Abstract
Combined use of nicotine and alcohol constitute a significant public health risk. An important aspect of drug use and dependence are the various cues, both external (contextual) and internal (interoceptive) that influence drug seeking/ taking behavior. The present experiments employed the use of Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) and complementary Pavlovian drug discrimination procedures (Feature Positive and Feature Negative training conditions) in order to examine whether medial prefrontal cortex (prelimbic; mPFC-PL) projections to the nucleus accumbens core (AcbC) modulate sensitivity to a nicotine+alcohol (N+A) interoceptive cue. First, we show neuronal activation in mPFC-PL and AcbC following treatment with N+A. Next, we demonstrate that chemogenetic silencing of projections from mPFC-PL to nucleus accumbens core decrease sensitivity to the N+A interoceptive cue, while enhancing sensitivity to the individual components, suggesting an important role for this specific projection. Furthermore, we demonstrate that clozapine-N-oxide (CNO), the ligand used to activate the DREADDs, had no effect in parallel mCherry Controls. These findings contribute important information regarding our understanding of the cortical-striatal circuitry that regulates sensitivity to the interoceptive effects of a compound N+A cue.
Keywords: addiction, chemogenetic, drug-discrimination, interoception, poly-drug
Introduction
Nicotine and alcohol are two of the most commonly co-abused substances and together constitute two of the greatest causes of preventable death world-wide. When used in combination, rates of heart disease and cancers of the mouth, esophagus and liver increase substantially (Castellsague et al. 1999; Franceschi et al. 1990; Olsen et al. 1985; Pelucchi et al. 2008). Considering the significant cost of nicotine and alcohol co-use, gaining a better understanding of the underlying neurobiology modulating behaviors relevant to seeking and taking nicotine and alcohol together is crucial for the development of more effective treatments.
Drug seeking behavior is influenced by salient cues, both external (contextual) and internal (interoceptive). Given that individuals who use nicotine and alcohol regularly consume both together, the interoceptive effects are experienced together. It has previously been demonstrated by our lab and others that the combined nicotine and alcohol (N+A) interoceptive effects can be trained as a cue that predicts reward and represents a more complex cue than either the nicotine or alcohol components on their own (Ford et al. 2013; Ford et al. 2012; Randall et al. 2016; Troisi et al. 2013). The present work seeks to build on these findings by exploring the role of two interconnected brain regions implicated in both associative learning (i.e., drug discrimination) and drug-seeking behavior: the medial prefrontal cortex (mPFC) and the nucleus accumbens core (AcbC). Of particular interest to the current studies is that mPFC has a high density of α4β2 nicotinic acetylcholine (ACh) receptors which have been shown to be affected by alcohol in addition to nicotine (Chatterjee and Bartlett 2010; Colombo et al. 2013; Dineley et al. 2015; Millar and Harkness 2008). Moreover, previous work from our lab has shown that mPFC and AcbC are both involved in aspects of alcohol-related behavior. For example, mPFC inactivation produces alcohol-like effects, substituting for alcohol under operant drug-discrimination conditions (Jaramillo et al. 2016). Moreover, AcbC plays an important role in modulating sensitivity to interoceptive drug cues and maintenance of alcohol self-administration (Bassareo et al. 2017; Besheer et al. 2014; Besheer et al. 2009; Bull et al. 2014).
In rodents, the mPFC can be functionally divided into dorsal and ventral sub-regions (prelimbic and infralimbic cortices). The prelimbic (PL) division has been shown to play an important role in numerous behaviors including action-selection, instrumental learning and drug seeking, aspects of associative learning, goal-directed behavior, and extinction (Miller and Marshall 2005; Peters et al. 2009; Zavala et al. 2007). mPFC-PL lesions or inactivation has been shown to produce deficits in attentional selectivity (Muir et al. 1996), and increases in perseverative responding (Chudasama and Muir 2001). In contrast, the infralimbic (IL) division of mPFC appears to play a role in behavioral inhibition and may be more involved in habitual responding than goal-directed behavior (Moorman et al. 2015). For example, inactivation of IL produces spontaneous recovery for cocaine and heroin following extinction or periods of abstinence (Ovari and Leri 2008; Peters et al. 2008). Furthermore, PL, but not the IL, sends projections to AcbC (Vertes 2004), as we have previously confirmed (Jaramillo et al. 2016), and is a region important for drug-seeking behavior. For example, PL, but not IL lesions block cocaine-induced increases in AcbC glutamate (Baker et al. 2003; Pierce et al. 1998).
In taking these differential roles of PL and IL into consideration, the current experiments are focused on mPFC-PL and its projections to AcbC and their role in modulating sensitivity to a N+A compound interoceptive drug cue and its individual components (i.e., nicotine or alcohol alone). As such, the first goal of this work was to demonstrate that these brain regions are activated by the N+A training dose selected for use in these studies. To do so, c-Fos expression in mPFC-PL and AcbC was examined following N+A treatment. Next, an important aspect of these assessments was to determine whether this circuitry is differentially recruited depending on whether the N+A compound cue is trained as a Feature Positive (FP) or Feature Negative (FN) drug cue. That is, is this circuitry differentially engaged when the N+A cue is excitatory (associated with sucrose reward; FP) or inhibitory (associated with the absence of sucrose reward; FN)? The use of these complementing procedures is important as they likely rely on different mechanisms (Bouton, 1998). FP relies on a direct stimulus-response circuit whereas FN relies on a context specific inhibitory circuit. As such, the current studies allow for a powerful assessment of the ability of N+A to both drive and inhibit reward-seeking behavior and for determination of the functional involvement of mPFC→AcbC projections in modulating these different behavioral responses. Furthermore, this work implements a chemogenetic approach by utilizing Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) in order to selectively silence mPFC-PL to AcbC projections. By taking this approach, we were able to observe the selective effects of silencing mPFC-PL projections to AcbC on sensitivity to the compound N+A interoceptive cue and the nicotine and alcohol components.
Materials and Methods
Animals
64 Adult male Long-Evans rats (Envigo-Harlan) were used for these experiments. The vivarium room was maintained on a 12-h light/dark cycle and experiments were conducted during the light portion of the cycle. Rats had ad libitum access to water in the home cage and were fed daily to maintain body weight. Animals were under continuous care and monitoring by veterinary staff from the Division of Comparative Medicine (DCM) at UNC-Chapel Hill. All animal procedures were approved by the UNC-Chapel Hill Institutional Animal Care and Use Committee (IACUC). All procedures were carried out in accordance with the NIH Guide to Care and Use of Laboratory Animals and institutional guidelines. UNC-Chapel Hill is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Drugs and Viral Constructs
Alcohol (95% w/v, Pharmaco-AAPER) was diluted in distilled water to a concentration of 20% (v/v). Nicotine tartrate (Sigma-Aldrich) was dissolved in 20% alcohol (v/v) for N+A experiments or distilled water for nicotine alone experiments and administered IG, with volumes varied by weight to obtain the desired doses (Randall et al. 2016). Nicotine doses are reported in base form. Clozapine-N-oxide hydrochloride (CNO; Research Triangle Institute, Durham, NC), was dissolved in aCSF. For DREADD experiments, AAV8-hSyn-DIO-hM4D(Gi)-mCherry or AAV8-hSyn-DIO-mCherry (control virus) + AAV8 Cre recombinase (UNC Vector Core, lot#4980D/4981C; Vector Biolabs, Lot#v4479/4481, respectively) were used. Sucrose (Great Value Brand granulated cane sugar) was dissolved in tap water to 26% (w/v).
Apparatus
Chambers (Med Associates) measuring 31 X 24 × 32 cm were located within sound attenuating cubicles and equipped with an exhaust fan that provided ventilation and masked external sounds. Two cue lights were located on one wall of the chamber adjacent to a liquid dipper receptacle equipped with a photobeam detector that was used to detect head entries into the receptacle. When activated, the dipper arm was raised for 4 sec and presented 0.1 ml of 26% sucrose (w/v). Chambers were also outfitted with infrared photobeams (that divided the chamber into 4 parallel zones) to measure locomotor activity during sessions (number of beam breaks).
Sucrose Access Training
Procedures were similar to those described in detail in (Besheer et al. 2012; Jaramillo et al. 2017; Randall et al. 2016). Briefly, rats had three 50-min sessions in which sucrose (26% w/v) was randomly presented across the session to train rats to approach the liquid receptacle. The probability of sucrose presentation decreased from the first to the last session and by the last 10 min of the final session rats received approximately 0.75 sucrose presentations/min.
Pavlovian Drug Discrimination Training Procedures:
Figure 1 depicts the Pavlovian training procedure. Training sessions were identical to those described in Randall et al. (2016). Briefly, training was conducted 5 days per week (M-F) during which a nicotine (0.4 mg/kg) + alcohol (1 g/kg) mixture (N+A) or water was administered by intragastric gavage (IG) prior to the start of the sessions. Immediately following N+A or water administration the rats were placed in the chambers. During this time no cue lights were illuminated, no sucrose was presented and head entries into the liquid receptacle were not recorded. The 15-min session began after a 10-min delay. For the Feature Positive (FP) groups, on N+A sessions, the offset of each of the 15-sec cue light presentations (10 total) was followed by sucrose presentation. On water sessions, no sucrose was delivered following the offset of the cue light presentations. For the Feature Negative (FN) groups, the reverse training occurred. That is, sucrose was presented following light presentations on water sessions, but not on N+A sessions. There were 10 cue light presentations (conditioned stimulus, CS) during each session. The onset of the first CS presentation varied from 45–75 s, and the inter-trial intervals (time from CS offset to the next CS onset) ranged from 30–105 s. Water and N+A training days varied on a double alternation schedule (W, W, N+A,N+A…). The primary dependent measure from training and later test sessions was the discrimination score. The discrimination score was calculated by subtracting the number of head entries that occurred in the 15 sec before cue light onset (i.e., pre-CS) from the head entries that occurred during the 15-sec cue light CS (Besheer et al. 2012; Randall et al. 2016). This score served as a measure of behavioral activation in response to the cue. The training sessions for both groups continued until the following acquisition criteria were met for both the first and the average discrimination score: For FP groups, the average of the discrimination score from the preceding two N+A sessions had to be ≥150% of the average of the discrimination scores from the preceding two water sessions. For FN groups, the average of the discrimination score from the preceding two water sessions had to be ≥150% of the average of the discrimination score from the preceding two N+A sessions. Testing began once these criteria were met.
Figure 1.
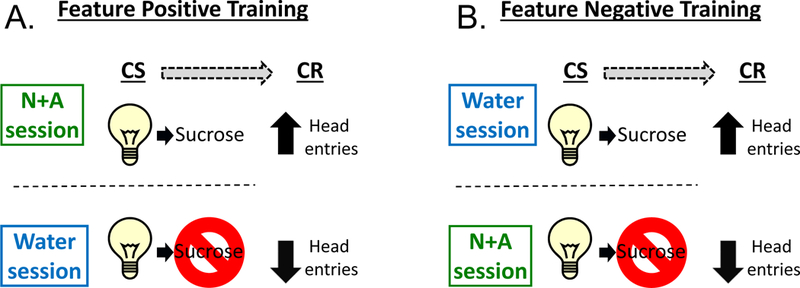
Diagram of Pavlovian discrimination training procedures. (A) Feature positive trained rats received 0.1 ml of 26% sucrose following stimulus light offset on N+A sessions. On water sessions, sucrose was not presented. (B) Feature negative trained rats received 0.1 ml of 26% sucrose following stimulus light offset on water sessions. On N+A session, sucrose was not presented.
Testing Procedures:
For experiment 3, individual test sessions were 2 min in duration (following the 10 min delay), with 1 light presentation that was followed by sucrose presentation. For these sessions, onset of light presentation was randomized and varied from 45–105 seconds into the 2-minute test period. Cumulative dosing procedures were used as we describe in (Besheer et al. 2012; Randall et al. 2016), in which 4 separate tests were conducted in succession such that testing of the stated dose curve was completed in approximately 48 min. This approach was used as it allowed for the fewest number of microinjections to limit tissue damage, while collecting a full dose response curve for N+A and nicotine and alcohol alone. The cumulative testing procedure has been shown to produce comparable results to traditional dose response curves that use discrete dosing in operant drug discrimination protocols (Hiltunen and Jarbe 1989). Test sessions were interspersed with training sessions. While uncommon, if an animal did not meet the criteria for testing (i.e., acquisition criteria above), the animal remained in the home cage on that test day. These animals continued with the testing schedule and made up any missed sessions at the end. For all behavioral experiments, testing occurred in a repeated measures (RM) design with all rats receiving all treatments in a randomized order.
Surgical Procedures (DREADD injection and AcbC cannulation)
Surgical and DREADD injection procedures were similar to those described in Jaramillo et al., 2017. Rats anesthetized with 3% isoflurane received a bilateral microinjection of AAV8-hSyn-hM4D(Gi)-mCherry or AAV8-hSyn-mCherry + AAV8 Cre recombinase (UNC Vector Core, lot#4980D/4981C; Vector Biolabs, Lot#v4479/4481) at a ratio of 7:3 into the mPFC-PL (AP +3.2, ML ±0.6, DV −3.0 from skull) at a volume of 2.0 μl/side across 10-min. The injector remained in place for an additional 10-min to allow for diffusion. 5 weeks post-infusion, anesthetized rats received bilateral implantation of 26-gauge guide cannulae (Plastics One, Roanoke, VA) aimed to terminate 2 mm above the AcbC (coordinates: AP +1.7, ML ±1.5 mm, DV −4.8 mm). Coordinates were based on (Paxinos & Watson, 2007). CNO microinjections were delivered through injectors extending 2 mm below the guide cannulae at a volume of 0.5 μl/side across 1 min. The injector(s) remained in place for an additional 1-min after the infusion to allow for diffusion. Additional microinjection procedures are described in detail in (Besheer et al. 2014; Cannady et al. 2011; Jaramillo et al. 2017). At the conclusion of Experiment 2, DREADD expression was verified (see procedures below). Additionally, brain tissue was also stained with cresyl violet to verify bilateral cannulae placement. Only data from rats with verified DREADD expression and bilateral cannulae/injector tracts determined to be in the target brain regions were used in the analyses.
DREADD Verification Procedures
Electrophysiological verification of DREADD function can be found in supplemental materials and Supplemental Figure 1. All experiments included verification of DREADD expression using IHC verification. At the end of the study, rats were anesthetized with pentobarbital (60 mg/kg, IP) and transcardially perfused with 0.1M PBS followed by 4% paraformaldehyde (4°C; pH=7.4). Brains were then extracted and stored in 4% paraformaldehyde overnight before being moved to 30% (w/v) sucrose in 0.1M PBS solution, and subsequently sliced on a freezing microtome into 40 μm coronal sections. Tissue was stored in cryoprotectant (−20°C) until ready for processing. Free-floating coronal sections (40 μm) were incubated in rabbit anti-DSRed (1:2500; Clontech, CA) for 24 h at 4 °C. Sections were then incubated at RT in fluorescent conjugated secondary antibody (goat anti-rabbit 594; Life Technologies, MA). hM4D-mCherry expression was confirmed by immunofluorescence (individual expression represented as 20% opacity) using a Nikon 80i Upright fluorescent microscope (Nikon Instruments, NY).
c-Fos immunohistochemistry and quantification procedures
90 minutes following N+A injection, rats were anesthetized with sodium pentobarbital and then transcardially perfused with ice cold 0.1M PBS followed by 4% paraformaldehyde. Brains were then extracted and stored in 4% paraformaldehyde overnight at 2oC. Brains were then moved to 30% sucrose (w/v) in 0.1M PBS and stored at 2oC. 40 micron coronal sections were taken on a freezing sliding microtome. IHC staining and quantification procedures were similar to those we have previously described (Besheer et al. 2014; Cannady et al. 2011; Jaramillo et al. 2016). Free-floating coronal sections were incubated in rabbit anti-c-Fos antibody (1:20,000; Millipore; lot #2905394) for 48 h at 4 °C with agitation. The brain regions examined were the mPFC-PL ( AP +4.2 to +3.2 mm) and AcbC and AcbSH(AP −2.3 to −1.3) according to (Paxinos and Watson 2007). Images were acquired utilizing Olympus CX41 light microscope (Olympus America) and analyzed utilizing Image-Pro Premier image analysis software (Media Cybernetics, MD). IR data (c-Fos positive pixels/mm2) were acquired from a minimum of three sections/brain region/animal, and the data were averaged to obtain a single value per subject. The quantification was conducted by an experimenter blind to experimental conditions.
Experiment 1: Verification of mPFC-PL and AcbC activation following N+A injection
In order to determine whether the N+A combination to be used as the training dose in these studies induces neuronal activation in the mPFC-PL, AcbC or nucleus accumbens shell (AcbSh) c-Fos immunoreactivity was assessed. Rats (n=12) trained on the FP condition described above (Acquisition and cumulative curve shown in Supplemental Figure 2) were injected with N+A (0.4 mg/kg nicotine + 1.0 g/kg alcohol, IG) or water and returned to the home cage (i.e., training session withheld). 90 min later rats were sacrificed and brains were processed and quantified for c-Fos immunoreactivity as previously described (Besheer et al. 2014; Cannady et al. 2011; Jaramillo et al. 2016). Given that the goal of this experiment was to determine whether the N+A training dose would induce neuronal activity in the brain circuitry of interest, this experiment was only carried out in rats trained on the FP procedure.
Experiment 2: Effects of chemogenetic silencing of mPFC-PL→AcbC projections on sensitivity to N+A and its components.
In order to determine whether silencing mPFC-PL→AcbC projections affected sensitivity to the N+A cue, two separate groups of rats were trained on the N+A Pavlovian discrimination, with one group (n=20) trained on the feature positive (FP) variant and one group (n=20) trained on the feature negative (FN) variant. Prior to training, rats were injected with hM4D (n=10 FP, 10 FN) or mCherry (n=10 FP, 10 FN). Following a week of recovery, rats underwent discrimination training. 5 weeks after the DREADD surgery, all rats underwent a second surgery to have bilateral microinjection cannulae targeting AcbC implanted. Following another week of recovery, all rats returned to training to re-establish baseline before testing began (i.e., at least 7 weeks from DREADD injection). For test sessions, both FP and FN groups received bilateral microinjections of CNO (0, 3 µM; 0.5 µL/side) into AcbC, 5 minutes prior to a cumulative N+A test session (0.1N+0.1A, 0.2N+0.3A, 0.4N+1A, 0.8N+1.7A mg/kg+g/kg, IG), nicotine alone (0.1, 0.2, 0.4, 0.8 mg/kg, IG), and alcohol alone (0.1, 0.3, 1, 1.7 g/kg, IG). All N+A testing was completed first and then component testing was completed in a counterbalanced manner.
Statistical Analyses
c-Fos quantification was analyzed using independent samples t-tests to compare expression between N+A and water pretreatments in each brain region. Given that the FP and FN groups have opposite training experience, these groups were analyzed separately. The number of head entries into the liquid receptacle was recorded in 15-s intervals throughout the training and testing sessions. The discrimination score was calculated by subtracting the number of head entries that occurred in the 15 sec before light onset (i.e., pre-CS) from the head entries that occurred during the 15-sec light CS (Besheer et al. 2012; Randall et al. 2016). The first head entry discrimination score (i.e. prior to feedback from sucrose delivery) was used as the primary dependent variable. PreCS head entries were examined for test sessions to determine whether there were changes in general head entry behavior. Locomotor rate (beam breaks/min) was analyzed for the entire session and served as a measure of non-specific motor activity. For the cumulative substitution tests, to confirm that the N+A training dose induced similar discrimination performance to that of N+A training session, a paired samples t-test was used to compare the discrimination score from the training dose (0.4N+1A) at the test to the average of the 2 N+A sessions prior to testing (i.e., baseline) (Jaramillo et al. 2017; Randall et al. 2016). All cumulative test curves were analyzed using factorial repeated measures analysis of variance (RM-ANOVA) with DREADD group as a between factor and CNO dose and cumulative testing dose as within factors. Where interactions exist, individual RM-ANOVA and post-hoc analysis (Tukey) were used to assess differences within DREADD conditions and CNO dose on each cumulative curve. In addition, substitution for the training condition was determined by comparing the training dose in the cumulative curve (0.4N+1.0A, 0.4N alone or 1.0A alone) following vehicle treatment to the average of the two previous N+A training sessions using a paired sampled t-test. Significance was set at p<0.05.
Results
Experiment 1: Verification of mPFC-PL and AcbC activation following N+A injection
Rats were trained on and acquired the N+A discrimination (See supplemental figure 2). Following N+A there was a significant increase in c-Fos immunoreactivity (IR) in mPFC-PL (t(12) = 3.102, p < 0.05; Figure 2A) and AcbC (t(12) = 2.982, p < 0.05; Figure 2B). There was no significant difference in c-Fos IR in AcbSh (Figure 2C).
Figure 2.
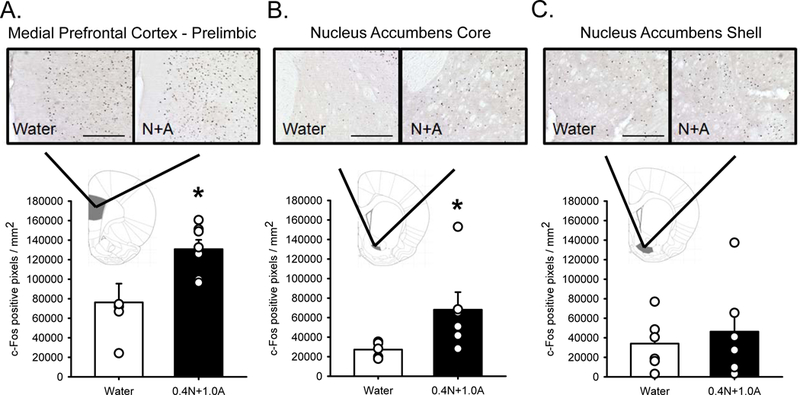
c-Fos expression in medial prefrontal cortex – prelimbic region (mPFC; A), nucleus accumbens core (AcbC; B) and nucleus accumbens shell (AcbSh; C) following water or 0.4N+1.0A (0.4 mg/kg nicotine + 1 g/kg alcohol, IG) in rats trained to discriminate N+A from water. Bars are mean (±SEM) c-Fos positive pixels/mm2. There was significantly more c-Fos expression in mPFC and AcbC following N+A compared to water. Representative photomicrographs appear above each figure. Scale bars = 250 µm. * - N+A treatment significantly greater than water treatment (p < 0.05).
Experiment 2: Effects of chemogenetic silencing of mPFC-PL→AcbC projections on sensitivity to N+A and its components.
FP Group
N+A Testing:
mCherry expression was confirmed by immunofluorescence and individual expression is represented by expression maps on Figure 3A. Figure 3B shows cannula placements and Figure 3C shows representative staining for hM4D/mCherry (upper panels) and cannulae (lower panels). 3 rats from the hM4D group and 1 rat from the mCherry control group were excluded due to inefficient or absent viral expression. 1 rat from the hM4D group and 2 rats from the mCherry control group were excluded due to inaccurate AcbC cannulae placements. As such, the following analyses and accompanying figures represent n=6 hM4D and n=7 mCherry control. Mean (±SEM) baseline N+A discrimination scores prior to testing were 5.2±0.17 and 4.9±0.30 for hM4D and mCherry groups, respectively. The training dose of N+A in the curve following vehicle treatment fully substituted for the N+A training condition in both the hM4D and mCherry control groups. As shown in Figure 3D, there was a significant main effect of N+A dose (F[3,66] = 16.983, p = 0.0001), a N+A dose by group interaction (F[3,66] = 3.057, p =0.034), a CNO by N+A dose interaction (F[3,66] = 3.130, p = 0.031). Post-hoc analysis showed that following intra-AcbC CNO, discrimination score at the training dose (0.4N+1.0A) was decreased in the hM4D group compared to vehicle or the mCherry control group, suggesting decreased sensitivity to the N+A training dose. There was no effect of CNO in the mCherry control group. There were no significant effects on locomotor rate (Table 1). In addition, there was no effect of CNO on PreCS head entries (Table S1), indicating that the effects observed on discrimination score were not the result of general changes in head entry activity but were specific to activation of the stimulus light. These results indicate that silencing the mPFC-PL to AcbC projection directly modulates response to the N+A cue, decreasing sensitivity.
Figure 3.
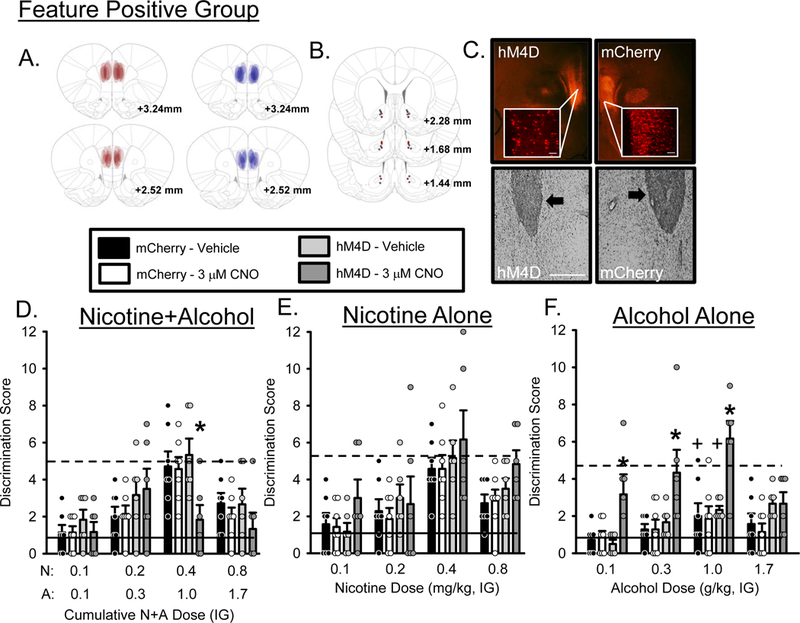
Substitution curves for the Feature Positive group following mPFC-PL→AcbC silencing. N = 6 for the hM4D group and n = 7 for the mCherry control group. (A) Representative expression maps for hM4D (red) and mCherry (blue), (B) cannulae placements for hM4D (red dots) and mCherry (blue dots) and (C) representative photomicrographs showing representative DREADD and mCherry (top panels, 2X and 20X images, scale bar is 1000 µm) and cannula placements (bottom panels, arrows indicate position of AcbC injector). (D) Mean(+S.E.M.) discrimination score (head entries during the single 15-s light CS minus head entries during 15 seconds before light onset) for the N+A substitution test. CNO significantly decreased discrimination score at the training dose (0.4N/1.0A) compared to vehicle and the mCherry control group suggesting that mPFC→AcbC projections are important for modulating sensitivity to N+A. (E) Mean(+S.E.M.) discrimination score for the nicotine only substitution test. (F) Mean(+S.E.M.) discrimination score for the alcohol only substitution test. CNO significantly increased discrimination score at 0.1, 0.3 and 1.0 g/kg alcohol doses compared to vehicle and mCherry control suggesting that the alcohol component of the compound cue is particularly sensitive to modulation by mPFC→AcbC projections. Solid lines indicate mean discrimination score from 2 water sessions prior to testing. Dashed lines indicate mean discrimination score from 2 N+A sessions prior to testing. +-denotes vehicle condition is significantly different from N+A baseline (p<0.05); *-denotes significant difference from vehicle and mCherry control (p < 0.05).
Table 1.
Mean(±SEM) locomotor rate (beam breaks per min) for all behavioral experiments. *-indicates significant main effect of cumulative dose, p<0.05.
| N+A (mg/kg+g/kg) | Nicotine (mg/kg) | Alcohol (g/kg) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1N+0.1A | 0.2N+0.3A | 0.4N+1.0A | 0.8N+1.7A | 0.1 | 0.2 | 0.4 | 0.8 | 0.1 | 0.3 | 1 | 1.7 | ||
| Feature Positive Group | |||||||||||||
| hM4D | VEH | 29.25±9.44 | 31.25±8.37 | 46.83±5.75 | 40.91±10.59* | 29.75±2.71 | 23.58±2.19 | 33.75±4.11 | 44.75±4.18* | 22.16±0.97 | 21.83±1.37 | 20.66±1.77 | 22.83±1.79 |
| CNO | 29.166±5.73 | 31.58±6.25 | 28.75±6.75 | 40.08±4.56* | 36.25±9.23 | 29.75±7.05 | 42.5±7.78 | 44.5±5.47* | 27.66±5.07 | 33.25±6.19 | 40.91±4.3 | 29±6.2 | |
| mCherry | VEH | 29.78±6.82 | 38.64±7.38 | 40.07±6.39 | 36.92±8.07* | 25.78±2.36 | 23.78±1.61 | 34.28±4.20 | 38.28±4.48* | 23.41±1.82 | 24±1.97 | 25.28±3.46 | 23.42±1.52 |
| CNO | 29.57±6.08 | 37.42±5.71 | 36.21±5.54 | 32.42±6.05* | 26.78±3.35 | 26.78±2.82 | 41±7.03 | 35.64±4.82* | 25.78±3.42 | 27.42±3.4 | 25.14±2.31 | 21.66±2.20 | |
| Feature Negative Group | |||||||||||||
| hM4D | VEH | 27.64±4.72 | 25.35±3.14 | 31.5±2.95 | 34.07±4.37 | 37.71±10.16 | 25.64±2.31 | 30.35±2.44 | 35.78±3.83* | 35.71±5.22 | 31.28±3.22 | 29.64±2.55 | 28.5±2.48* |
| CNO | 22.71±2.29 | 24.92±2.44 | 35.78±1.27 | 32.35±3.54 | 23.28±3.35 | 43.14±3.32 | 44.21±7.44 | 35.71±3.38* | 35.57±2.63 | 23.35±2.51 | 23.5±3.36 | 29.21±2.36* | |
| mCherry | VEH | 29.5±4.39 | 24.35±2.96 | 29.42±3.03 | 26.92±2.48 | 29.92±4.13 | 26.42±2.19 | 26.78±2.43 | 31.78±3.96* | 31.92±4.03 | 25.42±3.85 | 26.28±2.03 | 27.35±2.66* |
| CNO | 30±2.98 | 24.64±2.09 | 27.07±2.64 | 23.92±2.37 | 27.5±3.43 | 28.78±3.11 | 27.71±2.92 | 25.5±1.82* | 30.35±3.17 | 24.42±2.56 | 29.14±2.36 | 24.07±2.84* | |
Nicotine Alone Testing:
Mean (±SEM) baseline N+A discrimination scores prior to testing nicotine alone were 5.4±0.62 and 5.1±0.25 for hM4D and mCherry groups respectively. The training dose of nicotine (0.4 mg/kg) in the curve fully substituted for the N+A training condition in both the hM4D and mCherry control groups. As shown in Figure 3E, there was a main effect of nicotine dose on discrimination score (F[3,66] = 17.961, p = 0.0001) with discrimination score increasing as nicotine dose increased. There were no effects of CNO in the mCherry control group. Locomotor rate was not affected by CNO (Table 1). PreCS head entries were not affected by CNO (Table S1). These findings suggest that silencing mPFC-PL to AcbC projections does not affect sensitivity to the nicotine component in FP rats.
Alcohol Alone Testing:
Mean (±SEM) baseline N+A discrimination scores prior to testing alcohol alone were 4.8±0.61 and 5.3±0.34 for hM4D and mCherry groups respectively. The training dose of alcohol (1.0 g/kg) in the curve following vehicle treatment, did not substitute for the N+A training condition in either the hM4D or mCherry control groups. That is, discrimination score was significantly lower than the N+A baseline. As shown in Figure 3F, there was a main effect of alcohol dose (F[3,66] = 8.524, p = 0.0001), a CNO by alcohol dose interaction (F[3,66] = 3.221, p = 0.030) and a group by CNO interaction (F[1,22] = 7.777, p = 0.010). Post hoc analysis showed that following microinjection of 3µM CNO into AcbC, discrimination score significantly increased at 0.1 and 0.3 and 1.0 g/kg doses suggesting that silencing this projection made the alcohol component more similar to the N+A training dose. There were no effects of CNO in the mCherry control group. Locomotor rate was not affected (Table 1). PreCS head entries were not affected by CNO (Table S1).
FN Group:
N+A Testing:
mCherry expression was confirmed by immunofluorescence and individual expression is represented by expression maps on Figure 4A. Figure 4B shows cannula placements and Figure 4C shows representative staining for hM4D/mCherry (upper panels) and cannulae (lower panels). 3 rats from each group were excluded due to inefficient or absent viral expression. As such, the following analyses or accompanying figures represent n=7 hM4D and n=7 mCherry control. Mean (±SEM) baseline N+A discrimination scores prior to testing were 1.0±0.55 and 1.3±0.22 for hM4D and mCherry groups respectively. The training dose of N+A in the curve (0.4N+1.0A) following vehicle treatment fully substituted for the N+A baseline in both the hM4D and mCherry control groups. As shown in Figure 4D, there was a significant main effect of N+A dose (F[3,72] = 39.561, p = 0.0001), a N+A dose by group interaction (F[3,72] = 2.875, p =0.042), a CNO by N+A dose interaction (F[3,72] = 2.823, p = 0.044). Post-hoc analysis showed that following intra-AcbC CNO, discrimination score at the training dose (0.4N+1.0A) was significantly higher in the hM4D group compared to vehicle or the mCherry control group, suggesting decreased sensitivity to the N+A training dose. In addition, there was a main effect of N+A dose on locomotor rate (F[3,72] = 5.339, p = 0.002, Table 1). There was no effect of CNO on PreCS head entries (Table S1). These findings again suggest that silencing the mPFC-PL→AcbC projection directly modulates sensitivity to the N+A cue, reducing sensitivity.
Figure 4.
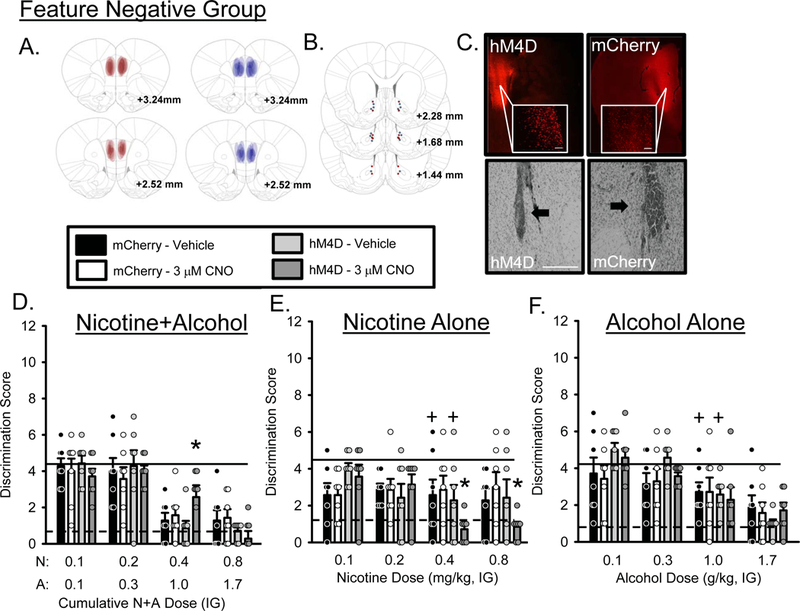
Substitution curves for the Feature Negative group following mPFC-PL→AcbC silencing. N = 7 for the hM4D group and n = 7 for the mCherry control group. A) Representative expression maps for hM4D (red) and mCherry (blue), B) cannulae placements for hM4D (red dots) and mCherry (blue dots) and (C) representative photomicrographs showing representative DREADD and mCherry (top panels, 2X and 20X images, scale bar is 1000 µm) and cannula placements (bottom panels, arrows indicate position of AcbC injector). (D) Mean (+S.E.M.) discrimination score (head entries during the single 15-s light CS minus head entries during 15 seconds before light onset) for the N+A substitution test. (E) Mean (+S.E.M.) discrimination score for the nicotine only substitution test. CNO significantly decreased discrimination score at the 0.4 and 0.8 mg/kg nicotine doses compared to vehicle and mCherry control suggesting that in FN trained rats, sensitivity to the nicotine component of the compound cue is sensitive to modulation by mPFC→AcbC projections. (F) Mean (+S.E.M.) discrimination score for the alcohol only substitution test. Solid lines indicate mean discrimination score from 2 water sessions prior to testing. Dashed lines indicate mean discrimination score from 2 N+A sessions prior to testing. +-denotes vehicle condition is significantly different from N+A baseline (p<0.05); *-denotes significant difference from vehicle and mCherry control (p<0.05).
Nicotine Alone Testing:
Mean (±SEM) baseline N+A discrimination scores prior to testing nicotine alone were 0.8±0.23 and 0.9±0.62 for hM4D and mCherry groups respectively. The training dose of nicotine in the curve (0.4 mg/kg) following vehicle treatment did not substitute for the N+A training condition in either the hM4D or mCherry control groups. That is, discrimination score was significantly higher for nicotine alone compared to N+A baseline. As shown in Figure 4E, there was a main effect of nicotine dose (F[3,72] = 3.655, p = 0.016), a nicotine dose by group interaction (F[3,72] = 2.919. p = 0.039) and a nicotine dose by CNO dose interaction (F[3,72] = 3.012, p = 0.035). Post-hoc analysis showed that following 3µM CNO, discrimination score at 0.4 and 0.8 mg/kg nicotine was significantly decreased compare to vehicle and mCherry controls suggesting that silencing this projection made the nicotine component more like the N+A training dose in the FN group. Furthermore, there were no effects of CNO in the mCherry control group. Locomotor rate was not affected (Table 1). PreCS head entries were not affected by CNO (Table S1).
Alcohol Alone Testing:
Mean (±SEM) baseline N+A discrimination scores prior to testing alcohol alone were 1.4±0.15 and 1.2±0.41 for hM4D and mCherry groups respectively. The training dose of alcohol (1.0 g/kg) in the curve following vehicle treatment did not substitute for the N+A training condition in either the hM4D or mCherry control groups. That is, discrimination score was significantly higher than N+A baseline. As shown in Figure 4F, there was a main effect of alcohol dose on discrimination score (F[3,72] = 19.041, p = 0.0001) with discrimination score decreasing as alcohol dose increased. There were no effects of CNO in the mCherry control group. In addition, there was a main effect of alcohol dose on locomotor rate (F[3,72] = 7.165, p = 0.0001, Table 1) with locomotor rate decreasing as alcohol dose increased. PreCS head entries were not affected by CNO (Table S1). This finding suggests that silencing this projection does not affect sensitivity to the alcohol component in the FN group.
Discussion
Here we demonstrate that the N+A training dose (0.4 mg/kg nicotine + 1.0 g/kg alcohol) induces neuronal activation in the mPFC-PL and AcbC. Moreover, sensitivity to a N+A interoceptive cue and its individual components are differentially modulated by mPFC-PL projections to AcbC. That is, silencing mPFC-PL projections to AcbC blocked sensitivity to the N+A cue and enhanced sensitivity to the components.
N+A increases neuronal activity in mPFC-PL and AcbC
The goal of the c-Fos experiment (Experiment 1) was to determine whether the N+A training dose would induce neuronal activation in the mPFC-PL and Acb circuitry. Because rats that undergo N+A discrimination training receive an extensive history of N+A exposure, it was important to use N+A discrimination-trained rats rather than N+A naïve rats. Therefore, rats received FP training history and on the terminal test received non-contingent treatment with N+A (i.e., in the home cage, no training session). We found increased neuronal activation relative to rats with the same N+A history that were treated with water. Importantly, increases in c-Fos were not observed in AcbSH, further supporting the specific role of mPFC-PL projections to AcbC. While this experiment was limited in scope, it would be interesting for future work to determine whether N+A training history would affect N+A neuronal activation. That is, to test the consequences of N+A prior to a behavioral session in FP and FN groups. Additionally, the finding of this pattern of neuronal activation is likely not unique to N+A, as it is probable that other drugs of abuse would activate these brain regions. However, this assessment was important to confirm that the N+A training dose would activate these brain regions.
Silencing mPFC-PL→AcbC Projections
When mPFC-PL→AcbC projections were selectively silenced, discrimination of N+A was disrupted. That is, sensitivity to the N+A training dose was blocked in both the FP and FN groups. Furthermore, the fact that FN rats also showed a disruption is important as it suggests that our findings under FP conditions are not simply the result of generally blunting the excitatory response, but that it is specific to disrupting the trained behavioral response. These data suggest that this projection likely plays a role in the expression of sensitivity to the N+A cue, and/or that this projection may be needed to drive goal-tracking behavior in the presence of the N+A cue. Interestingly, a previous study has shown that optogenetic inhibition of PL→NAc projections decreases reinstatement to cocaine seeking (Stefanik et al. 2013) and AcbC glutamate release induced by a cocaine prime is blocked by mPFC-PL inactivation (McFarland et al. 2003). Furthermore, it is important to note that silencing the mPFC-PL→AcbC projections did not affect PreCS head entries (Table S1). This is an important finding that demonstrates that the effects observed were specific to changes in behavioral activation in response to the stimulus light.
Another interesting finding from silencing mPFC-PL→AcbC projections was that when the individual components were tested, the effects were dependent on the training group (i.e., FP vs FN). In the FP group, when the components (alcohol or nicotine alone) were tested, silencing this projection increased sensitivity to the alcohol component, fully substituting for N+A. Again, there were no effects on PreCS head entries, suggesting that these effects were not the result of a general increase in head entry activity but instead specifically in response to the stimulus light. This pattern of increased head entries suggests that silencing mPFC→AcbC projections caused the alcohol component to be more N+A-like. This further suggests that the balance of the relative contribution of the nicotine and alcohol components may be disrupted following silencing of the mPFC-PL→AcbC projections. As such, it is possible that the enhanced sensitivity to the alcohol component may have contributed to the blunted sensitivity to the N+A compound, as under “normal” conditions the alcohol component plays a lesser role in the compound cue than nicotine (Randall et al., 2016). This finding also suggests that response to the alcohol component may be directly modulated by this projection. For example, Seif et al. (2013) showed that aversion-resistant alcohol intake depends on this projection. This is interesting in that it may suggest an important role for these projections in processing alcohol-related information. Indeed, our lab has previously shown that inactivation of mPFC produces alcohol-like effects in an operant discrimination model (Jaramillo et al. 2016) and modulation of glutamatergic transmission in AcbC affects sensitivity to alcohol (Besheer et al. 2014; Besheer et al. 2009).
In contrast, in the FN group, silencing the mPFC-PL→AcbC projection enhanced sensitivity to the nicotine component while not affecting the alcohol component. Again, this is in the absence of any changes in PreCS head entry activity, showing that these changes in behavior are specific to the CS onset. Importantly, the observed differences between the FP and FN groups are not necessarily surprising. As described by Bouton (1998), FP conditioning relies on a simple feature-US relationship in which the cue is directly associated with the reward. By contrast, the FN association is a more complex relationship to resolve in which the rat must learn the significance of both the feature (drug state) and the target (cue light) in order to determine the correct response (withholding behavior). Therefore, it is probable that slightly different neural circuitry is engaged under the two training conditions and this is supported by the present results.
An important avenue for future research will be to determine whether there are differences in brain-regional involvement in both training and response to FP vs FN cues. Given that the FN discrimination relies on a more complex inhibitory association, the role of attentional processes are likely greater in FN trained rats than FP, suggesting that silencing this projection would disrupt attentional processes needed for the FN association. As such, a possible explanation for the current findings is that nicotine alone increases attention, restoring inhibitory processes needed for the FN discrimination. Indeed, neurons in mPFC-PL are active during waiting periods of inhibitory response tasks (Narayanan and Laubach 2009) and inactivation of mPFC-PL has been shown to impair inhibitory control in such tasks (Bari et al. 2011; Broersen and Uylings 1999; Narayanan et al. 2006; Narayanan and Laubach 2006; Risterucci et al. 2003). Moreover, previous work from our lab has shown that the nicotinic partial agonist varenicline substitutes for N+A in both FP and FN conditions, which would suggest that activation of cholinergic systems (likely prefrontal cortical) plays a significant role in maintaining these associations (Randall et al., 2016).
Another interesting consideration in reference to our previous findings with varenicline (Randall et al., 2016), is that varenicline substitutes for the N+A compound cue, which suggests that the N+A compound is not a unique cue. However, there is growing evidence that varenicline decreases alcohol intake and may be effective in treating alcohol use disorders (Bowers et al. 2005; McKee et al. 2009). This would instead suggest that varenicline induces interoceptive effects that are similar to that of both nicotine and alcohol.
Methodological Considerations
Given that these experiments relied on repeated microinjections and the utilization of within subject testing, cumulative dosing testing procedures were used. These testing procedures allowed for the assessment of a full dose response curve while minimizing the total number of microinjections and minimizing the amount of damage done from repeated injector insertions. Importantly, in an operant drug discrimination task, the cumulative dosing strategy has been shown to produce results that do not differ from traditional discrete dose response curves (Hiltunen and Jarbe 1989). Furthermore, rats were extensively trained and received training sessions between all testing sessions so it is unlikely that conditioning was not supplanted by the testing procedures.
An important aspect of these experiments was the rigorous use of controls. Recently, it has been shown that CNO has low affinity for DREADD receptors and is rapidly converted into clozapine in vivo, which has high affinity for DREADD receptors (Gomez et al. 2017). Moreover, clozapine is detectable in plasma within 30 minutes following CNO injection (5 mg/kg, IP) in Long-Evans rats (MacLaren et al. 2016). With particular relevance to the current experiments, there is evidence that clozapine can produce discriminative stimulus effects (Goudie et al. 1998; Prus et al. 2016) and decrease alcohol-stimulated behavior (Thrasher et al. 1999), which has the potential to impact sensitivity to the N+A cue. In light of these studies, the inclusion of mCherry CNO-treated controls is crucial for data interpretation. In the current work, each behavioral experiment had a complementary mCherry control group that was trained and tested in parallel to verify that CNO, at the doses tested, was not modulating behavior on its own. As shown in the series of discrimination experiments, CNO did not change behavior in any of the mCherry control groups. These findings support that the observed results in the hM4D groups are the result of silencing the mPFC-PL→AcbC projections.
Another important feature of the present work was the functional validation of the DREADDs in the mPFC-PL using electrophysiology. Using immunohistochemistry we also showed robust DREADD expression in the mPFC-PL and in terminal fields of the AcbC, the site at which CNO was infused in the behavioral experiments. However, even though behavioral changes were observed following silencing of the mPFC-PL→AcbC projections, we did not directly determine whether CNO application in the AcbC inhibits activity of the mPFC-PL→AcbC projections. Silencing via activation of Gi DREADDs in axon terminals has been well demonstrated (Lichtenberg et al. 2017; Stachniak et al. 2014), but this would also be an important confirmatory experiment within the context of the present work.
An interesting question moving forward is whether or not DREADD activation produces interoceptive effects and whether or not this in and of itself can act as a cue in this task. Indeed, prior studies have shown that Gi DREADD activation in the insular cortex and insular cortex to AcbC projections produces alcohol-like effects in rats trained to discriminate 1 g/kg alcohol from water (Jaramillo et al. 2017). Moreover, this raises the question of whether silencing mPFC-PL→AcbC projections produce effects similar to N+A when intra-AcbC CNO is administered alone in the absence of the N+A compound or the components. However, given that discrimination performance was not potentiated (FN group) or inhibited (FN group) at the lowest nicotine (0.1 mg/kg) and alcohol (0.1 g/kg) doses when administered as part of the N+A compound or alone, makes such an explanation less tenable. However, this will be an important question to test with future experiments.
The current experiments utilized a single dose of nicotine and alcohol for training. This decision was based on previous work (Randall et al., 2016) and other literature in which these doses of nicotine and alcohol were found to produce stable discrimination behavior (Ford et al., 2012; Troisi et al., 2013). However, it would be valuable for future studies to assess different dose combinations of nicotine and alcohol as there may be differences in response following silencing of mPFC→AcbC. Additionally, comparing the current findings to rats trained on either nicotine or alcohol alone will be important for future studies as baseline discrimination behavior may differ in comparison to rats trained on the compound cue which may point to important circuit-related differences in the way that these cues are processed and learned.
Prelimbic vs. Infralimbic Considerations
The focus of the present work was the role of mPFC-PL, based on our interest in mPFC-PL to AcbC projections. However, there is great interest in the role of mPFC-IL in modulating drug-related behaviors. Similar to PL, the role of IL has been debated. For example, inactivation of IL has been shown to increase seeking behavior for heroin (Bossert et al. 2011; Bossert et al. 2012) while other studies show that lesion or inactivation of IL decrease seeking behavior for cocaine (Pelloux et al. 2013; Vassoler et al. 2013), suggesting a drug-type specific role for IL. Furthermore, there is some dispute over the anatomical division between PL and IL. For example, both IL and ventral PL (where PL meets IL) send projections to AcbSH (Heidbreder and Groenewegen 2003), which may account for overlap in function between these two divisions. It will be important for future studies to assess the role of IL in modulation of sensitivity to the N+A compound cue.
Conclusions
Taken together, the current findings show that mPFC-PL and its projections to AcbC play a role in modulating sensitivity to a N+A interoceptive drug cue and its components. This is important considering the powerful role that drug-related cues play in advancing the progression to addiction, influencing drug-motivated behavior, and inducing relapse to drug seeking (Bevins and Besheer 2014). Moreover, it will be important for future studies to examine other brain regions that receive input from mPFC-PL. Furthermore, considering that the N+A compound interoceptive cue represents a more complex cue than, made up of both nicotine and alcohol to varying degrees, future studies comparing rats trained on the components alone to those trained on the compound could offer important insights into the neurobiological underpinnings of combined nicotine-alcohol use and potentially lead to better treatments.
Supplementary Material
Acknowledgements:
This work was supported in part, by NIH/NIAAA grant (AA019682) to JB and NIH/NIAAA grant (AA024674) to PAR, and the Bowles Center for Alcohol Studies (AA011605). The authors declare no conflict of interest.
Abbreviations:
- (mPFC)
medial prefrontal cortex
- (mPFC-PL)
medial prefrontal cortex – prelimbic
- (mPFC-IL)
medial prefrontal cortex – infralimbic
- (PL)
prelimbic
- (IL)
infralimbic
- (Acb)
nucleus accumbens
- (AcbC)
nucleus accumbens core
- (AcbSH)
nucleus accumbens shell
- (N+A)
nicotine+alcohol
- (N)
nicotine
- (A)
alcohol
- (CNO)
clozapine-N-oxide
Footnotes
Data Accessibility Statement: Data will be made available via repository upon publication or by request.
References
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW (2003) Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 6: 743–9. [DOI] [PubMed] [Google Scholar]
- Bari A, Mar AC, Theobald DE, Elands SA, Oganya KC, Eagle DM, Robbins TW (2011) Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci 31: 9254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Cucca F, Frau R, Di Chiara G (2017) Changes in Dopamine Transmission in the Nucleus Accumbens Shell and Core during Ethanol and Sucrose Self-Administration. Front Behav Neurosci 11: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Durant B (2012) Assessment of the interoceptive effects of alcohol in rats using short-term training procedures. Alcohol 46: 747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Jaramillo AA, Frisbee S, Cannady R (2014) Stress hormone exposure reduces mGluR5 expression in the nucleus accumbens: functional implications for interoceptive sensitivity to alcohol. Neuropsychopharmacology 39: 2376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Grondin JJ, Salling MC, Spanos M, Stevenson RA, Hodge CW (2009) Interoceptive effects of alcohol require mGlu5 receptor activity in the nucleus accumbens. J Neurosci 29: 9582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Besheer J (2014) Interoception and learning: import to understanding and treating diseases and psychopathologies. ACS Chem Neurosci 5: 624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, Shaham Y (2011) Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci 14: 420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, Shaham Y (2012) Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci 32: 4982–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers BJ, McClure-Begley TD, Keller JJ, Paylor R, Collins AC, Wehner JM (2005) Deletion of the alpha7 nicotinic receptor subunit gene results in increased sensitivity to several behavioral effects produced by alcohol. Alcohol Clin Exp Res 29: 295–302. [DOI] [PubMed] [Google Scholar]
- Broersen LM, Uylings HB (1999) Visual attention task performance in Wistar and Lister hooded rats: response inhibition deficits after medial prefrontal cortex lesions. Neuroscience 94: 47–57. [DOI] [PubMed] [Google Scholar]
- Bull C, Freitas KC, Zou S, Poland RS, Syed WA, Urban DJ, Minter SC, Shelton KL, Hauser KF, Negus SS, Knapp PE, Bowers MS (2014) Rat nucleus accumbens core astrocytes modulate reward and the motivation to self-administer ethanol after abstinence. Neuropsychopharmacology 39: 2835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady R, Grondin JJ, Fisher KR, Hodge CW, Besheer J (2011) Activation of group II metabotropic glutamate receptors inhibits the discriminative stimulus effects of alcohol via selective activity within the amygdala. Neuropsychopharmacology 36: 2328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellsague X, Munoz N, De Stefani E, Victora CG, Castelletto R, Rolon PA, Quintana MJ (1999) Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int J Cancer 82: 657–64. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Bartlett SE (2010) Neuronal nicotinic acetylcholine receptors as pharmacotherapeutic targets for the treatment of alcohol use disorders. CNS Neurol Disord Drug Targets 9: 60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Muir JL (2001) Visual attention in the rat: a role for the prelimbic cortex and thalamic nuclei? Behav Neurosci 115: 417–28. [PubMed] [Google Scholar]
- Colombo SF, Mazzo F, Pistillo F, Gotti C (2013) Biogenesis, trafficking and up-regulation of nicotinic ACh receptors. Biochem Pharmacol 86: 1063–73. [DOI] [PubMed] [Google Scholar]
- Dineley KT, Pandya AA, Yakel JL (2015) Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol Sci 36: 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Davis NL, McCracken AD, Grant KA (2013) Contribution of NMDA glutamate and nicotinic acetylcholine receptor mechanisms in the discrimination of ethanol-nicotine mixtures. Behav Pharmacol 24: 617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, McCracken AD, Davis NL, Ryabinin AE, Grant KA (2012) Discrimination of ethanol-nicotine drug mixtures in mice: dual interactive mechanisms of overshadowing and potentiation. Psychopharmacology (Berl) 224: 537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi S, Talamini R, Barra S, Baron AE, Negri E, Bidoli E, Serraino D, La Vecchia C (1990) Smoking and drinking in relation to cancers of the oral cavity, pharynx, larynx, and esophagus in northern Italy. Cancer Res 50: 6502–7. [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M (2017) Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357: 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudie AJ, Smith JA, Taylor A, Taylor MA, Tricklebank MD (1998) Discriminative stimulus properties of the atypical neuroleptic clozapine in rats: tests with subtype selective receptor ligands. Behav Pharmacol 9: 699–710. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ (2003) The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev 27: 555–79. [DOI] [PubMed] [Google Scholar]
- Hiltunen AJ, Jarbe TU (1989) Discriminative stimulus properties of ethanol: effects of cumulative dosing and Ro 15–4513. Behav Pharmacol 1: 133–140. [PubMed] [Google Scholar]
- Jaramillo AA, Agan VE, Makhijani VH, Pedroza S, McElligott ZA, Besheer J (2017) Functional role for suppression of the insular-striatal circuit in modulating interoceptive effects of alcohol. Addict Biol [DOI] [PMC free article] [PubMed]
- Jaramillo AA, Randall PA, Frisbee S, Besheer J (2016) Modulation of sensitivity to alcohol by cortical and thalamic brain regions. Eur J Neurosci 44: 2569–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg NT, Pennington ZT, Holley SM, Greenfield VY, Cepeda C, Levine MS, Wassum KM (2017) Basolateral Amygdala to Orbitofrontal Cortex Projections Enable Cue-Triggered Reward Expectations. J Neurosci 37: 8374–8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, Espana RA, Clark SD (2016) Clozapine N-Oxide Administration Produces Behavioral Effects in Long-Evans Rats: Implications for Designing DREADD Experiments. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW (2003) Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23: 3531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E (2009) Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry 66: 185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar NS, Harkness PC (2008) Assembly and trafficking of nicotinic acetylcholine receptors (Review). Mol Membr Biol 25: 279–92. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF (2005) Altered Fos expression in neural pathways underlying cue-elicited drug seeking in the rat. Eur J Neurosci 21: 1385–93. [DOI] [PubMed] [Google Scholar]
- Moorman DE, James MH, McGlinchey EM, Aston-Jones G (2015) Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res 1628: 130–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW (1996) The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex 6: 470–81. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Horst NK, Laubach M (2006) Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience 139: 865–76. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M (2006) Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron 52: 921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M (2009) Methods for studying functional interactions among neuronal populations. Methods Mol Biol 489: 135–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J, Sabreo S, Fasting U (1985) Interaction of alcohol and tobacco as risk factors in cancer of the laryngeal region. J Epidemiol Community Health 39: 165–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovari J, Leri F (2008) Inactivation of the ventromedial prefrontal cortex mimics re-emergence of heroin seeking caused by heroin reconditioning. Neurosci Lett 444: 52–5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Academic Press/Elsevier, Amsterdam; Boston; [Google Scholar]
- Pelloux Y, Murray JE, Everitt BJ (2013) Differential roles of the prefrontal cortical subregions and basolateral amygdala in compulsive cocaine seeking and relapse after voluntary abstinence in rats. Eur J Neurosci 38: 3018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelucchi C, Gallus S, Garavello W, Bosetti C, La Vecchia C (2008) Alcohol and tobacco use, and cancer risk for upper aerodigestive tract and liver. Eur J Cancer Prev 17: 340–4. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ (2009) Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem 16: 279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW (2008) Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci 28: 6046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Reeder DC, Hicks J, Morgan ZR, Kalivas PW (1998) Ibotenic acid lesions of the dorsal prefrontal cortex disrupt the expression of behavioral sensitization to cocaine. Neuroscience 82: 1103–14. [DOI] [PubMed] [Google Scholar]
- Prus AJ, Wise LE, Pehrson AL, Philibin SD, Bang-Andersen B, Arnt J, Porter JH (2016) Discriminative stimulus properties of 1.25mg/kg clozapine in rats: Mediation by serotonin 5-HT2 and dopamine D4 receptors. Brain Res 1648: 298–305. [DOI] [PubMed] [Google Scholar]
- Randall PA, Cannady R, Besheer J (2016) The nicotine + alcohol interoceptive drug state: contribution of the components and effects of varenicline in rats. Psychopharmacology (Berl) 233: 3061–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risterucci C, Terramorsi D, Nieoullon A, Amalric M (2003) Excitotoxic lesions of the prelimbic-infralimbic areas of the rodent prefrontal cortex disrupt motor preparatory processes. Eur J Neurosci 17: 1498–508. [DOI] [PubMed] [Google Scholar]
- Stachniak TJ, Ghosh A, Sternson SM (2014) Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus-->midbrain pathway for feeding behavior. Neuron 82: 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, LaLumiere RT (2013) Optogenetic inhibition of cocaine seeking in rats. Addict Biol 18: 50–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher MJ, Freeman PA, Risinger FO (1999) Clozapine’s effects on ethanol’s motivational properties. Alcohol Clin Exp Res 23: 1377–85. [PubMed] [Google Scholar]
- Troisi JR 2nd, Dooley TF 2nd, Craig EM (2013) The discriminative stimulus effects of a nicotine-ethanol compound in rats: Extinction with the parts differs from the whole. Behav Neurosci 127: 899–912. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Hopkins TJ, Guercio LA, Espallergues J, Berton O, Schmidt HD, Pierce RC (2013) Deep brain stimulation of the nucleus accumbens shell attenuates cocaine reinstatement through local and antidromic activation. J Neurosci 33: 14446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51: 32–58. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, Neisewander JL (2007) Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience 145: 438–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW (2013) Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci 2013 Aug;16(8):1094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton MEN. Mechanisms of Feature-Positive and Feature-Negative Discrimination Learning in an Appetitive Conditioning Paradigm. In: Schmajuk NAHPC, editor. Occasion Setting: Associative Learning and Cognition in Animals. American Psychological Association; Washington DC: 1998. pp. 69–113. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


