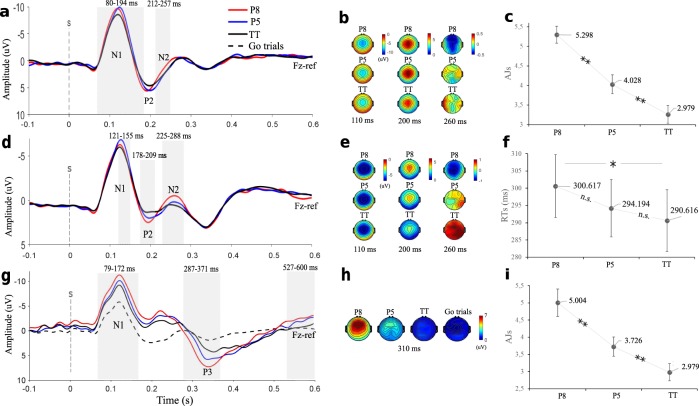
Aesthetic judgements (AJs) were significantly modulated by consonance (F = 45.682, p < 0.001, η2p = 0.685, observed-power = 1): more consonant intervals were, on average, more appreciated than more dissonant ones on a 1–9 Likert scale (5.298 for Octaves, 4.028 for Fifths and 3.26 for Tritones, Fig. 1c).
Figure 1.
AEPs and behavioural results. Panel a shows grand-average AEPs recorded at Fz during the Aesthetic Judgement task. AEPs elicited by different interval types during the Detection and Go-Nogo task are represented in panel d and g, respectively. Shaded areas represent significant time-clusters evidenced by the point-by-point ANOVA. Scalp-maps depict voltage distribution registered during the Aesthetic Judgement (panel b) and Detection task (panel e) at 110 ms (N1), 200 ms (P2), 260 ms (N2). Panel h shows voltages registered on the scalp at 310 ms post-onset (P3) during the Go-NoGo task. Panels c, f and i depict all subjects’ mean AJs from Experiment 1, RTs from Experiment 2 and AJs from Experiment 3, respectively. Bars represent standard errors. Asterisks represent significant differences in single subjects’ mean AJs and RTs between different interval types evidenced by two-tailed t-tests (*p < 0.05, **p < 0.001, n.s = not significant). P8 = perfect octaves, P5 = perfect fifth intervals, TT = tritone intervals.
Auditory evoked potential (AEP) results: The point-by-point ANOVA (corrected with 1000 permutations) highlighted two significant clusters with a fronto-central distribution. On Fz, the main effect of ‘Condition’ was a significant source of variance within the time window of 80–194 ms and 212–257 ms, corresponding to the latency of the N1/P2 complex and the N2 component, respectively (Fig. 1). Post-hoc pairwise comparisons (cluster corrected point-by-point t-tests comparing waveforms corresponding to the three different interval types) were performed given the significant effect of “Interval type”. The results of point-by-point t-tests are fully reported in Table 1.
Table 1.
Post-hoc point-by-point t-tests.
| (I) Interval type | (J) Interval type | Significant clusters latencies (ms post-onset) |
|---|---|---|
| Experiment 1 | ||
| P8 | P5 | 126–174 |
| 207–259 | ||
| P5 | TT | 78–135 |
| 160–193 | ||
| 229–258 | ||
| TT | 115–156 | |
| Experiment 2 | ||
| P8 | P5 | 126–158 |
| 222–281 | ||
| TT | 177–207 | |
| 235–291 | ||
| P5 | TT | 115–149 |
| 185–213 | ||
| Experiment 3 | ||
| P8 | P5 | 40–75 |
| 113–165 | ||
| TT | 82–186 | |
| 274–370 | ||
| 519–601 | ||
| P5 | TT | 544–623 |
This table reports the latencies of significant clusters evidenced at Fz by the point-by-point t-tests comparing waveforms registered during different interval types (I vs. J).
P8 = Octaves; P5 = Fifths; TT = Tritones.
Mean correlation coefficients (averaged across participants) between amplitudes at channel Fpz and AJs are depicted in Fig. 2. The point-by-point t-test on single subjects’ r values highlighted a significant positive correlation between trial-by-trial P2 amplitudes and AJs, as revealed by the presence of a significant cluster centred at the latency corresponding to the peak of the P2 component (150–189 ms). Moreover, a second significant time-cluster at 272–296 ms revealed a negative correlation between N2 amplitudes and AJs. As shown by scalpmaps in Fig. 2, r-values were peaking at Fpz at 180 and 280 ms post-onset.
Figure 2.
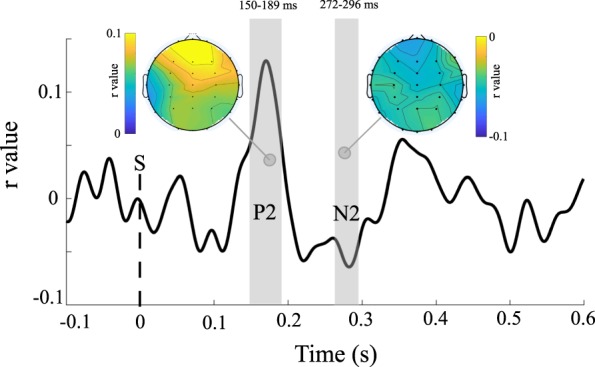
Point-by-point trial-by-trial correlation analysis. The graph shows mean (averaged across all participants) correlation coefficients between single subjects’AJs (Experiment 1) and signal amplitudes at channel Fpz. Shaded areas represent significant clusters evidenced by the point-by-point t-test comparing the 22 single subjects’ r values against 0. The t-test on r values between amplitudes and AJs revealed two significant clusters (150–189 and 272–296 ms) corresponding to P2 and N2. Scalpmaps represent the distribution of mean r values at 180 and 280 ms post-onset.
Experiment 2 (Detection task)
Behavioural results
Omission rate was 0% in the Detection task. On average, 8 responses per participant (2.85% of the total) were considered as outliers (i.e. RTs exceeded two standard deviations from the single subject’s mean) and were excluded from subsequent analyses. Outliers were equally distributed across interval types.
The repeated measures ANOVA performed on RTs failed to reveal a significant effect of the factor interval type (F = 2.678, p = 0.08, η2p = 0.113, observed-power = 0.502). Interestingly, however, RTs were on average slower for more appreciated intervals (300.617 ms for Octaves, 294.194 for Fifths and 290.616 for Tritones, Fig. 1f). Moreover, based on our hypothesis #1 and despite the fact that the main effect of interval type was not significant, we performed post-hoc analyses (two-tailed t-tests), to verify whether pair-wise comparisons between RTs belonging to different interval types yielded any significant result. Post-hoc comparisons revealed a significant difference between RTs to Octaves and Tritones (t = 2.066; p = 0.026; Cohen’s d = 0.075). Furthermore, results from the linear mixed-model analysis evidenced that AJs could significantly predict RTs (estimate of the effect = 3.133; 95% CI: 0.147 + 6.199; p = 0.04; t = 2.062). This result was significant after correction for multiple comparisons (Benjamini–Hochberg correction; false discovery rate: 10%; total number of tests in the study: 30). Crucially, as we expected, predicted RTs increased with AJs (see Fig. 3).
Figure 3.
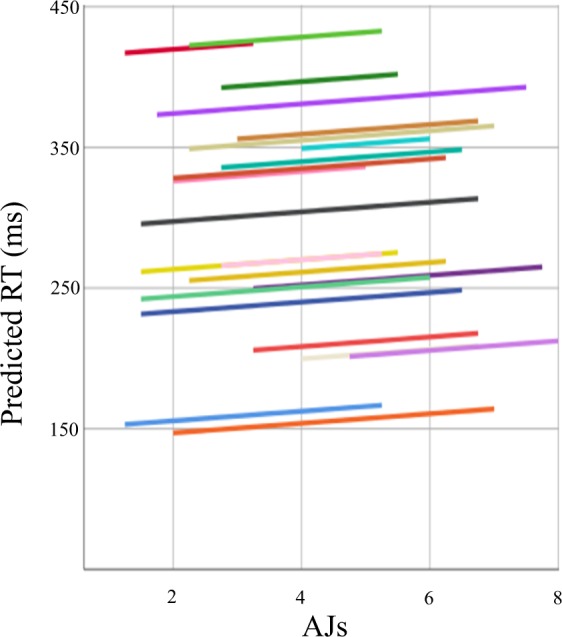
Linear mixed-model. The 22 coloured lines represent single participants’ predicted RTs, based on the parameters estimated by the mixed-model analysis (§ Data analysis, Behavioural data) and observed AJs. Predicted RTs were defined as a function of subjects’ ID and observed AJ. The positive slope of the lines indicates a positive statistically significant (§ Results, Experiment 2) relation between AJs and RTs.
AEP results
Similarly to Experiment 1, the point-by-point ANOVA (corrected with 1000 permutations) evidenced three significant clusters, with a wide fronto-central distribution. On Fz, the main effect of ‘Interval type’ was a significant source of variance within three time windows: 121–155 ms, coinciding with the latency of N1; 178–209 ms, corresponding to the latency of the P2 component; and 225–288 ms, coinciding with the N2 component (Fig. 1d). Therefore, the point-by-point analysis revealed that N1, P2 and N2 amplitudes were all significantly modulated by interval type during both the AJ and the Detection task.
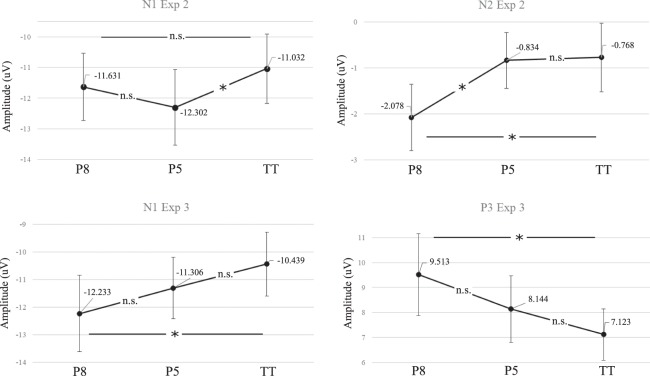
ANOVA results on peak amplitudes are fully reported in Table 2, where we also included pairwise comparisons between the levels of the factor interval type for components that were found to be significantly modulated by interval type. Results were corrected for multiple comparisons (Benjamini–Hochberg correction; false discovery rate: 10%; total number of tests in the study: 30). Overall peak analyses confirmed the findings highlighted by the point-by-point ANOVA, except for P2 peak in Experiment 2, where differences among interval types did not reach significance. In Experiment 2, N1 and N2 peak voltages were significantly modulated by interval type. Average peak amplitudes for significant components are represented in Fig. 4. With the exception of N1 in Experiment 2, average peak amplitudes generally showed the same trend as AJs, with greater peak voltages registered during the display of more appreciated consonant intervals.
Table 2.
Peak amplitudes ANOVA.
| ANOVAs on mean peak voltages | ||||||
|---|---|---|---|---|---|---|
| Task | Component | Mean voltage at peak (µv) | F-value | p-value | Effect-size (Eta partial square) | Observed power |
| Detection-Experiment 2 | N1 | P8: −11.63; P5: −12.30; TT: −11.03 | 6.091* | 0.005 | 0.225 | 0.865 |
| N2 | P8: −2.07; P5: −0.83; TT: −0.77 | 6.609* | 0.003 | 0.239 | 0.891 | |
| P2 | P8: 5.22; P5: 5.38; TT: 5.15 | 0.227 | 0.799 | 0.022 | 0.081 | |
| Go-NoGo-Experiment 3 | N1 | P8: −12.23; P5: −11.30; TT: −10.43 | 3.66* | 0.034 | 0.148 | 0.643 |
| P3 | P8: 9.51; P5: 8.14; TT: 7.12 | 3.85* | 0.029 | 0.155 | 0.666 | |
| Pairwise comparisons | ||||||
| (II) Interval type | (J) Interval type | Mean difference (I-J) | St. err. | p-value | 95% CI around Mean difference | |
| N1 Experiment 2 | ||||||
| P8 | P5 | 0.670 | 0.358 | 0.075 | −0.073; 1.414 | |
| TT | −0.599 | 0.351 | 0.103 | −1.329; 0.131 | ||
| P5 | TT | −1.270* | 0.382 | 0.003 | 0.475; 2.064 | |
| N2 Experiment 2 | ||||||
| P8 | P5 | −1.244* | 0.391 | 0.004 | −2.058; −0.431 | |
| TT | −1.311* | 0.440 | 0.007 | −2.226; −0.396 | ||
| P5 | TT | −0.066 | 0.385 | 0.865 | −0.867; 0.735 | |
| N1 Experiment 3 | ||||||
| P8 | P5 | −0.875 | 0.534 | 0.116 | −1.987; 0.236 | |
| TT | −1.746* | 0.791 | 0.039 | −3.391; −0.101 | ||
| P5 | TT | −0.870 | 0.582 | 0.149 | −2.08; 0.339 | |
| P3 Experiment 3 | ||||||
| P8 | P5 | 1.316 | 0.828 | 0.127 | −0.407; 3.039 | |
| TT | 2.248* | 0.870 | 0.017 | 0.438; 4.058 | ||
| P5 | TT | 0.932 | 0.738 | 0.221 | −0.603; 2.467 | |
Amplitudes at peak latencies at Fz were extracted from single subjects’ mean ERP for relevant components in Experiment 2 and 3. Peaks voltages were entered in a one-way repeated measures ANOVA with Interval type as a within-subject factor. P-values highlighted in bold are significant after Benjamini–Hochberg correction for multiple comparisons.
P8 = Octaves; P5 = Fifths; TT = Tritones.
Figure 4.
Mean peak amplitudes. The graphs show, for AEP components separately, all subjects’ mean peak amplitudes for the three interval types. Bars represent standard errors. Asterisks represent significant (p < 0.05) post-hoc pairwise comparisons between interval types (*p < 0.05, n.s = not significant). P8 = perfect octaves, P5 = perfect fifth intervals, TT = tritone intervals.
Experiment 3 (Go-NoGo task)
Behavioural results
One participant was excluded from subsequent analyses for a technical problem which hampered the recordings. The remaining 21 participants of Experiment 3 performed, on average, 4.95 errors in the Go-NoGo task (incorrect NoGo trials were 5.893% of the total). Average error rates were comparable across interval types (6.247% for Octaves, 5.71% for Fifths and 5.71% for Tritones) and did not significantly differ between interval types as evidenced by a repeated measure ANOVA (F = 0.106, p = 0.9, η2p = 0.005, observed-power = 0.065).
AEP results
Waveforms corresponding to the N1 (79–172 ms) and P3 (287–371 ms) components registered during the Go-NoGo task were significantly modulated by interval type (Fig. 1g), as evidenced by the point-by-point ANOVA (corrected with 1000 permutations). One additional significant time-cluster, centred around 550 ms post-onset, was evidenced by the ANOVA. This later cluster presumably corresponds to the negative rebound following the P3 oscillation. The ANOVA performed on peak voltages confirmed these results: N1 and P3 peak voltages were significantly modulated by interval type and were increased for more appreciated intervals (Table 2, Fig. 4).
Discussion
In this study we aimed to test the disinterested interest hypothesis in the auditory domain, namely that aesthetic appreciation for more consonant two-note intervals is associated with attentional enhancement and motor inhibition. Based on this hypothesis we predicted, for more appreciated intervals: (1) Slower response times in the Detection task; (2) Significantly larger motor-inhibition AEP responses; (3) More pronounced AEP components related to attention enhancement. The results substantially confirmed our predictions. In Experiment 1 and 3 we evidenced a subjective preference for more consonant intervals, thus replicating previous findings. Importantly, in Experiment 2, we showed that AJs predicted RTs in a simple detection task, as evidenced by the mixed-model analysis, with slower RTs for increasing AJs, thus confirming prediction #1. Moreover, results from Experiments 2 and 3 showed that attention and motor-inhibition related AEP components were significantly enhanced for more appreciated intervals, thus confirming predictions #2 and #3 (see also below). Overall, our behavioural and electrophysiological results seem to support the disinterested interest hypothesis. To our knowledge, this is the first empirical evidence of a direct link between aesthetic appreciation, attentional enhancement and motor-inhibition.
In the following paragraphs, we will discuss our electrophysiological results in relation to the existing literature, evidencing the possible evolutionary advantage of attentional enhancement and motor inhibition during aesthetic appreciation and the potential implications for basic and clinical research. We will discuss our results in the light of neuroimaging and behavioural results encompassing different sensory domains. This might be criticised, since some authors52 highlighted the need for domain-specific models of aesthetic judgements. It is possible that domain-specific models may be more appropriate to describe the processing of complex works of art52 (e.g. music pieces and paintings), which extends far beyond mere aesthetic pleasure and cannot be reduced to “core liking”18. Previous studies have demonstrated that, during the processing of works of art, domain specific processes normally apply to (low level) sensory processing, whereas domain general mechanisms apply to (higher level) central processing52. In our case, however, low-level perceptual correlates of “core liking”, triggered by basic stimuli (such as two-note intervals), might be predicted by domain-independent models as well. Indeed, neuroimaging and behavioural results in neuroaesthetics28,30,53 and neurocomputational models of aesthetic emtotions54,55 seem to suggest a common neurophysiological and behavioural pattern in the emergence of aesthetic appreciations across different sensory domains.
The N1/P2 complex amplitude has been frequently described as an index of attentional engagement31,56–61. In accordance with previous findings, in our study the N1/P2 complex amplitude was modulated by interval type in all three experiments, as evidenced by the point-by-point ANOVA, with larger amplitudes associated with preferred interval types. Interestingly, trial by-trial fluctuations in P2 voltages registered during Experiment 1 significantly correlated with single trial AJs (see Fig. 2). Moreover, N1 peak voltages from Experiment 3 were significantly larger for more appreciated intervals (see Fig. 4). Thus, overall, the point-by-point analyses on N1/P2 complex seem to indicate a significant enhancement of attentional-related responses for more appreciated interval types. Coherently, previous findings showed that expertise produced a similar effect: musical chords elicited larger P2 responses in professional musicians compared to laypersons, suggesting that experts develop specific abilities for music perception and cognition62.
It must be noticed, however, that peak analysis failed to find significant enhancement of N1/P2 complex for more appreciated intervals in Experiment 2, thus failing to replicate point-by-point results for what concerns N1 and P2. This apparently counterintuitive result might reflect the less ‘contemplative’ nature of the Detection task performed in Experiment 2, where participants had to respond to intervals as fast as possible. Task analysis, namely the comparison of contemplative vs. pragmatic responses to aesthetic stimuli, could inform us regarding the specific cognitive-affective processes underlying aesthetic judgement tasks63–65. This approach, however, might not be best suited to interpret our results because task demands and procedures among tasks are not directly comparable. Nevertheless, in the case of our study, more action-related neural resources were probably recruited in the Detection task: as a consequence, the expression of attentional-related components, was minimized (notice that N1 and P2 voltages were halved in the Detection task in respect to the AJ task and NoGo trials in the Go-NoGO task). Indeed, previous studies63–65 demonstrated that the amplitude of ERPs registered during the presentation of beautiful and ugly stimuli can be differently modulated when participants are judging stimulus beauty (i.e. contemplative condition) vs. more pragmatic aspects of the stimuli (i.e. non-contemplative condition). More pragmatic and action-finalized tasks (such as those requiring fast responses) seem to prevent the adoption of a contemplative attitude, typical of aesthetic judgements1,12,66, probably because motor preparation competes with perceptual mechanisms directing the attentional focus on stimulus features8. In other words, phenomenal and electrophysiological correlates of “core liking”18, i.e. embracing perception and sensations, might emerge only when perceivers are not goal-oriented (in the case of our study, when participants are not required to respond as fast as possible), thus allowing psychological distancing8.
In all our experiments, the amplitude of N2/P3 complex was systematically modulated by interval type, with larger amplitudes in response to more appreciated intervals. Crucially, N2/P3 amplitude enhancement is traditionally related to the recruitment of a “global suppression network”67 responsible for motor inhibition, and the slowing of motor output68. Apparently, N2 amplitude increases reflect early non-strictly motor aspects (i.e. cognitive) of inhibition50, finalized to overcome the usual stimulus-to-response mappings and to update the behaviour plan69,70. Therefore, N2 amplitude can be modulated even in tasks not directly requiring the inhibition of a motor response and, in such tasks, generally correlates with response times71,72. Consistently with this finding, we observed an increase of N2 voltages following the presentation of more appreciated intervals also in Experiment 1 and 2, where subjects were not required to inhibit their motor responses (i.e., they were not performing a Go-NoGo task). Notably, in Experiment 1, trial-by-trial fluctuations in N2 voltages were significantly correlated with AJs. P3 amplitude modulation, instead, has been traditionally related to later properly motoric stages of response inhibition33,50,51. As predicted by previous studies, we observed a significant P3 amplitude modulation only in Experiment 3 (the Go-NoGo task). Crucially, P3 average peak amplitudes registered during NoGo trials, were enhanced for more appreciated consonant intervals. Overall, the N2/P3 amplitude modulation observed in our experiments seem to indicate that aesthetic appreciation significantly fosters both non-motoric and strictly motoric stages of response inhibition, thus directly limiting motor activation. We propose that this inhibitory mechanism is finalized to support the contemplative “aesthetic attitude” (see also below).
Altogether, the enhancement of electrophysiological indexes of attentional engagement and motor inhibition might represent the neural counterpart of the neglect for self-referred concerns paired with an increment of attention for the stimulus perceptual features described by the theory of disinterest and distancing-embracing models of aesthetic experiences8 (see Introduction). However, it remains unclear what the evolutionary advantage of such a mechanism could be. Why do we divert attentional resources from action execution to the perception per se (i.e. contemplation) of more appreciated stimuli? This might be better understood within the theoretical framework of the free-energy principle73–75. Following the free-energy principle, agents select their action plans maximizing both expected utility (i.e. extrinsic value) and information gain or intrinsic epistemic value76–78. Since attention is a limited resource79, the most profitable strategy is probably to devote attentional resources, from time to time, either to maximize stimulus epistemic intrinsic value (i.e. updating and refining prior beliefs) or to maximize utilitarian extrinsic value based on prior beliefs80–82. But how does the nervous system choose where it should be most profitable to direct the attentional focus (toward perception vs toward action)? Previous theoretical models suggested that, in order to recognize stimuli which maximize epistemic value, intelligent systems (biological and artificial) have developed an intrinsic feedback on information gains (see Gottlieb et al.82 for a review). According to some authors15,83–85, the brain generates intrinsic rewards to stimuli with high informational content directly modulating the active sampling of sensory inputs. In accordance with this idea, we propose that aesthetic pleasure serves as an intrinsic reward in response to highly informative sensory interactions signaling to the nervous system the profitability of directing attention to present stimuli instead of modifying the environment through motor activation. This idea fits well with current models of aesthetic emotions, which posit that the intrinsic pleasantness of stimuli is of preeminent importance for their emergence14. Interestingly, previous research has already postulated the existence of a close link between aesthetic appreciation and stimulus informational value, with greater AJs for stimuli with the higher informational content86–91. Aesthetic pleasure has indeed been defined as a “meta-learning feedback”92 on successful perceptual learning dynamics5,55,86,87,92, i.e. when the cognitive system senses a progress in the refinement of mental representations and in the insightful93 creation of new ones87,89,92. Accordingly, the update of prior beliefs (which can be considered as an index of stimulus high informational value) was found to attract attention94,95 and to inhibit motor response68,96. Crucially, informational value per se also seems to trigger activations of midbrain reward-related areas97, which are usually found to correlate with aesthetic appreciation20,98,99. These data further support the presence of a direct link between aesthetic appreciation, stimulus information value, attentional enhancement and motor inhibition.
At first sight, the correlation between motor inhibition and aesthetic appreciation might seem at odds with other hypotheses which claim the active involvement of the mirror motor system in aesthetic appreciation, such as the “embodied simulation”100–102. According to this theory, the perception of beauty depends on the magnitude of empathic resonance with the content of works of art triggered by the activation of mirror neurons in motor103,104 and premotor105 areas. In our view, however, the two hypotheses are not mutually exclusive. Indeed, Gallese and colleagues argue that “embodied simulation” must be “liberated”, meaning that works of art (and the context in which they are perceived) must induce a potentiation of the mirroring mechanisms that are normally active in daily life102. According to Gallese, this potentiation is achieved via motor inhibition: “immobility, that is, a greater degree of motor inhibition, probably allows us to allocate more neural resources, intensifying the activation of bodily-formatted representations, and in so doing, making us adhere more intensely to what we are simulating”102 (p.48).
In our study, aesthetic preference, motor inhibition and attentional enhancement positively correlated with the consonance level of musical intervals. This result fits well with the hypothesis of a link between informational value and AJs, as discussed above. Aesthetic pleasure might be considered as a fundamental feedback to discriminate between fluently processed (i.e. informationally profitable) and noisy (i.e.“unlearnable”) signals. This argument might explain the preference for more consonant intervals given the evidence, well supported by behavioural and psychophysiological data, that consonant intervals are processed more fluently than dissonant intervals37,46,106–109. To this respect, it has been suggested that such processing efficiency enhancements might reflect the similarity between consonant intervals and conspecific vocalizations (which are mostly harmonic) to which the auditory system is tuned36,44,110.
In the present research, we propose that the experience of aesthetic appreciation might be considered as a cognitive state signalling to the system to refrain from acting in order to focus on present sensory stimulation to learn something new. Our results point to the possibility that the aesthetic value of stimuli can modulate cognitive functions, such as perceptual learning and memory retrieval (see also Lehmann & Seufert111 for a recent review), and future research should investigate this issue. Furthermore, the role of aesthetic emotions in automatically guiding attention toward perception and learning, instead of acting impulsively, is also potentially interesting for learning-oriented activities, such as teaching112,113, psychotherapy114,115 or communication more in general13. Moreover, the automatic attentional capture induced by aesthetic appreciation might be exploited in the design of experimental paradigms, where the attentional engagement of participants is crucial. Finally, the use of aesthetically more valuable stimuli might contribute to develop more effective neuropsychological rehabilitative protocols, for example with patients affected by mild cognitive impairments or dementia which manifest attentional and motivational deficits.
Although we consider our study an original contribution to the field of neuroaesthetic research, it presents a number of limitations that must be acknowledged.
First, the essentiality of two note intervals limits the range and the intensity of aesthetic responses. On the other hand, the use of richer stimuli would inevitably introduce potential confounds into the results, such as cognitive, perceptual, emotional, situational, socio-cultural, affiliation and historical factors116. Nevertheless, future studies should attempt testing the disinterested interest hypothesis employing more elaborated stimuli such as complex musical chords (rather than two-note intervals), photos or paintings. Secondly, although the occurrence of attentional enhancement and motor inhibition in the auditory modality are coherent with previous findings from neuroimaging studies20–22 investigating aesthetic appreciation across sensory modalities (see Nadal30 for a review), the issue of the level of generality of our results across sensory modalities has not been addressed in our study (i.e., modality dependence vs independence). Indeed, it was shown that more complex aesthetic experiences which extend beyond “core liking”, such as the appreciation of works of art (i.e. paintings and music pieces), entail both modality-independent and modality-specific processes52. Based on the evidence from previous studies20–22,30, we hypothesize that motor inhibition and attentional enhancement emerge during “core liking”18 independently from sensory modality, but additional research is needed to test this hypothesis. Thirdly, for technical constraints, our experimental design did not allow to collect AJs and RTs simultaneously while registering EEG activity: this prevented the investigation of the relationship between aesthetic appreciation and motor inhibition on a trial-by-trial basis, which would have increased the internal validity of our study. Lastly, the experimental procedures employed in our tasks differed too much to allow for a direct comparison of the results of the (more contemplative) AJ task and the (more pragmatic) Detection task. Future studies should specifically address the issue of the effect of the nature of the task (e.g. aesthetic judgement vs. pragmatic judgement) on motor and attentional responses to more appreciated stimuli.
Methods
Participants
Forty-four right-handed healthy volunteers participated to the study. Twenty-two participants (12 females; age: 24.45 ± 1.96; years of education: 16.45 ± 1.36) took part to Experiment 1 (AJ task) and 2 (Detection task). The remaining twenty-two (13 females; age: 25.75 ± 2.11; years of education: 16.71 ± 1.72) participated in Experiment 3 (Go-NoGo task). The order of Experiments 1 and 2 was counterbalanced among subjects: half of the participants started with Experiment 1, while the remaining half started with Experiment 2. All participants gave their written informed consent to participate to the study. The study conformed to the standards required by the Declaration of Helsinki and was approved by the local ethics committee (University of Turin).
Stimuli
Musical Intervals were created with Csound (https://csound.com/) software, which allowed to specify the frequency (Hz) of single notes composing the interval. Different types of two-note intervals were defined by the ratio between the frequency of the two notes. Although not exclusively117,118, consonance also depends on this ratio: the smaller the numbers that define the ratio, the more consonant will be the resulting interval36. Octaves (consonant) were composed by notes with a ratio of 2:1, fifth intervals (mildly dissonant) had a ratio of 3:2, while tritons (dissonant) were defined by a ratio of 45:32. We created seven intervals for each ratio type by varying the frequency of the first note from 200 Hz to 260 Hz (middle C) by steps of 10 Hz. The second note varied according to the ratio described above. In Table 3 we report the frequency of the notes of all the intervals we employed.
Table 3.
Stimuli.
| Frequency of the first note (Hz) | Frequency of the second note (Hz) | ||
|---|---|---|---|
| Octave | Fifth | Tritone | |
| 200 | 100 | 133.33 | 142.22 |
| 210 | 105 | 140 | 149.33 |
| 220 | 110 | 146.66 | 156.44 |
| 230 | 115 | 153.33 | 163.55 |
| 240 | 120 | 160 | 170.66 |
| 250 | 125 | 166.66 | 177.77 |
| 260 | 130 | 173.33 | 184.88 |
First and second notes were displayed simultaneously for 50 ms. Seven intervals with varying frequencies were displayed for each interval type.
In the Detection (Experiment 2) and in the Go-NoGo tasks (Experiment 3) intervals were intermixed with randomly generated white noise sounds (some trials contained intervals, others contained white noise). Both intervals and white noise were displayed via loudspeakers at the same output intensity (65 dB) for 50 ms. We chose short presentation durations to limit as much as possible the potentially detrimental effect of stimuli offset on EEG signals, given the fact that offset responses are inversely proportional to the duration of the prior sound119.
Apparatus
The set up was identical in the three experiments. Participants sat at a table in a fixed position, distant 60 cm from the loudspeakers and from a 53 cm (diagonal) computer screen, with the screen centre and loudspeakers placed one next to the other and aligned with the trunk midline. The participant’s left arm was resting on the corresponding leg, while the right arm was placed on the desk. Subjects had their index finger resting on the keyboard spacebar during the Detection and the Go-NoGo tasks. Response keys and subjects’ right hand were aligned with the trunk vertical axis. AEPs were registered during all experiments.
Experimental procedures
Experiment 1 (AJ task)
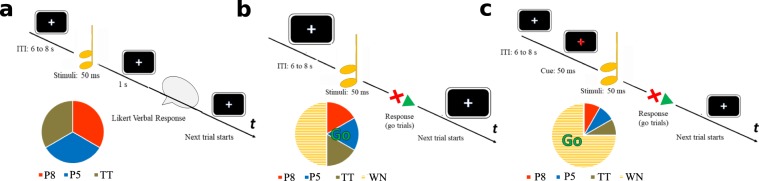
Experiment 1 consisted of two identical runs. In each run participants were asked to evaluate the beauty of musical intervals using a Likert scale ranging from 1 to 9 (1 = Most ugly, 9 = Most beautiful). Each of the 21 intervals we created was evaluated twice in each of the two runs (for a total of 28 judgements for each interval type in the whole experiment). The trial timeline is depicted in Fig. 5. Intervals were presented in a random order for 50 ms after a variable inter-trial interval (range: 6–8 s). Participants fixated a central white cross for the whole experiment. When they heard an interval, they were asked to wait 1 second until the cross changed into a question mark and then verbally report their evaluation. AJs were recorded by the experimenter using a keyboard and were automatically registered by E-Prime 2.0 software (Psychology Software Tools, Inc. USA). Participants had a five minutes break between runs. Each run lasted approximately 8 minutes.
Figure 5.
Trial timeline. Panel a shows the trial timeline for the AJ task: after the two-note interval was played participants remained still for one second and then verbally reported their answer. Panels b and c show the single-trial timeline of the Detection and Go-NoGo tasks, respectively: participants were instructed to press the spacebar only when hearing intervals in the Detection task. Contrarily they had to respond only when hearing white noise in the Go-NoGo task. Sounds were preceded (1 s) by a visual cue (fixation cross turning red for 50 ms) in the Go-NoGo task. Pie charts represent the proportion between perfect octave intervals (P8), perfect fifth intervals (P5), tritone intervals (TT) and white noise (WN) in each experiment.
Experiment 2 (Detection task)
Experiment 2 consisted of two runs of a simple detection task employing the same musical intervals of the AJ task. Intervals were intermixed with 50 ms of white noise. Each of the 21 intervals we created was presented twice in each of the two runs (for a total of 28 presentations for each interval type in the whole experiment). The white noise was presented 42 times in each run (white noise and interval trials were equally numerous). The trial timeline is depicted in Fig. 5. Intervals were presented in a random order after a variable inter-trial interval ranging from 6 to 8 s. Participants fixated a central white cross for the whole experiment. They were instructed to press the spacebar as fast as possible as soon as they heard an interval and to restrain from responding when they heard a white noise. Response time (RT) and response accuracy were automatically registered by the experimental software.
Experiment 3 (Go-NoGo task)
Experiment 3 consisted in a Go-NoGo task similar to Experiment 2 except that: 1) subjects had to respond to the white noise and refrain from responding when they heard an interval; 2) the fixation cross turned red for 50 ms (preparatory cue) 1 s before the sound (Go-Nogo signal) was played; 3) white noise and intervals were not equally numerous. Intervals (No-Go trials) were rarer than white noise (Go trials), with a proportion of one to three (28 intervals per interval type and 252 white noises for a total of 336 trials). In each run the 21 intervals were presented twice, randomly alternated with 126 white noise sounds. Additionally, after the Go-NoGo task participants of Experiment 3 performed a brief AJ task identical to Experiment 1 described above but with shorter ITI (2–3 s). AEPs were not registered during this second phase. The Go-NoGo task was devised to elicit those ERP components that are usually associated to the motoric stages of response inhibition (P3) during the presentation of intervals (NoGo stimuli), under the assumption that more appreciated intervals should facilitate the inhibition of motor response, therefore amplifying motor-inhibition-related components.
Electrophysiological recordings and preprocessing
EEG activity was recorded by 32 Ag-AgCl electrodes placed on the scalp of the participant according to the International 10–20 system and referenced to the nose. Electrode impedances were kept below 5 kΩ. The electro-oculogram (EOG) was recorded from two surface electrodes placed over the right lower eyelid and lateral to the outer canthus of the right eye. Signals were recorded and digitized by using a HandyEGG (Micromed, Treviso – IT) amplifier with a sampling rate of 1024 Hz.
EEG data were pre-processed and analysed with Letswave6 toolbox (Nocions, Ucl. BE) for Matlab (Mathworks, Inc. USA). Continuous EEG data were divided into epochs of 1.5 s (total duration), including 500 ms pre-stimulus and 1 s post-stimulus intervals. Epochs were band-pass filtered (1–30 Hz in Experiment 1 and 2) using a fast Fourier transform filter. In Experiment 3 epochs were band-pass filtered with a broader filter (0.5–30 Hz) in order to better evidence later components (expressed in lower frequencies), such as P300, that usually emerge in Go-Nogo tasks51. Filtered ERPs were baseline corrected using the interval from −0.5 to 0 s as a baseline. Artefacts due to eye movements were eliminated using Independent Component Analysis (ICA120). Epochs belonging to the same interval type were then averaged, to obtain three average waveforms (i.e. Octaves, Fifths, Tritones) for each subject. In the Go-NoGo task (Experiment 3) we additionally analysed epochs corresponding to Go-trials (white noise trials) which were averaged together.
Data analysis
Behavioural data
Outliers (RTs diverging more than 2.5 standard deviations from each single subject’s average value) in the Detection task (Experiment 2) were excluded from subsequent analyses121,122. AJs from Experiment 1 and outlier-corrected RTs from Experiment 2 were then averaged across trials with the same interval (each interval was presented 4 times in both experiments; § 2.4- Aesthetic judgement task) to obtain 21 average values per participant.
Single subjects’ averaged RTs were entered as a dependent variable in a linear mixed-model with subjects’ ID as a random-effect factor and AJs as a covariate (fixed-effect factor). This analysis was based on 462 observations (21 per each of the 22 participants).
In Experiment 3, omission error rates in the Detection task (i.e. incorrect Go trials) and commission error rates in the Go-NoGo task (i.e. incorrect NoGo trials) were computed for each interval type for each participant. Single subjects’ AJs from Experiment 3 were averaged across interval types to obtain three average values per participant.
EEG data
First, we were interested in identifying the waveform components modulated by interval type. To test for significant differences among AEP elicited by different interval types, we performed three (one per each experiment) one-way, repeated measures, point-by-point ANOVA123,124, with three levels corresponding to the three interval types. Correction for multiple comparisons was applied via clustersize-based permutation testing125 (1000 permutations; alpha level = 0.05; percentile of mean cluster sum = 95). Significant clusters were based on both temporal contiguity and spatial adjacency of a minimum of two electrodes.
Furthermore, to investigate the relation between EEG responses and AJs more directly and to further explore the reliability of the point-by-point ANOVA results, we computed a point-by-point trial-by-trial (i.e. considering each single epoch for each single subject separately) correlation analysis126 between the amplitude of the EEG responses from single trials (N = 84) registered during the AJ task and the corresponding AJ (§ 2.4- Aesthetic judgement task). The outcome of the correlation analysis was a 1.5 s (from 0.5 s pre-onset to 1 s post-onset) long time series of r-values for each channel for each subject. This constituted the input for a group-level two-tailed point-by-point t-test with permutation-based correction for multiple comparisons (1000 permutations; alpha level = 0.05; percentile of mean cluster sum = 95; minimum number of adjacent channels = 2). The test compared single subjects’ correlation coefficients against the constant 0 at each time point. This allowed to identify time-clusters containing signal amplitudes which significantly correlated with AJs.
To further test the presence of a possible enhancement in attention- and motor inhibition-related AEP components for more appreciated intervals, in Experiment 2 and 3, where it was not possible to compute point-by-point trial-by-trial correlations between AJs and voltages (since EEG responses and AJs were not simultaneously collected in Experiments 2 and 3), we extracted single-subjects’ peak amplitudes from relevant waveform components (N1, P2 and N2 in Experiment 2; N1 and P3 in Experiment 3). Peaks were extracted from single subjects’ average AEP corresponding to the three different interval types. Peaks were defined as the lowest or highest amplitude (for negative and positive components respectively) registered within significant time-cluster evidenced by the point-by-point ANOVA. For each component separately, we performed a one-way repeated measure ANOVA employing peak amplitude as dependent variable and interval type as a within-subject factor (3 levels: Octave, Fifths, Tritones).
Single subjects’ AEPs and correlations between trial-by-trial amplitudes and AJs are available at Mendeley.com.
Author contributions
S.P. and R.I. conceived the presented study; S.P., P.A. and F.E. collected data and performed the analyses; S.P., R.I. and N.M.M. wrote the manuscript. G.F. and R.R. reviewed the manuscript.
Data availability
Data are available at Mendeley.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Apter MJ. Reversal Theory, Cognitive Synergy and the Arts. Adv. Psychol. 1984;19:411–426. doi: 10.1016/S0166-4115(08)62361-4. [DOI] [Google Scholar]
- 2.Chatterjee A. Neuroaesthetics: A coming of age story. J. Cogn. Neurosci. 2011;23:53–62. doi: 10.1162/jocn.2010.21457. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee A, Vartanian O. Neuroscience of aesthetics. Ann. N. Y. Acad. Sci. 2016;1369:172–194. doi: 10.1111/nyas.13035. [DOI] [PubMed] [Google Scholar]
- 4.Cupchik, G. C. & Winston, A. S. Confluence and divergence in empirical aesthetics, philosophy, and mainstream psychology in Handbook of Perception & Cognition, CognitiveEcology (ed. Carterette, E. C. & Friedman, M. P.) 62–85 (Academic Press, 1990).
- 5.Marković S. Components of aesthetic experience: Aesthetic fascination, aesthetic appraisal, and aesthetic emotion. Iperception. 2012;3:1–17. doi: 10.1068/i0450aap. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramachandran VS, Hirstein W. The Science of Art A Neurological Theory of Aesthetic Experience. J. Conscious. Stud. 1999;6:15–51. [Google Scholar]
- 7.Shusterman R. The End of Aesthetic Experience. J. Aesthet. Art Crit. 1997;55:29–41. doi: 10.2307/431602. [DOI] [Google Scholar]
- 8.Menninghaus W, et al. The Distancing-Embracing model of the enjoyment of negative emotions in art reception. Behav. Brain Sci. 2017;40:e347. doi: 10.1017/s0140525x17000309. [DOI] [PubMed] [Google Scholar]
- 9.Hofstadter, A. & Kuhns, R. Philosophies of Art and Beauty: Selected Readings in Aesthetics from Plato to Heidegger 286 (University of Chicago Press, 2009)
- 10.Hofstadter, A. & Kuhns, R. Philosophies of Art and Beauty: Selected Readings in Aesthetics from Plato to Heidegger 447–448 (University of Chicago Press, 2009)
- 11.Kreitman, N. The Varieties of Aesthetic Disinterestedness. Contemporary Aesthetetics, https://contempaesthetics.org/newvolume/pages/article.php?articleID=390 (2006).
- 12.Stolnitz J. ‘The Aesthetic Attitude’ in the Rise of Modern Aesthetics. J. Aesthet. Art Crit. 1978;36:409–422. doi: 10.2307/430481. [DOI] [Google Scholar]
- 13.Stroud SR. Toward a Deweyan Theory of Communicative Mindfulness. Imagin. Cogn. Pers. 2010;30:57–75. doi: 10.2190/IC.30.1.d. [DOI] [Google Scholar]
- 14.Menninghaus W, et al. What are aesthetic emotions? Psychol. Rev. 2019;126:171–195. doi: 10.1037/rev0000135. [DOI] [PubMed] [Google Scholar]
- 15.Biederman I, Vessel E. Perceptual Pleasure and the Brain: A novel theory explains why the brain craves information and seeks it through the senses. Am. Sci. 2006;94:247–253. doi: 10.1511/2006.59.247. [DOI] [Google Scholar]
- 16.Cupchik GC, Vartanian O, Crawley A, Mikulis DJ. Viewing artworks: Contributions of cognitive control and perceptual facilitation to aesthetic experience. Brain Cogn. 2009;70:84–91. doi: 10.1016/j.bandc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Kubovy, M. On the Pleasures of the Mind in Well-being: The Foundations of Hedonic Psychology (ed. Kahneman, D., Diener, E. & Schwarz, N.) 134–150 (Russell Sage Foundation, 1999).
- 18.Brattico E, Bogert B, Jacobsen T. Toward a neural chronometry for the aesthetic experience of music. Front. Psychol. 2013;4:206. doi: 10.3389/fpsyg.2013.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Tommaso M, et al. Influence of aesthetic perception on visual event-related potentials. Conscious. Cogn. 2008;17:933–945. doi: 10.1016/j.concog.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Kawabata H, Zeki S. Neural Correlates of Beauty. J. Neurophysiol. 2004;91:1699–1705. doi: 10.1152/jn.00696.2003. [DOI] [PubMed] [Google Scholar]
- 21.Di Dio DC, Macaluso E, Rizzolatti G. The golden beauty: Brain response to classical and renaissance sculptures. PLoS One. 2007;2:e1201. doi: 10.1371/journal.pone.0001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy M, Mailhot JP, Gosselin N, Paquette S, Peretz I. Modulation of the startle reflex by pleasant and unpleasant music. Int. J. Psychophysiol. 2009;71:37–42. doi: 10.1016/j.ijpsycho.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Calvo-Merino B, Jola C, Glaser DE, Haggard P. Towards a sensorimotor aesthetics of performing art. Conscious. Cogn. 2008;17:911–922. doi: 10.1016/j.concog.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Cela-Conde CJ, et al. Sex-related similarities and differences in the neural correlates of beauty. Proc. Natl. Acad. Sci. 2009;106:3847–3852. doi: 10.1073/pnas.0900304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobsen T, Schubotz RI, Höfel L, Cramon DYV. Brain correlates of aesthetic judgment of beauty. Neuroimage. 2006;29:276–285. doi: 10.1016/j.neuroimage.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Vartanian O, Goel V. Neuroanatomical correlates of aesthetic preference for paintings. Neuroreport. 2004;15:893–897. doi: 10.1097/00001756-200404090-00032. [DOI] [PubMed] [Google Scholar]
- 27.Koelsch S, Fritz T, Cramon DYV, Müller K, Friederici AD. Investigating emotion with music: An fMRI study. Hum. Brain Mapp. 2006;27:239–250. doi: 10.1002/hbm.20180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirsch LP, Urgesi C, Cross ES. Shaping and reshaping the aesthetic brain: Emerging perspectives on the neurobiology of embodied aesthetics. Neurosci. Biobehav Rev. 2016;62:56–68. doi: 10.1016/j.neubiorev.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Leder H, Nadal M. Ten years of a model of aesthetic appreciation and aesthetic judgments: The aesthetic episode - Developments and challenges in empirical aesthetics. Br. J. Psychol. 2014;105:443–464. doi: 10.1111/bjop.12084. [DOI] [PubMed] [Google Scholar]
- 30.Nadal M. The experience of art. Insights from neuroimaging. In Prog. Brain Res. 2013;204:135–58. doi: 10.1016/B978-0-444-63287-6.00007-5. [DOI] [PubMed] [Google Scholar]
- 31.Fritz JB, Elhilali M, David SV, Shamma SA. Auditory attention - focusing the searchlight on sound. Curr. Opin. Neurobiol. 2007;17:437–455. doi: 10.1016/j.conb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Näätänen R. Selective attention and evoked potentials in humans. A critical review. Biol. Psychol. 1975;2:237–307. doi: 10.1016/0301-0511(75)90038-1. [DOI] [PubMed] [Google Scholar]
- 33.Huster RJ, Enriquez-Geppert S, Lavallee CF, Falkenstein M, Herrmann CS. Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. Int. J. Psychophysiol. 2013;87:217–33. doi: 10.1016/j.ijpsycho.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Wessel JR, Aron AR. It’s not too late: The onset of the frontocentral P3 indexes successful response inhibition in the stop-signal paradigm. Psychophysiology. 2015;52:472–80. doi: 10.1111/psyp.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramautar JR, Kok A, Ridderinkhof KR. Effects of stop-signal probability in the stop-signal paradigm: The N2/P3 complex further validated. Brain. Cogn. 2004;56:234–252. doi: 10.1016/j.bandc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Bowling DL, Purves D. A biological rationale for musical consonance. Proc. Natl. Acad. Sci. 2015;112:11155–11160. doi: 10.1073/pnas.1505768112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crespo-Bojorque P, Monte-Ordoño J, Toro JM. Early neural responses underlie advantages for consonance over dissonance. Neuropsychologia. 2018;117:188–198. doi: 10.1016/j.neuropsychologia.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Proverbio AM, Orlandi A, Pisanu F. Brain processing of consonance/dissonance in musicians and controls: a hemispheric asymmetry revisited. Eur. J. Neurosci. 2016;44:2340–2356. doi: 10.1111/ejn.13330. [DOI] [PubMed] [Google Scholar]
- 39.Virtala P, Huotilainen M, Partanen E, Tervaniemi M. Musicianship facilitates the processing of Western music chords-An ERP and behavioral study. Neuropsychologia. 2014;61:247–258. doi: 10.1016/j.neuropsychologia.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 40.Virtala P, Huotilainen M, Partanen E, Fellman V, Tervaniemi M. Newborn infants’ auditory system is sensitive to Western music chord categories. Front. Psychol. 2013;4:492. doi: 10.3389/fpsyg.2013.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner L, Rahne T, Plontke SK, Heidekrüger N. Mismatch negativity reflects asymmetric preattentive harmonic interval discrimination. PLoS One. 2018;13:e0196176. doi: 10.1371/journal.pone.0196176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lahdelma I, Eerola T. Mild dissonance preferred over consonance in single chord perception. Iperception. 2016;7:2041669516655812. doi: 10.1177/2041669516655812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Popescu T, et al. The pleasantness of sensory dissonance is mediated by musical style and expertise. Sci. Rep. 2019;9:1070. doi: 10.1038/s41598-018-35873-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bowling DL, Purves D, Gill KZ. Vocal similarity predicts the relative attraction of musical chords. Proc. Natl. Acad. Sci. 2017;115:216–221. doi: 10.1073/pnas.1713206115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cousineau M, McDermott JH, Peretz I. The basis of musical consonance as revealed by congenital amusia. Proc. Natl. Acad. Sci. 2012;109:19858–19863. doi: 10.1073/pnas.1207989109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crespo-Bojorque P, Toro JM. Processing advantages for consonance: A comparison between rats (Rattus norvegicus) and humans (Homo sapiens) J. Comp. Psychol. 2016;130:97–108. doi: 10.1037/com0000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDermott JH, Lehr AJ, Oxenham AJ. Individual differences reveal the basis of consonance. Curr. Biol. 2010;20:1035–1041. doi: 10.1016/j.cub.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pallesen KJ, et al. Emotion processing of major, minor, and dissonant chords: a functional magnetic resonance imaging study. Ann. N. Y. Acad. Sci. 2005;1060:450–453. doi: 10.1196/annals.1360.047. [DOI] [PubMed] [Google Scholar]
- 49.Sams M, Paavilainen P, Alho K, Näätänen R. Auditory frequency discrimination and event-related potentials. Electroencephalogr. Clin. Neurophysiol. 1985;62:437–48. doi: 10.1016/0168-5597(85)90054-1. [DOI] [PubMed] [Google Scholar]
- 50.Angelini M, et al. Motor inhibition during overt and covert actions: An electrical neuroimaging study. PLoS One. 2015;10:e0126800. doi: 10.1371/journal.pone.0126800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen AT, Moyle JJ, Fox AM. N2 and P3 modulation during partial inhibition in a modified go/nogo task. Int. J. Psychophysiol. 2016;107:63–71. doi: 10.1016/j.ijpsycho.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Jacobsen T, Beudt S. Domain generality and domain specificity in aesthetic appreciation. New Ideas Psychol. 2017;47:97–102. doi: 10.1016/j.newideapsych.2017.03.008. [DOI] [Google Scholar]
- 53.Pearce MT, et al. Neuroaesthetics: The Cognitive Neuroscience of Aesthetic Experience. Perspect. Psychol. Sci. 2016;11:265–279. doi: 10.1177/1745691615621274. [DOI] [PubMed] [Google Scholar]
- 54.Koelsch S, Vuust P, Friston K. Predictive Processes and the Peculiar Case of Music. Trends Cogn. Sci. 2019;23:63–77. doi: 10.1016/j.tics.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Van de Cruys S, Wagemans J. Putting reward in art: A tentative prediction error account of visual art. Iperception. 2011;2:1035–1062. doi: 10.1068/i0466aap. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alho K. Selective attention in auditory processing as reflected by event-related brain potentials. Psychophysiology. 1992;29:247–263. doi: 10.1111/j.1469-8986.1992.tb01695.x. [DOI] [PubMed] [Google Scholar]
- 57.Giuliano RJ, Karns CM, Neville HJ, Hillyard SA. Early auditory evoked potential is modulated by selective attention and related to individual differences in visual working memory capacity. J. Cogn. Neurosci. 2014;26:2682–2690. doi: 10.1162/jocn_a_00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Key APF, Dove GO, Maguire MJ. Linking brainwaves to the brain: An ERP primer. Dev. Neuropsychol. 2005;27:183–215. doi: 10.1207/s15326942dn2702_1. [DOI] [PubMed] [Google Scholar]
- 59.Oray S, Lu ZL, Dawson ME. Modification of sudden onset auditory ERP by involuntary attention to visual stimuli. Int. J. Psychophysiol. 2002;43:213–24. doi: 10.1016/S0167-8760(01)00174-X. [DOI] [PubMed] [Google Scholar]
- 60.Snyder JS, Alain C, Picton TW. Effects of attention on neuroelectric correlates of auditory stream segregation. J. Cogn. Neurosci. 2006;18:1–13. doi: 10.1162/089892906775250021. [DOI] [PubMed] [Google Scholar]
- 61.Wilkinson RT, Lee MV. Auditory evoked potentials and selective attention. Electroencephalogr. Clin. Neurophysiol. 1972;33:411–418. doi: 10.1016/0013-4694(72)90121-6. [DOI] [PubMed] [Google Scholar]
- 62.Müller M, Höfel L, Brattico E, Jacobsen T. Aesthetic judgments of music in experts and laypersons - An ERP study. Int. J. Psychophysiol. 2010;76:40–51. doi: 10.1016/j.ijpsycho.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Brattico E, Jacobsen T, De Baene W, Glerean E, Tervaniemi M. Cognitive vs. affective listening modes and judgments of music - An ERP study. Biol. Psychol. 2010;85:393–409. doi: 10.1016/j.biopsycho.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 64.Höfel L, Jacobsen T. Electrophysiological indices of processing aesthetics: Spontaneous or intentional processes? Int. J. Psychophysiol. 2007;65:20–31. doi: 10.1016/j.ijpsycho.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 65.Jacobsen T, Höfel L. Descriptive and evaluative judgment processes: Behavioral and electrophysiological indices of processing symmetry and aesthetics. Cogn. Affect. Behav. Neurosci. 2003;3:289–299. doi: 10.3758/CABN.3.4.289. [DOI] [PubMed] [Google Scholar]
- 66.Levinson J. Pleasure and the value of works of art. Br. J. Aesthet. 1992;32:293–389. doi: 10.1093/bjaesthetics/32.4.293. [DOI] [Google Scholar]
- 67.Wessel JR, Aron AR. On the Globality of Motor Suppression: Unexpected Events and Their Influence on Behavior and Cognition. Neuron. 2017;93:259–280. doi: 10.1016/j.neuron.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dutra IC, Waller DA, Wessel JR. Perceptual Surprise Improves Action Stopping by Nonselectively Suppressing Motor Activity via a Neural Mechanism for Motor Inhibition. J. Neurosci. 2018;38:1482–1492. doi: 10.1523/JNEUROSCI.3091-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burle B, Vidal F, Tandonnet C, Hasbroucq T. Physiological evidence for response inhibition in choice reaction time tasks. Brain Cogn. 2004;56:153–64. doi: 10.1016/j.bandc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 70.Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chernyshev, B. & Medvedev, V. Event-Related Potential Study of P2 and N2 Components on Fast and Slow Responses in the Auditory Condensation Task. HSE working papers https://ideas.repec.org/p/hig/wpaper/70psy2016.html
- 72.Dimoska A, Johnstone SJ, Barry RJ. The auditory-evoked N2 and P3 components in the stop-signal task: Indices of inhibition, response-conflict or error-detection? Brain Cogn. 2006;62:98–112. doi: 10.1016/j.bandc.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 73.Friston K. The free-energy principle: A unified brain theory? Nat. Rev. Neurosci. 2010;11:127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- 74.Friston K, Kilner J, Harrison L. A free energy principle for the brain. J. Physiol. Paris. 2006;100:70–87. doi: 10.1016/j.jphysparis.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Limanowski, J., Sarasso, P. & Blankenburg, F. Different responses of the right superior temporal sulcus to visual movement feedback during self-generated vs. externally generated hand movements. European Journal of Neuroscience47(4), 314–320 (2018). [DOI] [PubMed]
- 76.Friston KJ, et al. Active inference, curiosity and insight. Neural Comput. 2017;29:2633–2683. doi: 10.1162/neco_a_00999. [DOI] [PubMed] [Google Scholar]
- 77.Friston K, et al. Active inference and epistemic value. Cogn. Neurosci. 2015;6:187–214. doi: 10.1080/17588928.2015.1020053. [DOI] [PubMed] [Google Scholar]
- 78.Friston K, et al. Active inference and learning. Neurosci. Biobehav. Rev. 2016;68:862–879. doi: 10.1016/j.neubiorev.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ricci R, et al. Effects of attentional and cognitive variables on unilateral spatial neglect. Neuropsychologia. 2016;92:158–166. doi: 10.1016/j.neuropsychologia.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 80.Cohen JD, McClure SM, Yu AJ. Should I stay or should I go? How the human brain manages the trade-off between exploitation and exploration. Philos. Trans. R. Soc. B. Biol. Sci. 2007;362:933–942. doi: 10.1098/rstb.2007.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gottlieb J. Attention, Learning, and the Value of Information. Neuron. 2012;76:281–295. doi: 10.1016/j.neuron.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gottlieb J, Oudeyer PY, Lopes M, Baranes A. Information-seeking, curiosity, and attention: Computational and neural mechanisms. Trends Cogn. Sci. 2013;17:585–593. doi: 10.1016/j.tics.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaplan, F. & Oudeyer, P.-Y. Maximizing learning progress: An internal reward system for development in Embodied Artificial Intelligence (ed. Fumiya Iida, F., Pfeifer R., Steels, L. & Kuniyoshi, Y.) 259–270 (Springer, 2004).
- 84.Oudeyer PY, Gottlieb J, Lopes M. Intrinsic motivation, curiosity, and learning: Theory and applications in educational technologies. Prog. Brain Res. 2016;229:257–284. doi: 10.1016/bs.pbr.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 85.Oudeyer PY, Kaplan F, Hafner VV. Intrinsic motivation systems for autonomous mental development. IEEE Trans. Evol. Comput. 2007;11:265–286. doi: 10.1109/TEVC.2006.890271. [DOI] [Google Scholar]
- 86.Chetverikov A, Kristjánsson Á. On the joys of perceiving: Affect as feedback for perceptual predictions. Acta Psychol. (Amst). 2016;169:1–10. doi: 10.1016/j.actpsy.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 87.Consoli G. From beauty to knowledge: a new frame for the neuropsychological approach to aesthetics. Front. Hum. Neurosci. 2015;9:290. doi: 10.3389/fnhum.2015.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perlovsky L. Aesthetic emotions, what are their cognitive functions? Front. Psychol. 2014;5:98. doi: 10.3389/fpsyg.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schoeller F. Knowledge, curiosity, and aesthetic chills. Front. Psychol. 2015;6:1546. doi: 10.3389/fpsyg.2015.01546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schoeller F, Perlovsky L. Aesthetic chills: Knowledge-acquisition, meaning-making, and aesthetic emotions. Front. Psychol. 2016;7:1093. doi: 10.3389/fpsyg.2016.01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sarasso P., Ronga I., Kobau P., Bosso T., Artusio I., Ricci R., Neppi-Modona M. Beauty in mind: Aesthetic appreciation correlates with perceptual facilitation and attentional amplification. Neuropsychologia. 2020;136:107282. doi: 10.1016/j.neuropsychologia.2019.107282. [DOI] [PubMed] [Google Scholar]
- 92.Joffily M, Coricelli G. Emotional Valence and the Free-Energy Principle. PLoS Comput. Biol. 2013;9:e1003094. doi: 10.1371/journal.pcbi.1003094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muth C, Carbon CC. The Aesthetic Aha: On the pleasure of having insights into Gestalt. Acta Psychol. (Amst). 2013;144:25–30. doi: 10.1016/j.actpsy.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 94.Baldi P, Itti L. Of bits and wows: A Bayesian theory of surprise with applications to attention. Neural Networks. 2010;23:649–666. doi: 10.1016/j.neunet.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Itti L, Baldi P. Bayesian surprise attracts human attention. Vision Res. 2009;49:1295–1306. doi: 10.1016/j.visres.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wessel JR. Perceptual surprise aides inhibitory motor control. J. Exp. Psychol. Hum. Percept. Perform. 2017;43:1585–1593. doi: 10.1037/xhp0000452. [DOI] [PubMed] [Google Scholar]
- 97.Schwartenbeck P, FitzGerald THB, Dolan R. Neural signals encoding shifts in beliefs. Neuroimage. 2016;125:578–586. doi: 10.1016/j.neuroimage.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc. Natl. Acad. Sci. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cela-Conde CJ, et al. Activation of the prefrontal cortex in the human visual aesthetic perception. Proc. Natl. Acad. Sci. 2004;101:6321–6325. doi: 10.1073/pnas.0401427101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gallese V, Guerra M. Embodying movies: Embodied simulation and film studies. Cine. J. Philos. Mov. 2012;3:183–210. [Google Scholar]
- 101.Gallese V, Sinigaglia C. What is so special about embodied simulation? Trends in Cognitive Sciences. 2011;15:512–519. doi: 10.1016/j.tics.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 102.Gallese V. Visions of the body. Embodied simulation and aesthetic experience. Aisthesis. 2017;10:41–50. [Google Scholar]
- 103.Gallese V. Naturalizing aesthetic experience: The role of (liberated) embodied simulation. Projections. 2018;12:50–59. doi: 10.3167/proj.2018.120207. [DOI] [Google Scholar]
- 104.Gallese, V. Mirroring, a liberated embodied simulation and aesthetic experience in Mirror Images. Reflections in Art and Medicine (ed. Hirsch, H. & Pace, A.) 27–37 (Verlag für moderne Kunst, 2017).
- 105.Kornysheva K, Von Cramon DY, Jacobsen T, Schubotz RI. Tuning-in to the beat: Aesthetic appreciation of musical rhythms correlates with a premotor activity boost. Hum. Brain Mapp. 2010;31:48–64. doi: 10.1002/hbm.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.González-García N, González MA, Rendón PL. Neural activity related to discrimination and vocal production of consonant and dissonant musical intervals. Brain Res. 2016;1643:59–69. doi: 10.1016/j.brainres.2016.04.065. [DOI] [PubMed] [Google Scholar]
- 107.Komeilipoor N, Rodger MWM, Craig CM, Cesari P. (Dis-)Harmony in movement: effects of musical dissonance on movement timing and form. Exp. Brain Res. 2015;233:1585–1595. doi: 10.1007/s00221-015-4233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Masataka N, Perlovsky L. Cognitive interference can be mitigated by consonant music and facilitated by dissonant music. Sci. Rep. 2013;3:2028. doi: 10.1038/srep02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schellenberg EG, Trehub SE. Children’s discrimination of melodic intervals. Dev. Psychol. 1996;32:1039–1050. doi: 10.1037/0012-1649.32.6.1039. [DOI] [Google Scholar]
- 110.Gill KZ, Purves D. A biological rationale for musical scales. PLoS One. 2009;4:e8144. doi: 10.1371/journal.pone.0008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lehmann JAM, Seufert T. Can music foster learning - Effects of different text modalities on learning and information retrieval. Front. Psychol. 2018;8:2305. doi: 10.3389/fpsyg.2017.02305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Girod M, Wong D. An Aesthetic (Deweyan) Perspective on Science Learning: Case Studies of Three Fourth Graders. Elem. Sch. J. 2005;102:199–224. doi: 10.1086/499700. [DOI] [Google Scholar]
- 113.Uhrmacher PB. Toward a theory of aesthetic learning experiences. Curric. Inq. 2009;39:613–636. doi: 10.1111/j.1467-873X.2009.00462.x. [DOI] [Google Scholar]
- 114.Margherita Spagnuolo Lobb, PhD Aesthetic Relational Knowledge of the Field: A Revised Concept of Awareness in Gestalt Therapy and Contemporary. Psychiatry. Gestalt Rev. 2018;22:50–68. doi: 10.5325/gestaltreview.22.1.0050. [DOI] [Google Scholar]
- 115.Roubal J, Francesetti G, Gecele M. Aesthetic Diagnosis in Gestalt Therapy. Behav. Sci. 2017;7:70. doi: 10.3390/bs7040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jacobsen T. Individual and group modelling of aesthetic judgment strategies. Br. J. Psychol. 2004;95:41–56. doi: 10.1348/000712604322779451. [DOI] [PubMed] [Google Scholar]
- 117.Johnson-Laird PN, Kang OE, Leong YC. On musical dissonance. Music Percept. 2012;30:19–35. doi: 10.1525/mp.2012.30.1.19. [DOI] [Google Scholar]
- 118.Trulla LL, Di Stefano N, Giuliani A. Computational approach to musical consonance and dissonance. Front. Psychol. 2018;9:381. doi: 10.3389/fpsyg.2018.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kopp-Scheinpflug C, Sinclair JL, Linden JF. When Sound Stops: Offset Responses in the Auditory System. Trends Neurosci. 2018;41:712–728. doi: 10.1016/j.tins.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 120.Jung TP, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37:163–178. doi: 10.1111/1469-8986.3720163. [DOI] [PubMed] [Google Scholar]
- 121.Ronga I, et al. Everything is illuminated: Prismatic adaptation lowers visual detection threshold in normal subjects. J. Exp. Psychol. Hum. Percept. Perform. 2018;44:1619–1628. doi: 10.1037/xhp0000559. [DOI] [PubMed] [Google Scholar]
- 122.Sarasso P, et al. Everything is (still) illuminated: Dual right cathodal-left anodal tDCS of PPC prevents fatigue on a visual detection task. Brain Stimul. 2019;12:187–189. doi: 10.1016/j.brs.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 123.Bruno V, Ronga I, Fossataro C, Capozzi F, Garbarini F. Suppressing movements with phantom limbs and existing limbs evokes comparable electrophysiological inhibitory responses. Cortex. 2019;117:64–76. doi: 10.1016/j.cortex.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 124.Ronga I, Valentini E, Mouraux A, Iannetti GD. Novelty is not enough: laser-evoked potentials are determined by stimulus saliency, not absolute novelty. J. Neurophysiol. 2013;109:692–701. doi: 10.1152/jn.00464.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 126.Novembre G, et al. Saliency detection as a reactive process: unexpected sensory events evoke cortico-muscular coupling. J. Neurosci. 2018;8:2385–2397. doi: 10.1523/JNEUROSCI.2474-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at Mendeley.