Abstract
Previous studies suggested an association between heart failure (HF) and hepatic disorders. Liver function parameters have been shown to predict outcome in HF with reduced ejection fraction, but their impact in HF with preserved ejection fraction (HFpEF) has not yet been investigated. Between January 2011 and February 2017, 274 patients with confirmed HFpEF were enrolled (age 71.3 ± 8.4 years, 69.3% female) in a prospective registry. During a median follow-up of 21.5 ± 18.6 months, 97 patients (35.4%) reached the combined endpoint defined as hospitalization due to HF and/ or death from any cause. By multivariable cox regression, serum gamma-glutamyltransferase (GT) was independently associated with outcome (Hazard Ratio (HR) 1.002, p = 0.004) along with N-terminal pro brain natriuretic peptide (HR 2.213, p = 0.001) and hemoglobin (HR 0.840, p = 0.006). Kaplan-Meier analysis showed that patients with serum gamma-GT levels above a median of 36 U/L had significantly more events as compared to the remainder of the group (log-rank p = 0.012). By multivariable logistic regression, higher early mitral inflow velocity/ mitral peak velocity of late filling (Odds Ratio (OR) 2.173, p = 0.024), higher right atrial (RA) pressure (OR 1.139, p < 0.001) and larger RA diameter (OR 1.070, p = 0.001) were independently associated with serum gamma-GT > 36 U/L. Serum levels of gamma-GT are associated with both left and right-sided cardiac alterations and may serve as a simple tool for risk prediction in HFpEF, especially when further diagnostic modalities are not available.
Subject terms: Heart failure, Hepatology
Introduction
In patients presenting with heart failure (HF), left ventricular (LV) ejection fraction (EF) will be normal in 50% of cases. Together with elevated natriuretic peptides and structural changes, such as left atrial enlargement, the diagnosis of HF with preserved ejection fraction (HFpEF) can be made1. On a pathophysiological level, abnormal LV relaxation results in elevated filling pressures due to changes in cellular as well as collagen metabolism2–5. Affected patients suffer from dyspnea, exercise intolerance and impaired quality of life. In advanced stages, they also show signs and symptoms of central and/or peripheral congestion and face a dismal prognosis, similar to that of HF patients with reduced ejection fraction6,7.
Increasing evidence suggests an association between HF and hepatic disorders. In a meta-analysis published in 2000, Naschitz et al. classified this organ interaction according to etiology8. Heart diseases that are linked to liver alterations include congestive fibrosis or cardiogenic ischemic hepatitis. Liver disorders resulting in cardiac impairments are classified as cirrhotic and non-cirrhotic complications. Complications of cirrhosis include hepatopulmonary syndrome or pericardial effusion, whereas a non-cirrhotic complication may be high-output failure caused by intrahepatic arteriovenous fistulae. Combined disorders with common etiology may be caused by infectious, metabolic, immune or toxic conditions8.
In acutely decompensated HF (ADHF) patients with reduced EF and in patients with cardiogenic shock, abnormal liver function tests have previously been described and were independently associated with poor outcome9–15. For example, in the SURVIVE study observing ADHF patients elevated transaminases were found in 46% of patients - and were associated with a 2-fold increase in 31-day mortality14. In the EFICA trial studying patients with cardiogenic shock elevations in transaminases were an independent predictor of 4-week mortality15.
In addition, a prognostic value of serum bilirubin has been suggested in the acute16,17 and the chronic phase of HF18,19 as well as in patients with idiopathic pulmonary hypertension20.
Although there is some evidence that non-alcoholic fatty liver disease (NAFLD) is associated with LV diastolic dysfunction in patients with diabetes21, the prognostic role of liver enzymes in HFpEF as well as their pathophysiological correlates have not been investigated so far. Therefore, the aim of this study was to describe the association of commonly assessed liver enzymes with clinical outcome in patients with HFpEF. Furthermore, we sought to identify alterations of cardiac structure and function that may underlie deviations of liver function parameters.
Methods
Subjects and study design
This prospective, observational cohort-analysis was performed at the Division of Cardiology of the Medical University of Vienna, a tertiary referral center for HFpEF. Approval from the ethics committee from the Medical University of Vienna was obtained before study initiation (EK #796/2010). All procedures were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from all patients prior to enrollment and any study-related procedure.
Consecutive patients with HFpEF were included from the outpatient department. The following examinations were performed: physical examination including bioelectrical impedance spectroscopy, 12-lead electrocardiogram, laboratory assessment including serum amino N-terminal pro brain natriuretic peptide (NT-proBNP), transthoracic echocardiography (TTE) and right heart catheterization (RHC) followed by coronary angiography22. Cardiac magnetic resonance (CMR) imaging was performed in a subgroup of patients (n = 157). All baseline examinations were performed in clinically stable patients without any clinical signs of decompensation22.
Clinical endpoints
The primary endpoint was a combined endpoint defined as hospitalization due to HF and/or death from any cause. Secondary endpoints were hospitalization due to HF and all-cause mortality.
Diagnostic definitions
According to the guidelines of the American College of Cardiology Foundation and American Heart Association23 and the European Society of Cardiology24, the following criteria had to be fulfilled to confirm the diagnosis of HFpEF: symptoms/signs of HF, LV ejection fraction (LVEF) ≥50%, diastolic dysfunction and/ or structural alterations (LV hypertrophy, left atrial enlargement) by TTE and serum NT-proBNP levels >220 pg/ml.
Patients with significant coronary artery disease (CAD, stenosis ≥50%) and significant valvular heart disease other than tricuspid regurgitation were excluded from the registry. Arterial hypertension was defined according to the recent guidelines with a systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg25 or if the patient already received antihypertensive medication. Hyperlipidemia was defined as low-density lipoprotein cholesterol >100 mg/dl26 or if the patient was already on statins and/or ezetimibe prior to study inclusion.
Furthermore, patients with excessive alcohol intake were excluded and complete serologic assessment was performed in all patients to rule out any infectious, cholestatic or autoimmune hepatic disorder.
Bioelectrical impedance spectroscopy
Bioelectrical impedance spectroscopy was performed using a portable whole-body device, the Body Composition Monitor (Fresenius Medical Care, Bad Homburg, Germany)22. Patients were placed in supine position and electrodes were attached to the nondominant hand and the ipsilateral foot. Measurements were conducted according to the manufacturer’s manual. The following parameters were obtained: fat tissue index (kg/m2), total fat mass (kg), relative fat mass (%, total body weight divided by total fat mass). Body composition monitoring was performed in 161 patients.
Laboratory analysis
Complete blood count and blood chemistry including liver enzymes were performed as part of clinical routine assessment. Capillary blood from the earlobe was measured using an ABL 510 blood gas analyzer (Radiometer Medical ApS, Bronshoj, Denmark). Serum NT-proBNP was measured with an immunological test (Elecsys® Systems, Roche Diagnostics, Rotkreuz, Switzerland). Upper limit of normal serum gamma-glutamyltransferase (GT) levels was 60 units per liter (U/L).
Echocardiography
Board certified physicians performed TTE using high-end scanners such as GE Vivid 7 and E9 (GE Healthcare, Wauwatsa, WI, USA)22. All measurements were done according to the guidelines of the American Society of Echocardiography27. LVEF was assessed using the biplane Simpson’s method. Pulsed-wave Doppler was performed to obtain the early (E) to late (A) ventricular filling velocities. E´ (early diastolic mitral annular velocity) was assessed at the septal and lateral side of the mitral annulus with Tissue Doppler Imaging and averaged to calculate E/E’. Right ventricular (RV) function (RVF) was assessed by integrating visual assessment of contractility of the RV outflow tract, RV apex and interventricular septum from different views. Tricuspid regurgitation (TR) was quantified according to recent recommendations28. Moderate and severe TR were considered significant29.
Cardiac catheterization
For hemodynamic confirmation of HFpEF, a 7F Swan-Ganz catheter (Baxter, Healthcare Corp, Munich, Germany) was inserted via a femoral approach22. CathCorLX (Siemens AG, Erlangen, Germany) was used to measure pressures, which were recorded as average of eight measurements over eight recorded heart cycles. Cardiac output (CO) was assessed by thermodilution and by Fick’ s method. The transpulmonary pressure gradient (TPG) was calculated by subtracting pulmonary artery wedge pressure (PAWP) from mean pulmonary artery pressure (PAP) and pulmonary vascular resistance (PVR) was calculated by dividing TPG by CO.
Cardiac magnetic resonance
All CMR studies were performed by board-certified physicians on a 1.5-T cardiac-dedicated clinical magnetic resonance system (Avanto, Siemens Medical Solutions, Erlangen, Germany). The CMR protocol consisted of a functional study and late gadolinium enhancement (LGE) imaging and has been described previously by our group in more detail30. CMR was not performed in 117 patients due to claustrophobia, advanced stage renal disease, implanted pacemaker/implantable cardioverter defibrillator or other ferromagnetic implants.
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics 23.0 (New York, USA). P-values from two-sided tests <0.05 were considered statistically significant. Data were expressed as mean ± standard deviation, median (interquartile range), or frequency and percent. Continuous variables were compared using the Student’s t –test or Wilcoxon rank-sum test, as appropriate. Differences between dichotomous variables were assessed using the χ2 test.
Univariable and multivariable Cox-regression models were calculated to examine factors associated with adverse outcome. Predictors in the multivariable Cox model were selected from the set of variables that reached statistical significance in univariable analysis. Logarithmic transformation was done in not-normally distributed variables prior to univariable calculations. Results were expressed as hazard ratios (HR) with 95% confidence intervals (CI).
Survival curves were estimated with the Kaplan-Meier method and log rank test was applied to compare survival differences.
The influence of relevant parameters on gamma-GT levels was investigated first by univariable logistic regression. To identify the most relevant predictors for each category (clinical, echocardiographic, hemodynamic, magnetic resonance imaging), a separate multiple regression model was selected from all variables that reached statistical significance in univariable analysis in the respective category by a stepwise procedure. Results were expressed as odds ratio (OR) with 95% CI.
Results
Clinical and cardiac characteristics at baseline
Between January 2011 and February 2017, 334 patients were referred. Of these, 18 patients were excluded because of relevant CAD, 20 because of cardiac amyloidosis and 15 had NT-proBNP levels below the inclusion threshold of 220 pg/ml. Additionally, 7 patients were excluded because of excessive alcohol intake and the suspicion of hepatitis.
Finally, 274 patients with confirmed HFpEF were enrolled. Mean age was 71.3 ± 8.4 years and 69.3% were female (Table 1). After 21.5 ± 18.6 months of follow-up, 97 patients (35.4%) reached the combined endpoint. These patients presented with higher New York Heart Association (NYHA) functional class (p < 0.001) at baseline, had more prior HF hospitalizations (p < 0.001), more frequently suffered from atrial fibrillation (p = 0.022), diabetes (p = 0.028) and chronic obstructive pulmonary disease (p = 0.016) and had a higher intake of diuretics (p = 0.006) compared with the remainder of the group (n = 177).
Table 1.
Baseline characteristics.
| Total (n = 274) |
Event (n = 97) |
No Event (n = 177) | p-value | |
|---|---|---|---|---|
| Clinical parameters | ||||
| Age, years | 71.3 ± 8.4 | 71.9 ± 8.3 | 70.9 ± 8.5 | 0.330 |
| Female, n (%) | 190 (69.3) | 62 (63.9) | 128 (72.3) | 0.171 |
| Body Mass Index, kg/m2 | 30.5 ± 6.8 | 31.2 ± 7.7 | 30.1 ± 6.2 | 0.196 |
| Fat Tissue Index, kg/ m2 | 15.9 ± 6.4 | 16.1 ± 7.0 | 15.9 ± 6.1 | 0.877 |
| Total Fat Mass, kg | 32.7 ± 12.9 | 34.1 ± 14.4 | 31.8 ± 11.8 | 0.272 |
| Relative Fat Mass, % | 37.9 ± 9.7 | 37.2 ± 10.1 | 38.4 ± 9.4 | 0.418 |
| NYHA class III and IV, n (%) | 181 (66.1) | 80 (82.5) | 101 (57.1) | <0.001 |
| Prior HF hospitalization, n (%) | 81 (29.6) | 56 (57.3) | 25 (14.1) | <0.001 |
| Atrial fibrillation, n (%) | 158 (57.7) | 65 (67.0) | 93 (52.5) | 0.022 |
| Arterial hypertension, n (%) | 263 (96.0) | 93 (95.9) | 170 (96.0) | 1.000 |
| Hyperlipidemia, n (%) | 153 (55.8) | 51 (52.6) | 102 (57.6) | 0.447 |
| Diabetes mellitus, n (%) | 103 (37.6) | 45 (46.4) | 58 (32.8) | 0.028 |
| History of CAD, n (%) | 67 (24.5) | 24 (24.7) | 43 (24.3) | 1.000 |
| COPD, n (%) | 92 (33.6) | 42 (43.3) | 50 (28.2) | 0.016 |
| ACE-inhibitor, n (%) | 82 (29.9) | 34 (35.1) | 48 (27.1) | 0.214 |
| ATII-blocker, n (%) | 104 (37.9) | 33 (34.0) | 71 (40.1) | 0.363 |
| Calcium-antagonist, n (%) | 81 (29.6) | 28 (28.9) | 53 (29.9) | 0.702 |
| Beta-blocker, n (%) | 206 (75.2) | 77 (79.4) | 129 (72.9) | 0.246 |
| Oral anticoagulation, n (%) | 218 (79.6) | 81 (83.5) | 137 (77.4) | 0.274 |
| Diuretic, n (%) | 214 (78.1) | 85 (87.6) | 129 (72.9) | 0.006 |
| Statin, n (%) | 134 (48.9) | 44 (45.4) | 90 (50.8) | 0.449 |
| Echocardiographic parameters | ||||
| LA diameter, mm | 62.4 ± 7.9 | 63.1 ± 7.7 | 61.9 ± 8.1 | 0.254 |
| LA indexed for BSA, ml/m2 | 51.3 ± 19.9 | 53.2 ± 17.2 | 50.5 ± 21.1 | 0.579 |
| LVEDD, mm | 44.0 ± 5.5 | 44.1 ± 6.0 | 43.9 ± 5.1 | 0.834 |
| RA diameter, mm | 62.6 ± 8.9 | 63.6 ± 9.2 | 61.9 ± 8.6 | 0.148 |
| RVEDD, mm | 37.0 ± 7.6 | 39.0 ± 8.5 | 35.7 ± 6.6 | 0.001 |
| IVS, mm | 12.9 ± 2.6 | 12.9 ± 2.3 | 12.9 ± 2.9 | 0.797 |
| E/E’ ratio | 15.3 ± 6.3 | 16.4 ± 8.0 | 14.8 ± 5.4 | 0.318 |
| E/A ratio | 1.6 ± 1.1 | 1.8 ± 0.9 | 1.6 ± 1.1 | 0.469 |
| Significant TR, n (%) | 136 (49.6) | 60 (61.9) | 76 (42.9) | 0.108 |
| Hemodynamic parameters | ||||
| Systolic PAP, mmHg | 53.9 ± 17.5 | 61.4 ± 17.0 | 49.6 ± 16.4 | <0.001 |
| Diastolic PAP, mmHg | 22.3 ± 7.5 | 24.8 ± 6.7 | 20.9 ± 7.7 | <0.001 |
| Mean PAP, mmHg | 34.4 ± 10.2 | 38.4 ± 9.1 | 32.1 ± 10.0 | <0.001 |
| Mean RAP, mmHg | 12.6 ± 5.6 | 14.3 ± 6.2 | 11.6 ± 5.0 | 0.001 |
| PAWP, mmHg | 20.1 ± 5.9 | 21.8 ± 5.8 | 19.1 ± 5.7 | 0.001 |
| SaO2, % | 93.8 ± 4.7 | 92.8 ± 4.9 | 94.3 ± 4.5 | 0.018 |
| TPG, mmHg | 14.3 ± 7.5 | 16.6 ± 8.3 | 12.9 ± 6.6 | <0.001 |
| PVR, dynes.s.cm−5 | 228.1 ± 136.4 | 264.3 ± 151.3 | 206.8 ± 122.5 | 0.002 |
| SV, ml | 73.5 ± 21.4 | 75.7 ± 22.8 | 72.3 ± 20.6 | 0.260 |
| CO thermodilution, l/min | 5.3 ± 1.4 | 5.3 ± 1.4 | 5.2 ± 1.4 | 0.748 |
| CO Fick, l/min | 4.5 ± 1.3 | 4.4 ± 1.2 | 4.6 ± 1.3 | 0.401 |
|
Magnetic resonance imaging parameters (n = 157) (n = 59) (n = 98) | ||||
| LA, mm | 65.6 ± 9.4 | 67.8 ± 9.4 | 64.2 ± 9.2 | 0.021 |
| LVEDV, ml | 126.9 ± 44.8 | 131.2 ± 45.6 | 124.4 ± 44.4 | 0.371 |
| RA, mm | 65.6 ± 9.2 | 66.9 ± 10.2 | 64.8 ± 8.4 | 0.157 |
| RVEDV, ml | 160.7 ± 102.2 | 171.9 ± 72.5 | 153.9 ± 116.1 | 0.294 |
| IVS, mm | 11.2 ± 2.1 | 11.3 ± 1.7 | 11.1 ± 2.3 | 0.506 |
| LVEF, % | 53.3 ± 13.8 | 53.7 ± 14.3 | 53.0 ± 13.5 | 0.715 |
| RVEF, % | 51.8 ± 11.3 | 48.1 ± 11.6 | 53.9 ± 10.7 | 0.002 |
Continuous values are shown as mean ± standard deviation; A - mitral peak velocity of late filling, ACE – angiotensin converting enzyme, AT – angiotensin, BSA – body surface area, CAD - coronary artery disease, CO - cardiac output, COPD - chronic obstructive pulmonary disease, E - early mitral inflow velocity, E’ - early diastolic mitral annular velocity, ECV - extra cellular volume, HF – heart failure, IVS - interventricular septum, LA - left atrial, LVEDV - left ventricular end-diastolic volume, LVEF - left ventricular ejection fraction, NYHA - New York Heart Association, PAP - pulmonary artery pressure, PAWP - pulmonary artery wedge pressure, PVR - pulmonary vascular resistance, RA - right atrial, RAP - right atrial pressure, RVEDV - right ventricular end-diastolic volume, RVEF - right ventricular ejection fraction, SaO2 - arterial saturation of oxygen, SV - stroke volume, TPG - transpulmonary gradient, TR – tricuspid regurgitation.
RV end-diastolic diameter (RVEDD) as assessed by TTE was significantly larger in the event cohort (p = 0.001), which also displayed higher pulmonary artery pressures (e.g. mean PAP, p < 0.001), lower oxygen saturation (p = 0.018), higher TPG (p < 0.001) and higher PVR (p = 0.002).
In a subgroup of 157 patients who underwent CMR, left atrial (LA) size was larger in the event group (p = 0.021). Furthermore, on average, RV ejection fraction (RVEF) was significantly lower in this cohort (p = 0.002).
Liver enzymes, other laboratory parameters and outcome
Patients in the event group presented with lower hemoglobin levels (p = 0.001), lower serum iron levels (p = 0.001) and higher levels of C-reactive protein (p = 0.013). Furthermore, glomerular filtration rates were lower (p < 0.001) and NT-proBNP levels (p = 0.001) as well as serum gamma-GT levels (p < 0.001) were higher in patients who had reached the combined endpoint. No difference could be found with respect to other cholestatic parameters (p = 0.126 for alkaline phosphatase, p = 0.774 for bilirubin). Interestingly, patients in the event group showed lower levels of total cholesterol (p = 0.030).
Multivariable Cox-regression analysis was calculated using eight laboratory parameters that were statistically significant in univariable analysis. In the multivariable model, hemoglobin (p = 0.006), serum gamma-GT (p = 0.004) and serum NT-proBNP (p = 0.001) were associated with worse clinical outcome (Table 2).
Table 2.
Laboratory parameters and their association with clinical outcome.
| Total (n = 274) |
Event (n = 97) |
No Event (n = 177) | Univariable | Multivariable | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||||
| Hemoglobin, mg/dl | 12.5 ± 1.7 | 12.0 ± 1.8 | 12.7 ± 1.6 | 0.796 (0.704–0.899) | <0.001 | 0.840 (0.741–0.952) | 0.006 |
| Platelet count, G/L | 233.9 ± 75.1 | 244.9 ± 84.8 | 227.8 ± 68.7 | 1.002 (1.000–1.005) | 0.053 | ||
| White blood count, G/L | 7.1 (5.7–8.7) | 7.1 (5.7–9.6) | 7.1 (5.8–8.3) | 2.044 (0.493–8.472) | 0.324 | ||
| Serum iron, μg/dl | 66.0 (46.5–95.5) | 57.0 (42.0–79.0) | 74.5 (49.0–104.3) | 0.364 (0.196–0.675) | 0.001 | ||
| Total bilirubin, mg/dl | 0.6 (0.4–0.9) | 0.6 (0.5–0.9) | 0.5 (0.4–0.9) | 1.235 (0.559–2.725) | 0.602 | ||
| Albumin, mg/dl | 40.6 ± 4.9 | 40.1 ± 5.6 | 41.0 ± 4.4 | 0.964 (0.922–1.008) | 0.105 | ||
| Lipase, U/L | 31.0 (22.0–45.0) | 32.0 (21.0–43.0) | 31.0 (22.0–46.0) | 1.136 (0.517–2.498) | 0.751 | ||
| Alkaline phosphatase, U/L | 77.0 (62.0–98.0) | 81.0 (64.5–113.5) | 75.0 (61.0–92.5) | 1.770 (0.544–5.759) | 0.343 | ||
| ASAT, U/L | 25.0 (20.0–31.3) | 24.0 (19.5–31.0) | 25.0 (21.0–32.0) | 0.413 (0.105–1.624) | 0.205 | ||
| ALAT, U/L | 22.0 (16.0–29.0) | 19.0 (14.0–26.0) | 23.0 (18.0–31.0) | 0.175 (0.064–0.482) | 0.001 | ||
| Gamma-GT, U/L | 36.0 (22.0–66.0) | 49.0 (26.5–100.5) | 33.0 (21.0–51.0) | 2.499 (1.544–4.044) | < 0.001 | 1.002 (1.001–1.003) | 0.004 |
| LDH, U/L | 215.0 (185.0–253.0) | 222.0 (185.0–273.5) | 211.0 (185.0–246.5) | 3.546 (0.536–23.473) | 0.189 | ||
| C-reactive protein, mg/dl | 0.4 (0.2–0.9) | 0.6 (0.2–1.4) | 0.4 (0.2–0.8) | 1.814 (1.236–2.663) | 0.002 | ||
| Cholesterol, mg/dl | 171.1 ± 41.4 | 163.8 ± 43.3 | 175.2 ± 39.8 | 0.992 (0.988–0.997) | 0.001 | ||
| Triglycerides, mg/dl | 113.0 (83.0–155.0) | 113.0 (89.0–158.8) | 116.0 (81.0–153.0) | 0.758 (0.269–2.139) | 0.601 | ||
| GFR, ml/min/1.73 m2 | 59.4 ± 19.9 | 52.4 ± 17.0 | 63.2 ± 20.4 | 0.977 (0.967–0.987) | <0.001 | ||
| HbA1c, % | 5.9 (5.6–6.5) | 6.1 (5.7–6.7) | 5.9 (5.6–6.5) | 2.326 (0.573–9.442) | 0.238 | ||
| NT-proBNP quartile (pg/ml) | 3.015 (1.926–4.719) | <0.001 | 2.213 (1.373–3.569) | 0.001 | |||
| 0–600, n (%) | 89 (32.5) | 20 (20.6) | 69 (38.9) | ||||
| 601–1200, n (%) | 59 (21.5) | 11 (11.3) | 48 (27.1) | ||||
| 1201–1800, n (%) | 48 (17.5) | 22 (22.7) | 26 (14.7) | ||||
| >1800, n (%) | 78 (28.5) | 44 (45.4) | 34 (19.3) | ||||
Continuous values are shown as mean ± standard deviation or median (interquartile range); ALAT - Alanin Aminotransferase, ASAT - Aspartat Aminotransferase, GFR - Glomerular Filtration Rate, GT - Glutamyl Transferase, LDH - Lactatdehydrogenase, NT-proBNP - N-terminal pro Brain Natriuretic Peptide.
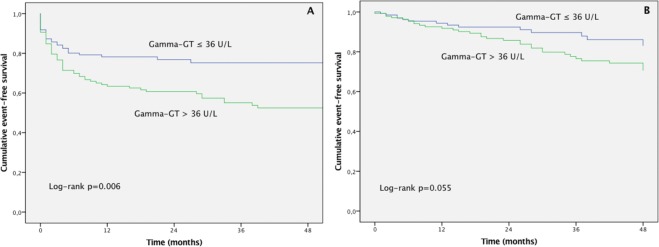
Kaplan-Meier analysis of the combined primary endpoint showed that patients with gamma-GT levels >36 U/L had significantly more events compared to patients with levels ≤ 36 U/L (log-rank p = 0.012, Fig. 1). Secondary endpoint analysis showed a significant difference in HF hospitalizations (log-rank p = 0.006) and a trend in all-cause mortality (log-rank p = 0.055, Fig. 2).
Figure 1.
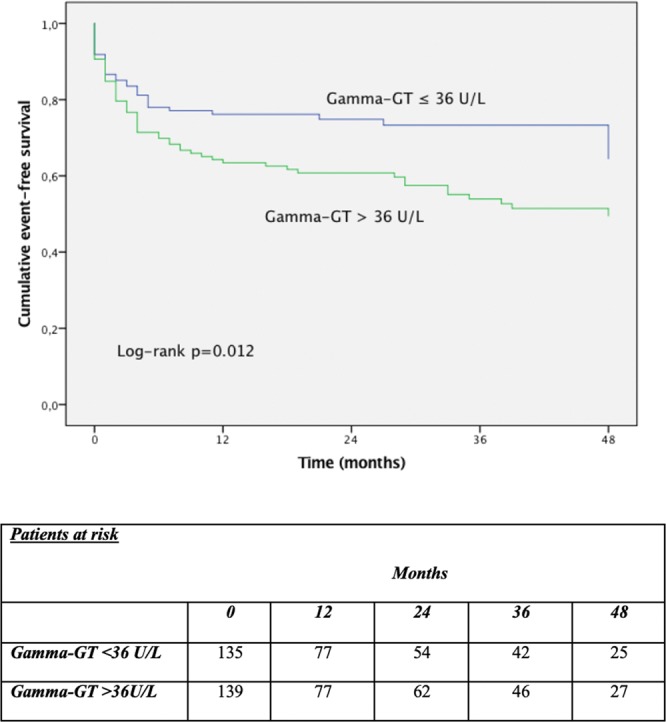
Kaplan-Meier event-free survival curve according to the gamma glutamyltransferase (GT) median of 36 U/L.
Figure 2.
Kaplan-Meier curves of hospitalization due to heart failure worsening (A) and all-cause mortality (B).
Determinants of gamma-glutamyltransferase
Univariable logistic regression was performed for each category to identify parameters associated with gamma-GT. The following variables were statistically significant in the univariable model: clinical: atrial fibrillation (OR 3.140, p < 0.001); echocardiographic: LA diameter (OR 1.061, p = 0.001), RA diameter (OR 1.064, p < 0.001), RVEDD (OR 1.060, p = 0.002), E/A ratio (OR 2.173, p = 0.024) and significant TR (OR 3.153, p < 0.001); hemodynamic: systolic PAP (OR 1.027, p = 0.001), diastolic PAP (OR 1.069, p = 0.001), mean PAP (OR 1.062, p < 0.001), mean RA pressure (OR 1.140, p < 0.001), PAWP (OR 1.110, p < 0.001), TPG (OR 1.044, p = 0.025), PVR (OR 1.002, p = 0.048); magnetic resonance imaging: LA diameter (OR 1.036, p = 0.050), RA diameter (OR 1.052, p = 0.009), RV end-diastolic volume (OR 1.012, p = 0.001) and RVEF (OR 0.963, p = 0.014).
By multivariable logistic regression analysis of each category, echocardiographic (5 variables): E/A ratio (OR 2.173, p = 0.024), hemodynamic (7 variables): mean RA pressure (OR 1.139, p < 0.001) and magnetic resonance imaging (4 variables): RA diameter (OR 1.070, p = 0.001) remained independently associated with gamma-GT levels (Table 3).
Table 3.
Parameters associated with gamma-glutamyltransferase (GT).
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | |
| Clinical parameters | ||||
| Age | 0.988 (0.960–1.016) | 0.397 | ||
| Female | 1.669 (0.992–2.809) | 0.054 | ||
| Body Mass Index | 1.021 (0.986–1.059) | 0.243 | ||
| Fat Tissue Index | 0.989 (0.942–1.038) | 0.641 | ||
| Total Fat Mass | 0.990 (0.967–1.015) | 0.438 | ||
| Relative Fat Mass | 0.975 (0.943–1.008) | 0.133 | ||
| Systolic blood pressure | 0.994 (0.982–1.006) | 0.308 | ||
| Diastolic blood pressure | 1.004 (0.985–1.024) | 0.661 | ||
| Prior HF hospitalization | 1.702 (0.950–3.049) | 0.074 | ||
| Atrial fibrillation | 3.140 (1.902–5.181) | <0.001 | ||
| Hypertension | 1.846 (0.528–6.4559 | 0.337 | ||
| Hyperlipidemia | 0.807 (0.500–1.301) | 0.379 | ||
| Diabetes mellitus | 1.263 (0.774–2.062) | 0.350 | ||
| History of CAD | 0.729 (0.419–1.268) | 0.263 | ||
| COPD | 1.420 (0.857–2.351) | 0.173 | ||
| Echocardiographic parameters | ||||
| LA diameter | 1.061 (1.024–1.099) | 0.001 | ||
| LA indexed for BSA | 1.023 (0.999–1.047) | 0.059 | ||
| LVEDD | 0.994 (0.948–1.042) | 0.801 | ||
| RA diameter | 1.064 (1.030–1.100) | <0.001 | ||
| RVEDD | 1.060 (1.021–1.101) | 0.002 | ||
| IVS | 1.009 (0.915–1.112) | 0.863 | ||
| E/E’ ratio | 1.033 (0.959–1.112) | 0.395 | ||
| E/A ratio | 2.173 (1.108–4.260) | 0.024 | 2.173 (1.108–4.260) | 0.024 |
| Significant TR | 3.153 (1.846–5.385) | <0.001 | ||
| Hemodynamic parameters | ||||
| Systolic PAP | 1.027 (1.011–1.044) | 0.001 | ||
| Diastolic PAP | 1.069 (1.029–1.112) | 0.001 | ||
| Mean PAP | 1.062 (1.031–1.094) | <0.001 | ||
| Mean RAP | 1.140 (1.078–1.206) | <0.001 | 1.139 (1.076–1.205) | <0.001 |
| PAWP | 1.110 (1.055–1.168) | <0.001 | ||
| SaO2 | 1.041 (0.982–1.102) | 0.176 | ||
| TPG | 1.044 (1.005–1.084) | 0.025 | ||
| PVR | 1.002 (1.000–1.004) | 0.048 | ||
| SV | 0.996 (0.983–1.008) | 0.496 | ||
| CO thermodilution | 1.037 (0.856–1.258) | 0.709 | ||
| CO Fick | 0.898 (0.714–1.130) | 0.358 | ||
| Magnetic resonance imaging parameters | ||||
| LA | 1.036 (1.000–1.073) | 0.050 | ||
| LVEDV | 1.006 (0.998–1.014) | 0.132 | ||
| RA | 1.052 (1.013–1.092) | 0.009 | 1.070 (1.026–1.115) | 0.001 |
| RVEDV | 1.012 (1.005–1.019) | 0.001 | ||
| IVS | 1.022 (0.879–1.188) | 0.779 | ||
| LVEF | 1.000 (0.983–1.017) | 0.982 | ||
| RVEF | 0.963 (0.935–0.992) | 0.014 | ||
A - mitral peak velocity of late filling, CAD - coronary artery disease, CO - cardiac output, COPD - chronic obstructive pulmonary disease, E - early mitral inflow velocity, E’ - early diastolic mitral annular velocity, ECV - extra cellular volume, HF – heart failure, IVS - inter-ventricular septum, LA - left atrial, LVEDV - left ventricular end-diastolic volume, LVEF - left ventricular ejection fraction, PAP - pulmonary artery pressure, PAWP - pulmonary artery wedge pressure, PVR - pulmonary vascular resistance, RA - right atrial, RAP - right atrial pressure, RVEDV - right ventricular end-diastolic volume, RVEF - right ventricular ejection fraction, SaO2 - arterial saturation of oxygen, SV - stroke volume, TPG - transpulmonary gradient, TR – tricuspid regurgitation.
Discussion
Our analysis of a well-characterized HFpEF patient cohort is the first to identify serum gamma-GT levels as an independent predictor of clinical outcome, besides hemoglobin and NT-proBNP.
Although we found a clear association between gamma-GT serum levels and the degree of LV diastolic function as well as blood pressures in the RA, the exact underlying pathophysiologic mechanisms remain yet unclear. It is not entirely possible to discern whether liver congestion as a result of elevated left and right-sided cardiac filling pressures or systemic low-grade inflammation with consecutive cardiac stiffness and pressure rise, or both mechanisms drive gamma-GT alteration in HFpEF patients.
Evidence supporting hepatic congestion as a determinant of serum gamma-glutamyltransferase levels
In 2000, Naschitz et al. systematically performed a survey of the Medline database and were the first to describe the close relationship between cardiac and hepatic disorders8. Over the past decades, there has been growing evidence on the importance of liver enzymes and abnormal liver function tests (LFT) in HF, especially in patients with reduced EF. Van Deursen and co-workers analyzed 234 ADHF patients and showed that abnormal LFTs were associated with an increased risk for mortality, rehospitalization, and in-hospital worsening of HF13. This finding was recently confirmed in the PROTECT study, where abnormal levels of Aspartat Aminotransferase (ASAT), Alanin Aminotransferase (ALAT) and bilirubin were associated with a higher-risk of in-hospital mortality and 180-day mortality11. In the larger ASCEND-HF trial, LFT data were obtained from 4.228 patients and more than 40% of study participants were out of range at the time of hospital admission, but only bilirubin was independently associated with worse clinical outcome12, which has also been confirmed in the CHARM program a few years before18 identifying bilirubin as a sensitive marker for congestion in HF with reduced EF. The crucial prognostic role of total bilirubin has also been described in the EVEREST trial in 201210, but Okada and the NaDEF investigators performed a more detailed assessment of bilirubin fractionation and found direct bilirubin superior to total bilirubin in predicting outcome in ADHF16.
Nevertheless, there is limited evidence on liver enzymes and LFTs in HF patients with preserved ejection fraction. In our cohort of 274 patients, we identified gamma-GT as an independent predictor of outcome after a mean follow-up of approximately two years and did not find any association with bilirubin or other LFTs. Interestingly, in previous observations gamma-GT was associated with in-hospital mortality in 183 ADHF patients with an ejection fraction < 50%31, but no association with long-term outcome has yet been reported, especially not in HFpEF patients. In 2002, Lau et al. showed that gamma-GT and alkaline phosphatase increased in direct proportion to the severity of TR in HF patients32. Significant TR is a common finding in HFpEF and was present in 49.6% of our patients and there was an association between TR and gamma-GT levels, if only in univariable analysis.
We were able to show that elevated RA filling pressures and higher RA diameters, as assessed by CMR, were associated with changes in gamma-GT. This is in accordance with a recently published study of Taniguchi and co-workers, who suggested that liver stiffness, reflecting right-sided filling pressures, provides important information regarding patients’ volume status33. Furthermore, the authors of this study concluded that liver congestion at hospital discharge is linked to HF hospitalization and death, although, once more, performed in patients with reduced systolic function33.
Evidence supporting low-grade inflammation as a determinant of serum gamma-glutamyltransferase levels
In 2011, Kanbay et al. evaluated the medical records of 166 patients with obstructive sleep apnea syndrome and found high serum gamma-GT levels as an independent predictor of cardiovascular disease34, supporting the theory of gamma-GT as a ‘novel’ cardiovascular biomarker35 and as a factor associated with subclinical inflammation36. In line with this, patients who have reached the combined endpoint in our analysis had slightly higher levels of C-reactive protein. These findings are in accordance with the hypothesis of Paulus and Tschöpe that chronic low-grade inflammation processes due to a variety of comorbidities lead to alterations in myocardial structure and function resulting in abnormal LV relaxation and consecutive diastolic dysfunction37. This theory was confirmed by Mantovani et al., who were able to show that in patients with type-2 diabetes, the inflammatory state of NAFLD was associated with early LV diastolic dysfunction21, which has been identified as an independent predictor of reduced functional capacity22.
Additionally, patients with NAFLD were more likely to be female and overweight/obese21 which is in line with a previous finding from our group supporting the HFpEF obesity phenotype22,38,39. A recently published analysis suggested that an increase in gamma-GT concentrations is a sensitive and early biomarker of unfavorable body fat distribution even in healthy individuals40. The median serum level in this study was 21.6 U/L compared to 36.0 U/L in our cohort, which is quite in the range of normal values. Thus, even gamma-GT levels within the normal range may provide important information concerning the prognosis of HF patients with preserved EF , although these results need to be confirmed in larger registries and clinical trials.
Limitations
Due to the single-center design, a center-specific bias cannot be excluded. In addition, liver sonographies and measurement of liver stiffness by elastography41 have not been systematically performed. Therefore, structural hepatic alterations and the specific impact of fatty liver disease42 cannot be accurately determined. However, we found a clear association between HFpEF-defining cardiac parameters and gamma-GT serum levels, while no such relation could be found with other liver function parameters. More information on the relation between gamma-GT and cardiac function as well as its association with other biomarkers reflecting pathophysiological processes is needed to further investigate its clinical utility.
Finally, due to the relatively small patient population, our results should be interpreted as hypothesis-generating and certainly need confirmation in larger patient collectives.
Conclusion
Our study demonstrates that serum levels of gamma-GT in HFpEF patients are multifactorial and largely related to LV diastolic dysfunction as well as right-sided heart alterations.
Serum gamma-GT levels may serve as an easily available tool to predict clinical prognosis, especially in the absence of further investigative modalities. Importantly, next to NT-proBNP and hemoglobin, serum gamma-GT levels predicted hospitalization and mortality in HFpEF patients.
Author contributions
D.D., S.A., A.K., T.R. and D.B. wrote the main text. C.B., F.D., J.M. and C.H. performed statistical analysis and wrote the Tables 1–3. D.D., T.R. and D.B. prepared Figures 1–2. Finally, all authors reviewed and approved the final version of the manuscript.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–327. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 2.Martos R, et al. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115:888–895. doi: 10.1161/CIRCULATIONAHA.106.638569. [DOI] [PubMed] [Google Scholar]
- 3.Mascherbauer J, et al. Cardiac magnetic resonance postcontrast T1 time is associated with outcome in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging. 2013;6:1056–1065. doi: 10.1161/CIRCIMAGING.113.000633. [DOI] [PubMed] [Google Scholar]
- 4.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure–abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 5.Zile MR, et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. 2015;131:1247–1259. doi: 10.1161/CIRCULATIONAHA.114.013215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia RS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 7.Smith GL, Masoudi FA, Vaccarino V, Radford MJ, Krumholz HM. Outcomes in heart failure patients with preserved ejection fraction: mortality, readmission, and functional decline. J Am Coll Cardiol. 2003;41:1510–1518. doi: 10.1016/S0735-1097(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 8.Naschitz JE, Slobodin G, Lewis RJ, Zuckerman E, Yeshurun D. Heart diseases affecting the liver and liver diseases affecting the heart. Am Heart J. 2000;140:111–120. doi: 10.1067/mhj.2000.107177. [DOI] [PubMed] [Google Scholar]
- 9.Ambrosy AP, et al. The predictive value of transaminases at admission in patients hospitalized for heart failure: findings from the RO-AHFS registry. Eur Heart J Acute Cardiovasc Care. 2013;2:99–108. doi: 10.1177/2048872612474906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambrosy AP, et al. Clinical course and predictive value of liver function tests in patients hospitalized for worsening heart failure with reduced ejection fraction: an analysis of the EVEREST trial. Eur J Heart Fail. 2012;14:302–311. doi: 10.1093/eurjhf/hfs007. [DOI] [PubMed] [Google Scholar]
- 11.Biegus J, et al. Abnormal liver function tests in acute heart failure: relationship with clinical characteristics and outcome in the PROTECT study. Eur J Heart Fail. 2016;18:830–839. doi: 10.1002/ejhf.532. [DOI] [PubMed] [Google Scholar]
- 12.Samsky MD, et al. Liver function tests in patients with acute heart failure and associated outcomes: insights from ASCEND-HF. Eur J Heart Fail. 2016;18:424–432. doi: 10.1002/ejhf.440. [DOI] [PubMed] [Google Scholar]
- 13.van Deursen VM, et al. Liver function, in-hospital, and post-discharge clinical outcome in patients with acute heart failure-results from the relaxin for the treatment of patients with acute heart failure study. J Card Fail. 2014;20:407–413. doi: 10.1016/j.cardfail.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Nikolaou M, et al. Liver function abnormalities, clinical profile, and outcome in acute decompensated heart failure. Eur Heart J. 2013;34:742–749. doi: 10.1093/eurheartj/ehs332. [DOI] [PubMed] [Google Scholar]
- 15.Zannad F, et al. Clinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: The EFICA study. Eur J Heart Fail. 2006;8:697–705. doi: 10.1016/j.ejheart.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Okada A, et al. Usefulness of the Direct and/or Total Bilirubin to Predict Adverse Outcomes in Patients With Acute Decompensated Heart Failure. Am J Cardiol. 2017;119:2035–2041. doi: 10.1016/j.amjcard.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 17.Shinagawa H, et al. Prognostic significance of increased serum bilirubin levels coincident with cardiac decompensation in chronic heart failure. Circ J. 2008;72:364–369. doi: 10.1253/circj.72.364. [DOI] [PubMed] [Google Scholar]
- 18.Allen LA, et al. Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail. 2009;11:170–177. doi: 10.1093/eurjhf/hfn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosoda J, et al. Significance of change in serum bilirubin in predicting left ventricular reverse remodeling and outcomes in heart failure patients with cardiac resynchronization therapy. J Cardiol. 2017;70:416–419. doi: 10.1016/j.jjcc.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Xu XQ, et al. Direct bilirubin: A new risk factor of adverse outcome in idiopathic pulmonary arterial hypertension. Int J Cardiol. 2017;228:895–899. doi: 10.1016/j.ijcard.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 21.Mantovani Alessandro, Pernigo Matteo, Bergamini Corinna, Bonapace Stefano, Lipari Paola, Pichiri Isabella, Bertolini Lorenzo, Valbusa Filippo, Barbieri Enrico, Zoppini Giacomo, Bonora Enzo, Targher Giovanni. Nonalcoholic Fatty Liver Disease Is Independently Associated with Early Left Ventricular Diastolic Dysfunction in Patients with Type 2 Diabetes. PLOS ONE. 2015;10(8):e0135329. doi: 10.1371/journal.pone.0135329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalos D, et al. Functional Status, Pulmonary Artery Pressure, and Clinical Outcomes in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2016;68:189–199. doi: 10.1016/j.jacc.2016.04.052. [DOI] [PubMed] [Google Scholar]
- 23.Mantovani Alessandro, Pernigo Matteo, Bergamini Corinna, Bonapace Stefano, Lipari Paola, Pichiri Isabella, Bertolini Lorenzo, Valbusa Filippo, Barbieri Enrico, Zoppini Giacomo, Bonora Enzo, Targher Giovanni. Nonalcoholic Fatty Liver Disease Is Independently Associated with Early Left Ventricular Diastolic Dysfunction in Patients with Type 2 Diabetes. PLOS ONE. 2015;10(8):e0135329. doi: 10.1371/journal.pone.0135329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponikowski P, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 25.Williams B, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 26.Catapano AL, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 27.Lang RM, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Lancellotti P, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease) Eur J Echocardiogr. 2010;11:307–332. doi: 10.1093/ejechocard/jeq031. [DOI] [PubMed] [Google Scholar]
- 29.Mascherbauer Julia, Kammerlander Andreas A., Zotter-Tufaro Caroline, Aschauer Stefan, Duca Franz, Dalos Daniel, Winkler Susanne, Schneider Matthias, Bergler-Klein Jutta, Bonderman Diana. Presence of ´isolated´ tricuspid regurgitation should prompt the suspicion of heart failure with preserved ejection fraction. PLOS ONE. 2017;12(2):e0171542. doi: 10.1371/journal.pone.0171542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duca, F. et al. Interstitial Fibrosis, Functional Status, and Outcomes in Heart Failure With Preserved Ejection Fraction: Insights From a Prospective Cardiac Magnetic Resonance Imaging Study. Circ Cardiovasc Imaging. e005277 (2016). [DOI] [PubMed]
- 31.Turfan M, et al. Serum gamma-glutamyl transferase levels and in-hospital mortality in patients with acute heart failure. Kardiol Pol. 2014;72:735–739. doi: 10.5603/KP.a2014.0048. [DOI] [PubMed] [Google Scholar]
- 32.Lau GT, Tan HC, Kritharides L. Type of liver dysfunction in heart failure and its relation to the severity of tricuspid regurgitation. Am J Cardiol. 2002;90:1405–1409. doi: 10.1016/S0002-9149(02)02886-2. [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi Tatsunori, Ohtani Tomohito, Kioka Hidetaka, Tsukamoto Yasumasa, Onishi Toshinari, Nakamoto Kei, Katsimichas Themistoklis, Sengoku Kaoruko, Chimura Misato, Hashimoto Haruko, Yamaguchi Osamu, Sawa Yoshiki, Sakata Yasushi. Liver Stiffness Reflecting Right-Sided Filling Pressure Can Predict Adverse Outcomes in Patients With Heart Failure. JACC: Cardiovascular Imaging. 2019;12(6):955–964. doi: 10.1016/j.jcmg.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 34.Kanbay A, et al. Serum gamma-glutamyl transferase activity is an independent predictor for cardiovascular disease in obstructive sleep apnea syndrome. Respir Med. 2011;105:637–642. doi: 10.1016/j.rmed.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Mason JE, Starke RD, Van Kirk JE. Gamma-glutamyl transferase: a novel cardiovascular risk biomarker. Prev Cardiol. 2010;13:36–41. doi: 10.1111/j.1751-7141.2009.00054.x. [DOI] [PubMed] [Google Scholar]
- 36.Ali SS, et al. Elevated gamma-glutamyl transferase is associated with subclinical inflammation independent of cardiometabolic risk factors in an asymptomatic population: a cross-sectional study. Nutr Metab (Lond). 2016;13:37. doi: 10.1186/s12986-016-0097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 38.Kitzman DW, Shah SJ. The HFpEF Obesity Phenotype: The Elephant in the Room. J Am Coll Cardiol. 2016;68:200–203. doi: 10.1016/j.jacc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 39.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure With Preserved Ejection Fraction. Circulation. 2017;136:6–19. doi: 10.1161/CIRCULATIONAHA.116.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coku V, Shkembi X. Serum Gamma-glutamyltransferase and Obesity: is there a Link? Med Arch. 2018;72:112–115. doi: 10.5455/medarh.2017.72.112-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reiberger T, et al. Noninvasive screening for liver fibrosis and portal hypertension by transient elastography - a large single center experience. Wien Klin Wochenschr. 2012;124:395–402. doi: 10.1007/s00508-012-0190-5. [DOI] [PubMed] [Google Scholar]
- 42.Scheiner Bernhard, Steininger Lisa, Semmler Georg, Unger Lukas W., Schwabl Philipp, Bucsics Theresa, Paternostro Rafael, Ferlitsch Arnulf, Trauner Michael, Reiberger Thomas, Mandorfer Mattias. Controlled attenuation parameter does not predict hepatic decompensation in patients with advanced chronic liver disease. Liver International. 2018;39(1):127–135. doi: 10.1111/liv.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).