Abstract
Background
Vascular complications are common during invasive cardiac electrophysiology procedures. This meta‐analysis compares outcomes following ultrasound and nonultrasound‐guided vascular access for these procedures.
Methods
PubMed, Embase and Cochrane 01/01/1980‐30/09/2018 were searched for relevant studies to meta‐analyse.
Results
Seven studies (6269 patients) were included. Pooled rates and odds ratio(95% confidence interval) for ultrasound and nonultrasound subgroups were 1.2% vs 3.0%, 0.32 (0.21‐0.49) for all vascular complications, with less hematomas and arterial punctures but similar arteriovenous fistulas, pseudoaneurysms or retroperitoneal bleeds.
Conclusion
Ultrasound guidance had less complications due to less hematoma and arterial puncture, and is generally recommended for electrophysiology procedures.
Keywords: arrhythmia, catheter ablation, electrophysiology, ultrasound, vascular access
We meta‐analysed the vascular complications' rates for ultrasound versus non‐ultrasound guidance for vascular access of electrophysiological procedures from 7 studies totalling 6,269 patients. We found ultrasound guidance was associated with significantly lower rates of composite vascular complications, including less haematomas and arterial punctures, and should be generally encouraged for these procedures.

1. INTRODUCTION
Invasive cardiac electrophysiology procedures have risen significantly over the last decade.1, 2 It is well‐established as curative for some arrhythmias, reducing burden and symptoms in others, and for selected patients with both atrial fibrillation and heart failure, potentially improving clinical outcomes including survival.1, 3 Periprocedural complications occur in 2%‐10% of these procedures, where vascular complications are most common.1, 4, 5 Ultrasound guidance for central vascular access has gained popularity over recent years but are rarely studied for invasive electrophysiology procedures.6, 7 We meta‐analysed the rates of vascular complications following cardiac electrophysiology procedures with ultrasound guidance vs a conventional nonultrasound approach for vascular access.
2. METHODS
Electronic databases Pubmed, Medline, Embase and Cochrane were searched from 1 January 1980 to 30 September 2018 for relevant studies and abstracts. The search terms used were “ultrasound”, “vascular” or “access” or “percutaneous”, and “catheter ablation” or “electrophysiology”. Original randomised trials and observational studies reporting vascular bleeding complication rates for both ultrasound‐guided and conventional nonultrasound‐guided access of electrophysiological procedures for any arrhythmias are included.
Data pertaining to study design, patient characteristics and outcomes of all included studies were then extracted. Review Manager Version 5.3 (Cochrane Collaboration, Oxford, England) was used. Pooled odds ratios (OR) with 95% confidence intervals (95% CI) and Forrest Plots were performed. We used random effects modeling to account for potential heterogeneity in study methodology and patient characteristics. Heterogeneity of studies was assessed using I 2 and publication bias using Funnel Plots for each outcome pooled. P‐value less than .05 was deemed statistically significant and all tests were two‐tailed.
3. RESULTS
The search yielded 229 articles, for which 41 duplicate studies and 173 unrelated studies were excluded after initial screening. Upon review of 15 full‐text articles, 4 were reviews and 2 were meta‐analyses without original data, and 2 were single‐arm.8, 9, 10, 11, 12, 13, 14 This resulted in seven studies being included for meta‐analysis, listed in Table 1. There was one randomised trial and six observational studies, totaling 6269 patients.
Table 1.
Characteristics of included studies
| Author/year | Design | Timeframe of procedures | Centers | Country | Cohort | Group | N | Age (years) | Male |
|---|---|---|---|---|---|---|---|---|---|
| Tanaka‐Esposito (2013)8 |
Retrospective cohort 2 phases |
January 2005‐December 2006 | 1 | United States | Atrial fibrillation | Conventional | 1909 | 62.9 | 63% |
| July 2008‐May 2010 | Ultrasound | 1511 | 63.1 | 60% | |||||
| Errahmouni (2014)9 |
Retrospective cohort Historic controls |
April 2012‐October 2012 | 1 | Monaco | All arrhythmias | Conventional | 150 | Not reported | 79% |
| November 2012‐June 2013 | Ultrasound | 150 | 77% | ||||||
| Wynn (2014)10 |
Prospective cohort 2 phases |
May 2012‐September 2012 | 1 | United Kingdom | Atrial fibrillation | Conventional | 146 | 58.7 | 68% |
| October 2012‐February 2013 | Ultrasound | 163 | 59.1 | 74% | |||||
| Rodriguez (2015)11 | Prospective Cohort | Not reported | 1 | Spain | All arrhythmias | Conventional | 12 | 57 | 61% |
| Ultrasound | 24 | 58 | 49% | ||||||
| Dussault (2016)12 | Retrospective cohort | January 2010‐October 2015 | 1 | United States |
Atrial fibrillation/ Atrial flutter |
Conventional | 757 | 65.4 | 67% |
| Ultrasound | 439 | 64.2 | 64% | ||||||
| Sharma (2016)13 |
Prospective Cohort 2 phases |
October 2014‐May 2015 | 1 | United States | All arrhythmias | Conventional | 349 | 65.4 | 42% |
| June 2015‐January 2016 | Ultrasound | 340 | 64.2 | 83% | |||||
| Yamagata (2018)14 | Randomised trial | March 2016‐November 2016 | 4 |
Czech Republic Japan |
Atrial fibrillation | Conventional | 160 | 61.2 | 72% |
| Ultrasound | 159 |
Forrest plots are illustrated in Figure 1A‐F. Ultrasound guidance had a significantly lower rate of composite vascular complications 1.2% vs 3.0%, OR 0.32 (95% CI 0.21‐0.49), P < .001; local hematoma 0.3% vs 1.4%, 0.20 (0.09‐0.42), P < .001; and inadvertent arterial puncture 6.4% vs 20.4%, OR 0.25 (0.11‐0.57). There were no statistically significant differences in the rates of pseudoaneurysm, arteriovenous fistula formation and retroperitoneal bleed (P > .05) although events were rare with either strategy (pooled rates <0.5%). No significant heterogeneity or publication bias was identified for all outcomes. The findings did not differ if only atrial fibrillation studies were meta‐analysed.8, 10, 12, 14
Figure 1.
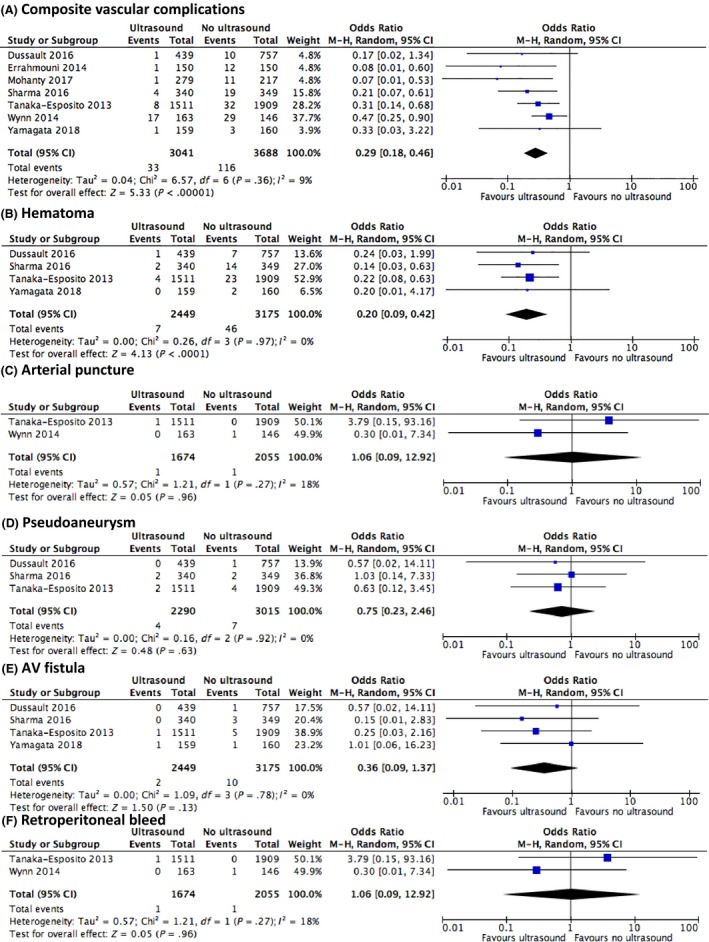
Forrest plots of pooled outcomes (A) composite vascular complications, (B) hematoma, (C) arterial puncture, (D) arteriovenous fistula, (E) pseudoaneurysm and (F) retroperitoneal bleed
4. DISCUSSION
Vascular complications are the commonest adverse event following invasive cardiac electrophysiology procedures, and rates vary based on the type of arrhythmia treated.1, 5 We found that ultrasound guidance for vascular access in these procedures was associated with reduction by two‐thirds in composite vascular complications and consistent across all studies.9, 10, 11, 12, 13, 14 The main strength of ultrasound is the ability to visualise vascular structures; in this setting, both the femoral vein and artery, as well as their size, depth and optimal route of access, rather than based on anatomical landmarks and palpation only. This explains the lower rate of inadvertent arterial punctures, and potentially lower risk of perforating the posterior venous wall, both of which could lead to hematomas.
Although we did not find statistically significant differences between the two access approaches for the rates pseudoaneurysm, arteriovenous fistula formation and retroperitoneal hematoma, the event rates of these major complications were extremely low, making the analysis underpowered. These events, particularly retroperitoneal bleeding, often require active management including blood transfusion, interventional radiology procedures, and occasionally vascular surgery. They are potentially fatal and inevitably lead to prolonged hospital stay. The low event rate is reassuring in the current era where an increasing number of electrophysiology procedures are undertaken with uninterrupted periprocedural anticoagulation.1 Pseudoaneurysms and arteriovenous fistula formation were in fact numerically lower in the ultrasound group (P > .05), so the lack of significant differences for major vascular complications does not go against recommendations for routine ultrasound use.
Pitfalls in using ultrasound for vascular access on a routine basis need to be considered.6, 15 There is cost associated with ultrasound machines, including having one readily available, and it requires another staff member to be present to operate it. Although puncture time may initially be lengthened, this tends to shorten with experience and may ultimately be a faster strategy for obtaining vascular access in experienced hands.8, 11 Additional training would be required for some operators, however, this is a useful and important skill, and routine application helps the operator attain the competency required for cases of varying complexity. Case complexity is often realized only after failed attempts for access where vascular spasm and hematoms may have developed complicating further visualisation and compromising patient safety. We therefore recommend having ultrasound available for all electrophysiology laboratories and at the discretion of operator, with encouraged use.
This meta‐analysis had some limitations. Only one study was randomised, whilst other observational studies had inherence biases that may influence outcomes. These include differences in baseline characteristics such as anticoagulation regimen, bleeding history and body mass index. Ultrasound guidance may in clinical practice be reserved for those with difficult access using the conventional approach, although this would only strengthen the differences in our findings. There were some differences in study design, patient characteristics and endpoint definitions, as well as risk of publication bias although neither was significant in our analysis. As patient‐level data were not available, subgroup and multivariable analyses could not be conducted. Some outcomes were very rare and so their analysis underpowered, but does present all the available comparative data in the literature to date. None of the studies evaluated the cost‐effectiveness of the use of ultrasound.
In summary, rates of vascular complications were significantly lower for ultrasound‐guided access strategy. These differences were driven primarily by reductions in minor complications such as local hematoma and inadvertent arterial puncture, while major and potentially fatal complications such as retroperitoneal hematoma had very low event rates to show statistically significant difference. These data suggest that routine ultrasound‐guided vascular access for invasive cardiac electrophysiology procedures is generally recommended.
CONFLICTS OF INTEREST
Authors declare no conflict of interests for this article.
Wang TKM, Wang MTM, Martin A. Meta‐analysis of ultrasound‐guided vs conventional vascular access for cardiac electrophysiology procedures. J Arrhythmia. 2019;35:858–862. 10.1002/joa3.12236
REFERENCES
- 1. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cappato R, Calkins H, Chen S‐A, Davies W, Iesaka Y, Kalman J, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–8. [DOI] [PubMed] [Google Scholar]
- 3. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al.; CASTLE‐AF Investigators . Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417–27. [DOI] [PubMed] [Google Scholar]
- 4. Gupta A, Perera T, Ganesan A, Sullivan T, Lau DH, Roberts‐Thomson KC, et al. Complications of catheter ablation of atrial fibrillation: a systematic review. Circ Arrhythm Electrophysiol. 2013;6(6):1082–8. [DOI] [PubMed] [Google Scholar]
- 5. Bohnen M, Stevenson WG, Tedrow UB, Michaud GF, John RM, Epstein LM, et al. Incidence and predictors of major complications from contemporary catheter ablation to treat cardiac arrhythmias. Heart Rhythm. 2011;8:1661–6. [DOI] [PubMed] [Google Scholar]
- 6. American Society of Anesthesiologists Task Force on Central Venous Access , Rupp SM, Apfelbaum JL, Blitt C, Caplan RA, Connis RT, et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539–73. [DOI] [PubMed] [Google Scholar]
- 7. Airapetian N, Maizel J, Langelle F, Modeliar SS, Karakitsos D, Dupont H, et al. Ultrasound‐guided central venous cannulation is superior to quick‐look ultrasound and landmark methods among inexperienced operators: a prospective randomized study. Intensive Care Med. 2013;39(11):1938–44. [DOI] [PubMed] [Google Scholar]
- 8. Tanaka‐Esposito CC, Chung MK, Abraham JM, Cantillon DJ, Abi‐Saleh B, Tchou PJ. Real‐time ultrasound guidance reduces total and major vascular complications in patients undergoing pulmonary vein antral isolation on therapeutic warfarin. J Interv Card Electrophysiol. 2013;37(2):163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Errahmouni A, Bun SS, Latcu DG, Saoudi N. Ultrasound‐guided venous puncture in electrophysiological procedures: a safe method, rapidly learned. Pacing Clin Electrophysiol. 2014;37(8):1023–8. [DOI] [PubMed] [Google Scholar]
- 10. Wynn GJ, Haq I, Hung J, Bonnett LJ, Lewis G, Webber M, et al. Improving safety in catheter ablation for atrial fibrillation: a prospective study of the use of ultrasound to guide vascular access. J Cardiovasc Electrophysiol. 2014;25(7):680–5. [DOI] [PubMed] [Google Scholar]
- 11. Rodríguez muñoz D, Franco díez E, Moreno J, Lumia G, Carbonell san román A, Segura de la cal T, et al. Wireless ultrasound guidance for femoral venous cannulation in electrophysiology: impact on safety, efficacy, and procedural delay. Pacing Clin Electrophysiol. 2015;38(9):1058–65. [DOI] [PubMed] [Google Scholar]
- 12. Dussault C, Baldinger S, Tdrow UB, Michaeud GF, Koplan BA, Stevenson WG. Preventing vascular access complications for ablation on uninterrupted anticoagulation: impact of ultrasound guidance and micro‐puncture needle. Heart Rhythm. 2016;13(5):S471. [Google Scholar]
- 13. Sharma PS, Padala SK, Gunda S, Koneru JN, Ellenbogen KA. Vascular complications during catheter ablation of cardiac arrhythmias: a comparison between vascular ultrasound guided access and conventional vascular access. J Cardiovasc Electrophysiol. 2016;27(10):1160–6. [DOI] [PubMed] [Google Scholar]
- 14. Yamagata K, Wichterle D, Roubíček T, Jarkovský P, Sato Y, Kogure T, et al. Ultrasound‐guided versus conventional femoral venipuncture for catheter ablation of atrial fibrillation: a multicentre randomized efficacy and safety trial (ULTRA‐FAST trial). Europace. 2018;20(7):1107–14. [DOI] [PubMed] [Google Scholar]
- 15. Weiner MM, Geldard P, Mittnacht AJ. Ultrasound‐guided vascular access: a comprehensive review. J Cardiothorac Vasc Anesth. 2013;27:345–60. [DOI] [PubMed] [Google Scholar]


