Abstract
Purpose
To assess the feasibility, safety and outcomes of an expedited One-Stop prostate cancer (PCa) diagnostic pathway.
Patients and Methods
We identified 370 consecutive patients who underwent multiparametric magnetic resonance imaging (mpMRI) and transrectal ultrasound fusion prostate biopsy (MRI/TRUS-PBx) from our institutional review board-approved database. Patients were divided according to diagnostic pathway: One-Stop (n=74), with mpMRI and same-day PBx, or Standard (n=296), with mpMRI followed by a second visit for PBx. mpMRIs were performed and interpreted according to Prostate Imaging-Reporting and Data System (PI-RADS v2). Grade Group ≥2 PCa defined clinically significant PCa (csPCa). Statistical significance was considered when p<0.05.
Results
Age (66 vs 66 years, p=0.59) and PSA density (0.1 vs 0.1ng/mL2, p=0.26) were not different between One-Stop vs Standard pathway, respectively. One-Stop patients lived further away from the hospital than Standard patients (163 vs 31Km; p<0.01), and experienced shorter time from mpMRI to PBx (0vs7 days; p<0.01). The number (p=0.56) and distribution of PI-RADS lesions (p=0.67) were not different between the groups. All procedures were completed successfully with similar perioperative complications rate (p=0.24). For patients with PI-RADS 3–5 lesions, the csPCa detection rate (49% vs 41%, p=0.55) was similar for One-Stop vs Standard, respectively. The negative predictive value of mpMRI (PI-RADS 1–2) for csPCa was 78% for One-Stop vs 83% for Standard (p=0.99). On multivariate analysis, age, prostate volume and PI-RADS score (p<0.01), but not diagnostic pathway, predicted csPCa detection.
Conclusion
A One-Stop PCa diagnostic pathway is feasible, safe and provides similar outcomes in a shorter time compared to the Standard two-visit diagnostic pathway.
Keywords: MRI, prostate cancer, prostate biopsy, MRI fusion biopsy, One-Stop
Introduction
Multiparametric magnetic resonance imaging (mpMRI) facilitates identification and imaging-guided targeted prostate biopsy (PBx) of suspicious prostate cancer (PCa) lesions. In fact, MRI-transrectal ultrasound fusion-guided prostate biopsy (MRI/TRUS-PBx) improves detection of clinically significant PCa (csPCa) while reducing detection of clinically insignificant PCa compared to systematic PBx [1].
MRI/TRUS-PBx requires mpMRI followed by PBx which are usually performed in two separate visits. This Standard diagnostic pathway may negatively impact patient experience, increase individual costs, prolong time to diagnosis, delay treatment with potential for progression, and increase the anxiety and psychological burden to the patient [2, 3]. As such, we proposed utilizing a same-day pathway to optimize our MRI-guided PBx protocol. The aim of this study is to assess the feasibility, safety and outcomes of an expedited One-Stop PCa diagnostic pathway with mpMRI followed by same-day MRI-TRUS fusion PBx.
Material and Methods
We identified, from our prospectively-maintained institutional review board-approved database (HS-03–00663), consecutive patients who underwent mpMRI followed by PBx between January of 2016 and June of 2018 at our institution. Inclusion criteria were, patients with clinical indication for prostate biopsy, including elevated PSA, abnormal digital rectal examination (DRE) or who were on active surveillance; and had mpMRI performed at our institution within six months prior to PBx. Exclusion criteria were, mpMRI performed outside of our institution, mpMRI performed longer than 6 months prior to PBx, or prior treatment for PCa.
Patients were offered either an expedited “One-Stop” or a two-visit “Standard” diagnostic pathway. The expedited, One-Stop pathway consisted of mpMRI followed by same-day MRI/TRUS-PBx. The Standard pathway consisted of mpMRI followed by MRI/TRUS-PBx on a separate day (Fig 1). A specific workflow protocol was developed for the One-Stop group, as follows: I) The radiology team was informed of One-Stop patients ahead of time; II) mpMRI acquisition was followed by stat expedited reading of the mpMRI; III) MRI/TRUS-PBx was performed within 3 hours after mpMRI, to allow for adequate imaging processing and interpretation of mpMRI. Both diagnostic procedures were performed at our institution. Patients living far from our facility or those who specifically requested an expedited diagnostic pathway, due to time constraints, were offered a One-stop pathway. There was no discrimination of any other demographic or clinical parameters for patients undergoing a One-stop diagnostic pathway. For patients undergoing repeat biopsy, only the most recent investigation (MRI and biopsy) was considered for this study.
Figure 1. Pathway workflows of mpMRI and MRI/TRUS-PBx.
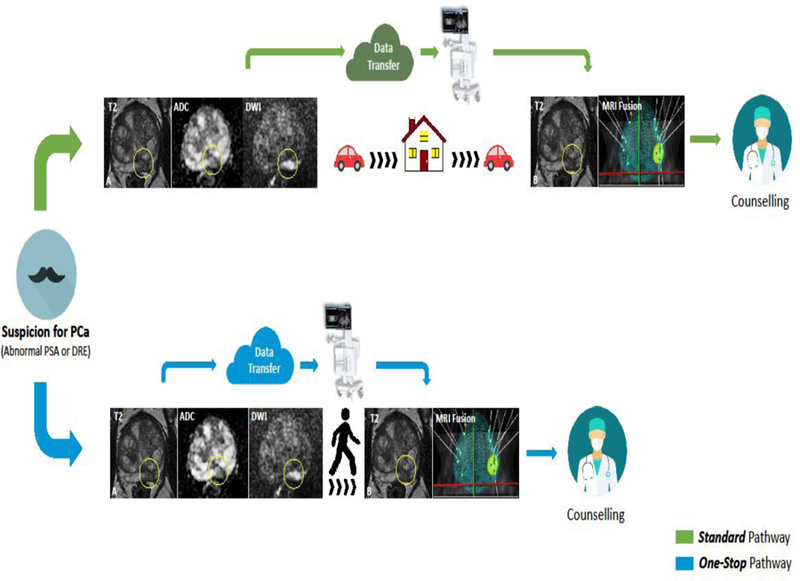
Patients were offered either: Standard pathway (green line), consisting of mpMRI aquisition (A) followed by MRI/TRUS-PBx (B) on separate days, or One-Stop pathway (blue line) consisting of mpMRI acquisition (A) followed by same-day MRI/TRUS-PBx (B). ADC: apparent diffusion coefficient; DRE: digital rectal examination; DWI: diffusion weighted images; mpMRI: Multiparametric magnetic resonance imaging; MRI/TRUS-PBx: MRI transrectal ultrasound fusion-guided prostate biopsy; PCa: prostate cancer; PSA: prostatic specific antigen.
Multiparametric MRIs were performed on a 3-Tesla MRI system (GE Healthcare, USA) using a multichannel phased-array abdominal coil. The MRI acquisition protocol included high resolution T2-weighted, diffusion weighted (DWI), and T1-weighted dynamic contrast-enhanced (DCE) sequences. Parametric apparent diffusion coefficient (ADC) maps were calculated from the diffusion-weighted images. The mpMRIs were interpreted by radiologists with more than 5 years of experience on MRI prostate, who assigned scores from 1 to 5 according with Prostate Imaging - Reporting and Data System Version 2 (PI-RADS v.2) standards [4].
MRI-TRUS-PBx was transrectally performed by the same experienced urologist under local anaesthesia, using an elastic image fusion software-assisted system (Koelis®, France) as previously described [5]. At least two cores for each mpMRI-detected PI-RADS 3–5 lesion followed by systematic extended sextant 12-core biopsies were performed. Patients with PI-RADS 1–2 underwent a 12-core systematic biopsy. In both cohorts, Bactrim or Ciprofloxacin were used for antibiotic prophylaxis. Augmentation prophylaxis (Gentamicin or Ceftriaxone) was administered within 1 hour prior to biopsy as clinically indicated [6]. Complications were recorded up to 90 days post biopsy or until initiation of any treatment for prostate cancer.
Histology was evaluated by an uro-pathologist according to International Society of Urological Pathology (ISUP) standards [7]. ISUP Grade Group ≥ 2 PCa was considered clinically significant PCa (csPCa).
Demographic, imaging characteristics, histology findings and perioperative complications, were analysed. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy were evaluated. Differences between groups were examined using the Kruskal-Wallis test for continuous variables and Pearson’s chi-squared or Fishers’ exact tests for categorical variables. Multivariable logistic regression analysis was used to identify predictors of PCa and csPCa. A p-value <0.05 was considered statistically significant. Statistical calculations were performed using SAS software version 9.4 (SAS Institute Inc, Cary, NC).
Results
Overall, 370 patients met the inclusion/exclusion criteria, with 74 patients in the One-Stop and 296 patients in Standard pathway groups. There were no significant differences of baseline characteristics between the two groups in terms of age (66 vs 66 years, p=0.59), PSA (5.4 vs 5.1ng/mL, p=0.1), PSA density (0.1 vs 0.1ng/mL2, p=0.26), suspicious DRE (26 vs 23%, p=0.65) and family history of PCa (22 vs 24%, p=0.76) for One-Stop vs Standard pathway, respectively (Table 1). The One-Stop pathway was associated with a shorter time from MRI to PBx (0 vs 7 days, p<0.01). One-Stop group patients lived further from the hospital (163 vs 31 km, p<0.01).
Table 1.
Demographics and MRI findings of MRI/TRUS fusion prostate biopsy.
| Variable | One-Stop Pathway |
Standard Pathway |
|
|---|---|---|---|
| Median (IQR) or n (%) | Median (IQR) or n (%) | p value | |
| N | 74 | 296 | - |
| Age, years | 66 (60–70) | 66 (61–71) | 0.59 |
| CCI score | 1 (0–2) | 2 (0–2) | 0.17 |
| PSA, ng/mL | 5.4 (2.8–7.8) | 5.1 (3.3–7.3) | 0.10 |
| PSA density, ng/mL2 | 0.1 (0.1–0.2) | 0.1 (0.1–0.2) | 0.26 |
| Family history of PCa | 16 (22) | 71 (24) | 0.76 |
| Suspicious DRE | 19 (26) | 66 (23) | 0.65 |
| Prostate biopsy history | |||
| Biopsy-naïve | 35 (47) | 141 (48) | 1.0 |
| Prior negative biopsy | 22 (30) | 86 (29) | 1.0 |
| Active Surveillance/restaging biopsy | 17 (23) | 69 (22) | 1.0 |
| Time between MRI and biopsy, days | 0 | 7 (3–19) | <0.01 |
| Home-hospital distance, km | 163 (58–237) | 31 (16–79) | <0.01 |
| MRI findings | |||
| Prostate volume, mL | 42 (27–62) | 53 (38–73) | <0.01 |
| PI-RADS 1–2 | 23 (31) | 84 (28) | |
| PI-RADS 3 | 36 (49) | 164 (55) | 0.67 |
| PI-RADS 4 | 8 (11) | 31 (11) | |
| PI-RADS 5 | 7 (9) | 17 (6) | |
| Total number of lesions | |||
| 1 lesion | 26 (51) | 112 (53) | |
| 2 Lesions | 21 (41) | 74 (35) | 0.56 |
| ≥ 3 Lesions | 4 (8) | 26 (12) |
CCI: Charlson comorbidity index; PCa, prostate cancer; DRE, digital rectal examination; MRI, magnetic resonance imaging; PI-RADS, Prostate Imaging and Reporting Data System; PSA, prostate-specific antigen.
MRI findings showed that prostate volume was smaller in One-Stop group (42 vs 53cc, p<0.01), but no differences were found in terms of number of lesions (p=0.56), and distribution of PI-RADS lesions (p=0.67) between the groups (Table 1).
The prostate biopsy findings were similar between the two groups (Table 2). All procedures were completed successfully; and no procedure was aborted. Perioperative complications were not significantly different between the two groups (4% vs 2%, p=0.24). Specifically, there were 3 complications in the One-Stop group (urinary tract infection (UTI), n=1; sepsis, n=1; and prolonged rectal bleeding during biopsy requiring local compression, n=1). Complications in the Standard pathway group were as follows: UTI, n=3; sepsis, n=2; urinary retention, n=2; prolonged rectal bleeding during biopsy requiring local compression, n=1 (Table 2). No patient died.
Table 2.
Histology outcomes and perioperative complications of MRI/TRUS fusion prostate biopsy.
| Variable | One-Stop Pathway(n=74) |
Standard Pathway (n=296) |
|
|---|---|---|---|
| Median (IQR) or n (%) | Median (IQR) or n (%) | p value | |
| Number of cores biopsy | 14 (13–16) | 14 (14–16) | 0.40 |
| Biopsy histology | |||
| Negative for PCa | 29 (39) | 142 (48) | 0.71 |
| Positive for PCa | 45 (61) | 154 (52) | 0.19 |
| Positive for csPCa | 30 (41) | 101 (34) | 0.34 |
| Grade Group | |||
| 1 | 15 (20) | 53 (18) | |
| 2 | 14 (19) | 53 (18) | |
| 3 | 5 (7) | 24 (8) | 0.71 |
| 4 | 7 (9.5) | 15 (5) | |
| 5 | 4 (5.5) | 9 (3) | |
| Complication, n % | 3 (4) | 7 (2) | 0.24 |
| Urinary tract infection | 1 | 3 | |
| Sepsis | 1 | 1 | |
| Urinary retention | 0 | 2 | |
| Rectal bleeding* | 1 | 1 |
PCa, prostate cancer; csPCa, clinically significant PCa
Prolonged bleeding during biopsy requiring local compression.
For patients with positive mpMRI (PI-RADS 3–5, n=263), the PCa (67% vs 59%, p= 0.20) and csPCa (49% vs 41%, p=0.55) detection rates were similar between One-Stop vs Standard groups, respectively. Specifically, the csPCa detection rate was 33% vs 33% (p=0.96) in patients with PI-RADS 3, 75% vs 65% (p=0.57) in those with PI-RADS 4, and 100% vs 76% (p=0.16) for patients with PI-RADS 5 lesion on mpMRI between the One-Stop vs Standard pathway, respectively (Table 3).
Table 3.
Prostate cancer detection according to multiparametric MRI findings.
| MRI findings | One-Stop pathway (n=74) | Standard pathway (n=296) | p-value |
|---|---|---|---|
| PI-RADS 1–2 (n=107) | 23 | 84 | |
| PCa | 11 (48%) | 28 (33%) | 0.2 |
| csPCa | 5 (22%) | 14 (17%) | 0.55 |
| PI-RADS 3–5 (n=263) | 51 | 212 | |
| PCa | 34 (67%) | 126 (59%) | 0.20 |
| csPCa | 25 (49%) | 87 (41%) | 0.55 |
| PI-RADS 3 (n=200) | 36 | 164 | |
| PCa | 20 (56%) | 88 (54%) | 0.84 |
| csPCa | 12 (33%) | 54 (33%) | 0.96 |
| PI-RADS 4 (n=39) | 8 | 31 | |
| PCa | 7 (88%) | 24 (77%) | 0.53 |
| csPCa | 6 (75%) | 20 (65%) | 0.57 |
| PI-RADS 5 (n=24) | 7 | 17 | |
| PCa | 7 (100%) | 14 (82%) | 0.23 |
| csPCa | 7 (100%) | 13 (76%) | 0.16 |
PCa, prostate cancer; csPCa, clinically significant PCa; MRI, magnetic resonance imaging; n= number of patients; PI-RADS, Prostate Imaging and Reporting Data System.
For patients with negative mpMRI (PI-RADS 1–2), the NPV of mpMRI for csPCa was 78% for One-Stop vs 83% for Standard pathway (p=0.99). Sensitivity, specificity, NPV, and PPV, for csPCa detection were not significantly different between both groups (Table 4).
Table 4.
Diagnostic performance characteristics of One-Stop versus Standard diagnostic pathways of MRI/TRUS fusion guided prostate biopsy for detecting clinically significant prostate cancer.
| Performance characteristics | One-Stop pathway | Standard pathway | p-value |
|---|---|---|---|
| Sensitivity | 83% | 86% | |
| Specificity | 41% | 36% | |
| Predictive positive value | 49% | 41% | 0.99 |
| Negative predictive value | 78% | 83% | |
| Accuracy | 58% | 53% |
In the univariate and multivariate analysis, age, prostate volume and PI-RADS score (p<0.01) were predictors of csPCa detection. The diagnostic pathway (One-Stop vs Standard) did not impact csPCa detection (Table 5).
Table 5.
Univariate and multivariable analyses of predictors for clinically significant prostate cancer detection on MRI/TRUS fusion guided prostate biopsy.
| Clinically Significant Prostate Cancer Detection | ||||
|---|---|---|---|---|
| Univariate | Multivariable | |||
| Variable | OR, CI 95% | p value | OR, CI 95% | p value |
| Age, years | 1.07 (1.04–1.10) | <0.01 | 1.08 (1.04–1.13) | <0.01 |
| Prostate volume, mL | 0.97 (0.96–0.98) | <0.01 | 0.97 (0.95–0.98) | <0.01 |
| PI-RADS lesion | ||||
| 3 | 2.28 (1.28–4.06) | 1.89 (1.00–3.72) | ||
| 4 | 9.26 (4.04–21.26) | 0.02 | 7.54 (2.94–20.63) | 0.01 |
| 5 | 23.16 (7.10–5.55) | 25.49 (6.94–120.36) | ||
| One-Stop vs Standard Pathway | 0.76 (0.45–1.28) | 0.30 | 0.99 (0.52–1.95) | 0.99 |
PI-RADS, Prostate Imaging and Reporting Data System
Discussion
In the present study, we report no statistically significant differences in terms of radiological, perioperative complications or pathologic outcomes between One-Stop vs Standard MRI/TRUS-PBx. The strength of our study is the size of our cohort, which to our knowledge, is the largest to date undergoing an MRI - based One-Stop pathway for PCa diagnosis. This study compares the One-Stop pathway to a contemporaneous control group of Standard two-visit mpMRI followed by PBx on separate days. Both groups underwent the same mpMRI and PBx protocols by the same experienced radiologists, urologist and pathologists at the same institution. The main difference between the groups was the specific workflow protocol developed for the One-Stop group. Although, patients undergoing a One-Stop pathway lived further from the hospital, they experienced shorter time from MRI to PBx. It is noteworthy that the outcomes herein reported correlate with existing literature [1, 5, 6, 8–10]. The multivariate analysis showed that age, prostate volume and PI-RADS score, but not the diagnostic pathway (One-Stop vs Standard), impact csPCa detection.
Often, patients with suspicion for PCa undergo diagnostic studies such as mpMRI and MRI-guided prostate biopsy on separate days. Although the use of pre-PBx mpMRI rapidly gained adoption [11], many community centers still do not have access to high-quality mpMRI and MRI/TRUS-PBx image fusion systems. Therefore, patients undergoing investigations with these specialized technologies may be required to, repeatedly, travel long distances to high-volume tertiary centers. The One-Stop pathway offers several key advantages: 1) it can potentially improve patient’s experience due to reduced wait and anxiety; 2) it can facilitate patient acceptance and logistics for undergoing MRI/TRSU-PBx, especially for those living far from these centers; 3) it reduces the opportunity for patients to be lost to follow-up between MRI and PBx; 4) it can reduces individual cost for biopsy.
Since 2010, we routinely perform office-based, transrectal systematic extended sextant 12-core and software-assisted (Koelis) MRI/TRUS fusion-guided PBx with, at least, 2 additional cores for each mpMRI-detected PI-RADS 3–5 lesion. As the use of sedation and operating room capabilities are not required, the One-Stop PCa diagnosis pathway was gradually introduced into our practice in January of 2016 to accommodate patients’ requests. We started offering an expedited One-Stop diagnostic pathway with same-day mpMRI and MRI/TRUS-PBx for patients traveling from far away or wishing to avoid any delay in biopsy. Currently, we routinely offer one-stop MRI/TRUS-PBx in our daily practice.
Although, One-Stop MRI and same-day MRI/TRUS-PBx seems to be easily feasible, it actually carries challenges that need to be addressed. First, to ensure patient satisfaction and avoid prolonged waiting times, the PBx should be performed as soon as possible after MRI acquisition. However, mpMRI requires imaging processing and expert interpretation, which requires time for proper imaging and reporting. Second, for MR imaging fusion and co-registration with TRUS, the MRIs should be uploaded onto the local intranet image system so it can, immediately, be available to the urologist performing the MRI/TRUS-PBx. Lastly, the imaging facility should be located close to the PBx outpatient clinic location, facilitating patient transit. On the other hand, a buffer for extra time should to be considered for possible issues with, the patient, imaging acquisition, and transferring. Therefore, coordination of these components is essential for a streamlined process. At our institution, the optimal time agreed amongst radiologist and urologist was a maximum of 3hs from MRI acquisition to PBx. With this workflow protocol, all procedures were successfully performed.
A recent, prospective, single-arm, pilot study evaluated suitability and feasibility of a “One-Stop” MRI-targeted biopsy pathway for patients with suspicious for PCa in “real-world” clinical practice in a tertiary referral centre in the United Kingdom. Of the 112 biopsy-naive men who presented with biochemical or clinical suspicion for PCa, 57 ultimately underwent transperineal cognitive-targeted PBx under local anesthesia. Patients with Likert score 1–2 on MRI (n=24; 22%) did not undergo PBx. Interestingly, a large proportion (n=15; 17%) of men wished to not undergo PBx under local anesthesia and underwent biopsy under sedation. The authors concluded that this approach greatly reduces the time to PCa diagnosis and the integration of mpMRI and MRI target biopsy has shown to be cost-effective in the long term. In fact, decreasing the number of visits during the diagnostic pathway of prostate cancer may reduce healthcare-related and patient incurred costs [10]. However, they utilized a transperineal approach, cognitive-targeted PBx without image-fusion software and 1.5T and 3T MRI. Also, they did not perform PBx in patients with negative MRI and had no control group. Nevertheless, these studies report corroborating evidence to show that One-Stop MRI and PBx using either approach (transrectal or transperineal) is feasible, safe and provides similar outcomes.
A prospective trial recently reported a median (IQR) of 53 (41–70) days between mpMRI and MRI/TRUS fusion PBx [9]. While delays in curative treatment may impact on the risk of biochemical recurrence after curative treatment, the results of retrospective single-centre studies are contradictory [3, 12–14]. Nevertheless, a meta-analysis showed that anxiety impacts 27% of the patients with PCa during the pre-treatment period and decreases after treatment [15]. In this context, by eliminating delays from MRI to PBx, the One-Stop pathway can help to reduce psychological burden on the patient and improve the patient experience.
It is debatable if patients with negative MRI (PI-RADS 1–2 lesions) should undergo systematic PBx. In fact, the PROMIS trial reported NPV of 76% for Grade Group ≥ 2 and stated that, by using MRI as a triage, 27% of PBx could be avoided [8]. We have previously reported the NPV for mpMRI in our institution [5]. Although, currently some patients meeting the criteria of negative MRI, PSA density <0.15ng/mL and previous negative PBx may defer repeat-PBx, for the present study, all patients with negative MRI underwent systematic PBx. Therefore, there was no selection bias and the NPV reported herein is 78% vs 83% for One-Stop vs Standard pathway respectively, similar to previously reported studies.
We performed transrectal biopsy with the patients in left lateral decubitus and under local anaesthesia without sedation or anxiolytic prior to the procedure. Empiric augmentation antibiotic prophylaxis was prescribed according to American Urological Association recommendations [6]. We report overall 1.1% of UTI with 0.5% of sepsis. A retrospective study reported on a large cohort of 15,236 patients undergoing transrectal PBx and showed 0.64% of sepsis. Interestingly, they showed that the augmented empirical prophylaxis was superior to prevent sepsis post PBx when compared with targeted prophylaxis antibiotics according to pre-PBx rectal swab culture or single agent empirical prophylaxis [16]. Another way to decrease chances of UTI/sepsis post-PBx is performing transperineal PBx. It seems that the main benefit of transperineal biopsy in comparison to transrectal biopsy is the lower risk of infection and sepsis [17, 18]. While the route of PBx, either transperineal or transrectal is debatable, patients undergoing transperineal biopsy still receive antibiotic prophylaxis and most of the centers still perform the procedure under sedation in the operating room, therefore increasing costs and operative time. Furthermore, transperineal biopsy is associated with greater rates of urinary retention [8, 19–21]. Two patients in our cohort presented with prolonged bleeding immediately after the PBx that required prolonged rectal compression. No transfusion, intravenous hydration or hospital admission was required.
The main limitation is the retrospective nature of this study; however, data were prospectively collected. Also, patients’ satisfaction was not analyzed. Nevertheless, our experience since 2016 shows that a One-Stop diagnostic pathway is safe and feasible with similar radiological and pathologic outcomes comparing to the Standard (two-visit) MRI/TRUS-PBx pathway. Additionally, the potential benefits of a One-Stop pathway include reduced patient anxiety, costs, time to diagnosis and time to treatment. Further, an expedited pathway may improve patient compliance and satisfaction by facilitating a single visit for both diagnostic studies [22, 23]. As a result, One-Stop pathway is, now, routinely offered to patients undergoing MRI/TRUS fusion biopsy at our institution.
In conclusion, the One-Stop pathway for prostate cancer diagnosis with same-day mpMRI and MRI/TRUS fusion-guided prostate biopsy is feasible, safe and provides similar cancer detection rate compared to the Standard (two-visit) diagnostic pathway, with the benefit of reduced time to diagnosis.
Acknowledgements and Funding
This study was funded by the R01 grant CA205058–01 from the National Institutes of Health/ National Cancer Institute (M.C.S, I.S.G. and A.L.D.C.A.) and in part by the Australasian Urological Foundation Scholarship (A.N.A).
Acronyms and abbreviations
- ADC
apparent diffusion coefficient
- AS
active surveillance
- CI
confidence interval
- csPCa
clinically significant prostate cancer
- DCE
dynamic contrast-enhanced
- DRE
digital rectal examination
- DWI
diffusion weighted images
- IQR
interquartile range
- ISUP
International Society of Urological Pathology
- mpMRI
Multiparametric magnetic resonance imaging
- MRI/TRUS-PBx
magnetic resonance imaging transrectal ultrasound fusion-guided prostate biopsy
- NPV
negative predictive value
- OR
odds ratio
- PBx
prostate biopsy
- PCa
prostate cancer
- PI-RADS v.2
Prostate Imaging - Reporting and Data System Version 2
- PPV
positive predictive value
- PSA
prostatic specific antigen
- UTI
urinary tract infection
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest
The authors declare that they have no conflict of interest. This study was funded by the R01 grant CA205058–01 from the National Institutes of Health/ National Cancer Institute (M.C.S, I.S.G. and A.L.D.C.A.) and in part by the Australasian Urological Foundation Scholarship (A.N.A). Dr. Vinay Duddalwar is a consultant for Intuitive Surgical and Radmetrix, sits on the advisory board for DeepTek, and received grant support from Samsung Healthcare.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. New England Journal of Medicine 2018;378(19):1767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh M, Maheu C, Brady T, Farah R. The psychological impact of the rapid diagnostic centres in cancer screening: A systematic review. Canadian Oncology Nursing Journal/Revue canadienne de soins infirmiers en oncologie 2017;27(4):348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg WT, Danzig MR, Pak JS, Korets R, RoyChoudhury A, Hruby G, et al. Delay from biopsy to radical prostatectomy influences the rate of adverse pathologic outcomes. The Prostate 2015;75(10):1085–91. [DOI] [PubMed] [Google Scholar]
- 4.Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS Prostate Imaging – Reporting and Data System: 2015, Version 2. European urology 2016;69(1):16–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oishi M, Shin T, Ohe C, Nassiri N, Palmer SL, Aron M, et al. Which Patients with Negative Magnetic Resonance Imaging Can Safely Avoid Biopsy for Prostate Cancer? The Journal of urology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liss MA, Ehdaie B, Loeb S, Meng MV, Raman JD, Spears V, et al. An update of the American Urological Association white paper on the prevention and treatment of the more common complications related to prostate biopsy. The Journal of urology 2017;198(2):329–34. [DOI] [PubMed] [Google Scholar]
- 7.Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. The American journal of surgical pathology 2016;40(2):244–52. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed HU, Bosaily AE-S, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. The Lancet 2017;389(10071):815–22. [DOI] [PubMed] [Google Scholar]
- 9.Wegelin O, Exterkate L, van der Leest M, Kummer JA, Vreuls W, de Bruin PC, et al. The FUTURE Trial: A Multicenter Randomised Controlled Trial on Target Biopsy Techniques Based on Magnetic Resonance Imaging in the Diagnosis of Prostate Cancer in Patients with Prior Negative Biopsies. European urology 2018. [DOI] [PubMed] [Google Scholar]
- 10.Bass EJ, Freeman A, Jameson C, Punwani S, Moore CM, Arya M, et al. Prostate cancer diagnostic pathway: Is a one-stop cognitive MRI targeted biopsy service a realistic goal in everyday practice? A pilot cohort in a tertiary referral centre in the UK. BMJ open 2018;8(10):e024941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oberlin DT, Casalino DD, Miller FH, Meeks JJ. Dramatic increase in the utilization of multiparametric magnetic resonance imaging for detection and management of prostate cancer. Abdominal radiology (New York) 2017;42(4):1255–8. [DOI] [PubMed] [Google Scholar]
- 12.Fossati N, Rossi MS, Cucchiara V, Gandaglia G, Dell’Oglio P, Moschini M, et al. , editors. Evaluating the effect of time from prostate cancer diagnosis to radical prostatectomy on cancer control: can surgery be postponed safely? Urologic Oncology: Seminars and Original Investigations; 2017: Elsevier. [DOI] [PubMed] [Google Scholar]
- 13.Korets R, Seager CM, Pitman MS, Hruby GW, Benson MC, McKiernan JM. Effect of delaying surgery on radical prostatectomy outcomes: a contemporary analysis. BJU international 2012;110(2):211–6. [DOI] [PubMed] [Google Scholar]
- 14.Morini MA, Muller RL, de Castro Junior PCB, de Souza RJ, Faria EF. Time between diagnosis and surgical treatment on pathological and clinical outcomes in prostate cancer: does it matter? World journal of urology 2018;36(8):1225–31. [DOI] [PubMed] [Google Scholar]
- 15.Watts S, Leydon G, Birch B, Prescott P, Lai L, Eardley S, et al. Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ open 2014;4(3):e003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang P, Liss MA, Szabo RJ. Targeted antimicrobial prophylaxis does not always prevent sepsis after transrectal prostate biopsy. The Journal of urology 2018;200(2):361–8. [DOI] [PubMed] [Google Scholar]
- 17.Xiang J, Yan H, Li J, Wang X, Chen H, Zheng X. Transperineal versus transrectal prostate biopsy in the diagnosis of prostate cancer: a systematic review and meta-analysis. World journal of surgical oncology 2019;17(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grummet JP, Weerakoon M, Huang S, Lawrentschuk N, Frydenberg M, Moon DA, et al. Sepsis and ‘superbugs’: should we favour the transperineal over the transrectal approach for prostate biopsy? BJU international 2014;114(3):384–8. [DOI] [PubMed] [Google Scholar]
- 19.Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, et al. Systematic review of complications of prostate biopsy. European urology 2013;64(6):876–92. [DOI] [PubMed] [Google Scholar]
- 20.Borghesi M, Ahmed H, Nam R, Schaeffer E, Schiavina R, Taneja S, et al. Complications After Systematic, Random, and Image-guided Prostate Biopsy. European urology 2017;71(3):353–65. [DOI] [PubMed] [Google Scholar]
- 21.Pinkhasov GI, Lin YK, Palmerola R, Smith P, Mahon F, Kaag MG, et al. Complications following prostate needle biopsy requiring hospital admission or emergency department visits - experience from 1000 consecutive cases. BJU international 2012;110(3):369–74. [DOI] [PubMed] [Google Scholar]
- 22.Nilbert M, Bläckberg M, Ceberg J, Hagberg O, Stenhoff R, Liedberg F. Diagnostic pathway efficacy for urinary tract cancer: population-based outcome of standardized evaluation for macroscopic haematuria. Scandinavian journal of urology 2018:1–7. [DOI] [PubMed] [Google Scholar]
- 23.Safir IJ, Gabale S, David SA, Huang JH, Gerhard RS, Pearl J, et al. Implementation of a tele-urology program for outpatient hematuria referrals: initial results and patient satisfaction. Urology 2016;97:33–9. [DOI] [PubMed] [Google Scholar]


