Abstract
Background/Objectives:
Defining common patterns of recovery after an acute health stressor (resiliency groups) has both clinical and research implications. We sought to identify groups of patients with similar recovery patterns across 10 outcomes following hip fracture (stressor) and to determine the most important predictors of resiliency group membership.
Design:
Secondary analysis of 3 prospective cohort studies.
Setting:
Participants were recruited from various hospitals in the Baltimore Hip Studies network and followed for up to 1 year in their residence (home or facility).
Participants:
Community-dwelling adults aged ≥65 with recent surgical repair of a hip fracture (n=541).
Measures:
Self-reported physical function and activity measures using validated scales were collected at baseline (within 15-22 days of fracture), 2, 6, and 12 months. Physical performance tests were administered at all follow-up visits. Stressor characteristics, co-morbidities, psychosocial and environmental factors were collected at baseline via participant report and chart abstraction. Latent class profile analysis was used to identify resiliency groups based on recovery trajectories across 10 outcome measures and logistic regression models to identify factors associated with those groups.
Results:
Latent Profile Analysis identified three resiliency groups that had similar patterns across the 10 outcome measures and were defined as “high resilience” (n=163, 30.1%), “medium resilience” (n=242, 44.7%), and “low resilience” (n=136, 25.2%). Recovery trajectories for the outcome measures are presented for each resiliency group. Comparing highest to the medium and low resilience groups, self-reported pre-fracture function was by far the strongest predictor of high resilience group membership with AUC=0.84. Demographic factors, co-morbidities, stressor characteristics, environmental factors, and psychosocial characteristics were less predictive, but several factors remained significant in a multivariable model (AUC=0.88).
Conclusions:
These three resiliency groups following hip fracture may be useful for understanding mediators of physical resilience, and provide a more detailed description of recovery patterns in multiple outcomes for use in clinical decision-making.
Keywords: Resilience, Groups, Hip Fracture
Introduction
Clinicians have long recognized the wide variability in how rapidly and completely older adults recover from a physical stressor such as an illness or injury. Those with rapid and complete recovery are said to be highly physical resilient, while those who do not decline at all may be considered robust;1,2 in this paper we refer to both concepts as “resilience” because our measurement approach considers all possible trajectories. The concepts of physical resilience and frailty, commonly defined as a state of physiological vulnerability to stressors resulting from age‐related decline in biological systems, are likely related but have potentially important distinctions.3 While frailty is a state that imparts a high likelihood of decline following a physical stressor, resilience measures the individual’s ability to recover from the stressor, which may depend on different mechanisms. Physical resilience is further likely impacted by psychological, social and environmental factors.
A better understanding of the factors associated with patterns of physical resilience is potentially useful for several reasons. First, clinical decision-making may be enhanced if clinicians are able to counsel patients and caregivers on what recovery pattern to expect so that they can better plan for their needs. Second, health service delivery and policy can be refined so that we target enhanced rehabilitation services and other interventions more efficiently. Finally, new research discoveries about mediators and biological pathways underlying physical resilience can be leveraged to develop resilience-promoting interventions.
Recovery from hip fracture is a logical condition in which to study physical resilience in older adults. It is clinically important, causing substantial morbidity, mortality and cost worldwide4. However, there is variation in the timing and completeness of recovery between individuals and across multiple outcomes.5-10 Prior longitudinal studies have defined average trajectories of recovery for individual outcomes9 and described outcomes for subgroups of patients with similar baseline characteristics.6 However, a description of common patterns of recovery across multiple outcomes simultaneously (i.e., resilience “group”) is not available. Once resilience groups after hip fracture are defined, we can then identify patient characteristics, stressor characteristics, and other factors associated with resilience.
Selection of factors associated with resilience should be guided by a conceptual model of physical resilience. Current models suggest that a person’s recovery group is determined by their underlying demographic and psychosocial characteristics, environmental factors, and physiologic reserve.1 Physiologic reserve is likely determined in part by co-morbidities11-13 and biological processes at the tissue, cellular, or subcellular level14 and may be manifest in the individual’s baseline functional status.13 The magnitude and duration of the stressor could also affect the subsequent recovery pattern.
The purpose of this paper is to: 1) identify groups of patients with similar recovery patterns across 10 outcome measures following hip fracture (resilience groups); 2) determine the most important predictors of resilience group group membership; and 3) estimate average trajectories for the outcome measures for each resilience group to assist with clinical decision-making.
Methods
This was a secondary analysis combining 3 cohorts of the Baltimore Hip Studies (BHS) (n=727) [BHS-4 (n=180 females), BHS-5 (n=208 females), BHS-7 (n=171 females and 168 males)]; a series of longitudinal studies enrolling older adults during or immediately following a hospitalization for a low trauma hip fracture during the years 1998-2011.10,15,16 Enrollment criteria, measures, data collection protocols, and follow-up times were similar for the 3 studies, with exceptions noted below. Two of the studies included randomization to an exercise intervention or control, however the two intervention groups did not differ in their functional outcomes.16,17 Nevertheless, all analyses are adjusted for cohort membership and intervention group assignment.
Subjects and Enrollment
Across the 3 cohorts, eligible subjects were community-dwelling adults aged 65 years and older admitted for surgical repair of a non-pathologic hip fracture to select hospitals within the BHS hospital network in the greater Baltimore area [BHS-4 3 hospitals, BHS-5 6 hospitals and BHS-7 8 hospitals] and were English-speaking, able to walk without human assistance prior to the fracture, and lived within 70 miles of the study center. The two intervention studies included only females and excluded patients with moderate or severe cognitive impairment as defined by a Mini-Mental Status Exam score ≤ 20, end stage renal disease, cirrhosis, or metastatic cancer. Two of the studies that measured bone mineral density (BHS4 and BHS7) excluded those with hardware in the contralateral hip or weight > 300 lbs.. For this analysis subjects from the 3 BHS studies were excluded if they did not have outcome data available at 2 months and at least one of the 6 or 12-month follow-up periods for a final analytic sample of n=541.
Eligible patients were approached for study enrollment within 15 days of the hip fracture or hospital admission. Informed consent was obtained from all participants or their legally authorized representative. The eligibility rate for the 3 cohort studies ranged from 18%-19% for the 2 RCTs (BHS-4 and BHS-5) to 54% for observational study (BHS-7). The proportion of eligible patients enrolled in the 3 studies ranged from 39% for BHS-7 and 69%-74% for the RCTs. All study procedures were approved by the Institutional Review Board at the University of Maryland and at the individual study hospitals where recruitment took place.
Data Collection
Study data were collected by trained research assistants in the hospital at baseline, and subsequently in the participant’s place of residence (private home, rehabilitation or skilled nursing facility, or long-term care facility) at 2, 6, and 12 months.
At baseline, subjects or their representatives provided demographic information and completed validated self-report measures of their pre-hip fracture functional status. These included ambulatory status, Instrumental Activities of Daily Living (IADL) 18, Physical Activities of Daily Living (PADL) 18, and the Yale Physical Activity Scale19. Chart abstractors recorded information about medical diagnoses, medications, hip fracture type, anesthesia, surgical approach, and rehabilitation received. Hospital complications, including incident delirium, cardiovascular events, infections, and surgical complications were recorded.
Follow-up visits at 2, 6, and 12 months post-fracture took place in the participant’s place of residence and self-reported functional measures were repeated. In addition, physical performance tests were administered including 3 meter gait speed, a balance test (Short Physical Performance Battery 20 or Tinetti Gait and Balance Test 21), grip strength using a hand dynamometer, timed single chair stand 22, and the Lower Extremity Gain Scale (LEGS) 23. Subjects wore activity monitors over 2 days.
Outcomes
The outcomes of interest were trajectories of physical activity, physical performance, and functional status. The scoring and interpretation of each scale is as follows:
Self-reported ambulatory status. Participants were asked to report if they needed help to walk a block, scored as 1) Needs Assistance; 2) Uses Device; 3) Independent.
Instrumental Activities of Daily Living (IADL).18 Participants were asked to rate themselves as fully independent (1), requiring assistance (2) or fully dependent (3) on the following tasks over the last 2 weeks: getting to places out of walking distance; shopping for groceries or clothes; preparing meals; and housecleaning. Scores ranged from 4-12 with higher scores indicating greater level of assistance.
Lower Extremity Physical Activities of Daily Living (LPADL). The LPADL is a measure of lower extremity disability specifically adapted for hip fracture patients from the Functional Status Index.24 Participants were asked to rate the level of assistance they required for each of 12 tasks over the past week. Tasks included walking various distances, transferring, bathing, dressing, toileting, and reaching to pick up an item from the ground. Responses for each activity included: no help, used equipment, used human assistance, used equipment and human assistance, did not perform due to health reasons, and did not perform due to non-health reasons.9 Higher total scores (0-12) indicate greater use of assistance.
Yale Physical Activity Scale.19 Participants were asked whether or not they had engaged in any of 31 physical activities in 5 categories during a typical week in the past month; housework, yardwork, caretaking, exercise, and recreational activities. For each activity, they noted how many times they did it and the duration (hrs/week). The total time was multiplied by a standard intensity code for the activity (kcal/min) and summed across all activities to provide a total kcal of physical activities over the past month.
Gait speed. Research assistants recorded average time to walk a 3-meter distance at usual pace.
Balance test. For one cohort the Short Physical Performance Battery20 was used to assess balance. Participants were asked to balance for 10 seconds with their feet in a side-by-side, semi-tandem, and tandem position. Scores ranged from 0-4 with higher scores indicating worse balance. In the other 2 cohorts, the Tinetti Gait and Balance Test 21 was completed. The participant’s balance was rated in 9 categories as they rose from a seated position, stood with and without eyes closed, were nudged, turned 360 degrees, and sat down. Scores ranged from 0-16 with higher scores indicating better balance. Because of the different scales used, the SPPB was reverse coded so that higher scores indicated better balance, and a Z score indicating standard deviations above/below the mean was used to compare across scales.
Grip strength was measured using a calibrated research grade dynamometer. Both hands were assessed and the maximal value across all trials was used.
Timed single chair stand.22 Research assistants recorded the average time to complete a single chair stand.
Lower Extremity Gain Scale (LEGS).23 Participants were asked to (1) walk 3m (10ft); put on a (2) sock and (3) shoe on the fractured side; (4) rise from an armless chair; step (5) up and (6) down 4 stairs; get (7) on and (8) off the toilet; and (9) reach for an item on the ground from a sitting position. Each item is scored 0-4 reflecting the time taken and whether they were able to complete it independently, with higher total scores (range 0-36) indicating better function.
Activity monitoring. Each participant wore a research grade activity monitor during waking and sleeping hours over 2 days, removing it for bathing. One cohort used an Actigraph™, one used a Step Activity Monitor (SMA), and the third used a Caltrac™.
Data Harmonization and Missing Data
Variable names and categories were harmonized to be consistent across the 3 cohorts. Where different scales or methods were used to measure the same construct (e.g., 2 different balance scales, steps/day vs. Kcal/day) outcomes were standardized by dividing by the pooled standard deviation. Missing data were common during follow-up especially for the physical performance tests. Where the reason for missing data was known (e.g., coded by the data collector as “unable” or “too sick to perform”) a logical value was imputed (e.g., gait speed = 0 m/sec). Where the reason for missing data was unknown, multiple imputation was used as described below.
Analysis
Latent Class Profile Analysis (LCPA), a form of Latent Growth Mixture Modeling, was used to define groups of patients with common recovery patterns.25-27 This method allows us to incorporate trajectories of multiple outcome measures simultaneously (e.g., gait speed, balance, strength, self-reported function, etc.). Groups or classes of individuals who have similar patterns of recovery are identified.
First, we constructed trajectories for each outcome within each individual. The intercept was defined as the 2-month value for the outcome, and the slope was calculated using the best fitting line from that point through the 6 and 12 month values. Only subjects with complete data for all 10 outcomes were considered in a latent growth mixture model using Mplus v.7.4 to define resilience groups. Bayes Information Criterion (BIC), and other indices of fit were used to determine the number of classes.
To incorporate subjects missing one or more measures, 10 iterations of multiple imputation using PROC MI in SAS v9.4 were performed for the missing outcomes. The latent class structure was computed and the class membership probability for each participant was estimated. The participant was assigned to the group based on their average probability over the imputed datasets.
Associations of demographic and clinical variables with group membership were estimated by ANOVA without Type-I error adjustment for multiple outcomes. In the next step, multinomial logistic regression models were constructed to identify variables associated with group membership. Domains of variables (demographic characteristics, comorbidities, pre-stressor status, stressor characteristics, psychosocial characteristics, and environmental characteristics) were then tested in aggregate in the prediction of group membership. The area under the curve was employed for discriminating group membership, combining adjacent ordinal groups to provide ease of contrast. In the final step, an adjusted model including all variables significant from the first step from each domain was conducted.
Results
Using latent class profile analysis, three groups in which subjects all had similar patterns of improvement across the 10 outcome measures (IADL disability, time to complete single chair stands, gait speed, grip strength, balance score, LPADL disability, steps/day, ambulatory status category, Yale Activity Scale) were identified using the Bayes Information Criterion (BIC). Participants on average had >95% probability of membership in assigned group, and the entropy estimate for the final model was 0.954. Entropy is a measure of certainty and information in a set of data. In latent class analysis under Mplus, it is a measure of likelihood of classification into a given class or class overlap. With a maximum value of 1, higher values indicate less overlap between classes. The complete case analysis included 93 individuals. For self-reported outcomes, missingness ranged from 1-8%. For physical performance tests, missingness ranged from 4% for grip strength to 38% for chair stands. There were similar rates of missing variables at the 6 and 12 month visit for each outcome. The complete case analysis included 93 individuals.
The latent class profile analysis method does not assume that the direction and rate of recovery is the same for all outcome measures. However, the three groups did exhibit roughly parallel trajectories within the 10 outcomes, with one group having low 2-month values and slow rate of recovery, another with intermediate 2-month values and intermediate rate of recovery, and the third with highest 2-month values and the fastest rate of recovery. These groups were therefore labelled as “low”, “intermediate”, and “high” resilience groups. Overall, 136 subjects (25.2%) were classified as low, 242 (44.7%) as intermediate, and 163 (30.1%) as highly resilient.
Characteristics of the entire cohort together and by resilience group are shown in Table 1. In general, individuals at the higher resilience levels were younger, less likely to be male, and had higher income and education level. More resilient subjects reported better self-rated health and functional status, and higher activity levels, but lower levels of depression before the hip fracture than less resilient subjects. Highly resilient subjects were less likely to have general anesthesia or a partial/total arthroplasty as their hip fracture repair, had shorter length of stay, fewer complications, and were more often discharged home from the hospital rather than to a rehabilitation or skilled nursing facility.
Table 1.
Descriptive characteristics of the overall study population and by recovery group.
| Variable n(%) unless noted | Overall Cohort N=541 |
Low Resilience N=136 |
Intermediate Resilience N=242 |
High Resilience N=163 |
Omnibus P value* |
|---|---|---|---|---|---|
| Demographics | |||||
| Age years, Mean (SD) | 80.7 (6.6) | 83.1 (6.7) | 81.1 (6.0) | 78.2 (6.6) | <.0001 |
| White Race | 517 (95.6) | 129 (94.9) | 230 (95.0) | 158 (96.9) | .59 |
| Male Sex | 102 (18.9) | 45 (33.1) | 35 (14.5) | 22 (13.5) | <.0001 |
| Psychosocial Characteristics | |||||
| High School Diploma or less education | 327 (60.4) | 91 (66.9) | 153 (63.2) | 83 (50.9) | .009 |
| Annual Household Income ≥$20,000 | 261 (48.2) | 60 (44.1) | 106 (43.8) | 95 (58.3) | .01 |
| Cognitive Impairment (3MS<80) | 46 (8.5) | 34 (25.0) | 12 (5.0) | 0 (0) | <.0001 |
| Depression screen positive | 215 (39.7) | 67 (49.3) | 91 (37.6) | 57 (35.0) | .028 |
| Pre-Stressor Status | |||||
| Mean # of Days too sick to do activities in last 6 months (SD) | 3.6 (15.3) | 4.9 (19.7) | 4.1 (16.1) | 1.6 (8.0) | .14 |
| Mean LPADL Disability Score (SD) | 2.0 (2.4) | 3.8 (3.0) | 1.9 (2.0) | 0.5 (1.1) | <.0001 |
| Self-rated health | |||||
| Excellent (score=1) | 82 (15.2) | 10 (7.4) | 31 (12.8) | 41 (25.2) | <.0001 |
| Poor (score=5) | 20 (3.7) | 11 (8.1) | 8 (3.3) | 1 (0.6) | |
| Mean IADL Disability Score (SD) | 1.1 (1.4) | 2.5 (1.5) | 1.0 (1.2) | 0.3 (0.8) | <.0001 |
| Ambulatory Status | <.0001 | ||||
| Independent | 371 (68.6) | 46 (33.8) | 167 (69.0) | 158 (96.9) | |
| Equipment only | 116 (21.4) | 53 (39.0) | 59 (24.4) | 4 (2.5) | |
| Human Assistance | 9 (1.6) | 8 (5.9) | 1 (0.4) | 0 (0) | |
| Non-ambulatory | 45 (8.3) | 29 (21.4) | 15 (6.2) | 1 (2.8) | |
| Mean Hospitalizations past year (SD) | 0.4 (3.1) | 0.5 (2.7) | 0.5 (4.1) | 0.1 (0.4) | .44 |
| Yale Self-Reported Physical Activity mean Kcal/week (SD) | 4554 (4773) | 2444 (2104) | 4629 (5800) | 6012 (3897) | <.0001 |
| Any weight loss in past year | 65 (12.0) | 27 (19.9) | 30 (12.4) | 8 (4.9) | .0004 |
| Mean Body Mass Index, kg/m2 (SD) | 24.6 (4.7) | 24.5 (4.7) | 25.3 (4.9) | 23.7 (4.1) | .002 |
| Comorbidities | |||||
| Diabetes | 101 (18.7) | 28 (20.6) | 52 (21.5) | 21 (12.9) | .08 |
| Chronic Lung Disease | 98 (18.1) | 26 (19.2) | 44 (18.2) | 28 (17.2) | .91 |
| Cardiovascular Disease | 311 (57.5) | 74 (54.4) | 157 (64.9) | 80 (49.1) | .005 |
| Renal Disease (mod-severe) | 11 (2.0) | 6 (4.4) | 5 (2.1) | 0 (0) | .03 |
| Cancer last 5 years | 85 (15.7) | 24 (17.7) | 39 (16.1) | 22 (13.5) | .60 |
| Cerebrovascular Disease | 83 (15.3) | 33 (24.3) | 38 (15.7) | 12 (7.4) | .0003 |
| Thyroid Disease | 121 (22.4) | 28 (20.6) | 58 (24.0) | 35 (21.5) | .71 |
| Rheumatoid Arthritis | 16 (3.0) | 2 (1.5) | 10 (4.1) | 4 (2.5) | .31 |
| Current smoker | 30 (5.6) | 10 (7.4) | 8 (3.3) | 12 (7.4) | .12 |
| Current alcohol user | 190 (35.1) | 54 (39.7) | 78 (32.2) | 58 (35.6) | .34 |
| Fracture site | .0012 | ||||
| Intracapsular | 279 (51.6) | 55 (40.4) | 122 (50.4) | 102 (62.6) | |
| Trochanteric | 226 (41.8) | 72 (52.9) | 99 (40.9) | 55 (33.7) | |
| Subtrochanteric | 36 (6.7) | 9 (6.6) | 21 (8.7) | 6 (3.7) | |
| Stressor Characteristics | |||||
| Anesthesia type | .80 (p-value for Spinal vs all others) | ||||
| Spinal/epidural | 106 (19.6) | 17 (12.5) | 50 (20.7) | 39 (23.9) | |
| General | 431 (79.7) | 118 (87.8) | 191 (78.9) | 122 (74.9) | |
| Other | 4 (0.7) | 1 (0.7) | 1 (0.4) | 2 (1.2) | |
| Duration of Surgery, min | 74.7 (38.5) | 78.1 (44.0) | 74.7 (37.1) | 70.8 (33.8) | .40 |
| Partial or Total arthroplasty vs. Other Fixation | 216 (40.0) | 44 (32.4) | 95 (39.3) | 77 (14.2) | .03 |
| Post-operative complication | |||||
| Surgical | 9 (1.7) | 3 (2.2) | 4 (1.7) | 2 (1.2) | .80 |
| Infection | 52 (9.4) | 23 (16.9) | 16 (6.6) | 12 (7.4) | .0025 |
| Cardiovascular | 11 (2.0) | 7 (5.2) | 4 (1.7) | 0 (0) | .006 |
| Neurologic or Other medical | 37 (6.8) | 18 (13.2) | 15 (6.2) | 4 (2.5) | .001 |
| Delirium | 26 (4.8) | 13 (9.6) | 6 (2.5) | 7 (4.3) | .008 |
| Environmental Factors | |||||
| Initial rehabilitation type after discharge | |||||
| Inpatient Rehab/ Skilled Nursing Facility | 502 (92.8) | 130 (95.6) | 232 (95.9) | 140 (85.9) | <.001 |
| Other | 39 (7.2) | 6 (4.4) | 10 (4.1) | 23 (14.1) | |
| Lives alone | 178 (48.2) | 52 (45.6) | 80 (50.0) | 46 (48.4) | .77 |
Omnibus p value calculated using Chi-square General Association test.
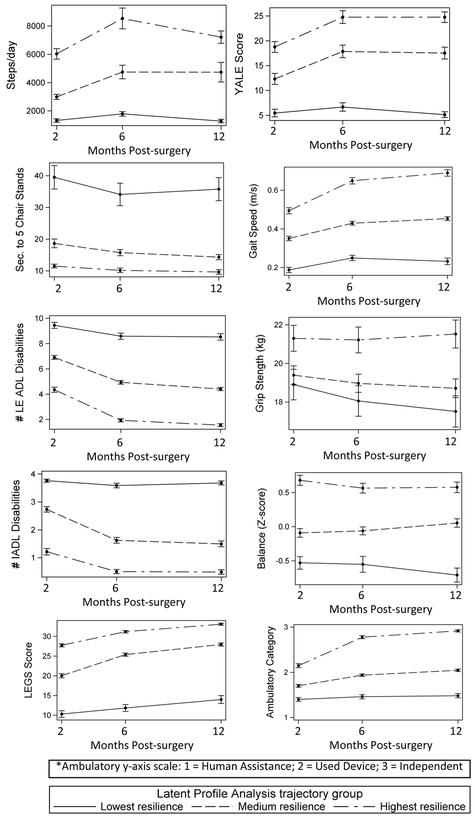
The average trajectories for all outcome variables by group are shown in Figure 1 as exemplars. The trajectories for LEGS, Ambulatory status category, and Yale Activity Scale were parallel to the gait speed trajectory, while the IADL trajectory was parallel to the lower extremity LPADL trajectory. Although the highest resilience group was fully recovered in LPADL and IADL by 12 months, on average they continued to have a slow gait speed (0.6 m/sec) and low daily step counts (2000 steps/day). Supplementary Table 1 shows the mean outcome measure values at each time point for each resilience group.
Figure 1.
Average recovery trajectories for selected outcomes in each of the 3 resilience groups.
The association of demographics, psychosocial characteristics, pre-stressor status, co-morbidities, stressor characteristics, and environmental factors on resilience group membership are shown in table 2. When variables were added to a multinomial logistic regression model in aggregate groups by type, the pre-stressor status variables were the most strongly predictive of the highest resilience group membership (as compared to the low or intermediate group), with an area under the curve (AUC) of 0.84. Demographic factors and co-morbidities were less associated with this group membership contrast, each with AUC of 0.67, followed by stressor characteristics (AUC=0.62), environmental factors (AUC=0.60), and psychosocial characteristics (0.59). An adjusted model in which significant variables from all groups were included had an AUC of 0.88 for the high vs. medium/low resilience contrast. In the fully adjusted model, factors that retained significance as predictors of high versus low/medium resilient recovery after hip fracture included younger age, lower BMI, better pre-fracture function, non-intracapsular fracture, and discharge home following hospitalization.
Table 2.
Variables associated with being in high vs. low/medium resilience recovery group in logistic model with the Area Under the Curve for each group of variables.
| Variable | Odds Ratio (95% CI) Adjusted for Group Variables |
Odds Ratio (95% CI) Full Adjusted Model (AUC 0.88) |
|---|---|---|
| Demographics | (AUC 0.67) | |
| Age, 1 year difference | 0.91 (0.89-0.94) | 0.94 (0.90-0.98) |
| White (vs. all other) | 2.34 (0.82-6.70) | |
| Male | 0.55 (0.32-0.94) | 0.88 (0.42-1.86) |
| Psychosocial Characteristics | (AUC 0.61) | |
| Education ≤ High School (vs. > HS Diploma) | 0.65 (0.44-0.96) | 0.68 (0.41-1.13) |
| Annual Income ≥ $20,000 | 1.60 (1.09-2.35) | 1.39 (0.84-2.31) |
| Depression | 0.77 (0.52-1.13) | |
| Pre-Stressor Status | (AUC 0.84) | |
| Days too sick to do activities in last 6 months, 1 day difference | 1.00 (0.98-1.02) | |
| LPADL 1 additional disability | 0.70 (0.56-0.87) | 0.73 (0.58-0.92) |
| Self-rated health, 1 point worse | 0.67 (0.52-0.85) | 0.71 (0.56-0.91) |
| IADL 1 additional disability | 0.58 (0.45-0.75) | 0.51 (0.38-0.69) |
| Needs Human Assistance or Non-Ambulatory (vs. independent) | 0.23 (0.09-0.56) | 0.25 (0.10-0.63) |
| Any Hospitalizations past | 0.82 (0.39-1.72) | |
| Self-reported Activity Level per 100 kcal/week | 1.00 (1.00-1.00) | |
| Weight loss in past year | 0.59 (0.24-1.47) | |
| Comorbidities | (AUC 0.67) | |
| Body Mass Index, 1 kg/m2 higher | 0.95 (0.91-0.99) | 0.93 (0.88-0.98) |
| Diabetes | 0.71 (0.41-1.23) | |
| Chronic Lung Disease | 0.98 (0.58-1.66) | |
| Cardiovascular Disease | 0.63 (0.42-0.93) | 0.78 (0.48-1.28) |
| Cancer last 5 years | 0.82 (0.47-1.43) | |
| Stroke | 0.42 (0.22-0.82) | 0.71 (0.31-1.63) |
| Thyroid Disease | 0.92 (0.58-1.47) | |
| Rheumatoid Arthritis | 0.87 (0.26-2.97) | |
| Current smoker | 1.14 (0.50-2.62) | |
| Current alcohol use | 1.68 (1.03-2.75) | 1.47 (0.83-2.61) |
| Stressor Characteristics | (AUC 0.60) | |
| Intracapsular fracture | 2.77 (1.07-7.22) | 3.02 (1.01-8.99) |
| General Anesthesia | 0.71 (0.45-1.12) | |
| Partial or Total arthroplasty | 1.01 (0.59-1.73) | |
| Any in-hospital complication | 0.53 (0.29-0.96) | 0.97 (0.45-2.09) |
| Delirium | 1.20 (0.47-3.03) | |
| Environmental Factors | (AUC 0.60) | |
| Inpatient Rehab /Skilled Nursing Facility (vs. home, outpatient or none) | 0.26 (0.13-0.51) | 0.24 (0.09-0.69) |
| Each Additional PT Session in Hospital | 1.03 (0.97-1.08) |
Discussion
Helping patients and families understand their likely time course for recovery in multiple outcomes following an acute health stressor is important in planning for the most appropriate rehabilitation setting, level of personal care assistance, and need for family medical leave. Prior studies have developed clinical prediction models for mortality after hip fracture 28, have reported average trajectories of functional recovery,9 or have used cluster analysis to define 6-month self-reported function based on common baseline characteristics.6 Our approach extends these findings by defining three common long-term recovery patterns for multiple outcomes simultaneously. Our approach accounts for the heterogeneity in the speed and completeness of recovery, and provides a rich description of recovery for clinicians, patients, and families as they plan for care after a hip fracture.
Our findings have several other clinical implications. First, we found that the rate of improvement in most outcomes (i.e., the “slope” of the recovery trajectory) is similar within the 3 resilience groups regardless of where they start, with the notable exception of activity levels. Therefore, clinicians should be concerned about any individual who is not improving at the expected rate, even if they are likely to be in the low resilience group. Early monitoring of the rate of recovery should be implemented for all hip fracture patients. Second, many of the variables demonstrated parallel recovery trajectories or had relatively flat slopes suggesting that clinicians and researchers may not need to complete as large a set of measures in order to monitor or classify recovery.. We suggest that baseline grip strength, and serial measures of gait speed, self-reported activity, and either IADL or LP ADL measures is sufficient to define resilience groups after hip fracture, as other measures have parallel slopes and measure similar domains. Self-reported measures could be substituted for gait speed or physical performance testing where the trajectories are parallel to reduce staff burden, because their recovery trajectories mirror the more burdensome performance tests.
Consistent with prior studies6,7,10,29,30, we found that baseline self-reported function is by far the strongest predictor of subsequent recovery; indeed, these measures by themselves provided excellent discrimination between the resilience groups with AUCs >0.80. It was relatively less informative to consider co-morbidities, psychosocial, environmental, and stressor characteristics (AUCs 0.60-0.67), although individual variables within these categories remained significantly associated with resilience group in multivariable models. This finding, combined with the observation noted above that recovery trajectories are frequently parallel between the resilience groups, suggests that these groups are likely a better reflection of underlying physiologic reserve rather than physical resilience.3 Other measures of physical resilience that describe how much better or worse an individual recovers than they are expected to based on their baseline health and functional status have been proposed, and may be more appropriate for mechanistic studies of physical resilience.31
These recovery groups following hip fracture can now be used in future research to identify factors that mediate or moderate resilience. For example, psychological factors such as self-efficacy32 and depression33, or environmental factors such as neighborhood34 may be modifiable mediators of resilience. We can now also identify biomarkers associated with resilience class membership, and this may generate hypotheses about underlying biological pathways that can be targeted.
Our work has several limitations that should be considered. As in most clinical research with older adults, there is likely a healthy volunteer bias which is further compounded by the exclusion of patients with significant cognitive impairment in 2 of the 3 cohorts. However, our goal was to identify factors associated with greater levels of resilience and this selection bias may have been helpful in enriching the sample for more resilient individuals. The BHS studies had limited racial and ethnic diversity, but represented the hip fracture population of the recruitment hospitals. Given the long length of follow-up and need for physical performance testing in this frail population, missing data was an issue and multiple imputation was used. In addition, our slopes were based on data collected at three time points and it is possible that more dynamic recovery groups may have been detected with more frequent measurements over a longer period of follow-up. Our existing datasets did not consistently include some outcome domains of interest, such as mood, cognition, or quality of life. Future research should determine whether recovery patterns are associated with these domains of health. Data on environmental and psychosocial factors were also limited. Finally, although our approach explicitly considers the heterogeneity in outcomes, there remains variability within the average trajectories for each resilience group which must be considered in making clinical decisions.
In summary, we identified three physical resilience groups following hip fracture in older adults depicting recovery trajectories in 10 outcomes of functional independence and physical performance. These can be defined in future studies with a more limited set of variables and may be useful for understanding mediators of physical resilience and clinical decision making. Pre-fracture self-reported function is the most important predictor of recovery patterns after hip fracture.
Supplementary Material
Supplementary Table 1. Mean outcome measure values at each time point for each resilience group.
Acknowledgements
This research was supported by grants from the National Institute on Aging (UH2 AG056925-01, AG049077-01A1, 2P30AG028716-11, R37 AG009901, R01 AG18668, R01 AG17082, R01 AG029315, P30 AG028747, and T32 AG000262).
This work was funded by the National Institutes of Health UH2 AG056925-02 mechanism awarded to Drs. Colon-Emeric and Whitson.
Footnotes
Conflicts of Interest. Colón-Emeric: During the past year, I consulted or served on advisory boards for: Novartis, Amgen, and Biscardia. There are no relevant conflicts of interest with this work. Magaziner: During the past year, I have consulted or served on advisory boards for the following entities: American Orthopaedic Association; Ammonett; Novartis; Pluristem; Viking. The following investigators have no conflicts and nothing to report: Whitson, Pieper, Crabtree, Huffman, Orwig, Parker, Gruber-Baldini, Prvu Bettger, and Sloane.
Sponsor’s Role. The funding agencies for this study played no role in the design, methods, subject recruitment, data collections, analysis or preparation of this paper.
References
- 1.Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colon-Emeric CS. Physical Resilience in Older Adults: Systematic Review and Development of an Emerging Construct. J Gerontol A Biol Sci Med Sci 2016;71:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varadhan R, Walston JD, Bandeen-Roche K. Can a Link Be Found Between Physical Resilience and Frailty in Older Adults by Studying Dynamical Systems? Journal of the American Geriatrics Society 2018;66:1455–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitson HE, Cohen HJ, Schmader KE, Morey MC, Kuchel G, Colon-Emeric CS. Physical Resilience: Not Simply the Opposite of Frailty. Journal of the American Geriatrics Society 2018;66:1459–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briggs AM, Cross MJ, March L, et al. Musculoskeletal Health Conditions Represent a Global Threat to Healthy Aging: A Report for the 2015 World Health Organization World Report on Ageing and Health. The Gerontologist 2016;56:S243–S55. [DOI] [PubMed] [Google Scholar]
- 5.Beaupre LA, Carson JL, Noveck H, Magaziner J. Recovery of Walking Ability and Return to Community Living within 60 Days of Hip Fracture Does Not Differ Between Male and Female Survivors. Journal of the American Geriatrics Society 2015;63:1640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eastwood EA, Magaziner J, Wang J, et al. Patients with Hip Fracture: Subgroups and Their Outcomes. Journal of the American Geriatrics Society 2002;50:1240–9. [DOI] [PubMed] [Google Scholar]
- 7.Hannan EL, Magaziner J, Wang JJ, et al. Mortality and locomotion 6 months after hospitalization for hip fracture: Risk factors and risk-adjusted hospital outcomes. JAMA 2001;285:2736–42. [DOI] [PubMed] [Google Scholar]
- 8.Magaziner J, Fredman L, Hawkes W, et al. Changes in Functional Status Attributable to Hip Fracture: A Comparison of Hip Fracture Patients to Community-dwelling Aged. American Journal of Epidemiology 2003;157:1023–31. [DOI] [PubMed] [Google Scholar]
- 9.Magaziner J, Hawkes W, Hebel JR, et al. Recovery From Hip Fracture in Eight Areas of Function. The Journals of Gerontology: Series A 2000;55:M498–M507. [DOI] [PubMed] [Google Scholar]
- 10.Orwig D, Hochberg MC, Gruber-Baldini AL, et al. Examining Differences in Recovery Outcomes between Male and Female Hip Fracture Patients: Design and Baseline Results of a Prospective Cohort Study from the Baltimore Hip Studies. J Frailty Aging 2018;7:162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu KC, D’Avanzo PA, Nesheiwat L, et al. Associations Between Neurocognitive Impairment and Biomarkers of Poor Physiologic Reserve in a Clinic-Based Sample of Older Adults Living with HIV. Journal of the Association of Nurses in AIDS Care 2017;28:55–66. [DOI] [PubMed] [Google Scholar]
- 12.Dolgin NH, Martins PNA, Movahedi B, Lapane KL, Anderson FA, Bozorgzadeh A. Functional status predicts postoperative mortality after liver transplantation. Clinical Transplantation 2016;30:1403–10. [DOI] [PubMed] [Google Scholar]
- 13.Hadley EC, Kuchel GA, Newman AB, et al. Report: NIA Workshop on Measures of Physiologic Resiliencies in Human Aging. The Journals of Gerontology: Series A 2017;72:980–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: Linking Aging to Chronic Disease. Cell 2014;159:709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Resnick B, Orwig D, Yu-Yahiro J, et al. Testing the effectiveness of the exercise plus program in older women post-hip fracture. Annals of Behavioral Medicine 2007;34:67. [DOI] [PubMed] [Google Scholar]
- 16.Orwig DL, Hochberg M, Yu-Yahiro J, et al. Delivery and Outcomes of a Yearlong Home Exercise Program After Hip Fracture: A Randomized Controlled Trial. Archives of internal medicine 2011;171:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resnick B, Orwig D, D’Adamo C, et al. Factors that influence exercise activity among women post hip fracture participating in the Exercise Plus Program. Clinical Interventions in Aging 2007;2:413–27. [PMC free article] [PubMed] [Google Scholar]
- 18.Doble SE, Fisher AG. The dimensionality and validity of the Older Americans Resources and Services (OARS) Activities of Daily Living (ADL) Scale. J Outcome Meas 1998;2:4–24. [PubMed] [Google Scholar]
- 19.Schuler PB, Richardson MT, Ochoa P, Wang MQ. Accuracy and Repeatability of the Yale Physical Activity Survey in Assessing Physical Activity of Older Adults. Perceptual and Motor Skills 2001;93:163–77. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik JM, Simonsick EM, Ferrucci L, et al. A Short Physical Performance Battery Assessing Lower Extremity Function: Association With Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. Journal of Gerontology 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 21.Mau-Roung L, Hei-Fen H, Ming-Hsia H, Isaac WH-D, Yi-Wei W, Fu-Chao H. Psychometric Comparisons of the Timed Up and Go, One-Leg Stand, Functional Reach, and Tinetti Balance Measures in Community-Dwelling Older People. Journal of the American Geriatrics Society 2004;52:1343–8. [DOI] [PubMed] [Google Scholar]
- 22.Jones CJ, Rikli RE, Beam WC. A 30-s Chair-Stand Test as a Measure of Lower Body Strength in Community-Residing Older Adults. Research Quarterly for Exercise and Sport 1999;70:113–9. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman S, Hawkes WG, Hebel JR, Fox KM, Lydick E, Magaziner J. The Lower Extremity Gain Scale: A Performance-Based Measure to Assess Recovery After Hip Fracture. Archives of Physical Medicine and Rehabilitation 2006;87:430–6. [DOI] [PubMed] [Google Scholar]
- 24.Jette A The Functional Status Index: reliability and validity of a self-report functional disability measure. J Rheumatol Suppl 1987;14:15–21. [PubMed] [Google Scholar]
- 25.Dziak J, Lanza S, Tan X. Effect Size, Statistical Power and Sample Size Requirements for the Bootstrap Likelihood Ratio Test in Latent Class Analysis. Structural Equation Modeling: a Multidisciplinary Journal 2014;21:534–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nylund K, Asparouhov T, Muthén B. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. . Structural Equation Modeling: a Multidisciplinary Journal 2007;14:535–69. [Google Scholar]
- 27.Zhang B, Chen Z, Albert PS. Latent class models for joint analysis of disease prevalence and high-dimensional semicontinuous biomarker data. Biostatistics (Oxford, England) 2012;13:74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang HX, Majumdar SR, Dick DA, et al. Development and Initial Validation of a Risk Score for Predicting In-Hospital and 1-Year Mortality in Patients With Hip Fractures. Journal of Bone and Mineral Research 2005;20:494–500. [DOI] [PubMed] [Google Scholar]
- 29.Dyer SM, Crotty M, Fairhall N, et al. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatrics 2016;16:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuman MD, Silber JH, Magaziner JS, Passarella MA, Mehta S, Werner RM. SUrvival and functional outcomes after hip fracture among nursing home residents. JAMA Internal Medicine 2014;174:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colon-Emeric C, Pieper CF, Schmader KE, et al. Two Approaches to Classifying and Quantifying Physical Resilience in Longitudinal Data. J Gerontol A Biol Sci Med Sci 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FORTINSKY RH, BOHANNON RW, LITT MD, et al. Rehabilitation therapy self-efficacy and functional recovery after hip fracture. International Journal of Rehabilitation Research 2002;25:241–6. [DOI] [PubMed] [Google Scholar]
- 33.Zimmerman SI, Smith HD, Gruber-Baldini A, et al. Short-term persistent depression following hip fracture: A risk factor and target to increase resilience in elderly people. Social Work Research 1999;23:187–96. [Google Scholar]
- 34.Foster JR. Successful Coping, Adaptation and Resilience in the Elderly: An Interpretation of Epidemiologic Data. Psychiatric Quarterly 1997;68:189–219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Mean outcome measure values at each time point for each resilience group.