Abstract
Fragile X syndrome (FXS) is characterized by hallmark features of gaze avoidance, reduced social approach, and social anxiety. The development of therapeutics to manage these symptoms has been hindered, in part, by the lack of sensitive outcome measures. This study investigated the utility of a novel eye tracking paradigm for indexing social avoidance-related phenotypes. Adolescent/young adult-aged males with FXS (n=24) and typical development (n=23) participated in the study. Participants viewed faces displaying direct or averted gaze and the first fixation duration on the eyes was recorded as an index of initial stimulus registration. Fixation durations did not differ across the direction of gaze conditions in either group, although the control group showed longer initial fixations on the eyes relative to the FXS group. Shorter initial fixation on averted gaze in males with FXS was a robust predictor of the severity of their social avoidance behavior exhibited during a social greeting context, whereas parent-reported social avoidance symptoms was not related to performance in the semi-naturalistic context. This eye tracking paradigm may represent a promising outcome measure for FXS clinical trials because it provides a quantitative index that closely maps onto core social avoidance phenotypes of FXS, can be completed in less than 20 minutes, and is suitable for use with individuals with low IQ.
Keywords: drug trials, social anxiety, gaze processing, gaze avoidance, eye contact
Fragile X syndrome (FXS) occurs when an expansion of >200 trinucleotide (CGG) repeats occurs on the 5’ untranslated region of the Fragile X Mental Retardation-1 (FMR1) gene, located on the X chromosome (Maddalena et al., 2001). This mutation results in hypermethylation, transcriptional silencing, and failure to express the Fragile X Mental Retardation Protein (FMRP) that is needed for brain development and function (Crawford, Acuna, & Sherman, 2001). FXS affects 1 in 7,000–10,000 individuals and represents the most common inherited cause of intellectual disability (ID; Hunter et al., 2014). Mild to moderate ID is seen in the majority of males with the syndrome, who are typically more severely affected than females who benefit from the protective effects of the second X chromosome (Hagerman & Hagerman, 2002). In addition to cognitive impairment, the FXS phenotype is characterized by high rates of co-occurring autism spectrum disorder (ASD), physiological hyperarousal, attention deficits, and communication impairment (Finestack, Richmond, & Abbeduto, 2009; Garcia-Nonell et al., 2008; Kaufmann et al., 2017; Klusek, Martin, & Losh, 2014b; Klusek, Roberts, & Losh, 2015; Munir, Cornish, & Wilding, 2000). Social withdrawal, shyness, and social avoidance are also core features of the phenotype, with as many as 60% of males with FXS meeting diagnostic criteria for social phobia (Budimirovic et al., 2006; Cordeiro, Ballinger, Hagerman, & Hessl, 2011; Kaufmann, Capone, Clarke, & Budimirovic, 2008).
Difficulty establishing and maintaining eye contact is one of the most ubiquitous behavioral features of FXS (Hall, DeBernardis, & Reiss, 2006; Hessl, Glaser, Dyer-Friedman, & Reiss, 2006; Reiss & Freund, 1992; Wolff, Gardner, Paccia, & Lappen, 1989). This characteristic is demonstrated by the classic example of the “fragile X handshake” in which individuals with FXS willingly accept a handshake during social greeting while physically turning away and avoiding eye contact (Wolff et al., 1989). Boys with FXS make eye contact less frequently than their typically developing peers and children with other neurodevelopmental disorders, such as Down syndrome (Hall et al., 2015; Hessl et al., 2006; Klusek, Martin, & Losh, 2014a; Murphy, Abbeduto, Schroeder, & Serlin, 2007). The degree of reduced eye contact is striking, with some studies documenting gaze aversion occurring 80–90% of the time during interaction with an examiner (Abbeduto et al., 2018; Hall et al., 2006; Hall et al., 2015; Hall, Lightbody, Huffman, Lazzeroni, & Reiss, 2009). Poor eye contact is also persistent over the course of an interaction; although eye contact may improve somewhat with time and familiarity, it typically does not normalize to the level that would be expected in typically developing individuals (Roberts, Weisenfeld, Hatton, Heath, & Kaufmann, 2007).
Poor eye contact in FXS is hypothesized to stem from heightened social anxiety, such that individuals with FXS actively avoid eye contact because they perceive it to be threatening (e.g., Cohen, Vietze, Sudhalter, Jenkins, & Brown, 1989). This hypothesis is based on an early behavioral observation that children with FXS seemed to actively avoid mutual eye contact with their parents by waiting until their parents looked away before returning their gaze (Cohen et al., 1989). Such active avoidance behavior is consistent with hypervigilant-avoidant patterns of attentional bias that have been observed in social anxiety in the general population, characterized by initial attention to threatening stimuli, followed by avoidance (Bögels & Mansell, 2004; Garner, Mogg, & Bradley, 2006). In FXS, physiological hyperarousal is thought to play a role in this process, contributing to anxiety and subsequent avoidance of social demands (see Cohen, 1995; Klusek et al., 2015). Consistent with this hypothesis, neuroimaging studies of gaze processing in males and females with FXS have documented atypical brain responses in regions involved in anxiety and arousal, such as the amygdala and insula (Dalton, Holsen, Abbeduto, & Davidson, 2008; Watson, Hoeft, Garrett, Hall, & Reiss, 2008).
Lower cortisol stress responses have also been detected in boys with FXS who make less frequent eye contact, supporting the notion that avoiding mutual gaze may reduce physiological stress (Hessl et al., 2006). Elevated skin conductance responses and heightened pupillary reactivity to emotional faces have also been documented in individuals with FXS (Farzin, Rivera, & Hessl, 2009; Williams, Langdon, & Porter, 2013). However, evidence of this relationship is mixed, as the extent of gaze avoidant behavior in FXS does not correlate with arousal level indexed by heart rate measures (Hall et al., 2009) and there is growing evidence to suggest that cardiac hyperarousal in FXS represents a generalized physiological state that is not specific to social contexts (Klusek, Martin, & Losh, 2013; see Klusek et al., 2015 for review).
Some recent evidence also suggests that gaze avoidance behavior is exhibited by individuals with FXS regardless of social tasks demands. Murphy et al. (2007) compared gaze avoidance of matched samples of boys with FXS, Down syndrome, and typical development during a naming task that was experimentally manipulated for social vs. nonsocial context. Gaze avoidance was uniformly elevated in the FXS group across both social and nonsocial conditions and, therefore, poor eye contact could not be attributed to social task demands. The contributors to poor eye contact in FXS are likely multifactorial, as other factors such as ASD symptoms (Roberts et al., 2007), language deficits (Hall et al., 2015), and social cognition (Holsen, Dalton, Johnstone, & Davidson, 2008) have also been implicated in the eye gaze behavior of individuals with FXS. Overall, there is a need for well-controlled investigations into the relationship between social avoidance and atypical gaze behavior in FXS, which has implications for theory and clinical practice.
In the present study, we used an experimental eye tracking paradigm to measure visual responses to direct and averted gaze exposure, as well as associations with social avoidance symptoms, in a sample of adolescent and young adult males with FXS. The study of differences in eye movement behavior in response to exposure to direct vs. averted gaze can provide insight into how individuals monitor and perceive interpersonal approach. Direct gaze signals intent to approach and, from an evolutionary perspective, heightened sensitivity to this signal may confer a survival advantage via enhanced detection of approaching predators (Emery, 2000). By contrast, averted gaze communicates motivational intent to avoid interaction and enhances the perception of withdrawal-related emotions, such as fear and sadness (Adams & Kleck, 2003). Because direct gaze provides a clear signal of another person’s intent to approach and initiate social interaction, socially anxious individuals may perceive direct gaze as a threat-related stimulus and respond with hypervigilance-avoidance behaviors. Exposure to direct gaze has also been shown to elicit heightened physiological arousal in socially anxious individuals relative to averted gaze (Wieser, Pauli, Alpers, & Mühlberger, 2009). Therefore, by contrasting responses to direct and averted gaze exposure, we aimed to shed light on how individuals with FXS process fundamental approach-avoidance signals, which will inform mechanisms underlying socially avoidant behavior in this group.
We also examined whether the looking behavior of males with FXS during the eye tracking paradigm was predictive of socially avoidant behavior in a semi-naturalistic context (i.e., during initial greeting with an unfamiliar adult). Because the lack of useful outcome measures has represented a significant barrier to the success of FXS clinical trials (Budimirovic et al. 2017), there is value in identifying accessible, objective measures that map onto core social phenotypes of FXS (Jacquemont et al., 2014). Eye tracking indices are especially promising in this regard as they are generally quick, objective, reliable, have automated data processing, and tap into fine-grained behaviors that may be overlooked by rating scales and other observational methods (e.g., Farzin, Scaggs, Hervey, Berry-Kravis, & Hessl, 2011). We posed the following research questions:
Does initial fixation on the eyes differ across FXS and age-matched controls, and do patterns differ following exposure to direct and averted gaze? In the males with FXS who were expected to show aversion to direct gaze (e.g., Cohen et al., 1989), we expected shorter initial fixation on direct gaze relative to averted gaze, marking avoidance of the direct gaze. We expected the age-matched control males to show longer initial fixation on the eyes relative to the males with FXS, with the former showing a preference for direct over averted gaze, consistent with a large body of evidence supporting attention to direct gaze as an innate, adaptive human trait (Baron-Cohen & Ring, 1994; Emery, 2000; Farroni, Csibra, Simion, & Johnson, 2002).
Can initial fixation in the eye region during the social gaze eye tracking paradigm predict the severity of socially avoidant behavior exhibited by males with FXS? We hypothesized that longer first fixation duration in response to eyes displaying direct, but not averted, gaze would predict more severe examiner-rated and parent-reported social avoidance behavior in the males with FXS.
Methods
Participants
The final participant sample included 24 males with FXS, aged 16–26 years (M = 19) and 23 typically developing males of a similar age (M=21 years, range = 19–26). Participant characteristics are described in Table 1. The typically developing control males were recruited from the local university community via word of mouth and flyers. Control males had no known history of developmental delays or disorders, per self-report, and scored below ASD cut-offs on the Social Responsiveness Scale-2 (Constantino & Gruber, 2012). All participants with FXS had ID, with the mean nonverbal IQ at 39 (IQ range = 36–56). Three additional males with FXS were recruited for the study but did not contribute data due to failure to complete >80% of the eye tracking trials (two males were unable to follow the instructions for calibration; one male refused to wear the head tracking sticker due to a sensory aversion). FXS data were collected as part of a longitudinal study focused on language development in males with FXS through adolescence and young adulthood (Abbeduto et al., 2019; Matherly et al., 2018), although previous publications from this study have not reported eye tracking data. Participants with FXS were recruited throughout the eastern and midwestern regions of the United States through social media, outreach to the National Fragile X Foundation Community Support Networks, and the Research Participant Registry Core of the Carolina Institute for Developmental Disabilities. All participants were native English speakers and able to use two to three-word phrases (according to parent report), which were part of the inclusionary criteria of the larger study. FXS (>200 CGG copies in the 5′-untranslated region of FMR1) was confirmed via DNA analysis of peripheral blood.
Table 1.
Demographics
| Variable | Group | |
|---|---|---|
| FXS n = 24 |
Typical Development n = 23 |
|
| Age in years | ||
| M (SD) | 19.28 (2.67) | 21.70 (1.96) |
| Range | 16.00–26.00 | 19.00–26.00 |
| Full Scale IQ | ||
| M (SD) | 39.42 (5.82) | n/a |
| Range | 36.00–56.00 | |
| Race n (%) | ||
| African American | 3 (12.5) | 0 (0) |
| Asian | 1 (4.17) | 0 (0) |
| Caucasian | 18 (75.00) | 22 (95.65) |
| Other | 0 (0) | 1 (4.35) |
| Not Specified | 2 (8.33) | 0 (0) |
Procedures
Data for the present study were collected in a research laboratory. Data for the FXS participants were collected at either Time 2 or Time 3 of participation in the larger four-year longitudinal study. Either Time 2 or Time 3 was chosen depending on staff availability and time constraints, given that this study was a “convenience” study conducted outside the scope of the original parent study. The eye tracking task was conducted in the morning of the second day of the two-day research protocol. The activities preceding the eye tracking tasks on the second day of testing consisted of about an hour of standardized language testing. Control participants were recruited for a one-time assessment that lasted about an hour and included the completion of a demographic form and the Social Responsiveness Scale-2 followed by administration of the eye tracking task. All procedures were approved by the Institutional Review Board (IRB) of the University of South Carolina and conform to the standards of the Declaration of Helsinki. Parental consent and participant assent were obtained according to IRB regulations.
Measures
Social Gaze Eye Tracking Paradigm.
The social gaze paradigm was programmed using the Experiment Builder software package (version 1.10, SR-Research Ltd, Ontario, Canada). This paradigm was adapted from Wieser et al. (2009) and the modified version has been previously described in detail in Klusek, Schmidt, Fairchild, Porter & Roberts (2017). Briefly, the eye gaze paradigm consists of a series of 3D computer graphics of neutral female faces that display gaze that is either directed at the participant or averted to the right or left. The trial began when participants were centrally fixated, which corresponded to the center of the eye region on the graphic of the female face which was initially presented with the eyes closed for 1200 ms. After that, the participant needed to look directly at the eye region for 300 ms before the trial would proceed and the eyes of the face would open. After the eyes open, the face displays either an averted or direct gaze for 6000 ms. In the unlikely event of track loss or excessive drift preventing the detection of gaze on the closed eyes, the experimenter could exit the trial and recalibrate as needed. In this case, the experiment continued with the next trial and the aborted trial was presented at the end of the block of trials.
At the beginning of the experiment, the participant is instructed: “You are going to see a series of faces on the screen. The faces will have their eyes closed. I want you to look within the eye region of the face until the eyes open. After the eyes open, you can look anywhere you want on the screen”. Stimuli included 16 different females faces, with condition (i.e., direct, averted) shown at random until 16 trials for each condition had been completed (32 trials total). Only female faces were included because females faces were expected to elicit more robust responses than male faces (Wieser et al., 2009), and sex-of-stimulus effects were not of particular interest in the present study. Example stimuli are shown in Figure 1.
Figure 1.
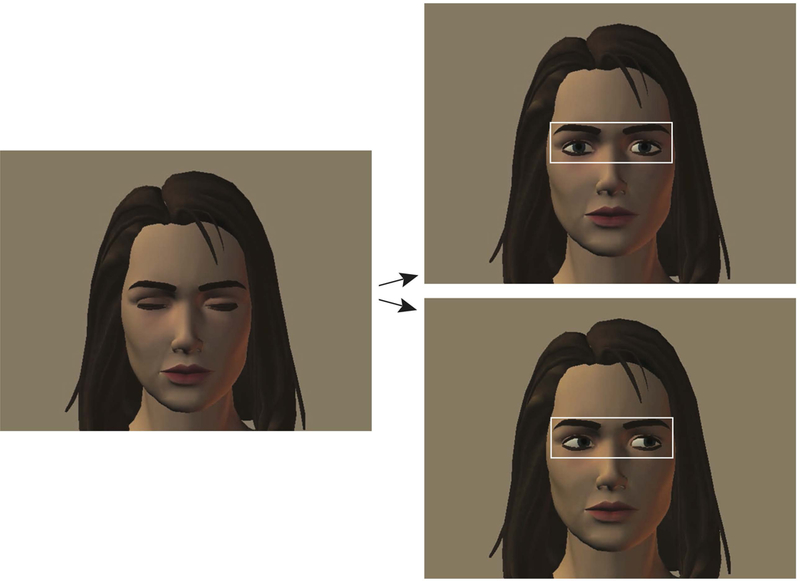
Example Stimuli from the Social Gaze Eye Tracking Paradigm
Note. The eye region area of interest is marked with a white rectangle. Trials begin with the eyes closed (leftmost image) for 1200 ms. After the participants looks in the eye region for 300 ms the trial proceeds and the eyes open to display either direct gaze (top right image) or averted gaze (bottom right image). Figure is reproduced from Klusek et al. (2017).
The Eyelink 1000 Plus (SR Research Ltd, Ontario, Canada) was used to record eye movements, which were monocularly sampled at 500 Hz in remote mode to allow for head movement. Stimuli were presented on a BEN-Q 2420T monitor (530mm x 300 mm, 1366 × 768 pixels, 60 Hz). Five-point calibration and validation were performed preceding the experiment. Calibrations were accepted if the average error was <.5° and the maximum error <1.00°. A single point drift check was presented before each trial and drifts in the data were recorded. To prevent drift during the study, recalibration was employed as needed. Data were collected in a well-lit room, with the participant seated approximately 750mm from the monitor. The Eyelink software default parameters were used to parse the online sample data into fixations, saccades, and blinks. The parser algorithm is designed to detect saccades by utilizing the instantaneous velocity and acceleration of the eye. A saccade signal is generated when the instantaneous acceleration exceeds 8000° per second or the instantaneous velocity exceeds 30° per second. Fixations are defined as periods without saccades but when the eye is present, whereas blinks are times when the pupil is undetected. Data were analyzed using Dataviewer (version 2.41 SR Research, Ontario, Canada).
An eye region area of interest was defined by a rectangle drawn around the eyes that subtended 8° horizontally and 3° vertically (see Figure 1). The average first fixation duration within the eye area of interest for each condition was extracted as the outcome variable. The first fixation duration reflects initial stimulus registration and is often used as an index of stimulus saliency (Henderson, 2003). This index does not require sustained attention to the stimulus, which is important given the low functioning level of the participants with FXS. The design of the eye gaze paradigm, which checked participant’s eye position online to ensure that participants were fixating within the eye region of the face when direct or averted gaze was initiated, ensured that the first fixation duration captured the initial viewing response following the opening of the eyes. Looking behavior that occurred after the first fixation on the eyes were not analyzed due to concerns regarding the validity of these data; many participants with FXS failed to attend to the facial stimuli for the full display time, resulting in significant missing data after the initial fixation on the eyes. All participants contributed valid data for the first fixation on the eyes for >80% of the trials. A programming error caused 1 missing trial for one participant with FXS, 6 missing trials for another participant with FXS, and 2 missing trials for one control participant; all other participants had complete data.
Parent-reported social avoidance behavior.
Social avoidance symptoms were measured in the FXS group using the social avoidance subscale of the Anxiety Depression and Mood Scale (ADAMS; Esbensen, Rojahn, Aman, & Ruedrich, 2003). The ADAMS is a 28-item caregiver report measure of mood and anxiety symptoms normed for populations with ID. The social avoidance subscale captures symptoms that align with the Diagnostic and Statistical Manual of Mental Health Disorder-5th Edition (DSM-5; American Psychiatric Association, 2013) classification of social anxiety disorder. This subscale includes questions such as “avoids others”, “spends much of time alone” and “avoids eye contact”. Items are scored on a scale of 0–3, with 0 signifying “not a problem” and 3 signifying “severe problem”. Possible raw scores for the subscale range from 0–21, with higher scores representing social avoidance behavior of increased severity. Cronbach’s alpha for the social avoidance subscale of the ADAMS for the present study was α = 0.66.
Examiner-rated social avoidance behavior.
Social avoidance behavior of the individuals with FXS was sampled from a semi-naturalistic context using the Social Avoidance Scale (formerly the Social Approach Scale; Roberts et al., 2009; Roberts et al., 2018; Roberts et al., 2007). The scale is rated by trained examiners based on the behaviors exhibited by the participants during the first minute of interaction with the examiner after arrival at the research laboratory on the first day of the two-day assessment. Behavior within the dimensions of physical approach, social shyness, and eye contact are coded on a scale of 0–4 based on operational definitions, with a higher score indicating greater avoidance. Physical approach is characterized by physical body movement towards and away from the examiner. Social shyness is characterized by the amount the participant speaks, their reflection of interest in the examiner, and subtle changes in the region of the face reflecting wariness. Eye contact is characterized by the estimated percentage of appropriate eye contact during the interaction.
The Social Avoidance Scale yields scores for each of the behavioral subdomains, as well as an overall rating (i.e., the average across the three subscales). The scale was coded via the consensus of two trained examiners who completed the scale immediately following the interaction with the participant. Cronbach’s alpha for the Social Avoidance Scale across the three dimensions (i.e. physical movement, facial expression, and eye contact) for the present study was α = 0.66. Inter-rater reliability prior to consensus was recorded for 20% of samples and Weighted Cohen’s Kappas were 0.86 for the Total Score, 0.90 for the physical approach subscale, 0.89 for the social shyness subscale, and 0.67 for the eye contact subscale. Kappa estimates ranging from 0.61–0.80 indicate “substantial” reliability and estimates ranging from 0.81–1.0 indicate “almost perfect” reliability (Cohen, 1960).
ASD symptom severity.
The Autism Diagnostic Observation Schedule-Second Edition (ADOS-2; Lord et al., 2012) was administered by research-reliable doctoral-level clinicians as an index of ASD symptom severity in the males with FXS. The ADOS-2 is a standardized, semi-structured assessment that allows opportunities for communication, social interaction, play, and restricted and repetitive behaviors. The comparison score was obtained as an index of overall symptom severity; scores range from 1–10, with scores above a 3 consistent with a diagnosis of ASD. Given that ASD symptoms in adolescents and adults with FXS show stability over time (Hernandez et al., 2009; Sabaratnam, Murthy, Wijeratne, Buckingham, & Payne, 2003), the ADOS-2 data were collected only at the first annual assessment for each participant in the larger longitudinal study. Thus, our measure of ASD symptom severity was collected 1–2 years prior to the eye tracking measure. Inter-rater reliability conducted on 15% of the administrations yielded 81% agreement for the algorithm items.
Nonverbal cognitive ability.
Nonverbal cognitive ability was assessed in participants with FXS using the Leiter International Performance Scale-Revised (Leiter-R) Brief IQ (Roid & Miller, 1997). The Brief IQ consists of 4 subtests of the broader Visualization and Reception battery and provides a reliable measure of nonverbal intelligence. The internal-consistency reliability coefficients for the Visualization and Reception battery subsets range from .75–.90 for the norming sample (Roid & Miller, 1997). Growth scale value scores were used to avoid floor effects associated with standard scores in this sample with ID.
Results
Descriptive Statistics
Analyses were conducted in SAS 9.4. First, descriptive statistics were computed and data were visually examined for normal distributions. The Box Cox transformation technique (Box & Cox, 1964) was applied to correct for positive skew in the first fixation duration variable (λ = −1.00). No other variables required transformation. Means, standard deviations, and ranges for each of the predictor and outcome variables are presented in Table 2.
Table 2.
Descriptive statistics
| Group | ||
|---|---|---|
| Variable | FXS | Typical Development |
| Mean (SD) Range |
Mean (SD) Range |
|
| First Fixation Duration in ms: Direct Condition (untransformed) | 372.17 (145.38) 212.50–937.75 | 925.30 (653.67) 255.75–2868.63 |
| First Fixation Duration in ms: Averted Condition (untransformed) | 380.51 (166.94) 184.38– 931.25 | 966.40 (751.38) 191.00–3568.88 |
| First Fixation Duration: Direct Condition (transformed) | 0.9971 (0.0008) 0.9953–0.9989 | 0.9984 (0.0010) 0.9961–0.9997 |
| First Fixation Duration: Averted Condition (transformed) | 0.9970 (0.0010) 0.9946–0.9989 | 0.9984 (0.0011) 0.9948–0.9997 |
| ADAMS, Social Avoidance Subscale | 4.50 (2.73) 0–9.00 | n/a |
| Social Avoidance Scale, Total Score | 2.18 (0.87) 1.00–3.67 | n/a |
| ADOS-2, Calibrated Severity Score | 5.38 (2.53) 1.00–10.00 | n/a |
| Leiter-R, Brief IQ Growth Scale Value | 466.48 (8.84) 448.00–484.00 | n/a |
Note. ADAMS= Anxiety Depression and Mood Scale; ADOS-2= Autism Diagnostic Interview Schedule-2; Leiter-R= Leiter International Performance Scale-Revised.
Comparison of Initial Fixation on the Eyes across Males with FXS and Typically Developing Males
The first analysis tested differences between the males with FXS and their age-matched typically developing peers on the duration of the first fixation within the eye region across conditions (averted gaze, direct gaze). A linear mixed effects model was fit using maximum likelihood estimation with an unstructured covariance matrix, specified using PROC MIXED in SAS 9.4 (SAS Institute, 2013). Condition was specified as a fixed effect, nested within the individual. Group membership and its interaction with condition were also included as fixed effects to test for their potential influences on looking behavior. Results indicated a significant group effect, with the males with FXS having shorter first fixation durations on the eyes relative to control participants (F [1, 44] = 28.36, p < .001). The main effect of condition was not significant (F [1, 44] = 0.06, p = .815) and neither was the group-by-condition interaction term (F [1, 44] = 0.02, p = .883), indicating that both groups responded to direct and averted gaze in a similar manner.
Performance on the Social Gaze Eye Tracking Task as a Predictor of Social Avoidance
Parent-reported Social Avoidance Symptoms.
General linear models were run to test the first fixation duration in each condition as a predictor of the ADAMS social avoidance subscale. Nonverbal cognitive level and ASD severity were covaried in these models. The model testing the first fixation duration in the direct gaze condition as a predictor of the ADAMS social avoidance subscale was not significant (F [3, 20] = 2.26, p = .113, R2 = .25). The overall model testing the first fixation duration in the averted condition was significant (F [3, 20] = 3.48, p = .035, R2 = .34). However, while the combined influence of the first fixation duration, ASD symptom severity, and nonverbal cognition accounted for significant variance in the ADAMS social avoidance score, no individual predictor accounted for significant variance on its own. A trend was observed for the first fixation duration on the averted eyes to account for unique variance in the ADAMS score (F [1, 20] = 3.02, p = .097), with a partial eta-squared effect size (η2p) of 0.13 consistent with a “large” effect (Cohen, 1988). Nonverbal cognitive ability did not account for significant variance in the ADAMS social avoidance score (F [1, 20] = 0.68, p = .419, η2p = 0.03), but a trend-level effect of a large effect size was observed for the influence of ASD severity in the model (F [1, 20] = 4.19, p = .054, η2p = 0.17).
Examiner-rated Social Avoidance Behavior.
Next, we tested whether looking behavior during the eye tracking paradigm could predict social avoidance behavior exhibited during initial greeting with an examiner. A general linear model tested the first fixation duration in each condition as predictors of the Total Score on the Social Avoidance Scale, controlling for nonverbal cognitive level and ASD severity. The Benjamini-Hochberg procedure (Benjamini & Hochberg, 1995) was applied to control for multiple comparisons. The overall model testing the first fixation duration in the direct gaze condition was not significant (F [3, 20] = 2,35, p = .103, R2 = .26). The model testing the first fixation duration in the averted gaze condition accounted for significant variance in the Total Score of the Social Avoidance Scale (F [3, 20] = 5.23, p = .008, R2 = .44). A significant effect for the first fixation duration in response to averted gaze was detected (F [1, 20] = 8.64, p = .008), with a shorter first fixation duration associated with increased socially avoidant behavior (see Figure 2). A partial eta-squared effect size (η2p) of 0.30 was detected for this effect, consistent with a “large” effect (Cohen, 1988). Neither nonverbal cognitive ability (F [1, 20] = 1.87, p = .187, η2p = 0.09) nor ASD severity (F [1, 20] = 0.95, p = .342, η2p = 0.05) accounted for significant variance in the outcome.
Figure 2.
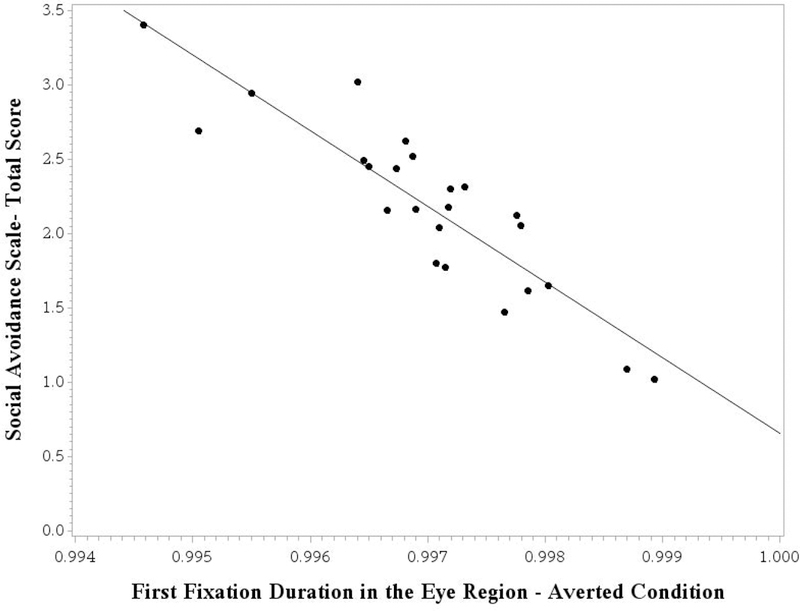
First Fixation Duration in the Eye Region as a Predictor of Social Avoidance Behavior Sampled from a Semi-Naturalistic Context
Note. Model-adjusted values are shown, controlling for nonverbal cognitive ability and ASD severity. A higher score on the Social Avoidance Scale reflects increased socially avoidant behavior. The first fixation duration variable relates to transformed data (power transformation of λ = −1.00).
Association between Parent-report and Examiner-Rated Indices of Social Avoidance
Finally, to shed light on the added value of eye tracking measures relative to parent-report measures of social avoidance that are commonly used in clinical trials, we conducted a follow-up analysis testing the ADAMS social avoidance subscale as a predictor of the Social Avoidance Scale. The combined effects of the ADAMS social avoidance subscale, nonverbal cognitive ability, and ASD severity did not account for significant variance in the Social Avoidance Scale-Total Score (F [3, 20] = 2.21, p = .118, R2 = .25). The ADAMS social avoidance subscale was not a significant predictor of the Social Avoidance Scale-Total Score (F [1, 22] = 1.38, p = .254), although the effect size was consistent with a medium effect (η2p = .06). Nonverbal cognitive ability (F [1, 22] = 2.07, p = .166, η2p = .09) and ASD severity (F [1, 22] = 0.81, p = .380, η2p = .04) did not contribute significantly to the model.
Discussion
FXS is characterized by gaze avoidance, reduced social approach, and social anxiety. The lack of sensitive measurement tools for these core phenotypic features has proven a major barrier to evaluating therapeutics in FXS clinical trials. This study investigated the utility of a novel experimental eye tracking paradigm for indexing social avoidance-related behavior in males with FXS. A key finding was that the social gaze eye tracking paradigm was successful at tapping subtle looking behaviors that mapped onto meaningful phenotypic variation in social avoidance behavior. Indeed, performance on the eye tracking paradigm was better able to predict avoidant behavior exhibited by the males in a semi-naturalistic context than were social avoidance symptoms captured by a parent-report questionnaire. We conclude that this eye tracking paradigm is sensitive to clinically meaningful variation in social avoidance behavior and may hold promise as an outcome measure for FXS clinical trials.
Utility of the Social Gaze Eye Tracking Paradigm as an Outcome Measure in FXS Clinical Trials
Progress in the development of pharmaceutical treatments for FXS has been blocked, in part, by the lack of appropriate outcome measures (Berry-Kravis et al., 2013; Budimirovic et al., 2017; Jacquemont et al., 2014). The measurement of social anxiety-related behavior, in particular, has proven challenging. A recent National Institutes of Health Working Group on outcome measures for FXS clinical trials concluded that few suitable anxiety measures are currently available for use in clinical trials (Berry-Kravis et al., 2013). The development of useful social anxiety outcome measure is a worthwhile objective because social anxiety represents a clinically impairing, neurobiologically-relevant aspect of the FXS phenotype. Social anxiety-related behaviors, such as social avoidance, represent common targets in FXS clinical trials (Wright, 2016). We propose that the social gaze eye tracking paradigm used in the present study could serve as a useful outcome measure for FXS clinical trials. We provide initial evidence of convergent validity, as the information gleaned from the social gaze eye tracking paradigm was highly predictive of examiner-rated social avoidance behavior sampled from a semi-naturalistic social greeting context.
Prior clinical trials focused on social avoidance outcomes in FXS have relied predominately on parent-report questionnaires. Overreliance on parent-report tools has been identified as a limiting factor in the success of extant FXS clinical trials because these types of measures are subject to rater bias and placebo effects and most capture gross behaviors that likely lack sensitivity to subtle behavioral improvement (Budimirovic et al., 2017). The ADAMS, which was examined as a parent-reported social avoidance measure in the present study, has been used as an outcome in several FXS clinical trials (e.g., Berry-Kravis et al., 2017; Ligsay et al., 2017). Our results suggest the social gaze eye tracking paradigm may be more sensitive to subtle variation in social avoidance behavior than the ADAMS. The first fixation duration in response to averted gaze was a robust predictor of social avoidance behavior exhibited by the participants during initial greeting with the examiner, with a large effect size. In contrast, parent-reported symptom severity on the ADAMS was not a significant predictor of performance in the semi-naturalistic context, with a small-to-medium effect size. Compared to the ADAMS, the social gaze eye tracking paradigm was better able to capture subtle behavioral information that mapped closely onto social avoidance behavior exhibited in a semi-naturalistic setting.
Another advantageous feature of the social gaze eye tracking paradigm is that it can be used across the full range of cognitive skills observed in the FXS population, at least in adolescent or older individuals. The present sample consisted of low-functioning males with FXS, demonstrating appropriateness for use with individuals with moderate ID. Overall, 89% of males with FXS who were initially recruited into the study were able to participate in the eye tracking assessment; higher success rates may be achieved in future work with the implementation of behavioral training techniques or the use of calibration targets with increased visual appeal (two participants were unable to contribute useable data because of failure to attend to the calibration targets). In our prior work, we have also demonstrated that the social gaze eye tracking paradigm is also sensitive to FMR1-related social phenotypes in individuals with the FMR1 premutation, providing evidence that the task is also valid and appropriate for individuals who do not have intellectual impairment (Klusek et al., 2017).
A final strength of the social gaze eye tracking paradigm is that it is feasible for wide-scale administration. Relatively little staff training is required to run the eye tracker, which is advantageous compared to traditional behavioral coding methods such as the Social Avoidance Scale, which necessitate time-consuming training and reliability procedures. Moreover, post-collection processing of the eye tracking data is essentially automated (the program was configured to automatically generate a results file containing all of the relevant data). The relatively quick administration time also improves feasibility. Average administration time in this study was about 15–20 minutes. However, our results suggest that simple modifications could shorten the administration time to about 5–10 minutes while maintaining validity. Specifically, the longest observed initial fixation duration was less than 1 second in length, suggesting that the 6-second display time could be shorted to about 2 seconds without data loss. Although cost has represented a barrier to the use of eye tracking technology in the past, advances in technology have allowed for the development of many affordable systems in the $1–10k range that offer acceptable accuracy and precision, such as the Gazepoint GP3 HD 105 Hz system and the Pupil-Labs Eye Camera.
Overall, our results are in line with others who have drawn attention to the promise of eye tracking indices to improve the measurement of outcomes for FXS clinical trials (e.g., Budimirovic et al., 2017; Farzin et al., 2009). For example, preliminary findings from a recent Novartis AFQ056 phase 2 FXS clinical trial suggest that eye tracking indices revealed treatment effects whereas traditional caregiver-reported behavioral ratings did not (Berry-Kravis et al., 2016; Hessl et al., 2019). In that clinical trial, the eye tracking paradigm described by Farzin et al. (2009) was used as an outcome measure. This paradigm consists of facial stimuli that are displayed for a 3-second interval, with the number of fixations on the eye region used as the outcome measure of interest. Compared to the Farzin protocol, the social gaze eye tracking paradigm employed here has some key differences. First, our eye tracking metric used a gaze-contingent trigger to initiate the onset of the trial, which guaranteed that participants were attending to the eyes at the onset of the trial. Additionally, the use of the first fixation duration on the eyes as the outcome variable ensured that sustained attention to the screen was not necessary for participants to contribute valid data. The first fixation is a relatively brief index, averaging at about one-third of a second in duration within the FXS group. In contrast, the Farzin protocol may be more vulnerable to missing data as participants must sustain attention to the facial stimuli for a 3-second interval in order to validly capture the total number of fixations on the eyes. In the present study, we were successful at obtaining valid data on >80% of trials for all participants who completed the eye tracking task, whereas the proportion of trials in which valid data were captured sometimes dipped below 80% in the Farzin protocol of the AFQ056 clinic trial (Hessl et al., 2019). A strength of the Farzin paradigm is that test-retest reliability has been established (Farzin et al., 2011) and preliminary data supports its sensitivity to subtle changes following mavoglurant treatment, which is significant (Hessl et al., 2019). Follow-up is needed to determine the test-retest reliability of the social gaze eye tracking paradigm used in the current report. Direct comparison of the social gaze eye tracking paradigm and the Farzin eye tracking paradigm in future work would be useful to inform the relative strengths and weaknesses of each methods.
Boys with FXS did not Show Avoidance of Direct Gaze Stimuli
Because direct eye contact is thought to represent a fear-associated stimulus in FXS (e.g., Cohen et al., 1989), we hypothesized that the males with FXS would show avoidance of the direct gaze stimuli, marked by shorter initial fixation on eyes displaying direct gaze relative to averted gaze. Contrary to expectations, males with FXS showed similar first fixation durations on the eyes across the direct and averted gaze conditions. The typically developing control males also did not differentiate between direct and averted gaze in their initial fixation times, suggesting that this was a “normal” response. Thus, we did not detect evidence that the males with FXS showed aversion to direct gaze, at least as indexed by performance on the social gaze eye tracking paradigm. It is possible that the animated facial stimuli were too far removed from real-life interaction to elicit the expected fear-related response.
We did find, however, that the males with FXS showed shorter initial fixation in the eye region in both conditions relative to their typically developing peers, which may suggest general avoidance of the eyes that occurs in FXS regardless of gaze direction. The lack of comparable social avoidance data in the control males is a limitation of the present study; measurement limitations prevented the inclusion of a social avoidance index that would be appropriate across the full range of cognitive abilities represented across both groups. In future work, the inclusion of a developmental delay comparison group would allow for both chronological and mental age matching and allow for further exploration into the specificity of the relationship between the fixation data and social avoidance symptoms.
It is notable that several recent behavioral and neuroimaging studies of FXS that have also failed to link social anxiety-related behavior with direct gaze processing. For example, in an fMRI study of gaze processing in individuals with FXS, (Bruno, Garrett, Quintin, Mazaika, & Reiss, 2014) detected similar neural habituation patterns following exposure to direct and averted gaze, suggesting that exposure to direct gaze did not elicit an exaggerated neural response in individuals with FXS. In another report, Holsen et al. (2008) failed to detect an association between social anxiety symptoms and the time that individuals with FXS spent fixating on the eyes or other parts of the face during a facial encoding eye tracking task. The neuroimaging data Holsen and colleagues collected during the task implicated dysfunctional social cognitive networks rather than anxiety networks in impaired face processing in FXS. Moreover, recent behavioral work has shown that the level of gaze avoidance exhibited by children with FXS is independent of social task demands (Murphy et al., 2007). In light of this growing evidence base, it may be informative to systematically detail how attentional responses to eye gaze in FXS vary according to factors such as exposure latency and stimulus valence, which would allow us to better place our understanding of gaze avoidance in FXS within cognitive-motivational frameworks of attentional bias. The possibility that attentional patterns may vary as a function of anxiety disorder type and severity, as has been suggested in some recent reports (e.g., Salum et al., 2013; Waters, Bradley, & Mogg, 2014), and is another prime avenue for investigation.
Shorter Initial Fixation on Averted Gaze Predicted More Severe Social Avoidance Behavior
Although we found limited evidence that initial attention to the direct gaze stimuli was associated with social avoidance symptoms, an important finding of this study is that initial fixation on the averted gaze stimuli was highly predictive of social avoidance behavior exhibited by the males with FXS during initial greeting with the examiner, as indexed by the Social Avoidance Scale. The association was of a large effect size and was present even after controlling for nonverbal cognitive level and ASD symptom severity. The fact that performance on this relatively artificial eye tracking task mapped onto real-life behavior so closely highlights the powerful salience of the eyes. Thus, we have demonstrated that initial fixation duration on averted gaze tapped subtle aspects of behavior that mapped closely onto the severity of directly observed social avoidance symptoms. A similar trend was also observed with the parent-report social avoidance measures in which participants with FXS who showed longer initial fixation on averted gaze tended to have lower scores on the ADAMS social avoidance subscale. While the association with the ADAMS did not reach statistical significance, a large effect size was observed suggesting that the model may have been underpowered. Nonetheless, the results call into question the sensitivity of parent-reported metric of social avoidance with young adult-males with FXS. In particular, performance on the eye tracking task more closely aligned with the examiner-rated social avoidance measure, and follow-up analyses indicated that parent-reported social avoidance symptoms on the ADAMS was not predictive of social avoidance behavior rated by the examiner.
It is curious that fixation during the averted gaze condition, but not direct gaze, was related to social avoidance severity. Increased salience of averted gaze has also been documented in women carriers of the FMR1 premutation (Klusek et al., 2017), suggesting that atypical social processing could be an FMR1-mediated feature. While direct gaze has clear evolutionary significance as a signal of intent to approach (Emery, 2000), the significance of averted gaze is context-dependent and thus may rely more heavily on social cognitive skills to interpret. For example, neural activity in response to averted gaze differs when the gaze is directed towards an object versus an empty space, suggesting that the perceived goal of averted gaze influences how it is processed (Pelphrey, Singerman, Allison, & McCarthy, 2003). Averted gaze is often associated with joint attention skills because the ability to follow others’ gaze shifts allows for the alignment of attentional focus between communication partners (Rothkirch, Madipakkam, Rehn, & Sterzer, 2015). It is possible that males with FXS who fixated longer on averted gaze were more aware of the social-cognitive implications of this signal, which translated to better performance during the initial greeting with the examiner. This is consistent with evidence that behaviors captured by the Social Avoidance Scale are influenced by the presence of ASD, a disorder that is characterized by central impairments in social cognition (Roberts et al., 2018; Roberts et al., 2007). Further highlighting the complex interlacing of social anxiety and social-cognitive features in FXS, some neuroimaging evidence suggests that social anxiety features of FXS could be linked to the failure to engage social cognitive networks during the initial phases of memory formation (Holsen et al., 2008). There is a need to better understand the interface between social cognitive impairment and social anxiety and their combined contributions to aberrant gaze processing in FXS in order to develop targeted treatments. Future investigations specifically incorporating direct measurement of social-cognitive skills would allow for better differentiation of the specific influences of social anxiety and social cognition on gaze processing in FXS. Additionally, future comparison with other neurodevelopmental groups characterized by ASD/ID could better inform aspects of atypical gaze behavior that are unique to the FXS phenotype.
Conclusion
The advancement of FXS therapeutics hinges upon the development of tools that are sensitive to changes in core behavioral aspects of the FXS phenotype. Social avoidance is a clinically relevant, biologically meaningful feature that is central to the FXS phenotype; yet, many existing measurement tools have proved insufficient for capturing subtle therapeutic changes within this symptom domain. Social avoidance and social anxiety-related behaviors are also prevalent in many other neurodevelopmental disorders, including ASD, and the development of tools that are sensitive to social avoidance-related phenotypes and are appropriate for use across a range of cognitive skills has implications for therapeutic advancement across neurodevelopmental disabilities more broadly. In this study, we presented a novel eye tracking paradigm that may serve as a useful outcome measure in clinical trials targeting social avoidance outcomes across individuals with a wide range of cognitive abilities.
Acknowledgments
We would like to thank the families who participated in this study. We also appreciate the assistance of Debra Reisinger, Emily Schworer, Sara Matherly, and Lisa Rague in conducting the participant assessments. This research was supported by the National Institutes of Health (F32DC013934; R01HD024356; U54HD079125, R01MH107573) and the Research Participant Registry Core of the Carolina Institute for Developmental Disabilities (P30HD03110).
Funding Sources
This research was supported by the National Institutes of Health (F32DC013934; R01HD024356; U54HD079125, R01MH107573) and the Research Participant Registry Core of the Carolina Institute for Developmental Disabilities (P30HD03110).
L.A. is currently serving as an advisor to Fulcrum Therapeutics and has received funding in the past to implement outcome measures in clinical trials from F. Hoffmann-La Roche Ltd., Roche TCRC, Inc., and Neuren Pharmaceuticals Ltd.
Footnotes
Conflicts of Interest
The authors have no others conflicts to disclose.
Contributor Information
Jessica Klusek, Department of Communication Sciences and Disorders, University of South Carolina.
Carly Moser, Department of Psychology, University of South Carolina.
Joseph Schmidt, Department of Psychology, University of Central Florida.
Leonard Abbeduto, Department of Psychiatry and Behavioral Sciences and MIND Institute, University of California, Davis.
Jane E. Roberts, Department of Psychology, University of South Carolina
References
- Abbeduto L, Thurman AJ, McDuffie A, Klusek J, Feigles RT, Brown WT, . . . Dobkins C. (2019). ASD comorbidity in fragile X syndrome: symptom profile and predictors of symptom severity in adolescent and young adult males. Journal of Autism and Developmental Disorders, 49(3), 960–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbeduto L, Thurman AJ, McDuffie A, Klusek J, Feigles RT, Ted Brown W, . . . Roberts JE (2018). ASD comorbidity in fragile X syndrome: Symptom profile and pedictors of symptom severity in adolescent and young adult males. Journal of Autism and Developmental Disorders, e-pub ahead of print. doi: 10.1007/s10803-018-3796-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RB, & Kleck RE (2003). Perceived gaze direction and the processing of facial displays of emotion. Psychological Science, 14(6), 644–647. doi: 10.1046/j.0956-7976.2003.psci_1479.x [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association; (2013). Diagnostic and statistical manual of mental disorders (5th ed.; DSM-5 ed.): American Psychiatric Publishing, Incorporated. [Google Scholar]
- Baron-Cohen S, & Ring H. (1994). A model of the mindreading system: Neuropsychological and neurobiological perspectives. Origins of an understanding of mind, 183–207. [Google Scholar]
- Benjamini Y, & Hochberg Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. doi:https://www.jstor.org/stable/2346101 [Google Scholar]
- Berry-Kravis E, Des Portes V, Hagerman R, Jacquemont S, Charles P, Visootsak J, . . . Zhu L. (2016). Mavoglurant in fragile X syndrome: results of two randomized, double-blind, placebo-controlled trials. Science Translational Medicine, 8(321), 321ra325–321ra325. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Hagerman RJ, Visootsak J, Budimirovic DB, Kaufmann WE, Bear MF, . . . Carpenter RL (2017). Arbaclofen in fragile X syndrome: Results of phase 3 trials. Journal of Neurdevelopmental Disorders, 9. doi: 10.1186/s11689-016-9181-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Abbeduto L, Reiss AL, Beckel-Mitchener A, & Urv TK (2013). Outcome measure for clinical trials in fragile X syndrome. Journal of Developmental and Behavioral Pediatrics, 34(7), 508–522. doi: 10.1097/DBP.0b013e31829d1f20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögels SM, & Mansell W. (2004). Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clinical Psychology Review, 24(7), 827–856. doi: 10.1016/j.cpr.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Box GE, & Cox DR (1964). An analysis of transformations. Journal of the Royal Statistical Society. Series B (Methodological), 211–252. [Google Scholar]
- Bruno JL, Garrett AS, Quintin E, Mazaika PK, & Reiss AL (2014). Aberrant face and gaze habituation in fragile X syndrome. American Journal of Psychiatry, 171(10), 1099–1106. doi: 10.1176/appi.ajp.2014.13111464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budimirovic DB, Berry-Kravis E, Erickson CA, Hall SS, Hessl D, Reiss AL, . . . Kaufmann WE (2017). Updated report on tools to measure outcomes of clinical trials in fragile X syndrome. Journal of Neurodevelopmental Disorders, 9(1), 14. doi: 10.1186/s11689-017-9193-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budimirovic DB, Bukelis I, Cox C, Gray RM, Tierney E, & Kaufmann WE (2006). Autism spectrum disorder in fragile X syndrome: Differential contribution of adaptive socialization and social withdrawal. American Journal of Medical Genetics Part A, 9999, 1–13. [DOI] [PubMed] [Google Scholar]
- Cohen IL (1995). A theoretical analysis of the role of hyperarousal in the learning and behavior of fragile X males. Mental Retardation and Developmental Disabilities Research Reviews, 1(4), 286–291. doi: 10.1002/mrdd.1410010410 [DOI] [Google Scholar]
- Cohen IL, Vietze PM, Sudhalter V, Jenkins EC, & Brown WT (1989). Parent-child dyadic gaze patterns in fragile X males and in non-fragile X males with autistic disorder. Journal of Child Psychology and Psychiatry and Allied Disciplines, 30(6), 845–856. doi: 10.1111/j.1469-7610.1989.tb00286.x [DOI] [PubMed] [Google Scholar]
- Cohen J. (1960). A Coefficient of Agreement for Nominal Scales. Educational and Psychological Measurement, 20(1), 37–46. [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences. Hillsdale, NJ: L. Erlbaum Associates. [Google Scholar]
- Constantino J, & Gruber C. (2012). The Social Responsiveness Scale Manual, Second Edition (SRS-2). In. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Cordeiro L, Ballinger E, Hagerman R, & Hessl D. (2011). Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: prevalence and characterization. Journal of Neurodevelopmental Disorders, 3, 57–67. doi: 10.1007/s11689-010-9067-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Acuna JM, & Sherman SL (2001). FMR1 and the fragile X syndrome: Human genome epidemiology review. Genetics in Medicine, 3, 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KM, Holsen L, Abbeduto L, & Davidson RJ (2008). Brain function and gaze fixation during facial-emotion processing in fragile X and autism. Autism Research, 1(4), 231–239. doi: 10.1002/aur.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery NJ (2000). The eyes have it: the neuroethology, function and evolution of social gaze. Neuroscience and Biobehavioral Reviews, 24(6), 581–604. doi: 10.1016/S0149-7634(00)00025-7 [DOI] [PubMed] [Google Scholar]
- Esbensen AJ, Rojahn J, Aman MG, & Ruedrich S. (2003). Reliability and validity of an assessment instrument for anxiety, depression, and mood among individuals with mental retardation. Journal of Autism and Developmental Disorders, 33(6), 617–629. doi: 10.1023/B:JADD.0000005999.27178.55 [DOI] [PubMed] [Google Scholar]
- Farroni T, Csibra G, Simion F, & Johnson MH (2002). Eye contact detection in humans from birth. Proceedings of the National Academy of Sciences, 99(14), 9602–9605. doi: 10.1073/pnas.152159999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Rivera SM, & Hessl D. (2009). Brief report: Visual processing of faces in individuals with fragile X syndrome: an eye tracking study. Journal of Autism and Developmental Disorders, 39(6), 946–952. doi: 10.1007/s10803-009-0744-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Scaggs F, Hervey C, Berry-Kravis E, & Hessl D. (2011). Reliability of Eye Tracking and Pupillometry Measures in Individuals with Fragile X Syndrome. Journal of Autism and Developmental Disorders. doi: 10.1007/s10803-011-1176-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finestack LH, Richmond EK, & Abbeduto L. (2009). Language development iniIndividuals with fragile X syndrome. Topics in Language Disorders, 29(2), 133–148. doi: 10.1097/TLD.0b013e3181a72016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Nonell C, Ratera ER, Harris S, Hessl D, Ono MY, Tartaglia N, . . . Hagerman RJ (2008). Secondary medical diagnosis in fragile X syndrome with and without autism spectrum disorder. American Journal of Medical Genetics Part A, 146A(15), 1911–1916. doi: 10.1002/ajmg.a.32290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M, Mogg K, & Bradley BP (2006). Orienting and maintenance of gaze to facial expressions in social anxiety. Journal of Abnormal Psychology, 115(4), 760. doi: 10.1037/0021-843X.115.4.760 [DOI] [PubMed] [Google Scholar]
- Hagerman R, & Hagerman P. (Eds.). (2002). Fragile X Syndrome: Diagnosis, Treatment, and Research (3rd ed.). Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- Hall S, DeBernardis M, & Reiss A. (2006). Social escape behaviors in children with fragile X syndrome. Journal of Autism and Developmental Disorders, 36(7), 935–947. doi: 10.1007/s10803-006-0132-z [DOI] [PubMed] [Google Scholar]
- Hall SS, Frank MC, Pusiol GT, Farzin F, Lightbody AA, & Reiss AL (2015). Quantifying naturalistic social gaze in fragile X syndrome using a novel eye tracking paradigm. American Journal of Medical Genetics: Part B, 168(7), 564–572. doi: 10.1002/ajmg.b.32331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Huffman LC, Lazzeroni LC, & Reiss AL (2009). Physiological correlates of social avoidance behavior in children and adolsecents with fragile X syndrome. Journal of the American Academy of Child and Adolescent Psychiatry, 48(3), 320–329. doi:0.1097/CHI.0b013e318195bd15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JM (2003). Human gaze control during real-world scene perception. Trends in cognitive sciences, 7(11), 498–504. doi: 10.1016/j.tics.2003.09.006 [DOI] [PubMed] [Google Scholar]
- Hernandez RN, Feinberg RL, Vaurio R, Passanante NM, Thompson RE, & Kaufmann WE (2009). Autism spectrum disorder in fragile X syndrome: A longitudinal evaluation. American Journal of Human Genetics, 149A(6), 1125–1137. doi: 10.1002/ajmg.a.32848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, & Reiss AL (2006). Social behavior and cortisol reactivity in children with fragile X syndrome. Journal Of Child Psychology And Psychiatry, And Allied Disciplines, 47(6), 602–610. doi: 10.1111/j.1469-7610.2005.01556.x [DOI] [PubMed] [Google Scholar]
- Hessl D, Harvey D, Sansone S, Crestodina C, Chin J, Joshi R, . . . Berry-Kravis E. (2019). Effects of mavoglurant on visual attention and pupil reactivity while viewing photographs of faces in Fragile X Syndrome. PloS One, 14(1), e0209984. doi: 10.1371/journal.pone.0209984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Dalton KM, Johnstone T, & Davidson RJ (2008). Prefrontal social cognition network dysfunction underlying face encoding and social anxiety in fragile X syndrome. Neuroimage, 43(3), 592–604. doi: 10.1016/j.neuroimage.2008.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J, Rivero-Arias O, Angelov A, Kim E, Fotheringham I, & Leal J. (2014). Epidemiology of fragile X syndrome: A systematic review and meta-analysis. American Journal of Medical Genetics Part A, 164(7), 1648–1658. doi: 10.1002/ajmg.a.36511 [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Berry-Kravis E, Hagerman R, von Raison F, Gasparini F, Apostol G, . . . Gomez-Mancilla B. (2014). The challenges of clinical trials in fragile X syndrome. Psychopharmacology, 231(6), 1237–1250. doi: 10.1007/s00213-013-3289-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Capone G, Clarke M, & Budimirovic DB (2008). Autism in Genetic Intellectual Disability: Insights into Idiopathic Autism In Zimmerman AW (Ed.), Autism: Current Theories and Evidence. (pp. 81–108). Totowa, N.J: The Humana Press Inc. [Google Scholar]
- Kaufmann WE, Kidd SA, Andrews HF, Budimirovic DB, Esler A, Haas-Givler B, . . . Sherman SL (2017). Autism spectrum disorder in fragile X syndrome: cooccurring conditions and current treatment. Pediatrics, 139(Suppl 3), S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Martin GE, & Losh M. (2013). Physiological arousal in autism and fragile X syndrome: Group comparisons and links with pragmatic language. American Journal on Intellectual and Developmental Disabilities, 118(6), 475–495. doi: 10.1352/1944.7558-118.6.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Martin GE, & Losh M. (2014a). A comparison of pragmatic language in boys with autism and fragile X syndrome. Journal of Speech, Language, and Hearing Research, 57, 1692–1707. doi: 10.1044/2014_JSLHR-L-13-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Martin GE, & Losh M. (2014b). Consistency between research and clinical diagnoses of autism among boys and girls with fragile X syndrome. Journal of Intellectual Disability Reasearch, 58, 940–952. doi: 10.1111/jir.12121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Roberts JE, & Losh M. (2015). Cardiac autonomic regulation in autism and fragile X syndrome: A review. Psychological Bulletin, 141, 141–175. doi:http://dx.doi.org.pallas2.tcl.sc.edu/10.1037/a0038237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Schmidt J, Fairchild AJ, Porter A, & Roberts JE (2017). Altered sensitivity to social gaze in the FMR1 premutation and pragmatic language competence. Journal of Neurodevelopmental Disorders, 9, 31. doi: 10.1186/s11689-017-9211-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligsay A, Van Dijck A, Nguyen DV, Lozano R, Chen Y, Bickel ES, . . . Hagerman RJ (2017). A randomized double-blind, placebo-controlled trial of ganaxolone in children and adolescents with fragile X syndrome. Journal of Neurodevelopmental Disorders, 9(1), 26–26. doi: 10.1186/s11689-017-9207-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddalena A, Richards CS, McGinniss MJ, Brothman A, Desnick RJ, Grier RE, . . . Popovich B. (2001). Technical standards and guidelines for fragile X: the first of a series of disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics. Genetics in Medicine, 3(3), 200–205. doi: 10.1097/00125817-200105000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matherly S, Klusek J, Thurman AJ, McDuffie A, Abbeduto L, & Roberts JE (2018). Cortisol profiles differentiated in adolescents and young adult males with fragile X syndrome versus autism spectrum disorder. Developmental Psychobiology, 60, 78–89. doi: 10.1002/dev.21578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir F, Cornish KM, & Wilding J. (2000). A neuropsychological profile of attention deficits in young males with fragile X syndrome. Neuropsychologia, 38, 1261–1270. doi: 10.1016/S0028-3932(00)00036-1 [DOI] [PubMed] [Google Scholar]
- Murphy MM, Abbeduto L, Schroeder S, & Serlin R. (2007). Contribution of social and information-processing factors to eye-gaze avoidance in fragile X syndrome. American Journal on Mental Retardation, 112(5), 349–360. doi: 10.1352/0895-8017(2007)112[0349:COSAIF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Singerman JD, Allison T, & McCarthy G. (2003). Brain activation evoked by perception of gaze shifts: the influence of context. Neuropsychologia, 41(2), 156–170. doi: 10.1016/S0028-3932(02)00146-X [DOI] [PubMed] [Google Scholar]
- Reiss AL, & Freund L. (1992). Behavioral phenotype of fragile X syndrome: DSM-III-R autistic behavior in male children. American Journal of Medical Genetics, 43, 35–46. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Clarke MA, Alcorn K, Carter JC, Long ACJ, & Kaufman WE (2009). Autistic behavior in boys with fragile X syndrome: social approach and HPA-axis dysfunction. Journal of Neurodevelopmental Disorders, 1(4), 283–291. doi: 10.1007/s11689-009-9028-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Ezell JE, Fairchild AJ, Klusek J, Thurman AJ, McDuffie A, & Abbeduto L. (2018). Biobehavioral composite of social aspects of anxiety in young adults with fragile X syndrome contrasted to autism spectrum disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. doi: 10.1002/ajmg.b.32674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Weisenfeld LH, Hatton D, Heath M, & Kaufmann WE (2007). Social approach and autistic behavior in children with fragile X syndrome. Journal of Autism and Developmental Disorders, 37, 1748–1760. doi: 10.1007/s10803-006-0305-9 [DOI] [PubMed] [Google Scholar]
- Rothkirch M, Madipakkam AR, Rehn E, & Sterzer P. (2015). Making eye contact without awareness. Cognition, 143, 108–114. doi: 10.1016/j.cognition.2015.06.012 [DOI] [PubMed] [Google Scholar]
- Sabaratnam M, Murthy NV, Wijeratne A, Buckingham A, & Payne S. (2003). Autistic-like behaviour profile and psychiatric morbidity in fragile X sydnrome: A prospective ten-year follow-up study. European Child and Adolescent Psychiatry, 12, 172–177. doi: 10.1007/s00787-003-0333-3 [DOI] [PubMed] [Google Scholar]
- Salum G, Mogg K, Bradley B, Gadelha A, Pan P, Tamanaha A, . . . Polanczyk G. (2013). Threat bias in attention orienting: evidence of specificity in a large community-based study. Psychological Medicine, 43(4), 733–745. doi: 10.1017/S0033291712001651 [DOI] [PubMed] [Google Scholar]
- SAS Institute. (2013). SAS Institute version 9.4. In: Cary NC. [Google Scholar]
- Waters AM, Bradley B, & Mogg K. (2014). Biased attention to threat in paediatric anxiety disorders (generalized anxiety disorder, social phobia, specific phobia, separation anxiety disorder) as a function of ‘distress’ versus ‘fear’diagnostic categorization. Psychological Medicine, 44(03), 607–616. doi: 10.1017/S0033291713000779 [DOI] [PubMed] [Google Scholar]
- Watson C, Hoeft F, Garrett AS, Hall SS, & Reiss AL (2008). Aberrant brain activation during gaze processing in boys with fragile x syndrome. Archives of General Psychiatry, 65(11), 1315–1323. doi: 10.1001/archpsyc.65.11.1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser MJ, Pauli P, Alpers GW, & Mühlberger A. (2009). Is eye to eye contact really threatening and avoided in social anxiety?—An eye-tracking and psychophysiology study. Journal of Anxiety Disorders, 23(1), 93–103. doi: 10.1016/j.janxdis.2008.04.004 [DOI] [PubMed] [Google Scholar]
- Williams TA, Langdon R, & Porter MA (2013). Hyper-reactivity in fragile X syndrome females: Generalised or specific to socially-salient stimuli? A skin conductance study. International Journal of Psychophysiology, 88(1), 26–34. doi: 10.1016/j.ijpsycho.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Wolff PH, Gardner J, Paccia J, & Lappen J. (1989). The greeting behavior of fragile X males. American Journal on Mental Retardation. [PubMed] [Google Scholar]
- Wright J. (2016, August 8 2017). Despite setbacks, fragile X drugs file into clinical trials. Spectrum News. Retrieved from https://www.spectrumnews.org/news/despite-setbacks-fragile-x-drugs-file-clinical-trials/ [Google Scholar]


