Abstract
Clinical evidence suggest that men are more sensitive than women to the abuse-related effects of mu-opioid agonists. In contrast, preclinical studies suggest the opposite sex difference. The aim of the present study was to clarify this discrepancy using a fentanyl vs. diluted Ensure® choice procedure to assess sex differences in opioid reinforcement. Sex differences in intravenous (IV) fentanyl self-administration were examined under a fixed-ratio (FR5) schedule, a multi-day progressive-ratio (PR) schedule for behavioral economic analysis, and a concurrent (choice) schedule of fentanyl and diluted Ensure® reinforcement in Sprague–Dawley male and female rats. The fentanyl dose-effect function under the FR5 schedule was significantly shifted upward in females compared to males. Similarly, the reinforcing effectiveness of both fentanyl (3.2 and 10 µg/kg per injection, IV) and diluted Ensure® (18 and 56%) were greater in females than in males as assessed using behavioral economic analysis, irrespective of dose or concentration. However, under a fentanyl vs. foodchoice procedure, males chose 3.2 µg/kg per injection fentanyl injections over 18%, but not 56%, diluted Ensure® at a higher percentage compared to females. Overall, these results suggest that the expression of sex differences in opioid reinforcement depends upon the schedule of reinforcement and that preclinical opioid vs. food choice procedures provide a translationally relevant measure (i.e., behavioral allocation) consistent with the direction of sex differences reported in the clinical literature.
Subject terms: Reward, Psychology, Addiction
Introduction
Drug overdose is now the leading cause of accidental death in the United States, with the majority of these fatalities involving mu-opioid receptor (MOR) agonists (e.g., fentanyl [1]). In addition, rates of opioid use disorder diagnosis have increased dramatically, with a recent report documenting a 493% increase from 2010 to 2016 [2]. Overall, these epidemiological data support the significance of opioid use disorder as a public health issue and highlight the need for preclinical research related to the expression and mechanisms of MOR agonist abuse-related effects.
Sex differences in opioid pharmacology have been observed on endpoints related to opioid abuse and opioid use disorder. For example, clinical evidence suggests that women are less likely than men to misuse opioids, although this gap appears to be narrowing [3, 4]. Sex differences in MOR agonist subjective effects have also been observed, with women reporting greater negative opioid effects than men [5–10]. In contrast to these human results, MOR agonists typically maintain higher rates of responding in female rats compared to male rats in preclinical drug self-administration studies under both fixed-ratio (FR) and progressive-ratio (PR) procedures [11–15], although see Stewart et al. [16]. However, one interpretive complication of these preclinical studies examining sex differences in opioid reinforcement is that the primary dependent measure, rate of operant responding, is an integration of both reinforcement-dependent and reinforcement-independent processes (see [17] for a review). For example, interpretation of a group difference under a rate-dependent drug self-administration procedure (i.e., FR or PR) cannot distinguish between differences in the reinforcing effects of the self-administered drug (reinforcement-dependent) and differences in the sensory, cognitive, or motor-impairing effects of the self-administered drug (reinforcement-independent). The distinction between these two processes may be important because male rats are more sensitive to MOR agonist-induced locomotor depression compared to female rats (see [18] for review).
The aim of the present study was to examine potential sex differences in opioid reinforcement in rats using an intravenous (IV) fentanyl vs. food choice procedure. A choice procedure was utilized for two main reasons. First, substance use disorders are increasingly recognized as mental health disorders of “choice” or behavioral allocation between the abused drug and competing nondrug alternative reinforcers [17, 19–22]. Second, the primary dependent measure of behavioral allocation between a drug and nondrug reinforcer is less sensitive to reinforcement-independent rate-altering effects than rate-dependent drug self-administration procedures [23–26]. Thus, the current study utilized an opioid vs. food choice procedure to examine sex differences in opioid reinforcement that is not solely dependent upon rates of responding.
Method
Subjects
Thirty-five Sprague–Dawley rats (18 males, 17 females) were acquired at 10 weeks of age (Envigo Laboratories, Frederick, MD, USA) and surgically implanted with custom-made jugular catheters and vascular access ports (Instech, Plymouth Meeting, PA, USA) as described previously [27]. Rats were singly housed in a temperature- and humidity-controlled vivarium that was maintained on a 12-h light/dark cycle (lights off at 6:00 p.m.). Water and food (Teklad Rat Diet, Envigo) were provided ad libitum in the home cage. Behavioral sessions were conducted 5–7 days per week from approximately 2:00–4:00 p.m. Estrous cycle was not monitored, as guidelines have suggested that estrous cycle monitoring is not essential for initial sex difference studies because sex differences when present are often sufficiently robust for detection without reference to estrous cycle phase [28]. Rat maintenance and research were conducted in accordance with the 2011 guidelines of the National Institutes of Health Committee on Laboratory Animal Resources and protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Apparatus and catheter maintenance
Twelve modular operant chambers located in sound-attenuating cubicles (Med Associates, St. Albans, VT, USA) were equipped with two retractable levers, a set of three LED lights (red, yellow, green) mounted above each lever, and a retractable “dipper” cup (0.1 ml) located between the levers for presenting diluted Ensure® (18 or 56% v/v vanilla flavor Ensure® in tap water; Abbott Laboratories, Chicago, IL, USA). Intravenous fentanyl was delivered by activation of a syringe pump (PHM-100, Med Associates) located inside the sound-attenuating cubicle as described previously [29]. After each behavioral session, catheters were flushed with gentamicin (0.4 mg), followed by 0.1 ml of heparinized saline (10 U/ml). Catheter patency was verified at the end of each experiment by instantaneous muscle tone loss following IV methohexital (0.5 mg) administration.
Experiment 1: Fentanyl self-administration under an FR schedule
Experiment 1 examined sex differences in the potency of fentanyl to function as a reinforcer under an FR schedule. Twelve male and eleven female rats were initially trained to respond for IV fentanyl (3.2 µg/kg per injection) under an FR5/20-s time-out schedule of reinforcement during daily 2-h sessions. Each session began with a non-contingent injection of the available fentanyl dose followed by a 60-s time out. The response period was signaled by the extension of only the right lever and illumination of the right green stimulus light. Following each response requirement completion, the lever was retracted, the green light was extinguished, and IV fentanyl was administered. This schedule was in effect until the number of fentanyl injections earned per session was within 20% of the running mean for three consecutive sessions with no upward or downward trends. Subsequently, saline was substituted for fentanyl every other session (i.e., SDSDS; S, saline; D, drug) until the number of earned saline injections earned was at least 75% lower than the number of fentanyl injections earned during the preceding fentanyl session for two consecutive alternations. The same experimental program was utilized during the saline substitution sessions, using the same infusion duration as a 3.2 µg/kg per injection of fentanyl of 5 s per 300 g of rat weight. Once training criteria were met, test sessions were inserted into the sequence (i.e., DTSTD or STDTS; T, test) to evaluate responding maintained by a range of fentanyl unit doses (i.e., saline, 0.32, 1, 3.2, or 10 µg/kg per injection fentanyl). Saline and each unit dose of fentanyl was tested once in each rat using a counterbalanced dosing order.
Experiment 2: Fentanyl- and liquid food-maintained responding under a multi-day progressive-ratio schedule for behavioral economic analysis
Experiment 2 examined sex differences in fentanyl reinforcement using behavioral economic procedures. Six male and six female rats, different from those in Experiment 1, were initially trained to respond under a similar FR5/20-s time-out procedure described above in Experiment 1, except that only the left lever was extended, diluted Ensure® availability was signaled by the illumination of the left red stimulus light, and response requirement completion resulted in a 5-s presentation of the 56% diluted Ensure® filled cup (0.1 ml). Once the number of diluted Ensure® presentations was within 20% of the running mean for three consecutive sessions with no upward or downward trends, the response requirement was decreased to FR1 until the same stability criteria were again met. Subsequently, the response requirement (i.e., 1, 3, 6, 10, 18, 32, 56, 100, 180, 320, 560, 1000) increased across consecutive sessions in a multi-day progressive-ratio (PR) schedule until each rat failed to complete the response requirement during the behavioral session. Next, the aforementioned sequence was repeated with 18% diluted Ensure® as the reinforcer. Rats were then implanted with intravenous catheters and trained to self-administer 3.2 µg/kg per injection fentanyl under the same FR5/20-s time-out procedure described in Experiment 1. Subsequently, the FR was decreased to FR1 until stable and then the multi-day PR schedule was in effect until each rat failed to complete the response requirement during the behavioral session. Finally, this sequence was repeated with 10 µg/kg per injection fentanyl as the reinforcer. The concentrations of diluted Ensure® and the unit doses of fentanyl used in these experiments were selected because they each functioned as reinforcers in preliminary pilot studies (data not shown) and spanned a 0.5 log unit of magnitude for either diluted Ensure® or fentanyl.
Experiment 3: Fentanyl- and liquid food-maintained responding under a concurrent (choice) schedule
Experiment 3 examined sex differences in fentanyl reinforcement under a concurrent FR5:FR5 schedule of diluted Ensure® and fentanyl availability. Twenty-eight rats (eight male and eight female rats from Experiment 1 and six male and six female rats from Experiment 2) were trained to respond under a fentanyl vs. diluted Ensure® choice procedure modified from a cocaine vs. food choice procedure developed for rats [26] and similar drug vs. food choice procedures in nonhuman primates [30, 31]. The behavioral session consisted of five 20-min response components each preceded by a 4-min “sample” component. Each sample component started with a non-contingent injection of the unit fentanyl dose available during the subsequent response component followed by a 2-min time out. Next, a 5-s presentation of diluted Ensure® was programmed followed by a 2-min time out. Following this second time out, the response component would begin. During each response component, both levers were extended, a red stimulus light above the left lever was illuminated to signal diluted Ensure® availability, and a green stimulus light above the right lever was illuminated to signal IV fentanyl availability. Response requirement (FR5) completion on the left lever resulted in a 5-s presentation of diluted Ensure®, whereas response requirement (FR5) completion on the right lever resulted in the delivery of the IV fentanyl dose available for that component. Responding on one lever reset the ratio requirement for the other lever. The liquid food concentration was held constant across components, and rats were initially trained and tested with 18% diluted Ensure®. A different fentanyl dose was available during each of the five successive response components (0, 0.32, 1.0, 3.2, and 10 µg/kg per injection during components 1–5, respectively). Fentanyl dose was varied by changing the infusion duration (300 g rat; 0, 0.5, 1.56, 5, and 15.6 s of pump activation during components 1–5, respectively) and visually signaled by the frequency of the flashing of the right green light above the drug-associated lever in 3-s cycles (component 1: off; component 2: on for 0.1 s and off for 2.9 s; component 3: on for 0.3 s and off for 2.7 s; component 4: on for 1 s and off for 2 s; component 5: on).
During each response component, rats could complete up to ten total ratio requirements between the food- and fentanyl-associated levers. Each ratio requirement completion initiated a 20-s time out, the retraction of both levers, and extinction of the red and green stimulus lights. If all 10 ratio requirements were completed before 20-min had elapsed, then both levers retracted, and stimulus lights were extinguished for the remainder of that response component. After at least five sessions under these conditions, choice was considered stable when the smallest unit dose of fentanyl that maintained at least 80% choice (typically 3.2 or 10 µg/kg per injection) was within a 0.5 log unit of the running mean for three consecutive days with no increasing or decreasing trends. After determination of fentanyl vs. food choice dose-effect functions during concurrent availability of 18% diluted Ensure®, the concentration was increased to 56%, and fentanyl vs. food choice dose-effect functions were re-determined.
Data analysis
For Experiment 1, the primary dependent measure was the number of injections earned per session and data were plotted as a function of fentanyl dose and sex. Results were analyzed between sexes using a two-way repeated-measures analysis of variance (ANOVA) with fentanyl dose as the within-subjects factor and sex as the between-subjects factor. In addition, results were analyzed within each sex using a one-way repeated-measures ANOVA. A significant effect of dose was followed by a Dunnett’s post-hoc test relative to saline. Comparison of the number of days to meet saline extinction criteria are presented in Supplemental Figure. 1.
For Experiment 2, the primary dependent measure was the number of reinforcers earned per session. Data were plotted as a function of FR value and fit using the Exponential Model of Demand [32] using a custom-designed GraphPad Prism 5.0 template (available from the Institutes for Behavior Resources, http://www.ibrinc.org) with the following equation:
Here, Q is number of earned reinforcers, Q0 is consumption as price approaches zero, k is a scaling variable defining consumption range in log units (fixed to 2.49), α or “demand elasticity” defines the rate of decline of consumption, and C is cost (FR value). Demand elasticity (α) values of aggregate demand curves were each compared between sexes using the extra sum-of-squares F test. Next, individual α values were transformed to “essential value” such that larger values reflect a greater reinforcing effectiveness using the following equation [33]:
Individually determined Q0 and essential values were compared between sexes for each reinforcer and reinforcer magnitude using unpaired t tests. These same analyses following normalizations of the x- and y-axis can be found in the Supplemental Figures. 2, 3, 4, and 5.
For Experiment 3, the primary dependent measures for each component were (1) percent fentanyl choice, defined as [(number of ratio requirements, or “choices,” completed on the fentanyl-associated lever/total number of choices completed on both the fentanyl- and food-associated levers) × 100], and (2) reinforcement rate defined as the total number of choices completed. Data were analyzed using two-way ANOVA with fentanyl dose and sex as the main factors. In addition, data were separated by training history (i.e., rats used in Experiment 1 vs. Experiment 2) and analyzed using two-way ANOVA with fentanyl dose and training history as the main factors. Significant interactions were followed by Sidak post-hoc tests.
Drugs
Fentanyl HCl was provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD, USA) and dissolved in sterile saline. All solutions were passed through a 0.22-μm sterile filter (Millex GV, Millipore Sigma, Burlington, MA, USA) before IV administration.
Results
Figure 1 shows fentanyl self-administration under an FR5 schedule of reinforcement. Under these conditions, fentanyl functioned as a reinforcer and displayed the prototypic inverted U-shaped dose-effect function in both male (F(1.16, 12.74) = 13.92, p = 0.002) and female (F(2.12, 21.21) = 25.5, p < 0.0001) rats. In addition, the dose-effect function was significantly shifted upward in females compared to males (sex: F(1,21) = 9.3, p = 0.006; dose: F(4, 84) = 14.49, p < 0.0001; sex × dose: F(4, 84) = 3.7, p = 0.008), with female rats earning a significantly greater number of reinforcers when 0.32 and 1 µg/kg per injection fentanyl were available as the unit dose.
Fig. 1.
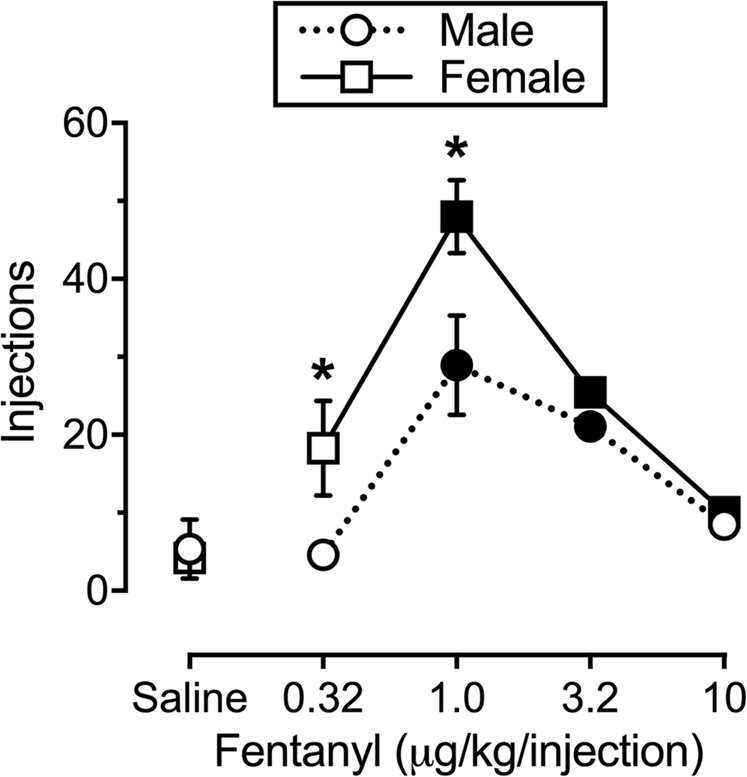
Fentanyl self-administration in male (circles; n = 12) and female (squares; n = 11) rats under a fixed-ratio 5 (FR5) schedule of reinforcement. Ordinate: number of fentanyl injections earned per 120-min behavioral session. Abscissa: unit dose of fentanyl in µg/kg per injection. All points depict mean ± SEM. Filled symbols denote significantly (p < 0.05) different from saline. * denotes a significant difference between sexes at a particular unit dose of fentanyl
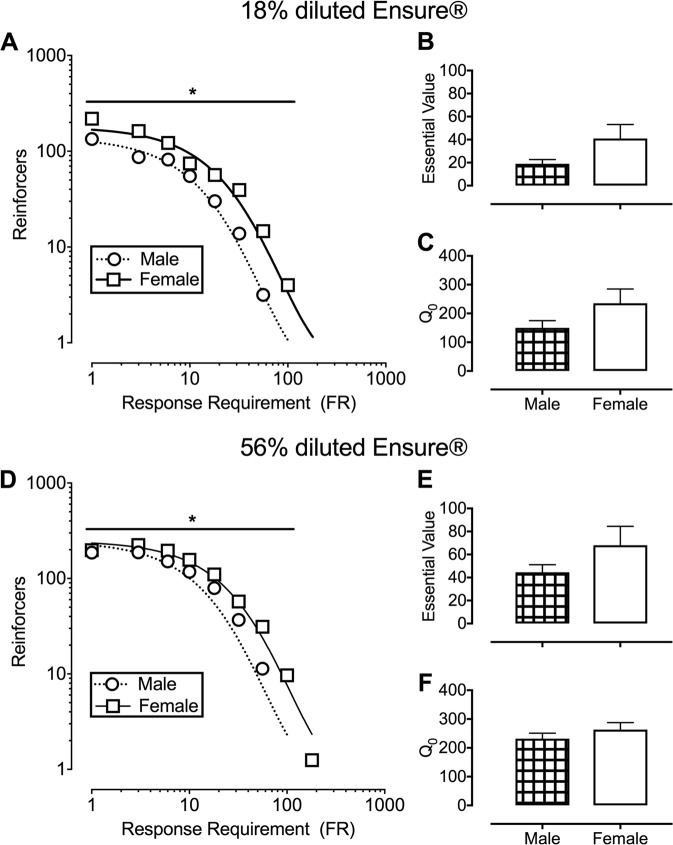
Figure 2 shows responding for 18% (top panels) or 56% (bottom panels) diluted Ensure® under the multi-day PR schedule in male and female rats. The exponential model provided a good fit for the aggregate functions of both 18% (R2 values of 0.98 for males and 0.97 for females) and 56% (R2 values of 0.91 for males and 0.98 for females) diluted Ensure®. Comparison of aggregate α values indicated that demand for 18 and 56% diluted Ensure® were each less elastic in female rats (18% diluted Ensure®: F(1,13) = 20, p = 0.0006; 56% diluted Ensure®: F(1,13) = 6.5, p = 0.024). Sex differences in individually determined essential values or Q0 values were not detected for diluted Ensure®.
Fig. 2.
Diluted Ensure®-maintained responding under a multi-day progressive-ratio schedule for 18% (top panel) or 56% (bottom panel) diluted Ensure® in male (circles; n = 6) and female (squares; n = 6) rats. a, d depict aggregate demand functions and nonlinear regression using the exponential model of demand for 18 and 56% diluted Ensure®, respectively. Ordinate: number of reinforcers earned per 120-min behavioral session. Abscissa: response requirement. Individually determined essential values for 18 and 56% diluted Ensure® are depicted in b and e, respectively. Individually determined Q0 values for 18 and 56% diluted Ensure® are depicted in c and f, respectively. *denotes significant (p < 0.05) difference between male and female rats
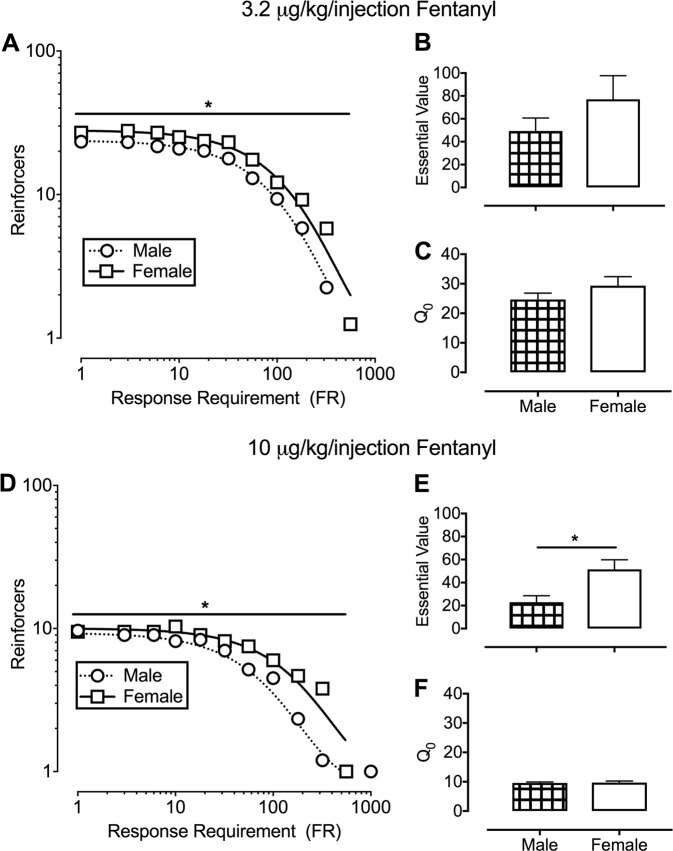
Figure 3 shows responding for 3.2 (top panels) or 10 (bottom panels) µg/kg per injection fentanyl under the multi-day PR schedule in male and female rats. The exponential model provided a good fit for the aggregate functions of both 3.2 µg/kg per injection (R2 values of 0.99 for males and 0.96 for females) and 10 µg/kg per injection (R2 values of 0.95 for males and 0.92 for females). Comparison of aggregate α values indicated that demand for fentanyl was less elastic in female rats at both doses (3.2 µg/kg per injection: F(1,17) = 15, p = 0.0012; 10 µg/kg per injection: F(1,19) = 17, p = 0.0005). Although individually determined essential values of 3.2 µg/kg per injection fentanyl did not differ between sexes, the essential value of 10 µg/kg per injection fentanyl was significantly greater in female rats (t(10) = 2.8, p = 0.018). Individually determined Q0 values were not significantly different between sexes for either unit dose of fentanyl.
Fig. 3.
Fentanyl-maintained responding under a multi-day progressive-ratio schedule for 3.2 (top panel) or 10 (bottom panel) µg/kg per injection fentanyl in male (circles; n = 6) and female (squares; n = 6) rats. a, d depict aggregate demand functions and nonlinear regression using the exponential model of demand for 3.2 or 10 µg/kg per injection fentanyl, respectively. Ordinate: number of reinforcers earned per 120-min behavioral session. Abscissa: response requirement. Individually determined essential values for 3.2 or 10 µg/kg per injection fentanyl are depicted in b and e, respectively. Individually determined Q0 values for 3.2 or 10 µg/kg per injection fentanyl are depicted in c and f, respectively. *denotes significant (p < 0.05) difference between male and female rats
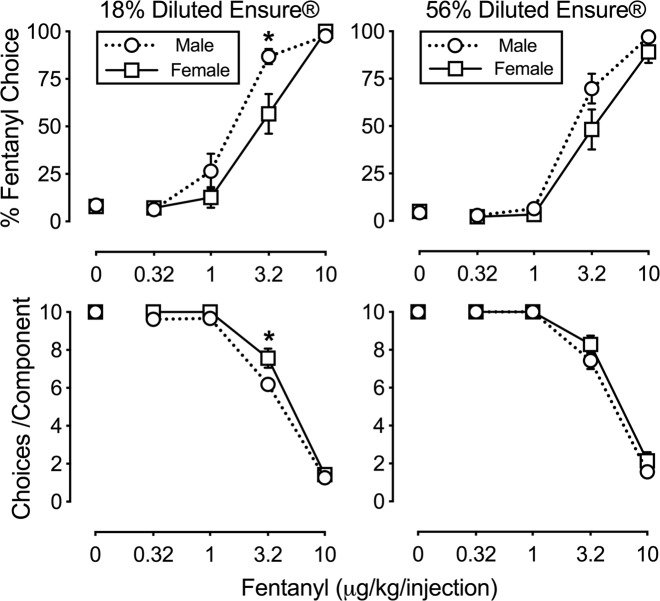
Figure 4 shows fentanyl- and diluted Ensure®-maintained behavior under a choice procedure in male and female rats. Choice data from rats used in Experiments 1 and 2 were combined due to a lack of a significant interaction between training history and unit dose of fentanyl for (1) percent fentanyl choice or (2) number of choices completed per component at either Ensure® concentration. Left panels show 18% diluted Ensure® availability and right panels show 56% diluted Ensure® availability. Both males and females primarily responded on the food-associated lever when either no fentanyl (0 µg/kg per injection) or small unit fentanyl doses (0.32–1 µg/kg per injection) were concurrently available (top panels). As the unit dose of fentanyl increased, behavior was allocated towards the fentanyl-associated lever such that 10 µg/kg per injection fentanyl maintained almost exclusive choice over either 18% (left) or 56% (right) diluted Ensure® (top panels). Under conditions of concurrent IV fentanyl and 18% diluted Ensure® availability, males chose 3.2 µg/kg per injection fentanyl over food (mean±SEM: 86.5±4.0% fentanyl choice) to a greater extent than females (56.7±10.5% fentanyl choice) (dose: F(4,104) = 169.1, p < 0.0001; sex × dose: F(4,104) = 4.6, p = 0.0118). Sex differences were not observed in percent fentanyl choice under conditions of concurrent IV fentanyl and 56% diluted Ensure® availability. Figure 4 bottom panels show the number of ratio requirements (i.e., choices) completed per component. Rats completed approximately ten choices when 0, 0.32, or 1 µg/kg per injection fentanyl was available, and the number of choices decreased during 3.2 or 10 µg/kg per injection fentanyl availability, irrespective of the alternative reinforcer concentration (18% diluted Ensure®: F(4,104 = 579, p < 0.001; 56% diluted Ensure®: F(4,104) = 536, p < 0.001). Females completed significantly more choices per component than males during the component where 3.2 µg/kg per injection fentanyl and 18% diluted Ensure® were concurrently available (sex × dose: F(4,104) = 3.1, p = 0.019).
Fig. 4.
Fentanyl self-administration under a fentanyl vs. food choice procedure in male (circles; n = 14) and female (squares; n = 14) rats. Left panels show results for intravenous fentanyl and 18% diluted Ensure®. Right panels show results for intravenous fentanyl and 56% diluted Ensure®. Abscissa: unit dose of fentanyl in µg/kg per injection. Top ordinate: percentage of completed ratio requirements on the fentanyl-associated lever. Bottom ordinate: number of choices completed per component. All points represent mean ± SEM obtained during three consecutive sessions. * denotes significant (p < 0.05) difference in the percent fentanyl choice at a given unit dose between sexes
Discussion
In the present study, sex differences in opioid reinforcement were detected at all three levels of behavioral analysis (i.e., FR, multi-day PR, and concurrent schedules of reinforcement). However, the direction of the sex difference depended upon the schedule of reinforcement. Specifically, female rats self-administered more fentanyl than males under the FR5 schedule and fentanyl was determined to be a more effective reinforcer in female rats when examined under the multi-day PR and analyzed using a behavioral economic framework. Similar sex differences were also observed with diluted Ensure®-maintained responding suggesting the sex difference was not specific to opioid reinforcement processes. However, when both fentanyl and diluted Ensure® were made concurrently available, choice of 3.2 μg/kg per injection fentanyl over 18% diluted Ensure® was greater in males compared to females, although no differences in fentanyl vs. food choice were observed when 56% diluted Ensure® was the alternative reinforcer. Overall, the present results demonstrate the utility of an opioid vs. food choice procedure in rats to dissociate processes that are reinforcement-dependent (i.e., allocation of behavior) and those that integrate reinforcement-independent factors (i.e., rate of behavior) and the influence of biological variables, such as sex.
Fentanyl and Ensure® responding under rate-based schedules
Under both FR and PR schedules of reinforcement, fentanyl functioned as a more effective reinforcer in females compared to males; results consistent with previous MOR agonist self-administration studies examining sex differences in MOR agonist reinforcement [11–15]. The present study extends these previous findings to the MOR agonist fentanyl and to the examination of sex differences in opioid reinforcement using a behavioral economic framework. Comparison of α values derived from aggregate demand functions found demand for both fentanyl doses to be less elastic in female than in male rats, suggesting that fentanyl was a more effective reinforcer in females. However, demand for both concentrations of diluted Ensure® were also found to be similarly less elastic in females compared to males under the same experimental conditions. Notably, although sex differences in aggregate α demand functions were detected for both fentanyl doses and both diluted Ensure® concentrations, essential values determined from individual functions were only significantly greater in female compared to male rats for 10 μg/kg per injection fentanyl. We interpret the totality of these results as a modest sex difference in rates of behavior for both fentanyl and diluted Ensure® under the multi-day PR procedure. Nevertheless, one implication of the present results is that sex differences in drug self-administration under rate-based schedules of reinforcement (i.e., FR or PR) may reflect sex differences in general operant behavior rather than selective sex differences in sensitivity to opioid reinforcement.
Fentanyl and Ensure® responding under a concurrent schedule
One method to minimize the influence of reinforcement-independent processes in drug self-administration procedures would be to measure behavioral allocation between two concurrently available reinforcers in addition to rates of behavior. The present study utilized an IV fentanyl vs. food choice procedure to examine potential sex differences in opioid reinforcement, based on IV drug vs. food choice procedures that we have previously validated in nonhuman primates and rats [26, 30, 31, 34]. Fentanyl maintained a dose-dependent increases in choice over diluted Ensure® in both female and male rats. This finding is consistent with previous rodent studies using similar procedures with cocaine as the drug reinforcer [26], nonhuman primate MOR agonist vs. food choice studies [31, 35–37], and human laboratory MOR agonist vs. money choice studies [38, 39]. However, the present results appear to be largely inconsistent with previous rat MOR agonist vs. food choice studies [40–44], but see [45]. Reconciliation of these perceived inconsistencies can be accomplished by comparing two experimental parameters. First, the magnitude of the drug and nondrug reinforcer are established independent variables known to alter behavioral allocation as demonstrated in the present study and these previous studies (for a review, see [17]). Second, manipulating the intertrial interval between reinforcer deliveries may also shift behavioral allocation between drug and nondrug reinforcers depending upon how the economic constraints are programmed [43, 46, 47]. Overall, the present results and the previously published literature are consistent in highlighting that drug dose, magnitude of the alternative reinforcer, and intertrial interval are important independent experimental parameters that influence behavioral allocation between drug and nondrug reinforcers.
Implications
Although sex differences in drug vs. food choice have been reported for other classes of abused drugs such as the monoamine transporter ligand cocaine [48, 49], to the best of our knowledge, sex differences in MOR agonist vs. food choice have not been previously reported. Expression of sex differences in fentanyl vs. food choice was only observed at a single fentanyl dose (3.2 µg/kg per injection) as the alternative to 18% diluted Ensure®, such that males chose fentanyl over diluted Ensure® to a greater extent than females. These choice results are not in agreement with data collected under FR or PR schedules of opioid reinforcement (including our own) wherein female rats self-administer MOR agonists to a greater extent than males. Thus, assessing behavioral allocation between IV fentanyl and diluted Ensure® “unmasked” a sex difference in the opposite direction of what would have been predicted when IV fentanyl or diluted Ensure® were examined in absence of a concurrently available reinforcer. Although the expression of sex differences in fentanyl vs. food choice was under a limited range of experimental conditions, the direction of the sex difference reported in the present study was consistent with the direction reported in the clinical literature [3, 4]. Taken together, our results provide further evidence for the usefulness of IV opioid vs. food choice procedures to interrogate both the biological and pharmacological mechanisms of opioid abuse and opioid use disorder.
Funding and disclosure
The research was supported by institutional professional development funds and the National Institute on Drug Abuse of the National Institutes of Health under Award Numbers UH3DA041146, F32DA047026, and T32DA007027. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Townsend’s, Negus’, and Banks’ research has been funded by the NIH. The authors declare no competing interests.
Supplementary information
Acknowledgements
We acknowledge the expert technical assistance of Kaycee Faunce. We also acknowledge Kevin Costa for writing the original version of the behavioral program used in the choice procedure.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-019-0356-1).
References
- 1.Rudd R, Seth P, D’avid F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States 2010–2015. Morb Mortal Wkly Rep. 2016;65:1445–52. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 2.Blue Cross and Blue Shield. America’s opioid epidemic and its effect on the nation’s commercially-insured population; 2017. https://www.bcbs.com/the-health-of-america/reports/americas-opioid-epidemic-and-its-effect-on-the-nations-commercially-insured.
- 3.Jones CM. The paradox of decreasing nonmedical opioid analgesic use and increasing abuse or dependence—an assessment of demographic and substance use trends, United States, 2003–2014. Addict Behav. 2017;65:229–35. doi: 10.1016/j.addbeh.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Marsh JC, Park K, Lin YA, Bersamira C. Gender differences in trends for heroin use and nonmedical prescription opioid use, 2007–2014. J Subst Abus Treat. 2018;87:79–85. doi: 10.1016/j.jsat.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zacny JP. Morphine responses in humans: a retrospective analysis of sex differences. Drug Alcohol Depend. 2001;63:23–28. doi: 10.1016/S0376-8716(00)00186-1. [DOI] [PubMed] [Google Scholar]
- 6.Zun LS, Downey LVA, Gossman W, Rosenbaum‡ J, Sussman§ G. Gender differences in narcotic-induced emesis in the ED. Am J Emerg Med. 2002;20:151–54. doi: 10.1053/ajem.2002.32631. [DOI] [PubMed] [Google Scholar]
- 7.Cepeda MS, Farrar JT, Baumgarten M, Boston R, Carr DB, Strom BL. Side effects of opioids during short-term administration: effect of age, gender, and race. Clin Pharmacol Ther. 2003;74:102–12. doi: 10.1016/S0009-9236(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 8.Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, et al. Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6:116–24. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Bijur PE, Esses D, Birnbaum A, Chang AK, Schechter C, Gallagher EJ. Response to morphine in male and female patients: analgesia and adverse events. Clin J Pain. 2008;24:192–8. doi: 10.1097/AJP.0b013e31815d3619. [DOI] [PubMed] [Google Scholar]
- 10.Comer SD, Cooper ZD, Kowalczyk WJ, Sullivan MA, Evans SM, Bisaga AM, et al. Evaluation of potential sex differences in the subjective and analgesic effects of morphine in normal, healthy volunteers. Psychopharmacology (Berl) 2009;208:45. doi: 10.1007/s00213-009-1703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein LC, Popke EJ, Grunberg NE. Sex differences in effects of predictable and unpredictable footshock on fentanyl self-administration in rats. Exp Clin Psychopharmacol. 1997;5:99–106. doi: 10.1037/1064-1297.5.2.99. [DOI] [PubMed] [Google Scholar]
- 12.Carroll ME, Campbell UC, Heideman P. Ketoconazole suppresses food restriction-induced increases in heroin self-administration in rats: sex differences. Exp Clin Psychopharmacol. 2001;9:307–16. doi: 10.1037/1064-1297.9.3.307. [DOI] [PubMed] [Google Scholar]
- 13.Cicero TJ, Aylward SC, Meyer ER. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav. 2003;74:541–49. doi: 10.1016/S0091-3057(02)01039-0. [DOI] [PubMed] [Google Scholar]
- 14.Lacy RT, Strickland JC, Feinstein MA, Robinson AM, Smith MA. The effects of sex, estrous cycle, and social contact on cocaine and heroin self-administration in rats. Psychopharmacology (Berl) 2016;233:3201–10. doi: 10.1007/s00213-016-4368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mavrikaki M, Pravetoni M, Page S, Potter D, Chartoff E. Oxycodone self-administration in male and female rats. Psychopharmacology (Berl) 2017;234:977–87. doi: 10.1007/s00213-017-4536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart J, Woodside B, Shaham Y. Ovarian hormones do not affect the initiation and maintenance of intravenous self-administration of heroin in the female rat. Psychobiology. 1996;24:154–59. [Google Scholar]
- 17.Banks ML, Negus SS. Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv Pharmacol Sci. 2012;2012:281768. doi: 10.1155/2012/281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craft RM. Sex differences in analgesic, reinforcing, discriminative, and motoric effects of opioids. Exp Clin Psychopharmacol. 2008;16:376–85. doi: 10.1037/a0012931. [DOI] [PubMed] [Google Scholar]
- 19.Heyman GH. Addiction: a disorder of choice. Cambridge: Harvard University Press; 2009. [Google Scholar]
- 20.Ahmed SH, Lenoir M, Guillem K. Neurobiology of addiction versus drug use driven by lack of choice. Curr Opin Neurobiol. 2013;23:581–87. doi: 10.1016/j.conb.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Freese L, Durand A, Guillem K, Ahmed SH. Pre-trial cocaine biases choice toward cocaine through suppression of the nondrug option. Pharmacol Biochem Behav. 2018;173:65–73. doi: 10.1016/j.pbb.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Banks ML, Negus SS. Insights from preclinical choice models on treating drug addiction. Trends Pharmacol Sci. 2017;38:181–94. doi: 10.1016/j.tips.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolverton WL, Johanson CE. Preference in rhesus monkeys given a choice between cocaine and d,l-cathinone. J Exp Anal Behav. 1984;41:35–43. doi: 10.1901/jeab.1984.41-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez TH, Meisch RA. Relation between choice of ethanol concentration and response rates under progressive- and fixed-ratio schedules: studies with rhesus monkeys. Psychopharmacology (Berl) 2003;170:1–8. doi: 10.1007/s00213-003-1489-8. [DOI] [PubMed] [Google Scholar]
- 25.Banks ML, Gould RW, Czoty PW, Nader MA. Relationship between response rates and measures of reinforcing strength using a choice procedure in monkeys. Behav Pharmacol. 2008;19:365–69. doi: 10.1097/FBP.0b013e32830990bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomsen M, Barrett AC, Negus SS, Caine SB. Cocaine versus food choice procedure in rats: environmental manipulations and effects of amphetamine. J Exp Anal Behav. 2013;99:211–33. doi: 10.1002/jeab.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huskinson SL, Naylor JE, Townsend EA, Rowlett JK, Blough BE, Freeman KB. Self-administration and behavioral economics of second-generation synthetic cathinones in male rats. Psychopharmacology (Berl) 2017;234:589–98. doi: 10.1007/s00213-016-4492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker JB, Koob GF. Sex differences in animal models: focus on addiction. Pharmacol Rev. 2016;68:242–63. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Townsend EA, Naylor JE, Negus SS, Edwards SR, Qureshi HN, McLendon HW, et al. Effects of nalfurafine on the reinforcing, thermal antinociceptive, and respiratory-depressant effects of oxycodone: modeling an abuse-deterrent opioid analgesic in rats. Psychopharmacology (Berl) 2017;234:2597–605. doi: 10.1007/s00213-017-4652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–31. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- 31.Negus SS. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther. 2006;317:711–23. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- 32.Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–98. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- 33.Hursh SR. Behavioral economics and the analysis of consumption and choice. The Wiley Blackwell handbook of operant and classical conditioning. Wiley-Blackwell; Chichester, West Sussex, UK 2014. p. 275–305. 10.1002/9781118468135.
- 34.Banks ML, Blough BE. Effects of environmental maniuplations and bupropion and risperidone treatments on choice between methamphetamine and food in rhesus monkeys. Neuropsychoharmacology. 2015;40:2198–206. doi: 10.1038/npp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spragg SDS. Morphine addiction in chimpanzees. comparative psychology monographs. Baltimore, MD: The John Hopkins Press; 1940. p. 1–132.
- 36.Griffiths RR, Wurster RM, Brady JV. Discrete-trial choice procedure: effects of naloxone and methadone on choice between food and heroin. Pharmacol Rev. 1975;27:357–65. [PubMed] [Google Scholar]
- 37.Maguire DR, Gerak LR, France CP. Delay discounting of food and remifentanil in rhesus monkeys. Psychopharmacology (Berl) 2013;229:323–30. doi: 10.1007/s00213-013-3121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heishman SJ, Schuh KJ, Schuster CR, Henningfield JE, Goldberg SR. Reinforcing and subjective effects of morphine in human opioid abusers: effect of dose and alternative reinforcer. Psychopharmacology (Berl) 2000;148:272–80. doi: 10.1007/s002130050051. [DOI] [PubMed] [Google Scholar]
- 39.Comer SD, Metz VE, Cooper ZD, Kowalczyk WJ, Jones JD, Sullivan MA, et al. Comparison of a drug versus money and drug versus drug self-administration choice procedure with oxycodone and morphine in opioid addicts. Behav Pharmacol. 2013;24:504–16. doi: 10.1097/FBP.0b013e328363d1c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology. 2013;38:1209–20. doi: 10.1038/npp.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madsen HB, Ahmed SH. Drug versus sweet reward: greater attraction to and preference for sweet versus drug cues. Addict Biol. 2015;20:433–44. doi: 10.1111/adb.12134. [DOI] [PubMed] [Google Scholar]
- 42.Tunstall BJ, Riley AL, Kearns DN. Drug specificity in drug versus food choice in male rats. Exp Clin Psychopharmacol. 2014;22:364–72. doi: 10.1037/a0037019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vandaele Y, Cantin L, Serre F, Vouillac-Mendoza C, Ahmed SH. Choosing under the influence: a drug-specific mechanism by which the setting controls drug choices in rats. Neuropsychopharmacology. 2016;41:646–57. doi: 10.1038/npp.2015.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz LP, Kim JS, Silberberg A, Kearns DN. Heroin and saccharin demand and preference in rats. Drug Alcohol Depend. 2017;178:87–93. doi: 10.1016/j.drugalcdep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panlilio LV, Secci ME, Schindler CW, Bradberry CW. Choice between delayed food and immediate opioids in rats: treatment effects and individual differences. Psychopharmacology (Berl) 2017;234:3361–73. doi: 10.1007/s00213-017-4726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elsmore TF, Fletcher GV, Conrad DG, Sodetz FJ. Reduction of heroin intake in baboons by an economic constraint. Pharmacol Biochem Behav. 1980;13:729–31. doi: 10.1016/0091-3057(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 47.Johnson AR, Banks ML, Blough BE, Lile JA, Nicholson KL, Negus SS. Development of a translational model to screen medications for cocaine use disorder I: choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend. 2016;165:103–10. doi: 10.1016/j.drugalcdep.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE. Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology. 2012;37:2605–14. doi: 10.1038/npp.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perry AN, Westenbroek C, Jagannathan L, Becker JB. The roles of dopamine and [alpha]1-adrenergic receptors in cocaine preferences in female and male rats. Neuropsychopharmacology. 2015;40:2696–704. doi: 10.1038/npp.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.