Abstract
Background
Fibroblast growth factors (FGFs) have a fundamental role in cancer. Sequestering FGFs with GSK3052230 (FP-1039) blocks their ability to activate FGFRs while avoiding toxicities associated with small molecule inhibitors of FGFR, including hyperphosphatemia and retinal, nail, and skin toxicities.
Methods
A multicenter, open-label, phase Ib study evaluated weekly GSK3052230 added to pemetrexed/cisplatin in patients with treatment-naive, unresectable malignant pleural mesothelioma. Doses were escalated according to a 3 + 3 design, followed by cohort expansion at the maximum tolerated dose (MTD). Endpoints included safety, overall response rate, progression-free survival, and pharmacokinetics.
Results
36 patients were dosed at 10, 15, and 20 mg/kg doses of GSK3052230. Three dose-limiting toxicities were observed at 20 mg/kg and one at 15 mg/kg. The MTD was defined as 15 mg/kg and used for cohort expansion. The most common treatment-related adverse events (AEs) were nausea (56%), decreased appetite (36%), infusion reactions (36%), decreased neutrophil counts (36%), and fatigue (33%). The confirmed ORR was 39% (95% CI: 23.1–56.5) (14/36 PRs) and 47% had stable disease (17/36), giving a disease control rate of 86%. At 15 mg/kg GSK3052230 (n = 25), the ORR was 44% (95% CI: 24.4–65.1), and the median PFS was 7.4 months (95% CI: 6.7–13.4). Four patients had disease control for over 1 year, and three were still ongoing.
Conclusion
At 15 mg/kg weekly, GSK3052230 was well tolerated in combination with pemetrexed/cisplatin and durable responses were observed. Importantly, AEs associated with small molecule inhibitors of FGFR were not observed, as predicted by the unique mechanism of action of this drug.
Keywords: FGF, Ligand trap, Mesothelioma, Combination therapy, Phase 1
Introduction
Malignant pleural mesothelioma (MPM) is a lethal cancer caused by malignant growth of mesothelial cells aligning the pleura and is strongly related to exposure to asbestos [1]. The only established and registered treatment regimen for MPM is combination chemotherapy with pemetrexed and cisplatin [2]. Despite this, the prognosis remains poor, its incidence is increasing worldwide, and alternative treatment options are scarce [1]. Therefore, identification of novel targets and treatment regimens is a medical need.
Preclinical studies have shown that fibroblast growth factor (FGF) signaling is involved in MPM based on overexpression of FGF2 protein and FGF2 and FGFR1 mRNA levels [3, 4]. The family of FGF proteins consists of 18 different soluble growth factors that activate FGF receptors (FGFRs) 1–4 [5]. FGF/FGFR signaling plays an important role in cancer by regulating cell growth and differentiation, angiogenesis, and tumor-stroma interactions [5–7]. Alterations in FGF signaling can occur by FGFR gene amplification, mutation, gene fusion, or by FGF/FGFR overexpression. The pathway is also known to be implicated in drug resistance to targeted therapies directed at other receptor tyrosine kinases [8]. To date, several pan-selective tyrosine kinase inhibitors of FGFR1–4 have been investigated across multiple cancers [8–11]. One observed limitation to the long-term clinical application of these molecules is on-target toxicities including hyperphosphatemia and tissue calcification resulting in retinal, nail and skin toxicities. These are the result of inhibiting hormonal (non-pathological) FGF signaling by FGF19, −21 and − 23 [8].
GSK3052230 is a soluble fusion protein consisting of the extracellular domains of human FGFR1 α-IIIc linked to the modified hinge and native Fc regions of human immunoglobulin G1 (IgG1) [12]. The molecule functions as a FGF ‘trap’ by binding to FGFs and reducing FGF/FGFR signaling. Because the FGFR1 α-IIIc domain has a relatively low affinity for FGF19, FGF21 and FGF23, the potential for toxicities observed with small molecule FGFR inhibitors is decreased [12]. This was confirmed in the first-in-human study of FP-1039 where doses were tolerable up to 20 mg/kg weekly as a monotherapy [13].
The role of FGFR inhibition has been studied extensively in MPM using small-molecule pan FGFR kinase inhibitors and with GSK3052230 resulting in suppression of FGF pathway signaling and antitumor activity in cell lines and human xenografts [3, 14–16]. Based on the efficacy of GSK3052230 observed in MPM preclinical models, a phase Ib study ( NCT01868022) was initiated to investigate the tolerability, safety, and preliminary signs of efficacy in combination with standard therapy pemetrexed and cisplatin in first-line treatment of MPM.
Methods
Participants and study oversight
Patients 18 years and older were enrolled after histological or cytological confirmation of unresectable, measurable MPM for which no prior systemic therapy was administered or after recurrence after local therapy and of which archival material was available for FGF2 protein expression analysis. Detailed inclusion and exclusion criteria information can be found in Online Resource 1. The study was performed according to Good Clinical Practice. All patients signed informed consent according to the Declaration of Helsinki, and no study procedure was started before this moment. The study protocol and amendments were approved by the investigational review boards at each participating site.
Study design
This was a nonrandomized, open-label, multicenter phase Ib trial ( NCT01868022) consisting of three arms. Arms A and B investigated GSK3052230 in FGFR1-amplified squamous non-small cell lung cancer (sqNSCLC) in combination with first- and second-line standard therapies, respectively, and Arm C investigated GSK3052230 with first-line therapy in mesothelioma. Results of Arm C are reported here. Doses of GSK3052230 were escalated according to a 3 + 3 design up to the maximum tolerated dose (MTD) at ≤1 out of 6 patients experiencing a dose-limiting toxicities (DLT) (pre-specified as in Online Resource 2). At the MTD, the cohort was to be expanded with 12–30 patients.
GSK3052230 was administered once weekly intravenously over 30 min in each 21-day cycle. Pemetrexed 500 mg/m2 and cisplatin 75 mg/m2 were administered per product label on day 1 of each cycle. Patients could continue GSK3052230 treatment until disease progression or intolerability. Pemetrexed and cisplatin could be continued if deemed safe by the investigator. Patients were hydrated and pre-medicated with folic acid and vitamin B12 as per institutional guidelines. The starting dose of GSK3052230 was 10 mg/kg. Dose-escalation was planned to 20 mg/kg, and an intermediate dose-level of 15 mg/kg was introduced after emerging safety data from Arms A and B of this study, investigating GSK3052230 in sqNSCLC patients.
Study procedures
For pharmacokinetic (PK) analyses, venous blood samples were drawn prior to the first infusion, at end of the GSK3052230 infusion, and post-infusion at 1 and 2 h and pre-dose on days 8 and 15. In cycle 2 on day 1, additional samples were drawn at the end of the pemetrexed infusion and 1, 2, and 5 h post-dose. In subsequent cycles, only pre- and end-of-infusion samples were collected. Plasma concentrations of GSK3052230 were determined using a validated FGF2 ligand-binding enzyme-linked immunosorbent assay in accordance with FDA’s published recommendations [17].
Samples for anti-GSK3052230 antibody analyses were drawn at baseline, day 15, and every two cycles starting from cycle 4 onwards. Detection was done using a tiered approach with electrochemiluminescence screening and confirmatory assays. Samples were acidified and neutralized with TRIS buffer containing equal concentrations of biotin-conjugate as capture agent and sulfo-Tag-drug conjugate as detection agent before transfer to a blocked MSD streptavidin coated plate. For both assays, samples were considered positive if the sample signal exceeds the background signal. For the confirmatory assay, excess drug was added to demonstrate specificity of the assay. Samples with a percent inhibition of the assay response due to the presence of excess drug were considered positive.
On archival tumor material, FGF2 protein expression was retrospectively evaluated by immunohistochemistry (IHC) analysis using the mouse anti-human FGF2/BFGF monoclonal (3D9) antibody (LifeSpan Biosciences) with UltraView DAB detection kit (Ventana Medical Systems Inc.) on the Ventana Benchmark XT instrument followed by counterstaining. Results were interpreted by a trained pathologist using light microscopy and expressed as H-score (percentage of positive cells (0–100) multiplied by staining intensity (0–3)).
Endpoints
Endpoints included safety by common terminology criteria for adverse events (CTCAE) version 4.03, overall response rate (ORR) by modified Response Evaluation Criteria in Solid Tumors (mRECIST) version 1.1, and progression-free survival (PFS) defined as the time from start of study treatment to the first reported disease progression. Tumor response was evaluated by computed tomography (CT) every two cycles during the first year and every four cycles thereafter. Patients were evaluable for efficacy if at least one on-treatment radiological evaluation was performed. Patients were evaluable for safety if they had received one dose of GSK3052230.
Statistical analysis
With a minimum of 12 patients at the MTD, the study was designed to demonstrate futility (ORR ≤ 40%) or efficacy (ORR ≥ 60%) with a power of 82% based on a previous phase III clinical trial in first-line MPM (2). PK, safety, and efficacy data were reported descriptively. The relation between FGF2 H-score and treatment outcome was analysed by a linear regression model based on the all-treated population.
Results
In total, 36 patients received study treatment as planned. Of these, 31 were evaluable for efficacy and 36 for PK and safety. Most patients were male (n = 25, 69%) and 65 years and older (n = 19, 53%), with ECOG performance status of 0 (n = 23, 64%) (Table 1). Patients did not receive previous systemic therapy for MPM with the exception of one patient who had received prior oxaliplatin and pemetrexed in the 15 mg/kg cohort. While this was not in line with the inclusion criteria, this patient was considered evaluable because treatment was completed 7 years before enrollment into this trial after achieving a complete response. As allowed per protocol, 56% of the patients had undergone one or more surgical procedures before start of the study.
Table 1.
Patient and disease characteristics at baseline
| 10 mg/kg GSK3052230 (n = 3) | 15 mg/kg GSK3052230 (n = 25) | 20 mg/kg GSK3052230 (n = 8) | Total (n = 36) | ||
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Female | 1 (33%) | 8 (32%) | 2 (25%) | 11 | (31%) |
| Male | 2 (67%) | 17 (68%) | 6 (75%) | 25 | (69%) |
| Age, mean (range), years | 72.3 | 57.1 | 70.9 | 61 | |
| [65–80] | [25–75] | [60–78] | [25–80] | ||
| Age group, n (%) | |||||
| 18–64 | 0 | 16(64%) | 1 (13%) | 17 | (47%) |
| 65–74 | 2 (67%) | 8 (32%) | 4 (50%) | 14 | (39%) |
| >74 | 1 (33%) | 1 (4%) | 3 (38%) | 5 | (14%) |
| ECOG PS, n (%) | |||||
| 0 | 1 (33%) | 17 (68%) | 5 (63%) | 23 | (64%) |
| 1 | 2 (67%) | 8 (32%) | 2 (25%) | 12 | (33%) |
| 2 | 0 | 0 | 1 (13%) | 1 | (3%) |
| Prior treatment, n (%) | |||||
| Surgery | 2 (67%) | 12 (48%) | 6 (75%) | 20 | (56%) |
| Chemotherapya | 0 | 1 (4%) | 0 | 1 | (3%) |
| Histology, n (%) | |||||
| Medullary | 0 | 10 (40%) | 2 (25%) | 12 | (33%) |
| Epithelioid | 2 (66%) | 4 (16%) | 0 | 6 | (16%) |
| Sarcomatoid | 0 | 3 (12%) | 1 (13%) | 4 | (11%) |
| Biphasic | 0 | 0 | 1 (13%) | 1 | (3%) |
| Otherb | 1 (33%) | 8 (32%) | 4 (50%) | 13 | (36%) |
| Ethnicity, n (%) | |||||
| White | 3 (100%) | 24 (96%) | 8 (100%) | 35 | (97%) |
| Asian | 0 | 1 (4%) | 0 | 1 | (3%) |
| Unknown | 0 | 0 | 0 | 0 |
ECOG PS, Eastern Cooperative Oncology performance status;
one patient received prior oxaliplatin/pemetrexed;
Histological subtype of 13 patients was not provided in the pathology report; however, their diagnosis of malignant pleural mesothelioma was confirmed histologically at screening, per protocol
At the time of data cut-off (17-March-2017) for final analysis, all but three patients had discontinued treatment. Reasons for discontinuation of treatment with GSK3052230 were disease progression (n = 25, 69%), adverse events (n = 4, 11%), investigators’ discretion (surgical intervention was performed) (n = 2, 5.5%), withdrawing of consent (n = 1, 2.8%) and site study closure (n = 1, 2.8%).
Pharmacokinetics and immunogenicity
The PK of GSK3052230 was assessed previously using preclinical models and in the first-in-human trial [12, 13]. In this study, a range of three doses based on prior data was used to identify a safe and efficacious dose that could be combined with standard therapy. Maximum concentration (Cmax) and time of maximum concentration (Tmax) after the first dosing day in cycle 1 to 6 are summarized for each dose-level in Table 2. A generally dose-proportional increase in Cmax was observed. There was no apparent accumulation in exposure between cycles. Median Tmax was comparable across all dose cohorts (0.4–1.1 h) and was observed at the end of the GSK3052230 infusion. Based on the limited data, there is a trend towards a lack of PK drug-drug interaction between GSK3052230 and pemetrexed and/or cisplatin. Of the 35 patients tested for anti-GSK3052230 antibodies, 14 (39%) tested positive after two administrations of GSK3052230, at cycle 1 day 15, decreasing to 14% at cycle 6 which suggests transient immunogenicity (not shown).
Table 2.
Pharmacokinetics of GSK3052230: maximum concentration (Cmax) and time of maximum concentration (Tmax) at Day 1 of the indicated cycles of therapy
| Cycle 1 Day 1 | Cycle 2 Day 1 | Cycle 4 Day 1 | Cycle 6 Day 1 | |
|---|---|---|---|---|
| 10 mg/kg GSK3052230 + 500 mg/m2 Pemetrexed + 75 mg/m2 Cisplatin (N = 3) | ||||
| Cmax (ng/mL) | ||||
| n | 3 | 3 | 2 | 1 |
| Geometric mean | 170,235.8 | 174,512.6 | 228,905.3 | 189,264.6 |
| CVb% | 29 | 14 | 25 | NC |
| Tmax (h) | ||||
| Median (Min - Max) | 0.60 (0.5–0.6) | 0.50 (0.5–1.0) | 0.55 (0.5–0.6) | 1.10 |
| 15 mg/kg GSK3052230 + 500 mg/m2 Pemetrexed + 75 mg/m2 Cisplatin (N =25) | ||||
| Cmax (ng/mL) | ||||
| n | 9 | 7 | 5 | 2 |
| Geometric mean | 205,104.9 | 221,082.8 | 173,559.0 | 300,070.8 |
| CVb% | 24 | 19 | 99 | 12 |
| Tmax (h) | ||||
| Median (Min - Max) | 0.60 (0.4–1.5) | 0.40 (0.0–1.0) | 0.90 (0.4–1.0) | 0.65 (0.4–0.9) |
| 20 mg/kg GSK3052230 + 500 mg/m2 Pemetrexed + 75 mg/m2 Cisplatin (N =8) | ||||
| Cmax (ng/mL) | ||||
| n | 8 | 6 | 3 | 2 |
| Geometric mean | 230,098.6 | 150,590.0 | 386,342.2 | 340,679.9 |
| CVb% | 119 | 901 | 11 | 22 |
| Tmax (h) | ||||
| Median (Min - Max) | 0.65 (0.4–1.6) | 1.05 (0.4–2.3) | 0.50 (0.4–0.9) | 0.50 (0.5–0.5) |
CVb% = between-subject coefficient of variation; NC = not calculated
Safety
Treatment-related adverse events (AEs) were reported in 97% of patients, with the most common being nausea (56%), decreased appetite (36%), infusion-related reactions (IRRs) (36%), decreased neutrophil counts (36%), and fatigue (33%) (Table 3). IRRs occurred mainly after the second infusion or later and did not lead to dose changes or interruptions in most cases. For one patient, treatment was discontinued after having a second grade 3 IRR. There was no clear relationship between IRR and anti-drug antibodies. At the highest dose-level of GSK3052230 (20 mg/kg), three grade 4 events, being neutropenia, respiratory failure, and thrombocytopenia (n = 1 each) occurred, of which only thrombocytopenia was related to GSK3052230, and one grade 5 event occurred, namely intestinal ischemia/intestinal perforation with bowel involvement. This grade 5 event was considered possibly related to study treatment, considering the potential anti-angiogenic properties of GSK3052230. Importantly, no AEs of hyperphosphatemia or any other toxicities associated with pan-FGFR kinase inhibitors were reported.
Table 3.
Treatment-related Adverse Events occurring in >2 patients, highest grade per patient
| Dose-level 1 (n = 3) | Dose-level 2 (n = 25) | Dose-level 3 (n = 8) | Total (N = 36) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GSK3052230 | 10 mg/kg | 15 mg/kg | 20 mg/kg | ||||||||||
| Adverse Event, (n %) | Grade 1/2 | Grade 3 | Grade 1/2 | Grade 3 | Grade 1/2 | Grade 3 | |||||||
| Nausea | 2 | (67%) | 11 | (44%) | 1 | (4%) | 6 | (75%) | 20 (56%) | ||||
| Decreased appetite | 3 | (100%) | 7 | (28%) | 3 | (38%) | 13 (36%) | ||||||
| Infusion related reaction | 1 | (33%) | 6 | (24%) | 1 | (4%) | 3 | (38%) | 2 | (25%) | 13 (36%) | ||
| Neutrophil count decreased | 1 | (33%) | 1 | (33%) | 3 | (12%) | 6 | (24%) | 2 | (26%) | 13 (36%) | ||
| Fatigue | 1 | (33%) | 7 | (28%) | 4 | (50%) | 12 (33%) | ||||||
| Asthenia | 0 | 5 | (20%) | 1 | (4%) | 3 | (38%) | 9 (25%) | |||||
| Blood creatinine increased | 1 | (33%) | 6 | (24%) | 2 | (25%) | 9 (25%) | ||||||
| Vomiting | 1 | (33%) | 4 | (16%) | 1 | (4%) | 3 | (38%) | 9 (25%) | ||||
| Anemia | 1 | (33%) | 4 | (16%) | 2 | (8%) | 0 | 7 (19%) | |||||
| Peripheral sensory neuropathy | 1 | (33%) | 6 | (24%) | 0 | 7 (19%) | |||||||
| Dysgeusia | 1 | (33%) | 3 | (12%) | 2 | (25%) | 6 (17%) | ||||||
| Hypertension | 0 | 4 | (16%) | 1 | (13%) | 1 | (13%) | 6 (17%) | |||||
| Lacrimation increased | 2 | (67%) | 4 | (16%) | 0 | 6 (17%) | |||||||
| Diarrhea | 1 | (33%) | 1 | (4%) | 1 | (4%) | 1 | (13%) | 4 (11%) | ||||
| Hiccups | 0 | 3 | (12%) | 1 | (13%) | 4 (11%) | |||||||
| Leukopenia | 0 | 4 | (16%) | 0 | 4 (11%) | ||||||||
| Tinnitus | 1 | (33%) | 2 | (8%) | 1 | (13%) | 4 (11%) | ||||||
| Abdominal pain upper | 0 | 3 | (12%) | 1 | (4%) | 0 | 3 (8%) | ||||||
| ALT increased | 3 | (12%) | 3 (8%) | ||||||||||
| Alopecia | 0 | 3 | (12%) | 0 | 3 (8%) | ||||||||
| AST increased | 0 | 3 | (12%) | 0 | 3 (8%) | ||||||||
| Constipation | 0 | 2 | (8%) | 1 | (13%) | 3 (8%) | |||||||
| Hypoesthesia | 0 | 2 | (8%) | 1 | (13%) | 3 (8%) | |||||||
| Mucosal inflammation | 0 | 2 | (8%) | 1 | (13%) | 3 (8%) | |||||||
| Paresthesia | 0 | 3 | (12%) | 0 | 3 (8%) | ||||||||
| Rash | 1 | (33%) | 2 | (8%) | 1 | (13%) | 3 (8%) | ||||||
| Weight decreased | 0 | 2 | (8%) | 1 | (13%) | 3 (8%) | |||||||
Abbreviations: ALT Alanine aminotransferase, AST Aspartate aminotransferase
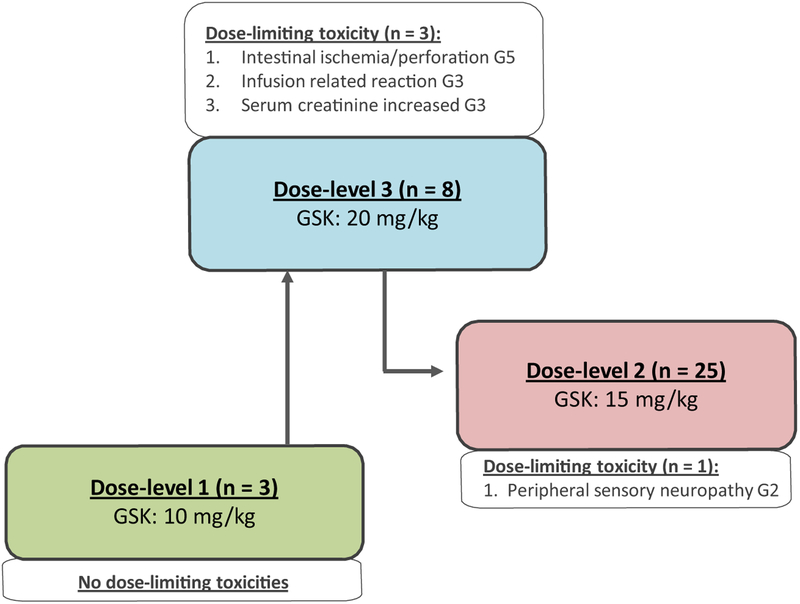
Four patients (11%) discontinued GSK3052230 due to creatinine increase (grade 1), intestinal ischemia/perforation (grade 5), IRR (grade 3), or acute kidney injury (grade 3) (n = 1 each). DLTs occurred mainly at 20 mg/kg (dose-level 3), in three out of eight patients and at 15 mg/kg (dose-level 2) in one out of 25 patients (Fig. 1). These included a grade 3 creatinine increase, grade 3 IRR, and a grade 5 intestinal ischemia/perforation at 20 mg/kg (n = 1 each), and a grade 2 peripheral sensory neuropathy at 15 mg/kg. Nine (25%) patients experienced serious adverse events (SAEs). All SAEs apart from IRRs (2 patients) occurred in 1 subject each. Six patients had nine SAEs that were considered to be related to study treatment (acute kidney injury, blood creatinine increased, diarrhea, infusion-related reaction, intestinal ischemia, intestinal perforation, and thrombocytopenia).
Fig. 1.
Overview of GSK3052230 dose-levels and observed dose-limiting toxicities (DLTs). At all dose-levels, the backbone chemotherapy was pemetrexed 500 mg/m2 and cisplatin 75 mg/m2 every 21 days. GSK, GSK3052230; G, grade
Efficacy
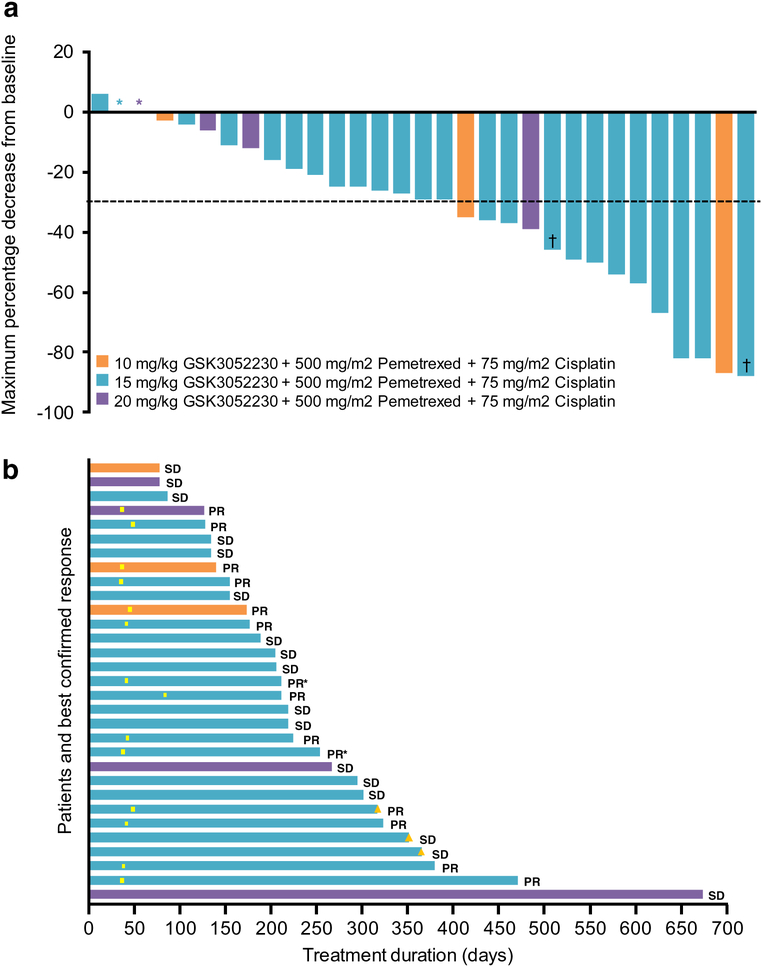
Out of 36 patients treated, 14 (39%) achieved a partial response (PR) and 17 (47%) had stable disease (SD) for a disease control rate (DCR) of 86% (Table 4). There were no complete responses. Four patients had tumor regression of more than 80% (Fig. 2a). Five patients (14%) were not evaluable for antitumor activity because they did not reach the first radiological evaluation after 8 weeks of study treatment due to clinical deterioration (n = 1) or treatment-related toxicity (n = 4).
Table 4.
Investigator-assessed best response with confirmation according to modified RECIST 1.1 criteria
| Dose-level 1 (n = 3) | Dose-level 2 (n = 25) | Dose-level 3 (n = 8) | Total (N =36) | |
|---|---|---|---|---|
| 10 mg/kg | 15 mg/kg | 20 mg/kg | ||
| Best response | ||||
| Partial response | 2 (67%) | 11 (44%) | 1 (13%) | 14 (39%) |
| Stable disease | ||||
| Discontinued | 1 (33) | 11 (44%) | 3 (38%) | 15 (42%) |
| Ongoing | 0 | 2 (8%) | 0 | 2 (6%) |
| Progressive disease | 0 | 0 | 0 | 0 |
| Not evaluable | 0 | 1 (4%) | 4 (50%) | 5 (14%) |
| Response ratea | 2 (67%) | 11 (44%) | 1 (13%) | 14 (39%) |
| [9.4–99.2] | [24.4–65.1] | [0.3–52.7] | [23.1–56.5] |
Defined as # of complete responses + partial responses/n*100%; [95% confidence interval]
Fig. 2.
Antitumor activity of GSK3052230 in combination with standard therapy in treatment-naive MPM patients. a Maximum percentage decrease from baseline in target lesions according to mRECIST 1.1 criteria. *Maximum percentage decrease from baseline = 0%. †Subject withdrawn from study for surgical resection. b Swimplot demonstrating duration of treatment and best confirmed response. PR, partial response; SD, stable disease; yellow box, first occurrence of PR; yellow triangle, ongoing patients as of 17-March-2017. *Subject withdrawn from study for surgical resection
At the recommended dose of 15 mg/kg GSK3052230, patients received a median of 11 treatment cycles with a maximum of 23. The ORR in the 15 mg/kg GSK3052230 group was 44% (Table 4) and the median PFS was 7.4 months. The median duration of treatment was 210 days [range: 78–673 days] with four patients receiving GSK3052230 for over 1 year (Fig. 2b). At the time of data cut-off, three patients were still ongoing.
FGF2 expression
Overexpression of FGF2 protein has been previously observed in MPM cell lines and tumor specimens [3, 15, 18–20]. To assess FGF2 protein expression in patients from this study, 27 archival formalin-fixed paraffin-embedded (FFPE) specimens were evaluated by IHC (Online Resource 3). There was no correlation between FGF2 protein expression and ORR observed (Online Resource 4a). However, despite the small sample size (n = 15), a statistically significant correlation between PFS and cytoplasmic FGF2 H-score was observed for the 15 mg/kg GSK3052230 group, p = 0.0157 (Online Resource 4b).
Discussion
GSK3052230 was designed to trap cancer-related FGFs selectively while sparing hormonal FGFs, thereby avoiding toxicities that have been observed with pan-selective small molecules that target the FGFR kinase domain [12]. This study confirmed what was observed in the GSK3052230 monotherapy study in that this selective binding modality indeed lowers the risk for these toxicities, even when combined with chemotherapy, since none of the patients reported any FGF-classspecific toxicities. Remarkably, the incidence of neutropenia, anemia, and increased blood creatinine levels were higher than expected. Since GSK3052230 had not been previously associated with these toxicities from prior preclinical and clinical studies, a contribution of pemetrexed and cisplatin seems likely [21].
In this study, we observed relevant and durable clinical activity in treatment-naive MPM patients when GSK3052230 was combined with standard first-line therapy. In contrast, the prior monotherapy study in all solid tumors did not yield objective responses [13]. The rationale to focus on mesothelioma patients was driven by prior data demonstrating high expression of FGF2 in MPM samples and reported sensitivity to GSK3052230 in preclinical models [3, 12]. Indeed, 100% of the evaluable patients showed response to treatment in terms of tumor regression or disease stabilization. In the 15 mg/kg GSK3052230 group, an ORR of 44% and a PFS of median 7.4 months was observed. Previously, the combination regimen of pemetrexed/cisplatin was reported to confer a 41% response rate, a median time to progression of 5.7 months, and a median OS of 12.1 months [2]. In the more recent Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS) trial, which compared the addition of bevacizumab to pemetrexed/cisplatin in first-line mesothelioma, the control group (pemetrexed/cisplatin) demonstrated a median time to progression of 7.3 months and a median OS of 16.1 months [22]. The ORR and PFS data obtained in the current study with the addition of GSK3052230 was comparable to these two previous studies. Similarly, the MAPS trial reported 62% grade 3–4 toxicities in patients treated with pemetrexed/cisplatin, while this study reported 56%. Thus, it is difficult to ascertain the actual contribution of GSK3052230 to the results observed. A randomized trial would be necessary to determine the benefits of GSK3052230.
While this study is the first to explore the potential of targeting the FGF/FGFR signaling pathway in patients with MPM, inhibition of FGF/FGFR signaling has been shown to be effective in several other tumor types using small molecules. For example, the FGFR1–3 inhibitors, BGJ398 and AZD4547, conferred partial responses in FGFR1-amplified squamous NSCLC and FGFR3-mutant bladder/urothelial cancer [10, 23]. Similarly, partial responses have been reported using the pan-FGFR inhibitor, erdafitinib, in urothelial cancer, glioblastoma, and endometrial cancer with FGFR1–4 or FGF3/4 alterations (amplifications/translocations/mutations), including FGFR3-TACC3 gene fusions [24]. Response to FGFR inhibition has also been observed in advanced cholangiocarcinomas, where FGFR2 gene fusions have been observed in 13–17% of patients [25]. This setting was the first to identify a genetic mechanism of resistance to FGFR kinase inhibition, as several patients acquired secondary FGFR2 mutations that rendered them resistant to BGJ398 treatment [26].
The above-mentioned mechanisms that drive FGF/FGFR signaling are considered to be FGF ligand-independent since these genomic alterations render the kinase activity of FGFRs to be constitutively active [8]. Alternatively, GSK3052230 targets ligand-dependent signaling in tumors where overexpression of FGFs or co-expression of FGFs and FGFRs occur. In this study, FGF expression was not used as a selection biomarker because FGFs, specifically FGF2, have been shown to be highly expressed in MPM [3, 15, 18–20]. The FGF2 expression analyses on archival material performed in this study confirmed that FGF2 protein levels are detectable in all patients. The potential of FGF2 as a biomarker for response in MPM may be further clarified in future research, as well as the use of FGFR1 expression which correlated with response in preclinical experiments in MPM cell lines [3]. Another potential biomarker that may help to select patients with optimal benefit may be BRCA1 Associated Protein 1 (BAP1). It has been shown that MPM cells with BAP1 protein loss are more sensitive to FGFR inhibition, which was related to increased expression of receptors FGFR1 and FGFR3 and ligands FGF9 and FGF18 [27].
A consequence of the selectivity of GSK3052230 for targeting FGFs that bind to the extracellular domain of FGFR1 α-IIIc could be that other FGFs that are not, or weakly, inhibited by GSK3052230 could still induce tumor growth. Preclinical models confirm that some signaling through the FGF pathway remains after treatment with GSK3052230, while complete suppression may be necessary for a more robust effect [3]. To overcome this, one approach could be to combine with other targeted agents that inhibit signaling pathways know to cross-talk with FGF/FGFRs, such as inhibitors of vascular endothelial growth factor (VEGF), the Ras-Raf-MEK-ERK pathway, or the PI3K-AKT pathway [8]. Another approach of potential interest could be to combine with immune checkpoint inhibitors. This is based on reported expression of PD-L1 on MPM cells, the observation of immune infiltrates in MPM tumor samples, and the preliminary efficacy observed with these agents [28–30].
In conclusion, this study demonstrates that GSK3052230 was well tolerated at a weekly dose of 15 mg/kg and that durable responses were achieved when combined with pemetrexed/cisplatin. The efficacy and safety profile of this molecule suggests the potential for its use in drug combination strategies and strongly supports its further clinical investigation.
Supplementary Material
Acknowledgements
We would like to thank all patients, together with their families and friends, who participated in the study. We would also like to thank the many investigators (along with individuals at the investigational sites who support the investigators), and the global and local study teams for their efforts for this study. Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Funding This work was supported by GlaxoSmithKline, Inc.
Footnotes
Conflict of interest
M. Dómine reports receiving speakers’ bureau honoraria and is a consultant/advisory board member for AstraZeneca (AZ), Bristol-Myers Squibb (BMS), Boehringer Ingelheim (BI), Celgene, MSD, Roche-Genentech, and AbbVie.
D.A. Fennell reports receiving commercial research grants from Astex, Bayer, and BI, speakers’ bureau honoraria from BI and BMS and is a consultant/advisory board member for Roche, Bayer, Aldeyra Therapeutics, AbbVie, and BMS.
H.L. Kindler reports receiving commercial research grants from Aduro Biotech, AZ, Bayer, GlaxoSmithKline (GSK), Merck, MedImmune, Verastem, BMS, Eli Lilly, Polaris, and Deciphera and is a consultant/advisory board member for Aduro Biotech, MedImmune, Bayer, Celgene, GSK, AZ, Merck, BMS, BI, Ipsen, Erytech Pharma, Five Prime Therapeutics, and Paredox Therapeutics.
S. Gadgeel is a consultant/advisory board member for AZ, Roche-Genentech, Takeda, BMS, and AbbVie.
P. Garrido Lopez reports receiving commercial research grants from GSK, Celgene, Sanofi, PharmaMar, Theradex, Roche, BMS, Lilly, Guardant, Sysmex, Takeda, AZ, BI, and Novartis and is a consultant/advisory board member for Roche, MSD, BMS, BI, Pfizer, Abbvie, Guardant,
Novartis, Lilly, AZ, Janssen, Sysmex, Blueprint Medicine, and Takeda.
D. Morgensztern is a consultant/advisory board member for Takeda, AbbVie, BMS, and PharmaMar.
M.G. Zauderer is an employee of MSKCC and MSKCC has an institutional collaboration agreement with IBM for Watson for Oncology and receives royalties from IBM, is the Chair of the Board of Directors of the Mesothelioma Applied Research Foundation, reports receiving commercial research grants from MedImmune, Epizyme, Polaris, Sellas Life Sciences, BMS, and Millenium, and is a consultant/advisory board member for Epizyme and Aldeyra Therapeutics.
J.F. Vansteenkiste reports receiving research funding/honoraria from MSD, AZ, BMS, BI, and Roche and is a consultant/advisory board member for AZ, BMS, BI, MSD, and Roche.
K. Baker-Neblett, X. Wang, L. Yan, I. Mitrica, and M.P. DeYoung are employees of and hold ownership interest in GlaxoSmithKline. J. Vasquez is an employee of GlaxoSmithKline.
D.I. Bellovin is an employee of and holds ownership interest in Five Prime Therapeutics.
J.H.M. Schellens is an employee of and holds ownership interest in Modra Pharmaceuticals and is a consultant/advisory board member for Debiopharm.
J.M. Trigo reports receiving speakers’ bureau honoraria from BMS and BI and is a consultant/advisory board member for BMS, BI, Merck, and Takeda.
No potential conflicts of interest were disclosed by the other authors.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
The online version of this article (https://doi.org/10.1007/s10637-019-00783-7) contains supplementary material, which is available to authorized users.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yap TA, Aerts JG, Popat S, Fennell DA (2017) Novel insights into mesothelioma biology and implications for therapy. Nat Rev Cancer 17:475–488 [DOI] [PubMed] [Google Scholar]
- 2.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P (2003) Phase III study of Pemetrexed in combination with Cisplatin versus Cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21:2636–2644 [DOI] [PubMed] [Google Scholar]
- 3.Blackwell C, Sherk C, Fricko M, Ganji G, Barnette M, Hoang B, Tunstead J, Skedzielewski T, Alsaid H, Jucker BM, Minthorn E, Kumar R, DeYoung M (2016) Inhibition of FGF/FGFR autocrine signaling in mesothelioma with the FGF ligand trap, FP-1039/GSK3052230. Oncotarget 7:39861–39871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stapelberg M, Gellert N, Swettenham E, Tomasetti M, Witting PK, Procopio A, Neuzil J (2005) α-Tocopheryl succinate inhibits malignant mesothelioma by disrupting the fibroblast growth factor Autocrine loop. J Biol Chem 280:25369–25376 [DOI] [PubMed] [Google Scholar]
- 5.Turner N, Grose R (2010) Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 10:116–129 [DOI] [PubMed] [Google Scholar]
- 6.Grose R, Dickson C (2005) Fibroblast growth factor signaling in tumorigenesis. Cytokine Growth Factor Rev 16:179–186 [DOI] [PubMed] [Google Scholar]
- 7.Itoh N (2007) The Fgf families in humans, mice, and zebrafish: their evolutional processes and roles in development, metabolism, and disease. Biol Pharm Bull 30:1819–1825 [DOI] [PubMed] [Google Scholar]
- 8.Dieci MV, Arnedos M, Andre F, Soria JC (2013) Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer Discov 3:264–279 [DOI] [PubMed] [Google Scholar]
- 9.Nishina T, Takahashi S, Iwasawa R, Noguchi H, Aoki M, Doi T (2018) Safety, pharmacokinetic, and pharmacodynamics of erdafitinib, a pan-fibroblast growth factor receptor (FGFR) tyrosine kinase inhibitor, in patients with advanced or refractory solid tumors. Investig New Drugs 36:424–434 [DOI] [PubMed] [Google Scholar]
- 10.Nogova L, Sequist LV, Perez Garcia JM, Andre F, Delord JP, Hidalgo M, Schellens JH, Cassier PA, Camidge DR, Schuler M, Vaishampayan U, Burris H, Tian GG, Campone M, Wainberg ZA, Lim WT, LoRusso P, Shapiro GI, Parker K, Chen X, Choudhury S, Ringeisen F, Graus-Porta D, Porter D, Isaacs R, Buettner R, Wolf J (2017) Evaluation of BGJ398, a fibroblast growth factor receptor 1–3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: results of a global phase I, dose-escalation and dose-expansion study. J Clin Oncol 35:157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michael M, Bang Y-J, Park YS, Kang YK, Kim TM, Hamid O, Thornton D, Tate SC, Raddad E, Tie J (2017) A phase 1 study of LY2874455, an Oral selective pan-FGFR inhibitor, in patients with advanced Cancer. Target Oncol 12:463–474 [DOI] [PubMed] [Google Scholar]
- 12.Harding TC, Long L, Palencia S, Zhang H, Sadra A, Hestir K et al. (2013) Blockade of nonhormonal fibroblast growth factors by FP-1039 inhibits growth of multiple types of Cancer. Sci Transl Med 5: 178ra39. [DOI] [PubMed] [Google Scholar]
- 13.Tolcher AW, Papadopoulos KP, Patnaik A, Wilson K, Thayer S, Zanghi J, Gemo AT, Kavanaugh WM, Keer HN, LoRusso PM (2016) A phase I, first in human study of FP-1039 (GSK3052230), a novel FGF ligand trap, in patients with advanced solid tumors. Ann Oncol 27:526–532 [DOI] [PubMed] [Google Scholar]
- 14.Pattarozzi A, Carra E, Favoni RE, Würth R, Marubbi D, Filiberti RA, Mutti L, Florio T, Barbieri F, Daga A (2017) The inhibition of FGF receptor 1 activity mediates sorafenib antiproliferative effects in human malignant pleural mesothelioma tumor-initiating cells. Stem Cell Res Ther 8:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schelch K, Hoda MA, Klikovits T, Münzker J, Ghanim B, Wagner C, Garay T, Laszlo V, Setinek U, Dome B, Filipits M, Pirker C, Heffeter P, Selzer E, Tovari J, Torok S, Kenessey I, Holzmann K, Grasl-Kraupp B, Marian B, Klepetko W, Berger W, Hegedus B, Grusch M (2014) Fibroblast growth factor receptor inhibition is active against mesothelioma and synergizes with radio- and chemotherapy. Am J Respir Crit Care Med 190:763–772 [DOI] [PubMed] [Google Scholar]
- 16.Marek LA, Hinz TK, von Massenhausen A, Olszewski KA, Kleczko EK, Boehm D et al. (2014) Nonamplified FGFR1 is a growth driver in malignant pleural mesothelioma. Mol Cancer Res 12:1460–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services FaDA, Center for Drug Evaluation and Research (CDER) and Center for Veterinary Medicine (CVM) (2001) Guidance for Industry: Bioanalytical Method Validation [Google Scholar]
- 18.Davidson B, Vintman L, Zcharia E, Bedrossian C, Berner A, Nielsen S, Ilan N, Vlodavsky I, Reich R (2004) Heparanase and basic fibroblast growth factor are co-expressed in malignant mesothelioma. Clin Exp Metastasis 21:469–476 [DOI] [PubMed] [Google Scholar]
- 19.Li Q, Wang W, Yamada T, Matsumoto K, Sakai K, Bando Y, Uehara H, Nishioka Y, Sone S, Iwakiri S, Itoi K, Utsugi T, Yasumoto K, Yano S (2011) Pleural mesothelioma instigates tumor-associated fibroblasts to promote progression via a malignant cytokine network. Am J Pathol 179:1483–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar-Singh S, Weyler J, Martin MJ, Vermeulen PB, Van Marck E (1999) Angiogenic cytokines in mesothelioma: a study of VEGF, FGF-1 and −2, and TGF beta expression. J Pathol 189:72–78 [DOI] [PubMed] [Google Scholar]
- 21.Hanigan MH, Devarajan P (2003) Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther 1:47–61 [PMC free article] [PubMed] [Google Scholar]
- 22.Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, Moro-Sibilot D, Molinier O, Corre R, Monnet I, Gounant V, Rivière F, Janicot H, Gervais R, Locher C, Milleron B, Tran Q, Lebitasy MP, Morin F, Creveuil C, Parienti JJ, Scherpereel A (2016) Bevacizumab for newly diagnosed pleural mesothelioma in the mesothelioma Avastin Cisplatin Pemetrexed study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 387: 1405–1414 [DOI] [PubMed] [Google Scholar]
- 23.Paik PK, Shen R, Berger MF, Ferry D, Soria JC, Mathewson A, Rooney C, Smith NR, Cullberg M, Kilgour E, Landers D, Frewer P, Brooks N, André F (2017) A phase Ib open-label multicenter study of AZD4547 in patients with advanced squamous cell lung cancers. Clin Cancer Res 23:5366–5373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabernero J, Bahleda R, Dienstmann R, Infante JR, Mita A, Italiano A, Calvo E, Moreno V, Adamo B, Gazzah A, Zhong B, Platero SJ, Smit JW, Stuyckens K, Chatterjee-Kishore M, Rodon J, Peddareddigari V, Luo FR, Soria JC (2015) Phase I dose-escalation study of JNJ-42756493, an Oral Pan–fibroblast growth factor receptor inhibitor, in patients with advanced solid tumors. J Clin Oncol 33:3401–3408 [DOI] [PubMed] [Google Scholar]
- 25.Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, Ramanathan RK, Goyal L, Sadeghi S, Macarulla T, el-Khoueiry A, Kelley RK, Borbath I, Choo SP, Oh DY, Philip PA, Chen LT, Reungwetwattana T, van Cutsem E, Yeh KH, Ciombor K, Finn RS, Patel A, Sen S, Porter D, Isaacs R, Zhu AX, Abou-Alfa GK, Bekaii-Saab T (2018) Phase II study of BGJ398 in patients with FGFR-altered advanced Cholangiocarcinoma. J Clin Oncol 36: 276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goyal L, Saha SK, Liu LY, Siravegna G, Leshchiner I, Ahronian LG, Lennerz JK, Vu P, Deshpande V, Kambadakone A, Mussolin B, Reyes S, Henderson L, Sun JE, van Seventer EE, Gurski JM Jr, Baltschukat S, Schacher-Engstler B, Barys L, Stamm C, Furet P, Ryan DP, Stone JR, Iafrate AJ, Getz G, Porta DG, Tiedt R, Bardelli A, Juric D, Corcoran RB, Bardeesy N, Zhu AX (2017) Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion - positive Cholangiocarcinoma. Cancer Discov 7:252–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quispel-Janssen JM, Badhai J, Schunselaar L, Price S, Brammeld J, Iorio F, Kolluri K, Garnett M, Berns A, Baas P, McDermott U, Neefjes J, Alifrangis C (2018) Comprehensive Pharmacogenomic profiling of malignant pleural mesothelioma identifies a subgroup sensitive to FGFR inhibition. Clin Cancer Res 24:84–94 [DOI] [PubMed] [Google Scholar]
- 28.Marcq E, Siozopoulou V, De Waele J, van Audenaerde J, Zwaenepoel K, Santermans E et al. (2017) Prognostic and predictive aspects of the tumor immune microenvironment and immune checkpoints in malignant pleural mesothelioma. Oncoimmunology 6:e1261241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alley EW, Lopez J, Santoro A, Morosky A, Saraf S, Piperdi B, van Brummelen E (2017) Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 18: 623–630 [DOI] [PubMed] [Google Scholar]
- 30.Zalcman G, Mazieres J, Greillier L, Lantuejoul S, Dô P, Bylicki O et al. (2017) LBA58_PR second or 3rd line Nivolumab (Nivo) versus Nivo plus Ipilimumab (Ipi) in malignant pleural mesothelioma (MPM) patients: up-dated results of the IFCT-1501 MAPS2 randomized phase 2 trial. Ann Oncol 28 Issue suppl_5, mdx440.074 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.