Abstract
A developing theory is that individuals with alcohol use disorder (AUD) display exaggerated reactivity to threats that are uncertain (U-threat), which facilitates excessive drinking as a means of avoidance-based coping. There is promising initial behavioral evidence supporting this theory; however, the neural bases of reactivity to U-threat in individuals with AUD has not been examined. The extent to which biomarkers of U-threat reactivity map onto drinking behaviors and coping motives for alcohol use is also unknown. The current study therefore examined group differences in behavioral and neural reactivity to U-threat in adults with and without AUD. The study also tested whether behavior and brain responses to U-threat correlate with problematic drinking and coping motivated drinking. Volunteers (n=65) with and without a history of AUD (38 AUD, 27 controls) were included and completed a well-validated threat-of-shock task to probe responses to U-threat and predictable threat (P-threat) while startle eyeblink potentiation was collected. Individuals also completed a newly-designed, analogous version of the task during functional magnetic resonance imaging (fMRI). Results indicated that individuals with AUD displayed greater startle magnitude during U-threat, but not P-threat, and greater right insula and dorsal anterior cingulate cortex (dACC) activation during both forms of threat compared with controls. Startle magnitude and insula activation during U-threat positively correlated with self-reported problem drinking and coping motives for alcohol use. Findings demonstrate that individuals with AUD display exaggerated sensitivity to U-threat at the behavioral and neural level, and that these multimethod biomarkers tap into negative reinforcement processes of alcohol abuse.
Keywords: uncertain threat, anticipatory anxiety, alcohol use disorder, coping motives
Introduction
The reduction of stress is a well-established, major motive for excessive alcohol use (Koob, 2003, 2013); although human laboratory studies have failed to consistently demonstrate that alcohol acutely dampens stress (Curtin and Lang, 2007). It is posited that alcohol is only stress-dampening for certain individuals under certain conditions. A series of studies conducted by Curtin and colleagues has shed light on the conditions impacted by alcohol by demonstrating that acute intoxication dampens aversive reactivity to uncertain, but not certain, threat/stress (U-threat; Bradford et al., 2013; Hachiya et al., 2010; Hefner et al., 2013; Moberg and Curtin, 2009). Indeed, studies show as threat uncertainty increases, so does the magnitude of alcohol’s stress-dampening effects and this association is both dose-dependent and generalizable across different forms of threat uncertainty (Bradford et al., 2013; Hefner et al., 2013). It is therefore concluded that alcohol selectively and effectively dampens aversive responding to U-threat and may target the biological, affective and/or cognitive processes engaged by threat uncertainty.
The distinction in threat type is important given that U-threat elicits a specific type of stress characterized by generalized feelings of apprehension and hypervigilance, referred to as anticipatory anxiety, whereas certain threat (or predictable threat; P-threat) elicits a phasic response to an identifiable stimulus called fear (Davis, 1998; Davis et al., 2010). U-threat and P-threat produce distinguishable aversive states and evidence confirms that they are pharmacologically distinct (Grillon et al., 2006) and mediated by overlapping, but separable, neural circuits (Alvarez et al., 2011). Reactivity to U-threat, specifically, has been proposed as a higher-order, fundamental individual difference factor underlying anxiety disorders, and several other psychopathologies (Carleton, 2016).
Based on this literature, an emerging theory is that individuals who are most sensitive to U-threat experience chronic anticipatory anxiety and are motivated to consume alcohol to dampen their distress, setting the stage for negative reinforcement processes to drive the onset and maintenance of alcohol use disorder (AUD). Findings from our laboratory corroborate this theory by demonstrating that when sober, problem drinkers exhibit exaggerated behavioral reactivity to U-threat, but not P-threat. Specifically, using a well-validated threat-of-shock-task we found that greater frequency of binge drinking and greater self-reported hazardous drinking scores were associated with greater reactivity to U-threat, but not P-threat, using startle eyeblink potentiation as an index of aversive reactivity (Gorka et al., 2016). We also demonstrated that individuals with current panic disorder (PD) and a past diagnosis of alcohol dependence exhibited greater startle eyeblink potentiation during U-threat (only) relative to healthy controls and individuals with PD-only (Gorka et al., 2013). Most recently, we found that both individuals with current and past AUD had greater startle eyeblink potentiation to U-threat, but not P-threat, compared with controls (Gorka and Shankman, 2017).
The studies reviewed above provide promising initial evidence to suggest that heightened reactivity to U-threat is an individual difference factor that facilitates excessive drinking and characterizes individuals with current and past AUD. However, there are several key questions that remain. For instance, little is known about the neural bases that underlie reactivity to U-threat, and the extent to which individuals with and without AUD differ in their neural response to U- (and P-) threat. Evidence from the anxiety disorder literature suggests that response to uncertainty may be mediated by a frontolimbic circuit referred to as the Anticipatory Anxiety Network (AAN; Grupe & Nitschke, 2013; Tovote et al., 2015), which includes affect-generating limbic regions such as the amygdala, insula, and bed nucleus of the stria terminalis (BNST), that interact with affect-modulating prefrontal regions such as the dorsolateral, ventrolateral and ventromedial prefrontal cortices, orbitofrontal cortex (OFC), and anterior cingulate cortex (ACC). Within the AAN, key nodes that have been shown to contribute to exaggerated subjective and psychophysiological response to U-threat are the anterior insula, amygdala and BNST (Avery et al., 2015; Craig, 2009; Singer et al., 2009; Somerville et al., 2010). Therefore, hyperactivity of the AAN limbic nodes may characterize individuals with AUD and contribute to excessive U-threat responding; however, very few studies have tested this hypothesis. To our knowledge, only one prior study has examined neural response to U-threat in individuals with AUD using a modified Pavlovian fear conditioning paradigm by which a conditioned-stimulus (CS) was paired with an unpredictable noxious stimulus (thermal pain). The results indicated that men with remitted AUD displayed reduced prefrontal cortex (PFC) activation during U-threat compared with controls (Yang et al., 2013). The authors concluded that AUD is associated with impaired emotion regulation which in-turn contributes to excessive aversive responding. However, given that this was one study, in a very specific sample, with a U-threat task that significantly differs from the startle/behavioral literature, much more research examining neural reactivity to U-threat in individuals with AUD is needed.
In addition to a better understanding of neural processes, it is also essential to test the core components of the U-threat theory in relation to AUD. More specifically, it is important to understand whether behavioral-brain measures of U-threat reactivity actually map onto the severity of AUD illness as well as negative reinforcement processes such as drinking to cope with negative affect. To date, two studies have shown that greater self-reported alcohol problems were related to greater startle potentiation to U-threat (Gorka et al., 2016; Moberg et al., 2017). It is important to extend this line of work to include U-threat measures beyond just startle. Moreover, it is assumed that individuals who are hypersensitive to U-threat are motivated to drink to reduce their negative affect and this motivation translates into real-world drinking behaviors but empirical data supporting these assumptions is necessary.
The study was designed to address three important questions: 1) Consistent with prior studies, do individuals with AUD display an exaggerated behavioral reactivity to U-threat measured via startle eyeblink potentiation?; 2) Do individuals with AUD display exaggerated neural reactivity to U-threat measured via functional magnetic resonance imaging (fMRI)?; 3) Do behavioral and brain responses to U-threat correlate with drinking behaviors and coping motives for drinking? Participants included adult volunteers with and without a lifetime diagnosis of moderate to severe AUD. Individuals completed our validated threat-of-shock task which probes responses to U-threat and P-threat during the collection of startle eyeblink potentiation (Gorka et al., 2013, 2016). Individuals also completed a newly-designed, analogous fMRI version of the same threat-of-shock task inside the scanner. Lastly, individuals were administered widely-used, self-report measures of drinking behaviors and motives. We anticipated that individuals with AUD would display greater startle potentiation and greater AAN limbic node reactivity during U-threat compared with individuals without AUD. We also hypothesized that startle potentiation and AAN limbic node reactivity would correlate with drinking behaviors and coping alcohol motives.
Methods
Participants
Participants were recruited via advertisements posted in the Chicago community, local psychiatric clinics, and nearby college campuses. To be included in the study individuals were required to: 1) have no personal or family history of AUD (i.e., controls) or 2) meet criteria for current or past AUD within the past two years. AUD psychopathology was assessed via the Structured Clinical Interview for DSM-5 Disorders (SCID-5; American Psychiatric Association [APA], 2015), in-person, by trained assessors, and supervised by a clinical psychologist. All participants were required to be between 21 and 30 years old and able to provide written informed consent. Exclusion criteria included any serious medical condition, psychotropic medication use, deafness, contraindication for neuroimaging, pregnancy, lifetime moderate or severe substance use disorder (other than alcohol and nicotine), and psychosis. The protocol was approved by the university Institutional Review Board and participants provided written informed consent. Individuals were instructed to abstain from drugs and alcohol at least 24-hours prior to the lab assessments which was verified via breath alcohol and urine screens. Participants were monetarily compensated for their time.
A total of 38 individuals with AUD (22 with current AUD and 16 with past AUD) and 27 controls enrolled in the study and completed the clinical interview, battery of questionnaires, and startle task. Six individuals dropped out of the study prior to their MRI scan (2 AUD, 4 controls). Therefore, the startle analyses include the full sample (n=65) whereas the fMRI analyses include a subset of the full sample (n=59).
Self-Report Measures
Participants completed the widely-used Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 1989), which was developed by the World Health Organization (WHO) to assess hazardous and harmful alcohol use. The AUDIT total score is a combined measure of alcohol consumption frequency, alcohol use problems, and dependence symptoms. It has been shown to be a sensitive measure in diverse populations and has demonstrated good internal consistency (Saunders et al., 1993a,b). Reliability within the current study was good (α=.81).
To assess drinking motives, participants completed the Modified Drinking Motives Questionnaire-Revised (M-DMQ-R; Grant et al., 2007). The M-DMQ-R includes 28-items in which participants respond using a 5-point Likert scale ranging from 1 (almost never/never) to 5 (almost always/always). Average scores for five motives subscales are computed: social, enhancement, conformity, coping-depression, and coping-anxiety. Reliability for each of the five subscales was good to excellent (α=.83 to .89).
All of the self-report measures and subscales were normally distributed except for the coping with depression motives subscale (skew=2.4; kurtosis=5.4). Given the content overlap between coping with anxiety and coping with depression we created a composite ‘coping with negative affect’ scale by averaging the two coping motive variables to normalize the distribution.
Startle Threat Task
The NPU startle task and laboratory procedures have been extensively described by our group (Gorka et al., 2013, 2016). In brief, shock electrodes were first placed on participants’ left wrist and a shock work-up procedure was completed to identify the level of shock intensity each participant described as “highly annoying but not painful” (between 1–5 mA). Participants then completed a 2-min startle habituation task to reduce early, exaggerated startle potentiation. The task itself was modeled after Grillon and colleagues NPU threat task and thus included no shock (N), predictable shock (P), and unpredictable shock (U) conditions. Text at the bottom of the computer monitor informed participants of the current condition. Each condition lasted 145-s, during which a 4-s visual countdown (CD) was presented six times. The interstimulus intervals (ISIs; i.e., time between CDs) ranged from 15 to 21-s during which only the text describing the condition was on the screen. No shocks were delivered during the N condition. A shock was delivered every time the CD reached 1 during the P condition. Shocks were delivered at random during the U condition (both during the CD and ISI). Startle probes were administered during both the CD and ISI, and there was always at least 10-s between two probes or a shock and a probe. Each condition was presented two times in a randomized order (counterbalanced). Participants received 24 total electric shocks (12 in P; 12 in U) and 60 total startle probes (20 in N; 20 in P; 20 in U).
Startle Data Collection and Processing
Startle data were acquired using BioSemi Active Two system (BioSemi, Amsterdam, The Netherlands) and stimuli were administered using Presentation (Albany, CA). Electric shocks lasted 400-ms and acoustic startle probes were 40-ms duration, 103-dB bursts of white noise with near-instantaneous rise time presented binaurally through headphones.
Startle responses were recorded from two 4-mm Ag/AgCl electrodes placed over the orbicularis oculi muscle below the left eye. The ground electrode was located at the frontal pole (FPZ) of an electroencephalography (EEG) cap that participants were wearing as part of the larger study. One startle electrode was placed 1-cm below the pupil and the other was placed 1-cm lateral of that electrode. Data were collected using a bandpass filter of DC-500-Hz at a sampling rate of 2000-Hz.
Blinks were processed (and scored) according to published guidelines (Blumenthal et al., 2005): applied a 28 Hz high-pass filer, rectified, and then smoothed using a 40 Hz low-pass filter. Peak amplitude was defined within 20–150-ms following the probe onset relative to baseline (i.e., average activity for the 50-ms preceding probe onset). Each peak was identified by software but examined by hand to ensure acceptability. Blinks were scored as non-responses if activity during the post-stimulus time frame did not produce a peak that is visually differentiated from baseline. Blinks were scored as missing if the baseline period was contaminated with noise, movement artifact, or if a spontaneous or voluntary blink began before minimal onset latency. Blink magnitude values (i.e., condition averages include values of 0 for non-responses) were used in all analyses.
fMRI Threat Task
Prior to the task, electrodes were placed on participants’ left foot and the same shock work-up was completed to identify the level of shock intensity each participant described as “highly annoying but not painful”. The task was designed to be analogous to the startle version though modifications were made for scanning compatibility. There were three, within-subjects conditions: N, P shock, and U shock. During each condition, participants viewed a numeric countdown that ranged between 3 and 8s (M=5s). Text at the bottom of the computer monitor informed participants of the current condition. During N, no shocks were delivered and the text read “No Shock.” During P, participants received a shock only when the countdown reached “1” and the text read “Shock at 1”. During U, participants received a shock at random, regardless of the number on the screen and the text read “Shock at Anytime.” Following each countdown individuals saw a fixation cross for 5 to 7s (M= 6s). N, P and U countdowns were presented in blocks of 6 and each condition/block was administered in a randomized order (counterbalanced) 6 times over the course of two runs. Participants received 10 electric shocks during P and 10 electric shocks during U, during each run. The rate of “Shock at 1” during the P condition was 60%, consistent with the NPU version used by Grillon and colleagues (Schmitz and Grillon, 2012).
fMRI Data Collection and Processing
Functional MRI was performed on a 3.0 Tesla GE MR 750 scanner (General Electric Healthcare; Waukesha, WI) using an 8-channel phased-array radio frequency head coil. A standard T2-sensitive gradient-echo echoplanar imaging (EPI) sequence was used (2s TR; 22.2ms TE; 90° flip; 64×64 matrix; 22cm FOV; 44 axial slices; 3.44 × 3.44 × 3.0 mm voxels; 336 volumes).
All fMRI data met criteria for high quality and scan stability with minimum motion correction (i.e., < 2mm displacement in any direction). Statistical Parametric Mapping software (SPM12, Wellcome Department of Imaging Neuro-Science, London, UK) was used to perform conventional preprocessing steps. Images were spatially realigned to correct for head motion, warped to standardized Montreal Neurological Institute (MNI) space using the participants’ T1 structural image, resampled to 2 mm3 voxels, and smoothed with an 8 mm3 kernel. The general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128-s high-pass filter. Condition effects for U, P, and N anticipation were separately estimated at each voxel for each subject. For each condition, the countdowns prior to the shock, or prior to trial termination in instances where there was no shock, were modeled. Importantly, number of data points (i.e., TRs/repetition times) were the same across the three conditions. Movement parameters obtained during realignment were included in the model as regressors-of-no-interest to account for motion-related effects on BOLD. Individual contrast maps for U-threat > No-threat and P-threat > No-threat were created for each subject.
Data Analysis Plan
We first confirmed that the startle and fMRI tasks elicited threat responding, as designed. For startle, we conducted a repeated measures analysis with task condition specified as a 3-level variable (N, P, or U). Only the NCD, PCD, and UCD startle condition averages were used in order to match the three conditions on visual stimuli and use variables most consistent with the design of the fMRI task (i.e., CDs were on the screen). For fMRI, we conducted a series of one-sample t-tests to detect the individual effects of UCD > NCD, PCD > NCD, UCD > PCD, and PCD > UCD.
In order to test for group differences, we conducted two group (AUD vs. no AUD) by threat condition analyses of variance (ANOVA). The startle model was a 2 (group) by 3-level (NCD, PCD, UCD) ANOVA conducted using SPSS (version 24; SPSS Inc., Chicago, IL). Statistical significance was set a p < .05. The fMRI model was a 2 (group) by 2-level (UCD > NCD, PCD > NCD) ANOVA conducted using SPM12. To determine a significance threshold, we applied an anatomically-derived (AAL atlas) partial brain mask of the AAN network including the bilateral insula, amygdala, BNST, midbrain, ACC, medial PFC, and OFC. Cluster-based significance thresholding was used to adjust for multiple comparisons within the AAN network using Monte Carlo simulations (10,000 iterations) performed with 3dClustSim, an adaptation of AlphaSim (https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html). The mixed autocorrelation function (acf) was utilized to give an accurate estimation of non-Gaussian noise structure (Cox et al. 2017). A family wise error correction at α<0.05 was achieved for voxel threshold of p<0.005 with minimum cluster size of 76 contiguous voxels. Parameter estimates from 8mm radius spheres surrounding peak activations within the AAN mask were extracted for further analysis (see below).
Lastly, we conducted Pearson’s correlations between the three startle condition averages, extracted peak fMRI activations, AUDIT total scores, and DMQ-R subscales across all subjects. Bonferroni correction for multiple comparisons was not applied in-order to comprehensively present all possible correlations and provide results for both U-threat and P-threat conditions.
Results
Descriptives and Clinical Characteristics
The two groups were matched on age, sex, ethnicity, race, and education level. The groups were also matched on rates of co-occurring depression and anxiety. As expected, the AUD group consumed more alcohol per month and reported higher AUDIT total scores than the control group (Table 1).
Table 1.
Participant Demographics and Clinical Characteristics
| AUD (n=38) | Controls (n=27) | ||
|---|---|---|---|
| Demographics | Mean (SD) or % | Mean (SD) or % | Comparison |
| Age (years) | 23.8 (3.0) | 24.3 (2.8) | F(1, 64) = 0.1, p = .68 |
| Sex (% female) | 42.1% | 44.4% | χ2 = 1.0, df = 1, p = .32 |
| Ethnicity (% Hispanic) | 23.7% | 11.1% | χ2 = 1.7, df = 1, p = .20 |
| Education Level (years) | 15.8 (1.8) | 16.1 (1.8) | F(1, 64) = 0.5, p = .50 |
| Race | |||
| White | 60.5% | 51.9% | χ2 = 0.5, df = 1, p = .49 |
| Black | 5.3% | 11.1% | p = .64 (Fisher’s exact) |
| Asian | 13.2% | 25.9% | p = .21 (Fisher’s exact) |
| American Indian or Pacific Islander | 5.3% | 0.0% | p = .59 (Fisher’s exact) |
| Biracial or Other | 15.7% | 11.1% | p = .72 (Fisher’s exact) |
| Other Current Diagnoses | |||
| Major Depressive Disorder | 5.3% | 7.4% | p = .56 (Fisher’s exact) |
| Generalized Anxiety Disorder | 10.5% | 3.7% | p = .39 (Fisher’s exact) |
| Panic Disorder | 2.6% | 0.0% | p = .59 (Fisher’s exact) |
| Social Anxiety Disorder | 0.0% | 0.0% | -- |
| Specific Phobia | 0.0% | 3.7% | p = .42 (Fisher’s exact) |
| Posttraumatic Stress Disorder | 0.0% | 3.7% | p = .42 (Fisher’s exact) |
| Eating Disorder | 2.6% | 3.7% | p = .66 (Fisher’s exact) |
| Substance Use | |||
| No. Drinks per Week in Past Month | 9.8 (7.3) | 2.8 (3.0) | F(1, 64) = 17.2, p < .00 |
| No. Binges in Past Month | 3.3 (2.7) | 0.6 (0.8) | F(1, 64) = 11.0, p < .00 |
| Daily Cigarette Smoker (Yes/No) | 0.0% | 0.0% | -- |
| No. Cigarettes Smoked in the Past Month | 1.4 (7.3) | 0.3 (1.2) | F(1, 64) = 0.7, p = .42 |
| Used Cannabis in Past Month (Yes/No) | 13.2% | 11.1% | χ2 = 0.4, df = 1, p = .51 |
| No. Times Used Cannabis in Past Month | 0.3 (0.4) | 0.2 (0.5) | F(1, 64) = 0.1, p = .72 |
| Used *Other Illicit Drugs in Past Month (Yes/No) | 5.3% | 0.0% | p = .51 (Fisher’s exact) |
| No. Times Used Other Illicit Drugs in Past Month | 0.2 (0.8) | 0.0 (0.0) | F(1, 64) = 1.2, p = .27 |
| Study Variables | |||
| AUDIT Total Score | 9.2 (4.7) | 3.3 (2.3) | F(1, 64) = 30.6, p < .00 |
| DMQ-R Social Motives | 3.8 (0.8) | 2.7 (1.2) | F(1, 64) = 15.4, p < .00 |
| DMQ-R Coping Motives | 1.8 (0.5) | 1.3 (0.3) | F(1, 64) = 17.5, p < .00 |
| DMQ-R Enhancement Motives | 2.7 (1.0) | 2.1 (1.0) | F(1, 64) = 8.1, p = .01 |
| DMQ-R Conformity Motives | 1.7 (0.9) | 1.2 (0.4) | F(1, 64) = 5.0, p = .03 |
Note. AUDIT = Alcohol Use Disorders Identification Test; DMQ-R Drinking Motives Questionnaire-Revised.
Other illicit drugs refers to any illicit drug other than cannabis (e.g., cocaine, heroin, nonmedical prescription medications).
Main Effects of Startle and fMRI Threat Tasks
For startle, across subjects, there was a significant main effect of task condition (F[2, 128] = 21.89, p<.01) such that startle magnitude was greater during PCD (F[1, 64] = 21.27, p< .01) and UCD (F[1, 64] = 26.52, p< .01) compared with NCD. Startle magnitude during UCD was also greater than startle magnitude during PCD (F[1, 64]=9.30, p <.01; i.e., U >P >N).
For fMRI, UCD > NCD significantly activated several areas of the AAN including the bilateral insula and dACC (Table 2). PCD > NCD also activated the bilateral insula but no other AAN regions. Results revealed that UCD activated the right insula to a greater extent than PCD. There were no other differences between P- and U-threat. All significant task activations within the AAN are presented in Table 2.
Table 2.
Main Effects of the Threat Task in the Anticipatory Anxiety Network
| Region |
MNI coordinates |
Volume (mm3) |
Z score | ||
|---|---|---|---|---|---|
| x | y | z | |||
| UCD > NCD | |||||
| R Insula | 36 | −16 | 12 | 37288 | 6.84 |
| L Insula | −36 | 4 | 8 | 9384 | 5.98 |
| Dorsal Anterior Cingulate Cortex | 4 | 0 | 40 | 6136 | 5.99 |
| PCD > NCD | |||||
| R Insula | 54 | 10 | 4 | 20024 | 6.35 |
| L Insula | −32 | 24 | 4 | 17728 | 6.25 |
| Midbrain | −2 | −42 | −20 | 25808 | 5.10 |
| PCD > UCD | |||||
| None | |||||
| UCD > PCD | |||||
| R Insula | 36 | −16 | 14 | 4176 | 4.81 |
Note. Reporting of all significant peak voxels within the Anticipatory Anxiety Network (AAN) mask at a voxel-wide threshold of p < .005 and a minimum cluster size of 76 voxels, corresponding to p < .05 corrected. R = Right; L = Left; MNI = Montreal Neurologic Institute.
Group Comparisons
Startle group comparisons indicated that there was a main effect of task condition, as described above. There was no main effect of Group (F[1, 63]= 3.04, p=.08) but there was a significant Group x Condition interaction (F[2, 126]= 7.79, p<.01). Follow-up analyses revealed that there was no effect of Group for NCD (F[1, 64]= 0.57, p=.45) or PCD (F[1, 64]= 2.42, p=.13). The groups did differ during UCD (F[1, 64]= 6.47, p<.05) such that the AUD group displayed greater startle magnitude than the non-AUD group.
The fMRI task main effects are presented above. There were no significant Group x Condition interactions; however, within the AAN there was a main effect of Group for the right INS (MNI cluster peak [40, 8, 2]; Z=3.37, k= 4707 mm3, p< .05) and dACC (MNI cluster peak [12, 4, 44]; Z= 3.18, k=672 mm3, p< .05). Although there were no significant Group x Condition interactions, given our strong a priori hypotheses regarding differences between U-threat and P-threat, we extracted parameter estimates from 8mm radius spheres surrounding the right INS and dACC clusters (identified above) for UCD > NCD and PCD > NCD contrasts to explore group comparisons using separate ANOVAs. Results revealed that individuals with AUD displayed greater insula reactivity compared with controls during U-threat (F[1, 59] = 5.23, p < .05). The insula difference during P-threat was only a trend (F[1, 59] = 2.53, p =.12). The same pattern was true for the dACC such that individuals with AUD displayed greater reactivity during U-threat (F[1, 59] = 4.25, p < .05), but not P-threat (F[1, 59] = 2.09, p = .15)1.
Although the two groups were matched on biological sex, we entered sex as a covariate, post-hoc, to our startle and fMRI ANOVAs to ensure the pattern of results remained the same. Findings indicated that the startle Group x Condition interaction and the fMRI main effects of Group for the right INS and dACC remained significant. There were also no main effects of sex or Group x Condition x Sex interactions (all ps > .12).
Correlations with Drinking Behaviors and Motives
Correlations between all startle, brain, and self-report measures are reported in Table 3, and significant U-threat findings are displayed in Figure 2. Startle magnitude during UCD was positively correlated with AUDIT total scores, coping with negative affect alcohol motives, and social alcohol motives. Insula reactivity during U-threat was positively correlated with AUDIT total scores and coping with negative affect alcohol motives. There were no significant correlations between dACC reactivity to U-threat and self-report measures. There were also no correlations between startle magnitude and neural activation. With regard to P-threat, greater insula reactivity during PCD was correlated with AUDIT total scores. There were no other startle, brain, and self-report correlations for P-threat.
Table 3.
Correlations between startle, brain, and self-report measures.
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. U Startle | 1.0 | |||||||||||
| 2. P Startle | .82* | 1.0 | ||||||||||
| 3. N Startle | .80* | .89* | 1.0 | |||||||||
| 4. U Insula | .20 | .08 | −.01 | 1.0 | ||||||||
| 5. P Insula | .25 | .19 | .15 | .54* | 1.0 | |||||||
| 6. U dACC | .11 | .11 | .05 | .81* | .55* | 1.0 | ||||||
| 7. P dACC | .16 | .19 | .17 | .49* | .73* | .74* | 1.0 | |||||
| 8. AUDIT Total | .30* | .22 | .15 | .28* | .29* | .18 | .19 | 1.0 | ||||
| 9. Social Motives | .28* | .21 | .10 | .07 | .22 | .04 | .13 | .70* | 1.0 | |||
| 10. Coping Motives | .30* | .24 | .09 | .31* | .18 | .20 | .15 | .61* | .36* | 1.0 | ||
| 11. Enhance Motives | .02 | −.02 | −.11 | .10 | .08 | .06 | .12 | .54* | .51* | .52* | 1.0 | |
| 12. Conformity Motives | .19 | .08 | −.01 | .25 | .19 | .24 | .12 | .55* | .45* | .51* | .49* | 1.0 |
Note.
p < .05;
U = Unpredictable; P = Predictable; N = No-shock; dACC = dorsal anterior cingulate cortex; AUDIT = Alcohol Use Disorders Identification Test. Motives were assessed via the Drinking Motives Questionnaire-Revised.
Fig. 2.
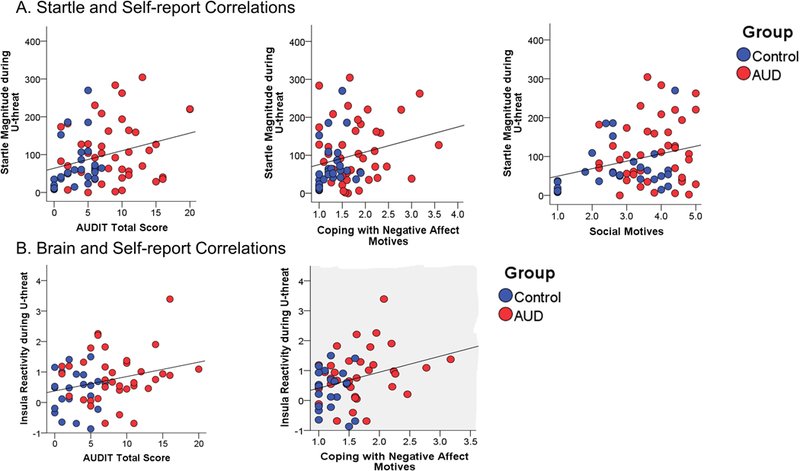
(A.) Scatter plots of the significant correlations between startle magnitude during U-threat and self-report measures. (B.) Scatter plots of the significant correlations between insula activation during U-threat and self-report measures. AUDIT = Alcohol Use Disorders Identification Test. Alcohol motives assessed via the Drinking Motives Questionnaire-Revised.
Post-hoc we examined whether any of the above significant correlations were moderated by group, which would indicate that the strength of the correlations differed between controls and individuals with AUD. Our results revealed no significant startle or brain activation by group interactions on self-report measures (all ps > .30). We also explored whether any of the significant correlations were moderated by biological sex and found no significant startle or brain activation by sex interactions on self-report measures (all ps > .14).
Discussion
The current study examined whether individuals with AUD display exaggerated reactivity to U-threat at both the behavioral and neural level, and whether behavioral and/or brain markers of U-threat sensitivity relate to drinking behaviors and alcohol motives. Our results were generally consistent with our hypotheses. We found that that individuals with AUD displayed greater startle magnitude to U-threat, but not P-threat, compared with individuals without AUD. Individuals with AUD also exhibited greater right insula and dACC reactivity during both U- and P-threat compared with individuals without AUD. Lastly, startle magnitude and insula reactivity to U-threat positively correlated with AUDIT total scores and coping motives for alcohol use. Startle magnitude to U-threat additionally correlated with social motives for alcohol use. Taken together, our findings reveal that individuals with AUD are hyper-sensitive to U-threat and that startle and brain biomarkers of this sensitivity can track the severity of problem drinking and patterns of alcohol use that are motivated by negative reinforcement processes such as coping with negative affect.
The current startle findings are consistent with prior studies examining reactivity to U-threat in relation to AUD and drinking behaviors. In four separate samples it has been shown that heavy drinkers and individuals with AUD (Gorka et al., 2013, 2016; Gorka and Shankman, 2017; Moberg et al., 2017) display increased startle to U-threat compared with healthy controls and individuals with anxiety disorders. It has also been previously demonstrated that startle magnitude to U-threat correlates with AUDIT scores (Gorka et al., 2016) and self-reported drinking problems (Moberg et al., 2017). This suggests that there is a consistent and reliable association between startle reactivity to U-threat and problematic drinking. The NPU startle paradigm may therefore be a feasible clinical tool for detecting AUD and tracking severity of illness. It could also be useful as an objective treatment target to test mechanisms for new and existing AUD interventions (Kaye et al., 2017). It is important to highlight that the NPU startle paradigm has excellent psychometric properties and is relatively cheap and easy to administer (Gorka et al., 2017, Kaye et al., 2016; Shankman et al., 2013), which is critical for its potential application to the clinic. In order to pursue this possibility, more research is needed to test whether startle magnitude values are clinically meaningful or diagnostic at the individual, patient-level.
Within the same sample we found that individuals with AUD also displayed exaggerated insula and dACC reactivity to threat. The fMRI results were less threat-type specific as there was a main effect of group rather than a threat condition (P vs. U) by group interaction. This may be related to thresholding and statistical power with fMRI as post-hoc comparisons indicated group differences during U-threat but not P-threat. Regardless, exaggerated limbic reactivity during threat within individuals with AUD is consistent with hypotheses and the broader literature. The insula and dACC are two of the primary nodes of the AAN and are heavily implicated in anxious responding. The insula is critical for interoceptive awareness and generates representations of current and future bodily states (Paulus and Stein, 2006; Craig, 2009, 2011). It also directly interacts with the dACC and other PFC nodes to form a ‘salience network’ that identifies and integrates emotionally salient information and makes inferences regarding subjective experiences, threat/reward value, and outcome probabilities (Seeley et al., 2007; Vytal and Hamann, 2010). The insula and dACC therefore underlie the subjective experience of affective states and other studies have shown that these regions are hyperactive in individuals with anxiety disorders, high trait anxiety, and high self-reported intolerance of uncertainty (Etkin and Wager, 2007; Shankman et al., 2014; Stein et al., 2007). Thus, exaggerated insula and dACC reactivity in individuals with AUD is posited to reflect increased anticipatory anxiety, consistent with the startle findings. The results of the study show for the first time exaggerated behavioral and neural reactivity and therefore provide convergence that individuals with AUD are characterized by heightened U-threat sensitivity.
The findings further indicate that both startle and brain biomarkers of U-threat sensitivity tap into drinking behaviors and motives across all participants (with and without AUD). Greater startle and insula reactivity to U-threat was associated with greater AUDIT total scores and greater coping with negative affect motives for alcohol use. This corroborates the emerging theory that individuals who are neurobiologically reactive to U-threat are motivated to dampen their distress via alcohol consumption. The negative reinforcement gained via alcohol consumption may in-turn facilitate continued alcohol use and problems. Notably, the concept of negative reinforcement (or ‘self-medication’) is not new (Baker et al., 2004; Khantzian et al., 1987); however, linking negative reinforcement processes to objective, neurobiological individual difference factors has been elusive. We argue that this is because ‘self-medication’ is more nuanced than originally theorized and that alcohol use is motivated by certain forms of distress more than others. Exaggerated anticipatory anxiety is one form of distress that has a clear association with drinking behaviors, which is further supported by the fact that acute intoxication selectively dampens startle magnitude to U-threat (but not P-threat; Bradford et al., 2013; Moberg and Curtin, 2009) and has been shown to exert its anxiolytic effects by disrupting cross-talk between the insula and dACC (Gorka et al., 2018).
Although startle magnitude and insula reactivity to U-threat were correlated with the same self-report measures, the two biomarkers themselves were not correlated. This is surprising if startle and brain are considered two indices of the same individual difference factor. At the same time it is important to consider method variance or the fact that the NPU startle and fMRI paradigms differed in certain ways that could have caused the variables to diverge. Even if startle and brain markers of U-threat reactivity are not identical it is still possible for them to both reflect increased anxious responding. In addition, the current results indicate that both markers relate to real-world drinking behaviors and motives.
The current study had several limitations. First, none of the AUD subjects were acutely intoxicated the day of the assessment, and none reported significant alcohol withdrawal symptoms; however, it is possible that at least some individuals were in a state of protracted withdrawal, which may have increased anxious responding. Second, the results are correlational and therefore causation cannot be inferred. Additional prospective studies which measure reactivity to U-threat prior to, and after, initiation of drinking and AUD are needed. Third, the two groups were matched on biological sex and all significant findings remained when sex was accounted for in our models; however, the study may not have been powered to detect nuanced sex differences (including three way interactions between threat condition, group, and sex). Biological sex should be therefore be taken into consideration in future studies.
The study provides convergent evidence to suggest that individuals with AUD are hyper-reactive to U-threat at the behavioral and neural level. Greater startle magnitude and insula reactivity to U-threat also correlated with drinking behaviors and coping motives for alcohol use, which supports new theories suggesting that heightened anticipatory anxiety contributes to negative reinforcement cycles of alcohol abuse. Exaggerated neurobiological reactivity to U-threat may be a novel phenotype for AUD prevention and/or intervention.
Supplementary Material
Fig. 1.
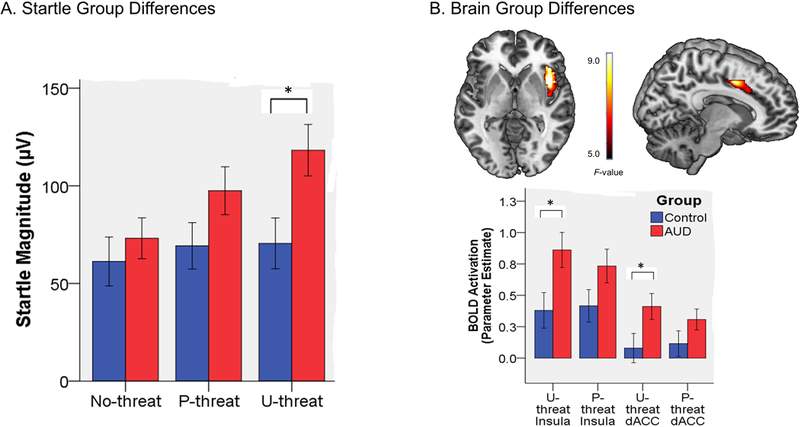
(A.) Bar graph illustrating group differences in startle magnitude during the no-threat, predictable threat (P-threat), and unpredictable threat (U-threat) task conditions. Bars reflect standard error. (B.) Top depicts voxel-wise statistical F-maps on a canonical brain displaying significant group differences within the Anticipatory Anxiety Network (AAN) during threat > no-threat at pcorrected < .05. Bottom reflects extracted parameter estimates of insula and dorsal anterior cingulate cortex (dACC) activation during both predictable (P-threat) and unpredictable (U-threat) threat. Bars reflect standard error.
Acknowledgements
Research reported in this paper was supported by the National Institute of Alcohol Abuse and Alcoholism (NIAAA) under awards P50AA022538 and K23AA025111. The study was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR002003.
Footnotes
This work was completed at the University of Illinois at Chicago, Department of Psychiatry, Chicago, IL 60612.
Individuals with current AUD and past AUD did not differ on startle potentiation, INS activation, or dACC activation to U-threat or P-threat (all ps > .11).
All authors declare no conflicts of interest.
References
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C (2011) Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage 55:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Blackford JU (2015) The Human BNST: Functional Role in Anxiety and Addiction. Neuropsychopharmacology 41:126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, De La Fuente JR, Saunders J, Grant M, 1989. AUDIT: The Alcohol Use Identification Test Guidelines for use in primary healthcare. World Health Organization Geneva, Switzerland. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC (2004) Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev 111:33–51. [DOI] [PubMed] [Google Scholar]
- Bradford DE, Shapiro BL, Curtin JJ (2013) How bad could it be? Alcohol dampens stress responses to threat of uncertain intensity. Psychol Sci 24:2541–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton RN (2016) Fear of the unknown: One fear to rule them all?. J Anxiety Disord 41:5–21. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA (2017). FMRI clustering in AFNI: false-positive rates redux. Brain Connect 7(3):152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2009) How do you feel — now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- Craig AD (2011) Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad of Sci 1225:72–82. [DOI] [PubMed] [Google Scholar]
- Curtin JJ and Lang AR (2007) Alcohol and emotion: Insights and directives from affective science In: Emotion and psychopathology: Bridging affective and clinical science. Rottenberg J, & Johnson SL (eds). Washington, DC: pp 191–213. [Google Scholar]
- Davis M (1998) Are different parts of the extended amygdala involved in fear versus anxiety?. Biol Psychiatry 44:1239–1247. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C (2010) Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35:105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A and Wager TD (2007) Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164:1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Pine DS, Lissek S, Lawley M, Ellis V, Levine J (2006) The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol Psychiatry 60:760–766. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Phan KL, Childs E (2018) Acute calming effects of alcohol are associated with disruption of the salience network. Addict Biol 23:921–930. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Phan KL, Shankman SA (2016) Association between problematic alcohol use and reactivity to uncertain threat in two independent samples. Drug Alcohol Depend 164:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Shankman SA, Phan KL (2017) Startle potentiation to uncertain threat as a psychophysiological indicator of fear-based psychopathology: An examination across multiple internalizing disorders. J Abnorm Psychol 126:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Nelson BD, Shankman SA (2013) Startle response to unpredictable threat in comorbid panic disorder and alcohol dependence. Drug Alcohol Depend 132:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM and Shankman SA (2017) Preliminary evidence that reactivity to uncertain threat is an endophenotype for alcohol use disorder. Drug Alcohol Depend 180:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant VV, Stewart SH, O’Connor RM, Blackwell E, Conrod PJ (2007) Psychometric evaluation of the five-factor Modified Drinking Motives Questionnaire—Revised in undergraduates. Addict Behav 32(11): 2611–2632. [DOI] [PubMed] [Google Scholar]
- Grupe DW and Nitschke JB (2013) Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci 14:488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya LY, Moberg CA, Curtin JJ (2010) Alcohol effects on affective response during variable and fixed duration threat. Alcohol Clin Exp Res, 34:117A. [Google Scholar]
- Hefner KR, Moberg CA, Hachiya LY, Curtin JJ (2013) Alcohol stress response dampening during imminent versus distal, uncertain threat. J Abnorm Psychol 122:756–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye JT, Bradford DE, Magruder KP, Curtin JJ (2017) Probing for neuroadaptations to unpredictable stressors in addiction: Translational methods and emerging evidence. J Stud Alcohol Drugs 78:353–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye JT, Bradford DE, Curtin JJ (2016) Psychometric properties of startle and corrugator response in NPU, affective picture viewing, and resting state tasks. Psychophysiology 53:1241–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ (1987) The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence In The cocaine crisis. Allen AF (eds). Springer: Massachusetts; pp 65–74. [DOI] [PubMed] [Google Scholar]
- Koob GF (2003) Alcoholism: allostasis and beyond. Alcohol Clin Exp Res 27:232–243. [DOI] [PubMed] [Google Scholar]
- Koob GF (2013) Negative reinforcement in drug addiction: the darkness within. Curr Opin Neurobiol 23:559–563. [DOI] [PubMed] [Google Scholar]
- Moberg CA, Bradford D, Kaye JT, Curtin JJ (2017) Increased startle potentiation to unpredictable stressors in alcohol dependence: Possible stress neuroadaptation in humans. J Abnorm Psychol 126:441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg CA and Curtin JJ (2009) Alcohol selectively reduces anxiety but not fear: startle response during unpredictable versus predictable threat. J Abnorm Psychol 118:335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP and Stein MB (2006) An insular view of anxiety. Biol Psychiatry 60:383–387. [DOI] [PubMed] [Google Scholar]
- Schmitz A and Grillon C (2012) Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test). Nature Protocols 7:527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Amundsen A, Grant M (1993a) Alcohol consumption and related problems among primary health care patients: WHO Collaborative Project on early detection of persons with harmful alcohol consumption I. Addiction 88: 349–362. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. (1993b) Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption II. Addiction 88:791–804. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Gorka SM, Nelson BD, Fitzgerald DA, Phan KL, O’daly O (2014) Anterior insula responds to temporally unpredictable aversiveness: an fMRI study. Neuroreport 25:596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K (2009) A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci 13:334–340. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, Kelley WM (2010) Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry 68:416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A (2015) Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16:317–331. [DOI] [PubMed] [Google Scholar]
- Vytal K and Hamann S (2010) Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. J Cogn Neurosci 22:2864–2885. [DOI] [PubMed] [Google Scholar]
- Yang H, Devous MD, Briggs RW, Spence JS, Xiao H, Kreyling N, Adinoff B (2013) Altered neural processing of threat in alcohol‐dependent men. Alcohol Clin Exp Res 37:2029–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


