Abstract
The rapidly increasing prevalence of carbapenem-resistant Enterobacteriaceae (CRE) over the past decade has increased concern in healthcare facilities and the impact on public health. The prevalence of blaKPC (KPC) in Thailand remains very low; only blaKPC-13 has been described previously. This study is the first to describe the characteristics of blaKPC-2-carrying Klebsiella pneumoniae, Escherichia coli, and Enterobacter asburiae in Thailand. The prevalence rate of blaKPC-2-carrying isolates was 0.13% among CRE isolates in our study. Based on carbapenem susceptibility testing, K. pneumoniae C1985 was resistant to meropenem and ertapenem, E. coli C1992 was resistant to meropenem, imipenem, and ertapenem, and E. asburiae C2135 was only resistant to imipenem. K. pneumoniae C1985 carried blaKPC-2, blaSHV-11, fosA, oqxA, and oqxB, while E. coli C1992 contained blaKPC-2 and mdf(A) and E. asburiae C2135 harbored blaKPC-2, blaACT-2, and qnrE1. The genetic features of blaKPC-2 in the 3 isolates revealed identical rearrangement and flanking regions. Analysis of genomic sequences from these 3 isolates revealed that the sequence types of K. pneumoniae C1985, E. coli C1992, and E. asburiae C2135 were ST4008, ST7297, and ST1249, respectively. The 3 blaKPC-2 isolates were from individual living cases. Two cases were colonization for K. pneumoniae C1985 and E. asburiae C2135 and the third case was hospital-acquired infection of E. coli C1992. Although the prevalence of blaKPC-2-carrying CRE is relatively low in this study, continued surveillance and close monitoring are warranted. In addition, prompt or early detection of CRE and strict implementation of infection control are essential to prevent outbreaks or rapid spread in hospitals.
Subject terms: Microbiology, Clinical microbiology
Introduction
The rapidly increasing prevalence of carbapenem-resistant Enterobacteriaceae (CRE) over the past decade has increased concern in healthcare facilities and public health communities worldwide1,2. CRE such as Escherichia coli, Klebsiella pneumoniae, Enterobacter spp., and Citrobacter spp. produce a variety of carbapenemases. There are three main groups of enzymes responsible for most carbapenem resistance: KPC (Klebsiella pneumoniae carbapenemase) (Ambler class A), Metallo-ß-Lactamases (Ambler class B) such as NDM and IMP, and OXA-48-like (Ambler class D)2. These enzymes are encoded by blaKPC, blaNDM, blaIMP, and blaOXA48-like, respectively, and are frequently found worldwide2. In Thailand, the prevalence of CRE during 2000–2018 increased from 1% to 10.2% for imipenem and from 1.2% to 10.2% for meropenem in K. pneumoniae, while the resistance rate in E. coli increased from 1% to 2.4% for imipenem and from 0.6% to 2.6% for meropenem, respectively (http://narst.dmsc.moph.go.th/). Carbapenemase genes have been detected in Enterobacteriaceae in Thailand including blaNDM-1, blaIMP-14a, blaKPC-13, blaOXA-48, blaOXA-181, and blaOXA-2323–6.
KPC was first reported in K. pneumoniae from the USA in 1996 and is endemic in the USA, Colombia, Argentina, Israel, Greece, Italy, and China2. In Thailand, KPC-13 (blaKPC-13) was first described in Bangkok in 2014; however, other KPC types have not been detected4. The current study is the first to describe the characteristics of KPC-2-carrying K. pneumoniae, E. coli, and Enterobacter asburiae from rural areas in Thailand.
Results
Clinical information of blaKPC-2-carrying patients
The 3 blaKPC-2 isolates were from individual living cases. K. pneumoniae C1985, E. coli C1992, and E. asburiae C2135 were isolated from urine of a 67-year-old female, from pus in the right arm of a 45-year-old male, and from sputum of a 43-year-old female, respectively. The diagnosis of the 45-year-old male was cellulitis in the right arm with septicemia; this case was classified as a hospital-acquired infection of E. coli C1992. Hospitalization of this case was at internal medicine for 14 days in September 2018. The diagnosis of the 67-year-old female was cholangiocarcinoma and this case was classified as colonization by K. pneumoniae C1985. This patient was admitted to the surgery ward during December 2018 (5 days). Finally, a breast cancer was diagnosed in the 43-year-old female and this case was classified as colonization by E. asburiae C2135 and the patient was admitted to the cancer ward for 14 days between March and April 2019. Meropenem treatment was used for the 45-year-old male (E. coli C1992), while the 67-year-old female (K. pneumoniae C1985) was treated with piperacillin-tazobactam and then this was changed to meropenem. No antibiotics were used to treat the E. asburiae C2135 case in the 43-year-old female.
Genomic characterization of blaKPC-2-carrying isolates
In September 2015, we established a surveillance network to detect carbapenemase genes in CRE isolates from hospitals in 11 provinces in Thailand consisting of: Surin, Udonthani, Sakhon Nakhon, Nakhon Phanom, Roi Et, Bueng Kan, Mukdahan, Tak, Phayao, Chumporn, and Surat Thani. The network is still operational. Of 2,245 CRE isolates, 3 isolates harboring blaKPC (0.13%) were detected using multiplex PCR. These isolates were from a hospital in Surin province, northeastern Thailand. Biochemical identification revealed 2 isolates were K. pneumoniae (strains C1985 and C2135) and the other isolate was E. coli (strain C1992). However, matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS Biotyper, Bruker, Daltonics, Germany) identification revealed isolates no. C1985, C1992, and C2135 were K. pneumoniae, E. coli, and E. asburiae, respectively. Comparison of genomic sequences using KmerFinder and the ANI calculator demonstrated that isolate C2135 was Enterobacter asburiae, while isolate C1985 was K. pneumoniae and isolate C1992 was E. coli.
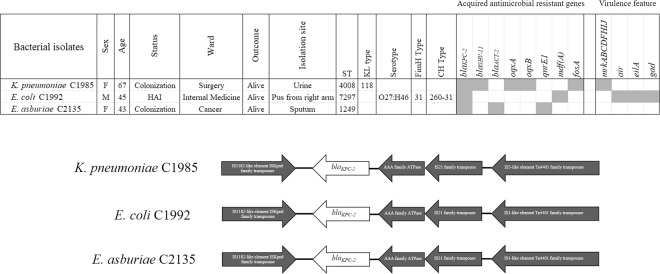
The MIC values for the 18 antimicrobial agents are shown in Table 1, K. pneumoniae C1985 was resistant to 4 agents (amoxicillin/clavulanic acid, cefotaxime, ceftriaxone, and piperacillin/tazobactam). This isolate was intermediately resistant to meropenem and ertapenem. E. coli C1992 was resistant to 8 antibiotics (amoxicillin/clavulanic acid, cefotaxime, ceftazidime, ceftriaxone, meropenem, imipenem, ertapenem, and piperacillin/tazobactam). Finally, E. asburiae C2135 was resistant to amoxicillin/clavulanic acid, cefotaxime, ceftriaxone, imipenem, and piperacillin/tazobactam. ResFinder and CARD analysis of all genomic isolates showed 5 acquired antimicrobial-resistant genes in K. pneumoniae C1985, 2 acquired antimicrobial-resistant genes in E. coli C1992, and 3 acquired antimicrobial-resistant genes in E. asburiae C2135, respectively (Fig. 1). K. pneumoniae C1985 carried blaKPC-2, blaSHV-11, fosA, oqxA, and oqxB, while E. coli C1992 contained blaKPC-2 and mdf(A). E. asburiae C2135 harbored blaKPC-2, blaACT-2, and qnrE1. The genetic features of blaKPC-2 in 3 isolates revealed identical rearrangement and flanking regions (Fig. 1). Analysis of serotype, FimH type, and CH type in E. coli C1992 revealed O27:H46, fimH31, and 260–31, respectively. The KL type (polysaccharide capsule and lipopolysaccharide O antigen) of K. pneumoniae C1985 showed KL118 using Kaptive software analysis. Virulence-associated genes analysis showed that K. pneumoniae C1985 contained only mrkABCDFHIJ, while E. coli C1992 carried air, eilA, and gad (Fig. 1).
Table 1.
Susceptibility profiles of all blaKPC-2-containing isolates.
| Antimicrobials | K. pneumoniae C1985 | E. coli C1992 | E. asburiae C2135 | |||
|---|---|---|---|---|---|---|
| MIC | Interpreted | MIC | Interpreted | MIC | Interpreted | |
| Amoxicillin/clavulanic acid | >16 | R | >16 | R | >16 | R |
| Piperacillin/tazobactam | >64 | R | >64 | R | >64 | R |
| Cefepime | 2 | S | 8 | SDD | 2 | S |
| Cefotaxime | 4 | R | 16 | R | 8 | R |
| Ceftazidime | 8 | I | 16 | R | 4 | S |
| Ceftriaxone | 16 | R | >32 | R | 32 | R |
| Ertapenem | 1 | I | 4 | R | ≤0.5 | S |
| Imipenem | 1 | S | 4 | R | 4 | R |
| Meropenem | 2 | I | 4 | R | 2 | I |
| Doripenem | 1 | S | 2 | I | ≤0.5 | S |
| Amikacin | ≤8 | S | ≤8 | S | ≤8 | S |
| Gentamicin | ≤2 | S | ≤2 | S | ≤2 | S |
| Ciprofloxacin | ≤0.06 | S | ≤0.06 | S | ≤0.06 | S |
| Levofloxacin | ≤0.06 | S | ≤0.06 | S | ≤0.06 | S |
| Netilmicin | ≤8 | S | ≤8 | S | ≤8 | S |
| Trimethoprim/sulfamethoxazole | ≤1 | S | ≤1 | S | ≤1 | S |
| Colistin | ≤1 | WT or S* | ≤1 | WT or S* | ≤1 | WT or S* |
| Tigecycline | ≤0.25 | S* | ≤0.25 | S* | 0.5 | S* |
R = resistance; I = intermediate; S = susceptible; SDD = susceptible dependent dose; WT = wild type.
*interpret following EUCAST, 2019.
Figure 1.
Characteristics of blaKPC-2-carrying Enterobacteriaceae isolates and genetic organization of blaKPC-2 in K. pneumoniae C1985, E. coli C1992, and E. asburiae C2135.
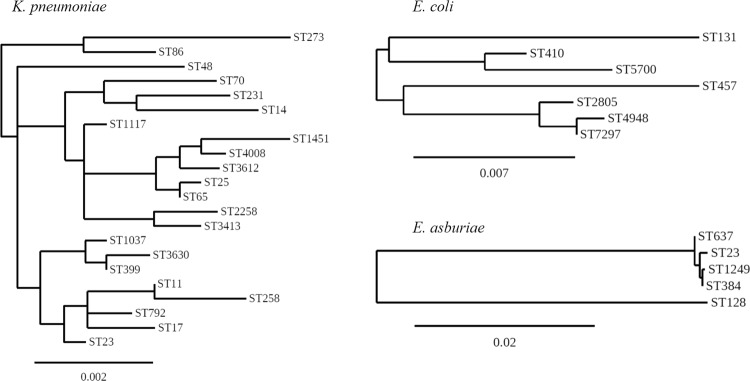
Analysis of genomic sequences from these 3 isolates revealed the sequence types (STs) of K. pneumoniae C1985, E. coli C1992, and E. asburiae C2135 were ST4008, ST7297, and ST1249, respectively. K. pneumoniae ST4008 and E. asburiae ST1249 are novel STs. Analysis based on the MLST database revealed that K. pneumoniae (C1985) ST4008 was a double-locus variant of gapA and tonB for ST399, ST3630 and ST3413, mdh and tonB for ST1037, phoE and rpoB for ST1451, phoE and tonB for ST2258, and gapA and rpoB for ST3612. The phylogenetic tree constructed using concatenated sequences of the 7 MLST genes showed that ST4008 was very close to ST1451 (Fig. 2). E. coli (C1992) ST7297 was closely related to ST4948 by a single locus variant (mdh) and this ST was a double locus variant (gyrB and icd) of ST2805 concordant with phylogeny analysis (Fig. 2). E. asburiae (C2135) ST1249 was closely related to E. cloacae ST384 with a single locus variant (dnaA) and this ST had no double-locus variant with any of the STs in the MLST database (Fig. 2). A triple locus variant of ST1249 was found in ST23 and ST637. The phylogenetic tree indicated that ST1249 was closely related to ST384 (Fig. 2).
Figure 2.
Unrooted tree based on the alignments of concatenated MLST allelic sequences in 3 species K. pneumoniae ST4008 (C1985), E. coli ST7297 (C1992), and E. asburiae ST1249 (C2135) using the neighbor-joining method. Scale bar indicates sequence dissimilarity.
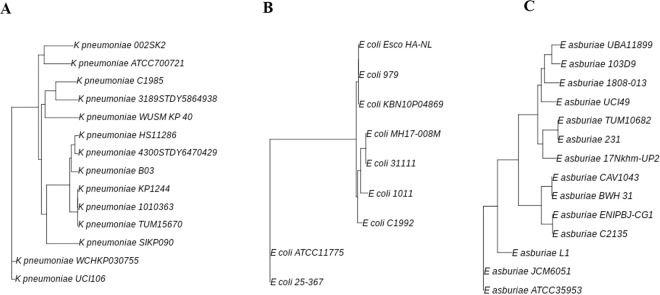
As shown in Fig. 3, based on a reference genome-based single nucleotide polymorphism (SNP) K. pneumoniae C1985 was related to K. pneumoniae strain 3189STDY5864938 (ST2258) isolated from human blood in Pakistan. E. coli C1992 was more related to E. coli strain 1011 than strains 31111 and MH17–008M. The strain 1011 was isolated from urine of patient with urinary infection in Germany, whereas strains 31111 and MH17–008M were from Vietnam, having been isolated from human feces and blood, respectively. E. asburiae C2135 isolate was related to E. asburiae strain ENIPBJ-CG1; this isolate was from a bone marrow transplant patient in China.
Figure 3.
Unrooted tree based on single nucleotide polymorphism (SNP) in 3 species K. pneumoniae ST4008 (C1985), E. coli ST7297 (C1992), and E. asburiae ST1249 (C2135) using the neighbor-joining method.
Discussion
Due to a rise in CRE globally, there are gradually fewer options for treatment. Carbapenemases is a major key mechanism for resistance to carbapenems and other β-lactam antimicrobial agents. The prevalence of CRE and the carbapenemase species such as KPC, NDM, IMP, and OXA-48 family enzymes is dependent on geographic region. KPC and its variants are mostly found in the USA, South America, and European countries7. NDM is concentrated in Asia (India, Pakistan, Bangladesh, China), and Southeast Asian countries7. The OXA-48-like enzymes are mainly found in European countries (France, Germany, the Netherlands, Italy, and the United Kingdom), in the Middle East (Turkey), and in Mediterranean countries including North Africa (mainly Morocco, Tunisia, Egypt, and Libya)7. In Thailand, blaNDM, blaOXA48-like and blaIMP-14 are frequently detected in clinical Enterobacteriaceae isolates3,6.
However, the prevalence of blaKPC (KPC) in Thailand remains very low; only blaKPC-13 has been described previously4,8, with the later study reporting rates of blaKPC-13-carrying isolates of 0.02% among Enterobacteriaceae and 1.7% among CRE isolates. This current study is the second report on KPC in Thailand and we discovered blaKPC-2 for the first time in Thai patients. The prevalence rate of blaKPC-2-carrying isolates was 0.13% among CRE isolates (n = 2,245) in our study, which was lower than in the previous report8. Other reports showed high prevalence rates of blaKPC-2-carrying Enterobacteriaceae of 14.6% in Taiwan9, 71.4% in Eastern China10, 25.3% in Australia11, and about 40–65% in Greece12. The blaKPC-2-carrying patients in the current study were separately admitted in different wards, indicating that there was no relationship or contact among them. Indeed, the genetic organization of blaKPC-2 was similar among the isolates even though they were different species. We hypothesized that these isolates may have received this gene from the same source. However, complete plasmid sequences should be done and aligned to confirm this hypothesis. In addition, further study should investigate for ability of blaKPC-2 conjugative transferable and enzyme kinetics.
The blaKPC-carrying K. pneumoniae strains can be characterized by their clonality, in that the majority of the strains circulating globally belong to clonal complex (CC) 258 as defined by MLST. The most common ST is ST258 in the USA, but other STs in CC258 such as ST11, ST340, ST437, and ST512 predominate in countries outside the USA1. In Singapore, blaKPC-2-carrying K. pneumoniae were associated with non-ST258 isolates, namely ST11, ST14, ST17, ST23, ST48, ST65, ST70, ST86, ST231, ST273, ST792, and ST111713. Vargas et al. (2019) reported 40% of K. pneumoniae ST25 (CC65) carried blaKPC-2 in Argentina. In China, blaKPC-2-carrying ST11 in K. pneumoniae isolates were the most frequent10,14. K. pneumoniae ST86 was reported to be carrying blaKPC-2 in Canada15. Our study was different from those reports, because we revealed a novel ST4008 of K. pneumoniae harboring blaKPC-2. This ST was closely related to ST1451, a strain susceptible to carbapenem and isolated from human blood in North America (https://bigsdb.pasteur.fr/cgi-bin/bigsdb/bigsdb.pl?db=pubmlst_klebsiella_seqdef&page=query). SNP phylogeny demonstrated that K. pneumoniae C1985 (ST4008) is related to strain 3189STDY5864938 from Pakistan; this is ST2258. MLST revealed that our isolate was closely related to ST1451; however, the genomic sequence of ST1451 is not available in the database yet, so comparison to these genomes is not possible at this time.
E. coli carrying blaKPC-2 has been reported in ST131, ST410, ST457, and ST5700 from Italy, Greece, China, Mexico, and Tunisia16–21, respectively, whereas our study involved detection in ST7297. This ST is closely related to ST4948 and both have the same ancestor similar with ST2805 according to the phylogenetic tree of concatenated MLST allelic sequences. SNP analysis revealed that E. coli C1992 (ST7297) was related to E. coli strain 1011 isolated from Germany; this strain is ST131. Due to there being no genomic sequence of ST4948 in the database yet, comparison between the genomes of these isolates is not possible at this time. Finally, blaKPC-2 was found in several species of Enterobacter such as E. asburiae, E. cloacae, and E. hormaechei22–24, similar to our study. SNP phylogeny revealed that E. asburiae in our study was related to the isolate from China.
In conclusion, we reported the emergence of blaKPC-2-harboring Enterobacteriaceae isolates. Although the prevalence of this type of CRE is relatively low, continued surveillance and close monitoring are warranted. In addition, prompt or early detection of CRE and strict implementation of infection control are essential to prevent outbreaks or rapid spread in hospitals.
Methods
Bacterial isolates and case information
Three isolates (K. pneumoniae C1985, E. coli C1992, and E. asburiae C2135) each carrying blaKPC-2 were used to characterize this study. Clinical information of patients with these isolates was reviewed by the attending physicians at the hospital.
Microbiological methods
Presumptive conventional biochemical tests described elsewhere were used for presumptive K. pneumoniae and Enterobacter spp., identification, including indole production, methyl-red reaction, Voges-Proskauer reaction, citrate utilization, and urea hydrolysis25. An E. coli identification kit (U & V Holding, Thailand) was used for confirmation of E. coli according to the manufacturer’s instruction. MALDI-TOF MS Biotyper (Bruker, Daltonics, Germany) was used to confirm E. coli, K. pneumoniae, and E. asburiae. Carbapenemase genes were detected using multiplex PCR26.
The minimal inhibition concentrations (MICs) for 18 antimicrobial agents (amoxicillin/clavulanic acid, amikacin, cefepime, cefotaxime, ceftazidime, ceftriaxone, ciprofloxacin, doripenem, ertapenem, meropenem, imipenem, gentamicin, levofloxacin, netilmicin, piperacillin/tazobactam, trimethroprim/sulfamethoxazole, tigecycline, and colistin) were determined among 3 isolates using broth microdilution and the antimicrobial susceptibility profile (resistant, intermediate, and susceptible) with interpretation according to the Clinical Laboratory Standards Institute27, except for tigecycline which was interpreted using European Committee on Antimicrobial Susceptibility Testing (EUCAST)28.
Whole genome sequencing and analysis
The whole genome sequencing of the three blaKPC-2 isolates was carried out on the Illumina platform with massively parallel sequencing Illumina technology at Beijing Novogene Bioinformatics Technology Co., Ltd. (Beijing, China). A-tailed, ligated to paired-end adaptors and PCR amplified with a 500 bp insert and a mate-pair library with an insert size of 5 kb were used for the library construction.
Illumina PCR adapter reads and low-quality reads from the paired-end and mate pair library were filtered by the step of quality control using the company compiling pipeline. All good quality paired reads were assembled using the SOAP de novo (http://soap.genomics.org.cn/soapdenovo.html) into a number of scaffolds. The assembly result was gap-filled and optimized by the company using software such as krskgf and gapclose.
Analysis of whole genome sequence data was performed for confirmation of the species using KmerFinder 3.1 (https://cge.cbs.dtu.dk/services/KmerFinder/) and the ANI calculator (https://www.ezbiocloud.net/tools/ani)29,30. Antimicrobial resistance genes were identified using ResFinder 3.1 (https://cge.cbs.dtu.dk/services/ResFinder/) and The Comprehensive Antibiotic Resistance Database (CARD) (https://card.mcmaster.ca/)31,32. Serotypes, CH type, FimH type, KL type, and virulence genes were analyzed using SerotypeFinder 2.0 (https://cge.cbs.dtu.dk/services/SerotypeFinder/), CH typer (https://cge.cbs.dtu.dk/services/CHTyper/), FimTyper 1.0 (https://cge.cbs.dtu.dk/services/FimTyper/), Kaptive (http://kaptive.holtlab.net/), and VirulenceFinder 2.0 (https://cge.cbs.dtu.dk/services/VirulenceFinder/), Institut Pasteur (https://bigsdb.pasteur.fr/klebsiella/klebsiella.html), respectively33–37.
Multilocus sequence typing (MLST) analysis for sequence types of E. coli, E. cloacae complex, and K. pneumoniae used MLST 2.0 (https://cge.cbs.dtu.dk/services/MLST/)38 and Institut Pasteur MLST (https://bigsdb.pasteur.fr/klebsiella/klebsiella.html), respectively. Construction of phylogenetic trees for all STs in this study was performed via Phylogeny.fr39. The genomic comparison of these isolates was conducted using a reference genome-based single nucleotide polymorphism (SNP) strategy with BacWGSTdb40 (http://bacdb.org/BacWGSTdb/).
Accession number
The genomic sequences assembled were deposited under the Bioproject accession number of PRJNA525849 with Sequence Read Archive (SRA) accession numbers of SRR8690013 (plasmid contigs) and SRR9722215 (chromosomal contigs) for K. pneumoniae C1985. The genomic sequence of E. coli C1992 was deposited under the Bioproject accession number of PRJNA525942 with SRA accession numbers of SRR8693447 (plasmid contigs) and SRR9722217 (chromosomal contigs), respectively. The genomic sequence of E. asburiae C2135 was deposited under the Bioproject accession number of PRJNA555780 with the SRA accession number of SRR9729532.
Ethical approval
This study was reviewed and approved by the Surin Hospital Ethics Review Board (ERB). The medical records of these 3 cases were reviewed by attending physicians at the hospital using the clinical case record form approved by the ERB. The ERB waived requirement for informed consent as the study satisfied the conditions of the policy statement on ethical conduct for research involving humans. This study was conducted according to the principles expressed in the Declaration of Helsinki.
Acknowledgements
We would like to thank the Kasetsart University Research and Development Institute (KURDI), Bangkok, Thailand for a grant and for English-editing assistance. We thank the team of curators of the Institut Pasteur MLST for curating the whole genome MLST databases and making them publicly available at http://bigsdb.pasteur.fr/.
Author contributions
A.K., D.T., Y.A., K.T. and S.H. conceived and designed this study. S.D. and S.C. collected the clinical data. A.K., S.D., R.H. and P.C. conducted bacterial identification, PCR detection of carbapenemase genes and antimicrobial susceptibility testing. A.K. analyzed the sequence data. A.K. wrote the manuscript. All authors have reviewed and approve of the final submitted version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Iovleva A, Doi Y. Carbapenem-Resistant Enterobacteriaceae. Clin. Lab. Med. 2017;37:303–315. doi: 10.1016/j.cll.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suay-García B, Pérez-Gracia MT. Present and future of carbapenem-resistant Enterobacteriaceae (CRE) infections. Antibiotics (Basel). 2019;19:8. doi: 10.3390/antibiotics8030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rimrang B, et al. Emergence of NDM-1- and IMP-14a-producing Enterobacteriaceae in Thailand. J Antimicrob Chemother. 2012;67:2626–2630. doi: 10.1093/jac/dks267. [DOI] [PubMed] [Google Scholar]
- 4.Netikul T, Sidjabat H, Paterson D, Kiratisin P. Emergence of novel bla(KPC-13) among carbapenem-resistant Enterobacteriaceae in Thailand. Int J Antimicrob Agents. 2014;44:568–569. doi: 10.1016/j.ijantimicag.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Sakamoto N, et al. Genomic Characterization of carbapenemase-producing Klebsiella pneumoniae with chromosomally carried bla (NDM-1) Antimicrob Agents Chemother. 2018;62:e01520–18. doi: 10.1128/AAC.01520-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laolerd W, Akeda Y, Preeyanon L, Ratthawongjirakul P, Santanirand P. Carbapenemase-Producing Carbapenem-resistant Enterobacteriaceae from Bangkok, Thailand, and their detection by the Carba NP and Modified carbapenem inactivation method tests. Microb Drug Resist. 2018;24:1006–1011. doi: 10.1089/mdr.2018.0080. [DOI] [PubMed] [Google Scholar]
- 7.Cui X, Zhang H, Du H. Carbapenemases in Enterobacteriaceae: Detection and Antimicrobial Therapy. Front Microbiol. 2019;10:1823. doi: 10.3389/fmicb.2019.01823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Netikul T, Kiratisin P. Genetic Characterization of carbapenem-resistant Enterobacteriaceae and the spread of carbapenem-resistant Klebsiella pneumoniae ST340 at a University hospital in Thailand. PLoS One. 2015;10:e0139116. doi: 10.1371/journal.pone.0139116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng IL, et al. Emergence of Carbapenemase producing Klebsiella pneumoniae and spread of KPC-2 and KPC-17 in Taiwan: A nationwide study from 2011 to 2013. PLoS One. 2015;10:e0138471. doi: 10.1371/journal.pone.0138471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu M, et al. High prevalence of KPC-2-producing hypervirulent Klebsiella pneumoniae causing meningitis in Eastern China. Infect Drug Resist. 2019;12:641–653. doi: 10.2147/IDR.S191892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherry NL, et al. Genomics for molecular epidemiology and detecting transmission of carbapenemase-producing Enterobacterales in Victoria, Australia, 2012 to 2016. J Clin Microbiol. 2019;57:e00573–19. doi: 10.1128/JCM.00573-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giakkoupi P, et al. An update of the evolving epidemic of blaKPC-2-carrying Klebsiella pneumoniae in Greece (2009-10) J Antimicrob Chemother. 2011;66:1510–1513. doi: 10.1093/jac/dkr166. [DOI] [PubMed] [Google Scholar]
- 13.Octavia, S., et al. Klebsiella pneumoniae and Klebsiella quasipneumoniae define the population structure of blaKPC-2Klebsiella: a 5 year retrospective genomic study in Singapore. J Antimicrob Chemother. pii: dkz332, 10.1093/jac/dkz332 (2019). [DOI] [PubMed]
- 14.Fu P, et al. Pandemic spread of blaKPC-2 among Klebsiella pneumoniae ST11 in China is associated with horizontal transfer mediated by IncFII-like plasmids. Int J Antimicrob Agents. 2019;54:117–124. doi: 10.1016/j.ijantimicag.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Mataseje LF, Boyd DA, Mulvey MR, Longtin Y. Two hypervirulent Klebsiella pneumoniae isolates producing a blaKPC-2 carbapenemase from a Canadian patient. Antimicrob Agents Chemother. 2019;63:e00517–19. doi: 10.1128/AAC.00517-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ripabelli, G., Sammarco, M.L., Scutellà, M., Felice, V. &Tamburro, M. Carbapenem-resistant KPC- and TEM-producing Escherichia coli ST131 isolated from a hospitalized patient with urinary tract infection: first isolation in Molise region, Central Italy, July 2018. Microb Drug Resist. 10.1089/mdr.2019.0085 (2019). [DOI] [PubMed]
- 17.Simoni S, et al. Increase and diversity of carbapenemase-producing Escherichia coli isolates, Italy. Future Microbiol. 2019;14:1035–1042. doi: 10.2217/fmb-2019-0069. [DOI] [PubMed] [Google Scholar]
- 18.Mavroidi A, et al. Emergence of Escherichia coli sequence type 410 (ST410) with KPC-2 β-lactamase. Int J Antimicrob Agents. 2012;39:247–250. doi: 10.1016/j.ijantimicag.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Zhang F, et al. Molecular epidemiology of carbapenemase-producing Escherichia coli and the prevalence of ST131 subclone H30 in Shanghai, China. Eur J Clin Microbiol Infect Dis. 2015;34:1263–1269. doi: 10.1007/s10096-015-2356-3. [DOI] [PubMed] [Google Scholar]
- 20.Aquino-Andrade, A., Merida-Vieyra, J., Arias de la Garza, E., Arzate-Barbosa, P. & De Colsa Ranero, A. Carbapenemase-producing Enterobacteriaceae in Mexico: report of seven non-clonal cases in a pediatric hospital. BMC Microbiol. 18, 38, 10.1186/s12866-018-1166-z (2018). [DOI] [PMC free article] [PubMed]
- 21.Ben Tanfous F, et al. first description of KPC-2-producing Escherichia coli and ST15 OXA-48-positive Klebsiella pneumoniae in Tunisia. Microb Drug Resist. 2017;23:365–375. doi: 10.1089/mdr.2016.0090. [DOI] [PubMed] [Google Scholar]
- 22.Li X, et al. Draft genome sequence of Enterobacter cloacae HBY, a ST128 clinical strain co-producing KPC-2 and NDM-1 carbapenemases. J Glob Antimicrob Resist. 2018;12:1–2. doi: 10.1016/j.jgar.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Bennett JW, Herrera ML, Lewis JS, Wickes BW, Jorgensen JH. KPC-2-producing Enterobacter cloacae and Pseudomonas putida coinfection in a liver transplant recipient. Antimicrob Agents Chemother. 2009;53:292–294. doi: 10.1128/AAC.00931-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira PS, et al. Coproduction of NDM-1 and KPC-2 in Enterobacter hormaechei from Brazil. Microb Drug Resist. 2015;21:234–236. doi: 10.1089/mdr.2014.0171. [DOI] [PubMed] [Google Scholar]
- 25.Abbott, S. Klebsiella, Enterobacter, Citrobacter, Serratia, Plesiomonas, and other Enterobacteriaceae, p639–657. In Versalovic, J, Carroll, K. C, Funke, G, Jorgensen J. H, Landry, M. L,Warnock, D. W (ed), Manual of Clinical Microbiology. 10thed,vol 2. ASM Press,Washington,DC. (2011).
- 26.Hatrongjit R, Kerdsin A, Akeda Y, Hamada S. Detection of plasmid-mediated colistin- resistant and carbapenem-resistant genes by multiplex PCR. MethodsX. 2018;5:532–536. doi: 10.1016/j.mex.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinical Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing—29th Edition. CLSI document M100–S29. Wayne, P. A: Clinical and Laboratory Standards Institute; (2019).
- 28.EUCAST. Breakpoint table for bacteria. http://www.eucast.org/clinical_breakpoints/, (accessed 21.08.19). (2019)
- 29.Larsen, M.V., et al. Benchmarking of methods for genomic taxonomy. J Clin Microbiol. 52, 1529-1539 (2014). [DOI] [PMC free article] [PubMed]
- 30.Yoon SH, Ha SM, Lim J, Kwon S, Chun J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek. 2017;110:1281–1286. doi: 10.1007/s10482-017-0844-4. [DOI] [PubMed] [Google Scholar]
- 31.Zankari E, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia B, et al. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017;45(D1):D566–D573. doi: 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carattoli A, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joensen KG, et al. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J Clin Microbiol. 2014;52:1501–1510. doi: 10.1128/JCM.03617-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joensen KG, Tetzschner AM, Iguchi A, Aarestrup FM, Scheutz F. Rapid and easy in silico serotyping of Escherichia coli using whole genome sequencing (WGS) data. J Clin.Microbiol. 2015;53:2410–2426. doi: 10.1128/JCM.00008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camacho C, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wick RR, Heinz E, Holt KE, Wyres KL. Kaptive Web: User-friendly capsule and lipopolysaccharide serotype prediction for Klebsiella genomes. J Clin Microbiol. 2018;56:e00197–18. doi: 10.1128/JCM.00197-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsen MV, et al. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol. 2012;50:1355–1361. doi: 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dereeper A, et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36:W465–469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhi R, Ye F. BacWGSTdb, a database for genotyping and source tracking bacterial pathogens. Nucleic Acids Res. 2016;44(D1):D682–D687. doi: 10.1093/nar/gkv1004. [DOI] [PMC free article] [PubMed] [Google Scholar]