Abstract
Background
Psychologically resilient persons persist despite obstacles and bounce back after adversity, leading to better outcomes in non-neurologic populations. It is unknown whether psychological resilience relates to objective functional outcomes in multiple sclerosis (MS).
Objective
To determine whether psychological resilience explains differential objective cognitive and motor functioning in persons with early MS.
Methods
Psychological resilience was assessed in 185 patients with early MS and 50 matched healthy controls with the Connors-Davidson Resilience Scale (CDRS-10). Subjects completed the MS Functional Composite (MSFC) and a comprehensive neurobehavioral evaluation. Correlations assessed links between CDRS-10 and MSFC, motor indices (Total, Fine Motor, Gross Motor), and cognitive indices (Total, Cognitive Efficiency, Memory).
Results
Higher CDRS-10 among patients was linked to better MSFC and motor outcomes (but not cognition), with the most robust relationships for gross motor function (grip strength, gait endurance). Findings were independent of mood and fatigue. CDRS-10 was unrelated to MS disease burden. CDRS-10 was also specifically linked to motor outcomes in healthy controls.
Conclusions
Functional outcomes vary across persons with MS, even when disease burden and neurologic disability are low. These findings identify high psychological resilience as a non-disease-specific contributor to motor strength and endurance, which may explain differential outcomes across patients.
Keywords: multiple sclerosis, psychological resilience, cognitive performance, motor function
INTRODUCTION
Multiple sclerosis (MS) is a chronic unpredictable disease typically diagnosed during early adulthood, a time when persons are striving to manage multiple responsibilities (educational, occupational, social, familial, etc.). Management of these goals is encumbered by physical and cognitive decline, which often begins early in disease.(1, 2) Advances in disease modifying treatments for MS have led to improved disease control and more favorable prognoses for our patients;(3, 4) however, in our clinical experience, we encounter patients who still develop substantial cognitive and physical disability in everyday life despite adequate relapse control, relatively low disease burden (e.g., T2 lesion volume), and only minimal disability on neurologic exam (which leads to poor outcomes, e.g., unemployment, social isolation). This contrasts with other patients who remain cognitively, physically, and socially active despite comparable disease burden.
This disconnect between disease burden and outcomes was the impetus for the Reserve Hypothesis,(5–8) which in part seeks to identify risk and protective factors for functional expression (i.e., disability) of neurologic disease burden. Herein we investigate whether divergent outcomes may relate in part to differences in reactions to (and management of) stress. Psychiatric research has linked certain interpersonal differences to greater ability to overcome stress (and even flourish) when faced with challenging situations, including disease.(9–11). This “psychological resilience” can be defined as a dynamic process encompassing positive adaptation within the context of substantial adversity.(12) More generally, psychologically resilient people are self-confident; view obstacles as surmountable, can deal with strong emotions, and can see humor in challenging situations.
The construct of psychological resilience is potentially important but relatively understudied in MS. Previous work has linked psychological resilience with better patient-reported outcomes (e.g. social function, mental health);(13–18) however, our study examines relationships between psychological resilience and objective measures of cognitive and physical function: assessed with validated measures used in MS clinical trials, as well as domains of cognitive and motor function derived from a comprehensive behavioral assessment. We also evaluate whether psychological resilience contributes to objective functional outcomes (cognition, motor) independent of mood and disease burden (e.g., T2 lesion volume).
MATERIALS AND METHODS
Participants
The Reserve against Disability in Early MS (RADIEMS) Cohort is a longitudinal study of risk and protective factors for cognitive decline in persons aged 20 to 50 years and within five years of relapsing-remitting MS or clinically isolated syndrome diagnosis. Key exclusions include other neurologic or neurodevelopmental conditions, dyslexia, pregnancy, major psychiatric illness (e.g., bipolar disorder, schizophrenia, current major depressive episode), and clinical MS relapse within the past six weeks. All subjects were English language proficient. Baseline data from 185 persons with MS and matched 50 healthy controls are presented here. Healthy controls were friends or non-first-degree relatives of patients. Demographic characteristics of patients and controls are displayed in Table 1.
Table 1.
Sample Characteristics
| MS | Healthy Controls | Statistic, P-Value | |
|---|---|---|---|
| Sample SizeA | 185 | 50 | |
| Age (mean ± sd) | 34.4 ± 7.5 | 32.9± 7.5 | t=1.24, p=.216 |
| Sex (% Female) | 66.5 | 64.0 | X2=0.11, p=.742 |
| Race | X2=0.53, p=.766 | ||
| Caucasian (%) | 71.4 | 76.0 | |
| African American (%) | 20.5 | 16.0 | |
| Other (%) | 8.1 | 8.0 | |
| Ethnicity (% Hispanic) | 22.7 | 8.0 | X2=5.41, p=.020 |
| Maternal Education (% ≥16 years)B | 52.4% | 60.0% | X2=0.91, p=.341 |
| Verbal IQ (mean ± sd) | 108.2 ± 9.2 | 110.9 ± 9.1 | t=1.86, p=.064 |
| Disease Course (RRMS / CIS) | 165 / 20 | ||
| Years since Diagnosis (mean ± sd) | 2.2 ± 1.4 (median=2.0) | ||
| Years since 1st Symptom (mean ± sd) | 3.5 ± 2.9 (median=2.8) | ||
| EDSS (median; interquartile range) | 1.0; 0.0–1.5 | ||
| EDSS score (% of sample): | 0.0 (34.1), 1.0 (28.1), 1.5 (18.4), 2.0 (11.3), ≥2.5 (8.1) | ||
Two enrolled patients were not permitted to undergo research MRIs due to metal in their bodies. These patients had not reported this contraindication prior to enrollment because the metal had not precluded previous clinical MR imaging. The sample size for imaging analyses was therefore 183.
Maternal education is a better marker of socioeconomic status than a person’s own education because (a) it represents socioeconomic status during childhood and adolescence and (b) the current sample was relatively young, with 15% of the sample younger than 25 years of age.
This study was approved by the institutional review board of the Icahn School of Medicine at Mount Sinai and all participants provided informed consent.
Psychological Resilience
Personality attributes of psychological resilience were measured with the Connor-Davidson Resilience Scale (CDRS-10): a validated ten-item self-report measure on which subjects report their perceived ability (0=not true at all, 4=true nearly all the time; maximum raw=40) to deal with stressful and challenging situations and to overcome obstacles (e.g., seeing oneself as a strong person when faced with challenges, bouncing back after setbacks). Higher scores indicate a stronger belief that one can overcome obstacles, and even grow in the process. Psychometric properties of the CDRS-10, an abbreviated version of the original 25-item version, have been established in different populations,(19) with high internal consistency and convergent validity relative to the 25-item version.(20)We chose the CDRS-10 for the best combination of reliability / validity and feasibility.
Mood & Fatigue
Mood was assessed with the Mental Health Inventory (MHI) and the Beck Depression Inventory-Fast Screen (BDI-FS), which were considered as covariates given a previous association between mood and resilience in newly diagnosed MS patients (21). The Fatigue Severity Scale (FSS) was also considered as a covariate given links between fatigue and both mood and functional outcomes in MS.
Neurologic Disability
We assessed links between the CDRS and the Expanded Disability Status Scale (EDSS) to investigate differences in MS-related basic neurologic disturbance. The EDSS is a neurologic exam of seven functional systems: vision, brainstem, pyramidal, sensory, cerebellar, bowel / bladder, and ambulation. Disability was minimal in this sample of early patients (Table 1).
Functional Outcomes
MS Functional Composite (MSFC)
The MSFC was developed as a quantitative assessment of continuous cognitive, upper extremity, and gait function: Symbol Digit Modalities Test (SDMT) is a 90-second cognitive test requiring subjects to quickly state the digit that corresponds to rows of symbols based on a key of digit-symbol pairings (number of correct responses); Nine-Hole Peg Test (NHPT) requires subjects to quickly place pegs into nine holes and then remove them one at a time, with two trials performed with each upper extremity (mean time across four trials); Timed 25-Foot Walk (T25FW): subjects walk flat 25-foot course as quickly as possible (mean time across two trials). Raw scores were adjusted for age and sex, and converted to z-scores based on normative data. An MSFC Composite was derived as the mean z-score across tasks.
Comprehensive Neurobehavioral Evaluation
Patients and controls completed a comprehensive neurobehavioral evaluation, including eight cognitive and six motor tasks used herein. All tasks were administered by a licensed psychologist (PI: JFS) and/or trained research staff.
Cognitive tasks
(1) SDMT (described above); (2) Stroop Color-Word Test (Stroop) during which subjects rapidly named the ink color (red, green, blue) of non-matching printed words (e.g., “red” written in green ink)(22); (3) NIH Toolbox Pattern Comparison (PC) task is a tablet-based task requiring subjects to rapidly decide whether two presented pictures are the same or different(23); (4) Decision Speed (DS) during which subjects are presented with sixty rows each containing pictures of four common objects (e.g., dress, car, book, paper clip) and they have 100 seconds to quickly circle the object in each row that is largest in real life (e.g., car); (5) CANTAB Paired Associate Learning (PAL; Cambridge Cognition, Cambridge, UK) required subjects to recall the locations where visual stimuli were presented; (6) Brief Visuospatial Memory Test, Revised (BVMT-R) and (7) Selective Reminding Test (SRT) are the nonverbal (geometric shapes and locations) and verbal (word list learning) memory tests of the Brief Repeatable Battery for MS(24); (8) Verbal Paired Associate Learning (V-PAL) task required subjects to learn 12 unrelated word pairs across four trials. Cognitive tasks were regression-adjusted for age, sex, estimated premorbid verbal intelligence (Wechsler Test of Adult Reading, WTAR), and whether English was a primary or secondary language. Adjusted scores were converted to z-scores based on the mean and standard deviation (SD) of the matched healthy control group.
Motor tasks
(1) Grooved Pegboard (G-Pegs) and (2) NHPT (described above) are both timed motor tasks requiring rapid placement of pegs in holes; (3) electronic Finger Tapping Test (FTT) during which subjects press a button with their index finger as quickly as possible for ten seconds. This is performed five times with the dominant and five times with the non-dominant hands, and then averaged into one score. (4) Grip Strength (Grip) uses a hand dynamometer to measure maximal strength exerted by dominant and nondominant hands, and then averaged into one score; (5) the T25FW (described above) and the (6) Two-Minute Walk Test (2MWT) assesses the maximal distance a person can walk across a flat surface within a two-minute time limit. Motor tasks were regression-adjusted for age, sex, height, and weight. Adjusted scores were converted to z-scores based on the mean and SD of the matched healthy control group. (T25FW was not administered to healthy controls, so the mean of patients was used to derive z-scores for patients. Mean raw T25FW scores were normal [4.1±0.5s]. Controls were given z-scores of 0.)
A Total Cognitive composite was derived as the mean of all cognitive tasks; a Total Motor composite was derived as the mean of all motor tasks. All 14 tasks were entered into a principal components analysis (PCA, direct oblimin rotation, eigenvalues ≥1.0) to identify finer-grained sub-components for further analysis. PCA yielded four unique sub-components (Table 2): Cognitive Efficiency (SDMT, Stroop, PC, DS), Memory (SRT, BVMT-R, PAL, V-PAL), Gross Motor (T25FW, 2MWT, Grip), and Fine Motor (FTT, NHPT, G-Pegs). Z-scores of tasks within each component were averaged to derive mean z-scores for the four sub-components.
Table 2. Principal Components Analysis Validating Composites.
The following pattern matrix was derived from the principal components analysis, which yielded four independent components (eigenvalues ≥ 1.0) validating our four sub-composites: (1) Cognitive Efficiency, (2) Memory, (3) Gross Motor, (4) Fine Motor. Loadings ≥ .40 are shown.
| Pattern Matrix | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| PC | 0.846 | |||
| DS | 0.689 | |||
| SDMT | 0.614 | |||
| Stroop | 0.540 | |||
| SRT | 0.774 | |||
| V-PAL | 0.762 | |||
| PAL | 0.714 | |||
| BVMT-R | 0.621 | |||
| T25FW | 0.885 | |||
| 2MWT | −0.793 | |||
| Grip | −0.546 | |||
| FTT | −0.697 | |||
| NHPT | 0.658 | |||
| G-Pegs | −0.623 | |||
MS Disease Burden
Normalized grey matter volume (nGM) and T2 lesion volume (T2LV) were assessed in patients who underwent 3D T1 and 3D T2 3.0T MR imaging (Siemens Skyra, n=183) to assess whether psychological resilience differed as a function of MS disease burden. T2LV was quantified using a local thresholding segmentation technique (Jim 6.0) and log-transformed. nGM was measured with SIENAX using lesion-filled T1 images, and regression-adjusted for age and sex.
Statistical Analysis
Preliminary Analyses: Independent t-tests (two-tailed) assessed differences in CDRS between patients and controls; relationships between CDRS and demographic and disease variables were assessed to identify covariates for subsequent analyses. Primary Analyses: Pearson correlations (two-tailed) investigated relationships between CDRS and MSFC, composite motor outcomes (Total Motor, Fine Motor, Gross Motor), and composite cognitive outcomes (Total Cognitive, Cognitive Efficiency, Memory) in patients. Then, in a more conservative approach, partial correlations assessed links between outcomes and CDRS controlling for all identified covariates. To investigate specificity to MS, we performed aforementioned simple and partial correlations within the healthy control group. Secondary Analyses: To further characterize results, the aforementioned approach was performed for each of the individual tasks (e.g., FTT) composing composite outcomes (e.g., Fine Motor) significantly related to CDRS.
RESULTS
Preliminary Analyses
There were no differences in psychological resilience between patients (CDRS: 29.95±5.98) and healthy controls (31.10±4.85, t[233]=1.26, p=.210), and scores were comparable to a community-based sample (Memphis n=764, 31.77±5.47).(25) There were no outliers (99% CI) Among patients, there were no relationships between CDRS and age, sex, ethnicity, maternal education, premorbid intellectual function (WTAR), or EDSS (Ps>.50), time since diagnosis (p>.10), or disease burden assessed radiologically (T2LV & nGM, Ps>.25). There was a difference across races, with lower CDRS among African-Americans (27.16±5.23) than non-African-Americans (30.73±5.79, t[181]=3.42, p<.001). Lower CDRS was also associated with worse mood (MHI r=.620, p<.001; BDI-FS r=−.586, p<.001) and worse fatigue (FSS r=−.449, p<.001). To identify independent predictors of CDRS (and avoid multicollinearity among covariates), stepwise regression (entry p=.05, removal p=.10) predicted CDRS with MHI, BDI-FS, FSS, and African-American race. MHI (rp=.533, p<.001) and FSS (rp=−.203, p=.006) were retained, and were therefore selected as covariates in subsequent analyses. African-Americans reported worse mood (MHI, p=.002) and fatigue (p=.001), which mediated the aforementioned link between race and CDRS (Sobel Zs=≥2.93, Ps≤.002; race did not mediate links between CDRS and MHI or FSS, Ps>.05). These preliminary results suggest that any subsequent links between psychological resilience and functional outcomes are not due to differences in demographics, basic neurologic disturbances, or disease burden, especially in models adjusted for mood and fatigue.
Primary Analyses
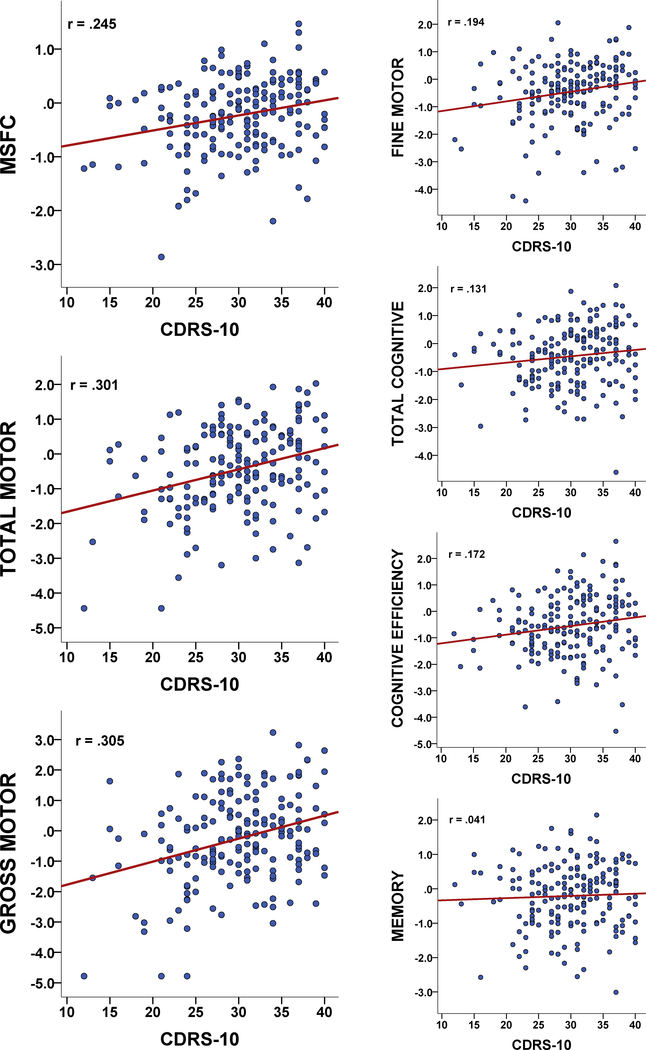
Correlations: Higher psychological resilience was linked to better MSFC performance, as well as better performance on Total Motor, Fine Motor, and Gross Motor composites (Table 3, Figure 1). There was a small reliable link between higher psychological resilience and better Cognitive Efficiency, but no links to Memory and only a trend for the Total Cognitive composite. Links between higher psychological resilience and better MSFC, Total Motor, and Gross Motor withstood correction for multiple comparisons (Bonferroni-adjusted p≤.007). Fully-Adjusted Partial Correlations: Of the three composites withstanding multiple comparisons, Total Motor and Gross Motor (but not MSFC) remained significant when adjusting for mood and fatigue, and both withstood correction for multiple comparisons (Bonferroni-adjusted p≤.017). Results for all outcomes are reported (Table 3). Specificity to MS: The same pattern of results was found in matched healthy controls, with higher psychological resilience linked to better motor outcomes, with the largest relationship for Gross Motor (Table 3). Consideration of Outliers: Aforementioned results for Pearson correlations were not unduly influenced by outliers, as (a) there were no extreme outlier values for any metric and (b) reanalysis with Spearman correlations yielded comparable results. For instance, after adjusting values for mood and fatigue, higher CDRS-10 was still related to better Gross Motor in patients (rho=.187, p=.011 vs rp=.184, p=.012).
Table 3. Correlations between Psychological Resilience and Composite Outcomes.
Relationships between CDRS and composite outcomes among patients are first reported as unadjusted correlations, and then as partial correlations controlling for mood and fatigue. Significant correlations are in bold. The same relationships are then reported for healthy controls, with correlations in bold if they are significant or if they would be significant in a sample with n=185 (to allow comparisons with the MS groups). Exact p-values are reported. (As stated in the preliminary analysis section, FSS and MHI were empirically identified as important covariates. If we added BDI-FS as an additional covariate, the pattern of results remains the same: specific significant links between CDRS and both Total Motor and Gross Motor, with no significant links to any other outcomes.)
| MSFC | MOTOR Composite | Fine Motor | Gross Motor | COGNITIVE Composite | Cognitive Efficiency | Memory | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| MULTIPLE SCLEROSIS | Unadjusted Correlations | CDRS | r | 0.245 | 0.301 | 0.194 | 0.305 | 0.131 | 0.172 | 0.041 |
| p | <0.001 | <0.001 | 0.008 | <0.001 | 0.077 | 0.019 | 0.577 | |||
| Fully Adjusted Correlations | CDRS | rp | 0.128 | 0.199 | 0.143 | 0.184 | 0.021 | 0.039 | −0.005 | |
| Controlling for MHI & FSS | p | 0.083 | 0.007 | 0.053 | 0.013 | 0.780 | 0.602 | 0.951 | ||
| HEALTHY CONTROLS | Unadjusted Correlations | CDRS | r | n/a | 0.320 | 0.181 | 0.409 | −0.011 | 0.110 | −.108 |
| p | 0.023 | 0.209 | 0.003 | 0.941 | 0.447 | 0.455 | ||||
| Fully Adjusted Correlations | CDRS | rp | n/a | 0.194 | 0.091 | 0.274 | 0.043 | 0.024 | 0.046 | |
| Controlling for MHI & FSS | p | 0.186 | 0.537 | 0.059 | 0.770 | 0.870 | 0.757 | |||
Figure 1. Correlations between Psychological Resilience and Outcomes.
Scatterplots depict simple correlations between psychological resilience (CDRS-10) and MSFC, Total Motor, and Gross Motor (left), as well as Fine Motor and cognitive outcomes (right).
Secondary Analyses
Individual Tasks: To further characterize findings, we examined individual tasks composing composites related to psychological resilience (Table 3). Higher psychological resilience was related to better Grip, 2MWT, NHPT, T25FW, FTT, and SDMT, of which Grip and 2MWT withstood correlation for multiple comparisons (Bonferroni-adjusted p≤.007), and both Grip strength and gait endurance during the 2MWT remained significant when controlling for mood and fatigue (Table 3). (FTT was the only other task remaining significant after controlling for mood and fatigue.) The same pattern was shown for healthy controls (Table 3). As with composite scores, there were no extreme outliers for any of the individual tasks, and reanalysis with Spearman correlations did not change the results. For instance, after adjusting values for mood and fatigue, higher CDRS-10 was still related to stronger Grip in patients (rho=.211, p=.004 vs rp=.223, p=.002).
DISCUSSION
In our cohort of 185 patients with early MS, high psychological resilience was associated with better objective performance on the MSFC and motor outcomes, especially gross motor function (i.e., grip strength, gait endurance). This relationship of psychological resilience to outcomes was independent of mood and fatigue, and psychological resilience was unrelated to disease burden (T2LV, nGM) or traditional metrics of neurologic disturbance (EDSS), thereby arguing against worse overall disease as a mediator between psychological resilience and outcomes. In fact, high psychological resilience was also related to better motor performance in healthy controls, suggesting that the effect of psychological resilience is not disease-related. Instead, patients with higher psychological resilience may come to the disease with a motor advantage, supporting psychological resilience as a potential explanation for the functional disparities seen among patients with similar MS disease burden and neurologic symptoms.
Our work utilizing objective measures of cognitive and motor functioning adds to previous research in MS linking higher psychological resilience to better patient-reported outcomes.(14–18) The positive effect of psychological resilience herein was specifically related to motor functioning. One possible explanation is that higher psychological resilience may translate to greater drive / motivation, which might impact the physical force exerted during a grip strength task. In contrast, sheer drive or motivation may not help a person remember more information, as demonstrated by the lack of association between psychological resilience and the overall cognitive composite, and memory specifically (although explanations of negative results are speculative). In everyday life, this greater drive / motivation may translate to functional differences, such as pushing oneself to leave the house and engage in work or social pursues despite disease-related challenges. Importantly, although we often attribute “motivation” and “drive” to a person’s character, we must remember that these attributes are products of brain function (rather than resorting to mind-body dualism). Indeed, a growing field of preclinical and human research focuses on the neurobiology of psychological resilience,(11, 26–28) which explores complex interactions among genetic, environmental, neurobiological, and psychosocial traits to help explain differences in psychological resilience against poor outcomes in the context of stress (e.g., traumatic stress, chronic emotional stress, adjustment to disease). Note also that measurable differences in strength and endurance were also observed across healthy controls with differing psychological resilience, thereby suggesting that at least some functional differences are present in the absence of traumatic stress.
Patients with higher psychological resilience appear to come to the disease with advantages in their capacity to muster strength and physical endurance, which may allow patients to maintain performance closer to premorbid levels in the face of disease. More investigation is needed to understand these relationships, especially the observed specific link between psychological resilience and gross motor function. Preclinical work aims to elucidate biological bases of resilience against stress (e.g.,(29)), but work is needed to understand a potential shared basis for psychological resilience and motor drive outside of traumatic or chronic stress.
The current results were shown in a cohort of patients diagnosed for less than five years; future research is needed to examine whether our findings translate to patients with more advanced disease. There may be a threshold effect not captured in early patients with overall low disease burden: patients with more severe cerebral and infratentorial dysfunction may not overcome challenges to motor function despite high psychological resilience. On the other hand, the effect of psychological resilience may be even more pronounced among patients with more disease to overcome. As our data are both cross-sectional and observational, limited conclusions can be drawn about underlying causative mechanisms of the reported relationship between psychological resilience and outcomes in MS or about the potential protective effect of psychological resilience over MS disease course. Note also that psychological resilience was only measured with one questionnaire, so a broader exploration is necessary to better explain the exact role of resilience in MS.
Though high psychological resilience was associated with better motor performance in our cohort, it is unclear how this translates into a patient’s everyday functioning. There is some evidence that psychological resilience may be bolstered through interventions (e.g.(30, 31)), which may improve outcomes. Conversely, given that the directionality of our findings is unknown, it may be that improving motor strength and endurance could enhance psychological resilience. As noted, work has explored the complex neurobiology of psychological resilience in nonhuman and human mammals,(11, 26–28) but how and whether these processes interact with MS disease specifically represents another area of future inquiry, which may ultimately inform psychological resilience interventions.
Table 4. Correlations between Psychological Resilience and Individual Tasks.
Relationships between CDRS and individual tasks (composing composites related to psychological resilience) among patients are first reported as unadjusted correlations, and then as partial correlations controlling for mood and fatigue. Significant correlations are in bold. The same relationships are then reported for healthy controls, with correlations in bold if they are significant or if they would be significant in a sample with n=185 (to allow comparisons with the MS groups). Exact p-values are reported. (As stated in the preliminary analysis section, FSS and MHI were empirically identified as important covariates. If we added BDI-FS as an additional covariate, the pattern of results remains the same: specific significant links between CDRS and both Grip and FTT, with a trend for 2MWT.)
| SDMT | NHPT | G-Pegs | FTT | Grip | T25FW | 2MWT | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| MULTIPLE SCLEROSIS | Unadjusted Correlations | CDRS | r | 0.166 | −0.194 | 0.098 | 0.183 | 0.307 | −0.192 | 0.238 |
| p | 0.024 | 0.008 | 0.185 | 0.013 | <0.001 | 0.009 | 0.001 | |||
| Fully Adjusted Correlations | CDRS | rp | 0.082 | −0.144 | 0.014 | 0.192 | 0.223 | −0.072 | 0.150 | |
| Controlling for MHI & FSS | p | 0.268 | 0.052 | 0.852 | 0.009 | 0.002 | 0.334 | 0.042 | ||
| HEALTHY CONTROLS | Unadjusted Correlations | CDRS | r | 0.099 | −0.110 | 0.098 | 0.224 | 0.382 | n/a | 0.275 |
| p | 0.495 | 0.445 | 0.496 | 0.118 | 0.006 | 0.053 | ||||
| Fully Adjusted Correlations | CDRS | rp | 0.201 | −0.012 | −0.012 | 0.203 | 0.274 | n/a | 0.157 | |
| Controlling for MHI & FSS | p | 0.170 | 0.934 | 0.935 | 0.166 | 0.059 | 0.286 | |||
Acknowledgments
FUNDING
This study was funded by the National Institutes for Health (R01 HD082176 to JFS).
Footnotes
DISCLOSURES
S. Klineova reports advisory board work with Genentech and Biogen Idec and has given non-promotional lectures with Biogen Idec. M.T. Fabian has given non-promotional lectures with Biogen Idec. S. Krieger reports consulting or advisory work with Acorda, Bayer, Biogen, Celgene, EMD Serono, Genentech, Genzyme, Mallinckrodt, MedDay, Novartis, Teva, and TG Therapeutics, and non-promotional speaking with Biogen. F.D. Lublin reports funding for research from Novartis Pharmaceuticals Corp, Teva Neuroscience, Actelion, Transparency Life Sciences, NMSS, and Sanofi and consulting agreements/advisory boards/DSMB fees from Bayer HealthCare, Biogen, EMD Serono, Novartis, Teva; Actelion; Sanofi/Genzyme; Acorda; Roche/Genentech; MedImmune; Receptos/Celgene; Forward Pharma; TG Therapeutics; Abbvie; Regeneron; Medday; Atara Biotherapeutics; Polpharma; Mapi Pharma; Innate Immunotherapeutics; Apitope; and Orion Biotechnology. A. E. Miller reports consulting or advisory work with Accordant Health Services (Caremark), Adamas, Biogen Idec, Celgene, Corrona, EMD Serono, Genzyme/Sanofi, Mallinckrodt, Mapi-Pharma, Novartis, Roche/Genentech and research support from Biogen Idec, Genzyme/Sanofi, Mallinckrodt, Novartis, Roche/Genentech, MedDay. C.S. Riley reports consulting or advisory work with Biogen Idec, Celgene, Genentech/Roche, Genzyme, TG Therapeutics. J. F. Sumowski reports consulting or advisory work with Biogen Idec and Sanofi Genzyme. R. Brandstadter, I. Katz Sand, V. M. Leavitt, C. Lewis report no disclosures.
REFERENCES
- 1.Mitchell AJ, Benito-Leon J, Gonzalez JM, Rivera-Navarro J. Quality of life and its assessment in multiple sclerosis: integrating physical and psychological components of wellbeing. The Lancet Neurology. 2005;4(9):556–66. [DOI] [PubMed] [Google Scholar]
- 2.Sumowski JF, Benedict R, Enzinger C, Filippi M, Geurts JJ, Hamalainen P, et al. Cognition in multiple sclerosis: State of the field and priorities for the future. Neurology. 2018;90(6):278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.University of California SFMSET, Cree BA, Gourraud PA, Oksenberg JR, Bevan C, Crabtree-Hartman E, et al. Long-term evolution of multiple sclerosis disability in the treatment era. Annals of neurology. 2016;80(4):499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sormani MP, Arnold DL, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Annals of neurology. 2014;75(1):43–9. [DOI] [PubMed] [Google Scholar]
- 5.Stern Y What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society : JINS. 2002;8(3):448–60. [PubMed] [Google Scholar]
- 6.Stern Y Cognitive reserve in ageing and Alzheimer’s disease. The Lancet Neurology. 2012;11(11):1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumowski JF, Rocca MA, Leavitt VM, Dackovic J, Mesaros S, Drulovic J, et al. Brain reserve and cognitive reserve protect against cognitive decline over 4.5 years in MS. Neurology. 2014;82(20):177–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumowski JF, Rocca MA, Leavitt VM, Meani A, Mesaros S, Drulovic J, et al. Brain reserve against physical disability progression over 5 years in multiple sclerosis. Neurology. 2016;86(21):2006–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depression and anxiety. 2003;18(2):76–82. [DOI] [PubMed] [Google Scholar]
- 10.Ong AD, Bergeman CS, Bisconti TL, Wallace KA. Psychological resilience, positive emotions, and successful adaptation to stress in later life. Journal of personality and social psychology. 2006;91(4):730–49. [DOI] [PubMed] [Google Scholar]
- 11.Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science (New York, NY). 2012;338(6103):79–82. [DOI] [PubMed] [Google Scholar]
- 12.Lutha SS, Cicchetti D. The construct of resilience: implications for interventions and social policies. Development and psychopathology. 2000;12(4):857–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battalio SL, Silverman AM, Ehde DM, Amtmann D, Edwards KA, Jensen MP. Resilience and Function in Adults With Physical Disabilities: An Observational Study. Archives of physical medicine and rehabilitation. 2017;98(6):1158–64. [DOI] [PubMed] [Google Scholar]
- 14.Koelmel E, Hughes AJ, Alschuler KN, Ehde DM. Resilience Mediates the Longitudinal Relationships Between Social Support and Mental Health Outcomes in Multiple Sclerosis. Arch Phys Med Rehabil. 2017;98(6):1139–48. [DOI] [PubMed] [Google Scholar]
- 15.Rainone N, Chiodi A, Lanzillo R, Magri V, Napolitano A, Morra VB, et al. Affective disorders and Health-Related Quality of Life (HRQoL) in adolescents and young adults with Multiple Sclerosis (MS): the moderating role of resilience. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2017;26(3):727–36. [DOI] [PubMed] [Google Scholar]
- 16.Senders A, Bourdette D, Hanes D, Yadav V, Shinto L. Perceived stress in multiple sclerosis: the potential role of mindfulness in health and well-being. J Evid Based Complementary Altern Med. 2014;19(2):104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverman AM, Molton IR, Alschuler KN, Ehde DM, Jensen MP. Resilience predicts functional outcomes in people aging with disability: a longitudinal investigation. Arch Phys Med Rehabil. 2015;96(7):1262–8. [DOI] [PubMed] [Google Scholar]
- 18.Silverman AM, Verrall AM, Alschuler KN, Smith AE, Ehde DM. Bouncing back again, and again: a qualitative study of resilience in people with multiple sclerosis. Disabil Rehabil. 2017;39(1):14–22. [DOI] [PubMed] [Google Scholar]
- 19.Campbell-Sills L, Stein MB. Psychometric analysis and refinement of the connor–davidson resilience scale (CD-RISC): Validation of a 10-item measure of resilience. Journal of Traumatic Stress. 2007;20(6):1019–28. [DOI] [PubMed] [Google Scholar]
- 20.Kuiper H, van Leeuwen CCM, Stolwijk-Swuste JM, Post MWM. Measuring resilience with the Connor-Davidson Resilience Scale (CD-RISC): which version to choose? Spinal cord. 2019. [DOI] [PubMed] [Google Scholar]
- 21.Tan-Kristanto S, Kiropoulos LA. Resilience, self-efficacy, coping styles and depressive and anxiety symptoms in those newly diagnosed with multiple sclerosis. Psychol Health Med. 2015;20(6):635–45. [DOI] [PubMed] [Google Scholar]
- 22.Golden CJ, Freshwater S. Stroop Color and Word Test, Revised 2002 Adult Manual for Clinical and Experimental Uses.: Wood Dale, IL: Stoelting Co.; 2002. [Google Scholar]
- 23.Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80(11 Suppl 3):S54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41(5):685–91. [DOI] [PubMed] [Google Scholar]
- 25.Campbell-Sills L, Forde DR, Stein MB. Demographic and childhood environmental predictors of resilience in a community sample. Journal of psychiatric research. 2009;43(12):1007–12. [DOI] [PubMed] [Google Scholar]
- 26.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nature reviews Neuroscience. 2009;10(6):446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nature neuroscience. 2012;15(11):1475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker FR, Pfingst K, Carnevali L, Sgoifo A, Nalivaiko E. In the search for integrative biomarker of resilience to psychological stress. Neuroscience and biobehavioral reviews. 2017;74(Pt B):310–20. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Chaudhury D, Nectow AR, Friedman AK, Zhang S, Juarez B, et al. alpha1- and beta3-Adrenergic Receptor-Mediated Mesolimbic Homeostatic Plasticity Confers Resilience to Social Stress in Susceptible Mice. Biological psychiatry. 2019;85(3):226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunwasser SM, Gillham JE, Kim ES. A meta-analytic review of the Penn Resiliency Program’s effect on depressive symptoms. Journal of consulting and clinical psychology. 2009;77(6):1042–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chmitorz A, Kunzler A, Helmreich I, Tuscher O, Kalisch R, Kubiak T, et al. Intervention studies to foster resilience - A systematic review and proposal for a resilience framework in future intervention studies. Clinical psychology review. 2018;59:78–100. [DOI] [PubMed] [Google Scholar]