Supplemental Digital Content is Available in the Text.
Key Words: HIV/AIDS, ART, postpartum, adherence, viremia
Background:
HIV-infected, postpartum women on antiretroviral therapy (ART) have high rates of viremia. We examined predictors of postpartum viremia in the PROMISE study.
Methods:
Women with pre-ART CD4+ T-cell counts ≥400 cells/mm3 who started ART during pregnancy were randomized postpartum to continue ART (CTART) or discontinue ART (DCART). Viral load and self-reported adherence were collected every 12 weeks, up to 144 weeks. Women in DCART reinitiated therapy when clinically indicated. Viremia was defined as 2 consecutive viral loads >1000 copies/mL after 24 weeks on ART. Adherence was dichotomized as missing versus not missing ART doses in the past 4 weeks. Predictors of viremia were examined using Cox proportional hazards regression with adherence as a time-varying covariate.
Results:
Among 802 women in the CTART arm, median age at entry was 27 years and median CD4+ T-cell count 696 cells/mm3. Of 175 women in CTART with viremia (22%), 141 had resistance data, and 12% had resistance to their current regimen. There was an estimated 0.12 probability of viremia by week 48 and 0.25 by week 144. Predictors of viremia included missed ART doses within the past 4 weeks, younger age, shorter duration of pre-entry ART, and being from the South American/Caribbean region. Of 137 women in DCART who reinitiated therapy, probability of viremia was similar to CTART (0.24 by week 96; 0.27 by week 144).
Conclusions:
Rates of postpartum viremia are high and viremia is more likely in younger postpartum women who start ART later in pregnancy. Interventions should target these higher-risk women.
INTRODUCTION
More than 1 1/2 million HIV-infected women become pregnant and deliver annually, and most of these women receive antiretroviral therapy (ART) antepartum and are expected to continue lifelong ART after delivery.1 Previous studies have shown high rates of nonadherence and loss of virologic control among postpartum HIV-infected women.2,3 A systematic review and meta-analysis of over 20,000 postpartum women from 51 studies ranging from the US to Africa found that only 53% [95% confidence interval (CI): 32.8% to 79.7%] of postpartum women had optimal adherence.2 Predictors of poor adherence and viremia have included younger age,3,4 nondisclosure/stigma,5 recent HIV diagnosis,4 substance use,6 and a low level of health literacy about HIV and ART.5
The clinical benefits of postpartum ART were reported from the “HAART (highly active antiretroviral therapy) Standard (HS)” component of the Promoting Maternal and Infant Safety Everywhere (PROMISE HS) study, a trial of women with pre-ART CD4+ T-cell counts of ≥400 cells/mm3 randomized to continue or discontinue three-drug ART after delivery, conducted in settings where women did not breastfeed after delivery.7 The study was initiated before the results of the Strategic Timing of AntiRetrovial Treatment (START) study, which showed the clinical benefits of immediate treatment of HIV regardless of clinical stage and CD4+ T-cell count.8 Despite improved clinical outcomes with continued ART, PROMISE 1077HS revealed high rates of viremia (23%) alongside low rates of resistance-associated mutations, suggesting nonadherence.7 The PROMISE 1077HS study design provides a unique opportunity to explore predictors of postpartum viremia in women on ART, and to compare differences among women who remained on ART postpartum to those who stopped and reinitiated later for clinical indications. We hypothesized that women who continued ART postpartum would have higher rates of viremia because of high self-rated health compared with women who discontinued ART postpartum and started later for clinical indications. We also explore rates of viral resuppression following loss of virologic control among women randomized to continue ART postpartum.
METHODS
PROMISE HS was conducted by the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network in collaboration with the AIDS Clinical Trials (ACTG) Network. PROMISE HS was a randomized strategy trial conducted among clinically stable HIV-infected pregnant women, antiretroviral-naïve except for previous ART in pregnancy, without other indications for ART based on local guidelines, who received triple drug ART during pregnancy for the purpose of preventing perinatal HIV infection. Women were enrolled from 56 sites in Argentina, Botswana, Brazil, China, Haiti, Peru, Thailand, and the US between December 2011 and November 2014. Women ≥18 years or who had attained the minimum age of independent consent as defined by the local institutional review board were eligible to enroll if they had documentation of a CD4+ T-cell count of ≥400 cells/mm3 within 120 days of the start of ART during the current pregnancy and evidence that CD4+ T-cell count remained ≥400 cells/mm3 within 45 days before entry, while on ART. Participants could not have a clinical indication for ART, including any World Health Organization (WHO) clinical stage 3 or 4 condition, or any clinically significant illness within 30 days before entry. Detailed study methods, including a CONSORT diagram, have been published with primary outcome data.7 The study was approved by the institutional review board or ethics committee at each participating site and written informed consent was obtained from all participants.
The trial evaluated 2 strategies for the management of ART among postpartum women within 42 days after delivery: continuing ART (CTART) or discontinuing ART (DCART) and restarting when clinically indicated. Participants randomized to DCART reinitiated treatment if they met one of the following criteria: (1) developed an AIDS-defining/WHO Stage 4 illness, (2) had a confirmed CD4+ T-cell count <350 cells/mm3, (3) developed a clinical condition considered an indication for ART by country-specific guidelines (including subsequent pregnancy), or (4) otherwise required ART as determined in consultation with the study clinical management committee.
The preferred study-supplied ART regimen was lopinavir/ritonavir (LPV/RTV) plus fixed-dose combination emtricitabine/tenofovir disoproxil fumarate (FTC/TDF). This regimen was chosen because it was the regimen recommended for use in pregnancy by the US Department of Health and Human Services guidelines at the time the study was designed.8
Participants were evaluated at 4, 12 weeks, and every 12 weeks thereafter. For all participants on ART, self-reported adherence was collected at entry, weeks 12, 24, and then every 24 weeks thereafter using adherence measures previously validated by the ACTG.9 Women were asked “When was the last time you missed any of your medications?” with the following responses offered: 1–2 weeks ago; 2–4 weeks ago; 1–2 months ago; more than 2 months ago; never skip medications. For all participants self-rated health (measured as excellent, very good, good, fair, or poor) was collected at entry, weeks 12, 24, and then every 24 weeks thereafter. HIV-1 RNA was collected every 12 weeks and used to maximize the benefits of ART and to determine when ART should be changed. The absence of virologic control was defined as 2 successive measurements of plasma HIV-1 RNA >1000 copies/mL, with the first measurement taken at or after at least 24 weeks on ART. For the remainder of the manuscript, the term viremia will be used to define the absence of virologic control, as defined by the criteria above.
Women with an elevated VL were asked to return within 4 weeks for confirmatory testing. Women with confirmed viremia were offered intensive adherence counseling and followed monthly with repeat viral load assessment. Regimen switching was done at the discretion of the provider and with support from the study's clinical management committee. Genotypes were sent in real-time and any woman with resistance to her current regimen was switched. Viral resuppression was defined as VL ≤1000 copies/mL at 2 consecutive visits after the first episode of confirmed viremia.
This analysis includes all women in the CTART arm and women in the DCART arm who reinitiated ART during follow-up. For inclusion, women had to be on ART for at least 24 weeks and have both viral load and adherence data available during the 144 weeks of study follow-up.
We evaluated rates of grade 3 and 4 signs and symptoms and chemistry abnormalities for women with versus without viremia within each arm during the first 24 weeks on ART. Later time points were excluded because of the differential follow-up time of women with and without viremia. Women with a single VL >1000 copies/mL were not categorized as having viremia, given their elevations were unconfirmed (either no subsequent viral load measured or on repeat found to be ≤1000 copies/mL). The analyses include data obtained before July 7, 2015, when participants were informed about the Strategic Timing of AntiRetroviral Treatment (START) trial results10 and all participants were offered ART.
Statistical Methods
Self-reported adherence responses were dichotomized as missing versus not missing any ART doses in the past 4 weeks. Predictors of viremia and resuppression were examined using Cox proportional hazards regression with adherence as a time-varying covariate. If participants did not complete the adherence questionnaire for a particular time interval, that time interval was excluded from the Cox regression. The predictors used were values at study entry for the CTART group and those at ART reinitiation for the DCART group. ART regimen was excluded as a predictor, because most participants were on PI-based ART, limiting power to detect differences. For analyses of viral re-suppression, time started at the time of the first episode of confirmed viremia.
There were significant differences between regions at baseline with more women from Thailand/China and Botswana with CD4+ T-cell counts in the lowest range (400 to <500 cells/mm3). Fewer women from Botswana were on PI-based ART at entry relative to other regions (see Table 1, Supplemental Digital Content, http://links.lww.com/QAI/B394). Given these differences, region was included in all multivariable models. The time-to-event distributions were summarized using Kaplan–Meier estimators. P-values ≤0.05 were considered statistically significant. Analyses used the principle of intention-to-treat according to assigned treatment arm and were conducted using SAS 9.2 software.
RESULTS
There were 1653 women randomized, 828 in the CTART arm and 825 in the DCART arm. In the CTART arm, 802 women (97%) had at least 24 weeks of adherence and VL data available and were included in the analysis. In the DCART arm, 199 women (24%) reinitiated ART during study follow-up and of these, 137 (69%) had at least 24 weeks of adherence and virologic data available and were included in the analysis (Fig. 1, CONSORT). There were 104 participants who had an unconfirmed VL >1000 copies/mL (95 in CTART and 9 in DCART). Of these, 26 had no subsequent VL measured, 64 had a subsequent VL ≤400 copies/mL, and 14 had a VL between 400 and 1000 copies/mL.
FIGURE 1.
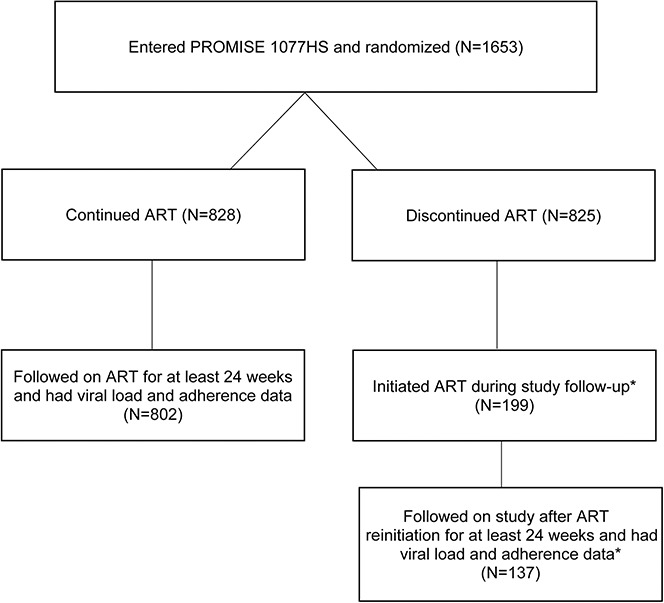
CONSORT diagram for women in PROMISE included in the analysis of viremia and resuppression. *Per protocol, ART was initiated for any of the following: (1) AIDS-defining/WHO stage 4 illness, (2) confirmed CD4+ count <350 cells/mm3, (3) clinical condition considered an indication for ART by country-specific guidelines or (4) otherwise required ART as determined in consultation with the study clinical management committee.
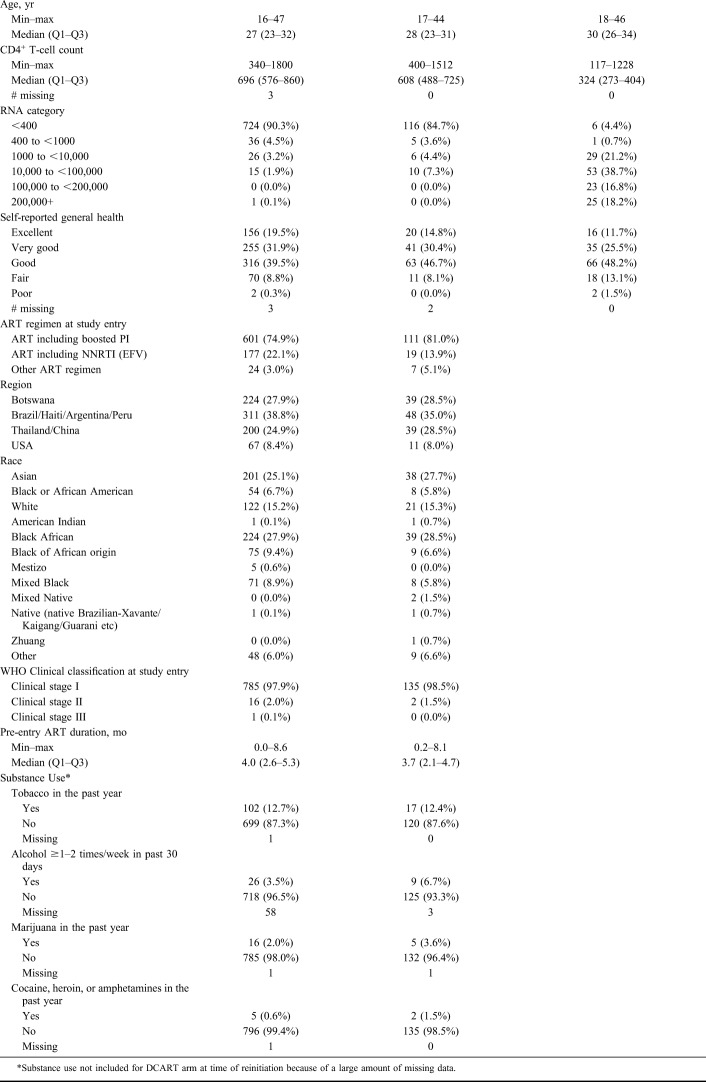
Characteristics of women included in the analysis are summarized in Table 1, including entry data by arm and selected characteristics of women in DCART at the time they reinitiated therapy. Among the 939 women included in the analysis (802 in the CTART arm and 137 in the DCART arm), the median age at entry was 27 years [interquartile range (IQR) 23–32], median CD4+ T-cell count at entry 683 cells/mm3 (IQR 561–835), and median time on ART during pregnancy 4.0 months (2.6–5.3). At study entry, 3% of women reported drug use in the past year (cocaine, heroin, amphetamines, and/or marijuana). Baseline self-rated health was high in both arms at study entry, with 90.9% of women in the CTART arm and 85.4% in the DCART arm rating themselves as having excellent, very good, or good health (compared to fair or poor). In the DCART arm, at the time of ART reinitiation, the median age was 30 (IQR 26–34) and median CD4+ T-cell count 324, (IQR 273–404). Self-rated health remained high in the DCART arm at the time of ART reinitiation, with 85% of women rating their health as good, very good, or excellent.
TABLE 1.
Participant Characteristics at Entry for Each Arm and at the Time of ART Reinitiation for Those in the Discontinue Arm
Viremia in the CTART Arm
Among women who were randomized to CTART, 90.3% had virologic suppression at study entry. Of the 802 women in the CTART arm, 175 women (21.8%) experienced at least one episode of viremia over 144 weeks of follow-up. Twenty-six of these women (14.9%) never achieved viral suppression during the first 24 weeks of follow-up. There was an estimated 0.04 probability of viremia by week 24, 0.12 by week 48, 0.20 by week 96, and 0.25 by week 144 (Fig. 2).
FIGURE 2.
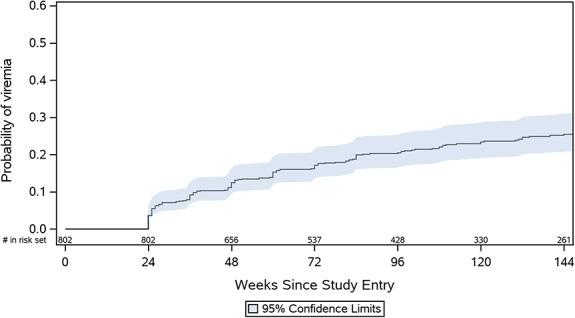
Estimated probability of viremia after the first 24 weeks on study through 144 weeks of follow-up in the CTART arm.
At the time of viremia, 126 (72.0%) women were on PI-based therapy, 11 (6.3%) were on NNRTI-based therapy, 3 (1.7%) were on other regimens (integrase or combination PI/NNRTI), and 35 (20.0%) were off ART. Of those off ART, the median number of days off treatment was 83 (IQR 38–165) and the most common reasons included participant decision (33.3%), “temporary hold” for unknown reason (26.7%), nonadherent to ART (16.7%), nonadherent to study visits (10.0%), diarrhea (3.3%), and rash (3.3%). Rates of grade 3 and 4 signs and symptoms were low in women with and without viremia during the first 24 weeks on therapy, with <1% of women having any grade 3 or 4 gastrointestinal or liver sign or symptom. Likewise, grade 3 and 4 chemistry abnormalities were also low in both groups, including for liver function tests (<1% in women without viremia and none in women with viremia) (see Table 2, Supplemental Digital Content, http://links.lww.com/QAI/B394).
One hundred forty-one women in the CTART arm had resistance data available. Of these, 17 (12%) had resistance selected by their current regimen. Viremia with resistance to the current regimen was more common in women on NNRTI-based therapy (6/11, 54.5%) compared with PI-based therapy (11/126, 8.7%). Detailed resistance mutations have been published in the primary PROMISE 1077HS paper.7
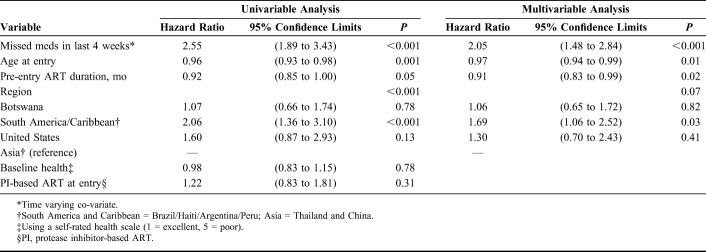
There were differences in rates of viremia by region, with participants from South America and the Caribbean having the highest estimated probability of viremia at 144 weeks (0.34) (Fig. 3). In the multivariable analysis, missed ART doses within the past 4 weeks [adjusted hazard ratio (aHR) 2.05, 95% CI: 1.48 to 2.84] and region (South America/Caribbean) (aHR 1.69, 95% CI: 1.06 to 2.52) were associated with increased risk of viremia, whereas older age (aHR 0.97, 95% CI: 0.94 to 0.99) and longer duration of pre-entry ART (aHR 0.91, 95% CI: 0.83 to 0.99) were associated with reduced risk of viremia (Table 2). Regarding age, the probability of viremia at week 144 was 0.36 for women <20 years, 0.30 for 20–24 years, 0.25 for 25–29 years, 0.22 for 30–34 years, and 0.14 for ≥35 years.
FIGURE 3.
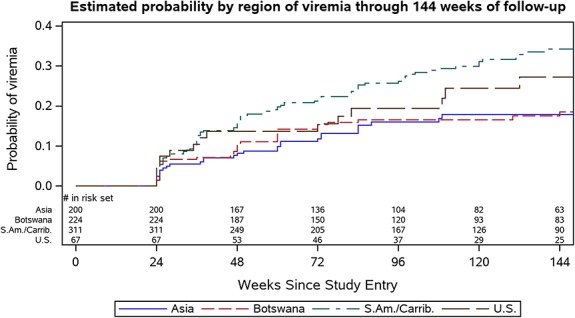
Estimated probability by region of viremia through 144 weeks of follow-up in the CTART arm.
TABLE 2.
Univariable and Multivariable Analyses for Viremia Among Women in the CTART Arm (N = 802)
A sensitivity analysis excluding women if they were on a “temporary ART hold” or if they were experiencing side effects did not significantly change the probability of viremia results.
Viral Resuppression in the CTART Arm
Among the 175 women with viremia in the CTART arm, there was 0.20 probability of resuppression 24 weeks after first confirmed viremia, 0.37 probability at 48 weeks, 0.48 at 96 weeks, and 0.57 at 144 weeks (see Figure 1, Supplemental Digital Content, http://links.lww.com/QAI/B394). There were no statistically significant predictors of resuppression.
Viremia in the DCART Arm
Among the 137 women in the DCART arm who reinitiated ART during follow-up for whom at least 24 weeks of both VL and adherence data were available, the most common reasons for reinitiation were decline in CD4+ T-cell count to treatment threshold (86%, n = 118), thrombocytopenia (3%, n = 4), and WHO stage 3 or 4 clinical condition (2%, n = 2). From this group, 31 women (22.6%) experienced viremia, of whom 14/31 (45%) never achieved viral suppression during the first 24 weeks on ART. Women were on the following regimens: 71% on PI-based therapy, 6.5% on NNRTI-based therapy with efavirenz, and 3.2% on integrase-based therapy with raltegravir. Nineteen percent of these women (n = 6) were not on ART at the time of viremia (median number of days off treatment 41, IQR 28–57) for either “temporary hold” for unknown reason (66.7%) or nonadherence to ART (33.3%). Rates of grade 3 and 4 signs and symptoms were low in women with and without viremia (<1%). Likewise, grade 3 and 4 chemistry abnormalities were also low in both groups, including for liver function tests (<1%) (see Table 3, Supplemental Digital Content, http://links.lww.com/QAI/B394). Resistance data were only available on 16 women, and of these, none had resistance selected by their current ART regimen. There was a 0.01 probability of viremia 24 weeks after ART reinitiation, 0.17 at 48 weeks, 0.24 at 96 weeks, and 0.27 at 144 weeks. Among the 31 women in the DCART arm with viremia after starting ART, there was a 0.22 probability of resuppression by 48 weeks.
In multivariable analysis, self-report of missing doses in the last 4 weeks was significantly associated with virema (aHR 2.01, 95% CI: 1.49 to 2.72). Women from the South America/Caribbean region had a significantly higher risk of viremia than women from Asia (aHR = 1.84, 95% CI: 1.23 to 2.76), and older women were at reduced risk of viremia (aHR 0.96, 95% CI: 0.94 to 0.98).
DISCUSSION
Our data are consistent with other studies that show high rates of nonadherence and viremia in postpartum women.11,12 In a recent study of 522 South African women who started ART during pregnancy and achieved suppression, 22% had a viremic episode postpartum (>1000 copies/mL), a rate identical to that found in PROMISE HS. In this South African study, each additional month postpartum was associated with an 11% increase in viremia (95% CI: 1.07 to 1.15).13 The rate of viremia with resistance to the current regimen was lower in our study (12%) compared with other reports of women in resource-limited settings, with 2 studies showing up to one-third of women developing significant resistance mutations by 24 months postpartum.11,14 The differential rates are likely due to the high frequency of PI-based ART in our population compared with NNRTI-based ART in other studies. In addition, a high number of women reported not taking ART at the time of viremia. Tolerability and toxicity can be an issue with PI-based regimens, particularly lopinavir/ritonavir; however, we did not find differences in grade 3 and 4 signs/symptoms or chemistry abnormalities in women with versus without viremia during the first 24 weeks on therapy in either arm, and rates of these events were extremely low overall.
We saw very low rates of viral resuppression among women in the CTART arm who had a first episode of viremia. Similarly, in the aforementioned South African study, 58% of women had more than one viremic episode,13 with the incidence of viremic episodes associated with younger age, ART initiation during the third trimester, and previous loss to follow-up on ART. In a recent study of women in Zimbabwe starting ART during pregnancy, only 39% of women demonstrated adequate adherence one year after delivery, with similar risk factors for poor adherence as seen in our study, including younger age, new HIV diagnosis, and late presentation/ART initiation during pregnancy.15 Despite an increasing and consistent body of evidence about risk factors for nonadherence in pregnant and postpartum women, rigorous interventional studies have demonstrated negative or modest results, particularly for community health worker and other facility and community-based peer support.16 More promising outcomes have been seen with differentiated models of care, such as adherence clubs for pregnant women in South Africa.17,18 Further study is needed to find low cost, logistically feasible models that will work in high prevalence, resource-limited settings.
Previous studies have shown that adherence to ART is high during pregnancy and decreases postpartum.11,15,19,20 Women in the CTART arm of our study had high self-rated health status and may have felt that ART was not critical to their health relative to women in the DCART arm who were reinitiated on ART for specific clinical indications. However, probabilities of viremia were similar in these 2 groups and self-reported health ratings were also high among women in the DCART arm at the time of ART reinitiation. Previous data have demonstrated perceived good health is a barrier to ART continuation in postpartum women21 and small studies from nonpregnant women and men have revealed similar findings.22,23,24 Understanding the relationship between health perceptions and adherence and retention will be critical to achieving broad population coverage and epidemic control.
Our data revealed regional differences in the probability of viremia, with higher rates in South America and the Caribbean. Given the small sample size, we could not analyze differences between individual countries and were under-powered to detect differences by ART regimens, which were predominately PI-based in all regions. Publications on adherence and viremia in postpartum women suggest a range of rates of nonadherence and/or viremia, with higher rates from Africa and the US (16%–86%)2,11,12,13,14,25 compared with Latin America (10%–20%),26,27 and with substantial variability based on the VL threshold evaluated and on timing of measurements postpartum (early versus late). Our findings are unique in that we are evaluating rates of postpartum viremia across women enrolled in the same study, undergoing standardized visit procedures, and this may allow for an improved ability to detect regional differences. Although there may be similarities in challenges faced by postpartum women, there are also likely to be differences by geographic setting related to socio-cultural differences, particularly for beliefs about health and health seeking (nontraditional approaches), norms around health care, use of substances (alcohol/drugs), and challenges with stigma. In addition, PROMISE did not assess postpartum depression, which is an important and under-recognized factor in resource-limited settings28,29,30 and may contribute to poor adherence and episodes of viremia.
Limitations
We do not have complete adherence and VL data for all women in PROMISE and it is possible that women for whom data are missing are different from women included in these analyses. Although most women were viremic without resistance mutations attributable to their current regimen, participants were predominately on PI-based ART, and viremia without resistance is more commonly seen in this setting.31,32,33 There were a high number of women initiated on ART in both arms who were off ART at the time of viremia as a temporary hold, and the specific reasons ART was held could not be further defined from study records. There are many important factors that influence adherence in postpartum women that were not collected in our study and therefore not included in our analyses, including disclosure, depression, and intimate partner violence. Finally, the most common ART regimen used was lopinavir/ritonavir, which is no longer used as standard of care in pregnancy/postpartum. Newer integrase regimens are now available as single tablet-fixed dose combinations, have a high barrier to resistance, and a favorable tolerability profile. It is possible that postpartum women with access to integrase-based regimens will have improved adherence and be at lower risk for episodes of viremia.
CONCLUSIONS
Among postpartum women in PROMISE HS, rates of viremia were high and not explained by resistance mutations, and the probability of viremia was similar among women in the CTART arm compared with those in the DCART arm who reinitiated later for clinical indications. Postpartum women with a first episode of viremia were at high risk for continued viremia. Interventions to support postpartum women should focus on younger women, those with shorter durations of ART in pregnancy, and be contextualized by region taking into account the socio-cultural factors that influence adherence. Supporting ART adherence among postpartum women remains a critical gap in efforts to optimize outcomes along the maternal–child health continuum.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge all of the participants in the study as well as the PROMISE 1077HS Investigators: M. Losso, J. de Menezes, R. Sperhacke, J. Pinto, R. Kreitchman, B. Santos, L. Wei, J.W. Pape, J. Sanchez, E. Sandoval, G. Halue, P. Yuthavisuthi, S. Prommas, C. Bowonwatanuwong, V. Sirisanthana, S. Riddler, P. Kumar, W. Shearer, R. Yogev, S. Spector, C. Cunningham, M. Bamji, E. Cooper, A. Wiznia, J. Hitti, P. Emmanuel, R. Scott, M. Acevedo, S. Nachman, T. Jones, S. Rana, M. Keller, M. Rathore, E. McFarland, A. Puga, A. Agwu, T. Chen, R. Van Dyke, J. Deville, M. Purswani, P. Tebas, P. Flynn, and M. Fischl.
The authors would also like to acknowledge the following individuals without whom the study and these analyses would not be possible: Statistical and Data Analysis Center: Paula Britto, Sean Brummel, David Shapiro. Data Management Center: Michael Basar, Amy Gonzalez, Linda Marillo, Adam Manzella. Laboratory Center: Susan Fiscus. Operations Center: Katie McCarthy, Melissa Allen. US NIH: Renee Browning, Devasena Gnanashanmugam, Karin L. Klingman, Lynette Purdue, Lynne Mofenson, George Siberry.
Footnotes
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). Overall support for the AIDS Clinical Trials Group (ACTG) 5UM1AI068636. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The study products were provided free of charge by AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV, and Merck and Company.
Presented at the Conference on Retroviruses and Opportunistic Infections; March 4–7, 2018; Boston, MA; Abstract no. 812 Link: http://www.croiconference.org/sessions/predictors-virologic-failure-postpartum-women-art-promise-1077hs.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Prevention of mother-to-child transmission (PMTCT): situation and trends. Global Health Observatory (GHO) Data. World Health Organization; Available at: http://www.who.int/gho/hiv/epidemic_response/PMTCT_text/en/. Accessed August 28, 2018. [Google Scholar]
- 2.Nachega JB, Uthman OA, Anderson J, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS. 2012;26:2039–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas AD, Msukwa MT, Egger M, et al. Adherence to antiretroviral therapy during and after pregnancy: cohort study on women receiving care in Malawi's option B+ program. Clin Infect Dis. 2016;63:1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onoya D, Sineke T, Brennan AT, et al. Timing of pregnancy, postpartum risk of virologic failure and loss to follow-up among HIV-positive women. AIDS. 2017;31:1593–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodgson I, Plummer ML, Konopka SN, et al. A systematic review of individual and contextual factors affecting ART initiation, adherence, and retention for HIV-infected pregnant and postpartum women. PLoS One. 2014;9:e111421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vitalis D. Factors affecting antiretroviral therapy adherence among HIV-positive pregnant and postpartum women: an adapted systematic review. Int J STD AIDS. 2013;24:427–432. [DOI] [PubMed] [Google Scholar]
- 7.Currier JS, Britto P, Hoffman RM, et al. Randomized trial of stopping or continuing ART among postpartum women with pre-ART CD4 ≥ 400 cells/mm3. PLoS One. 2017;12:e0176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-Infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. May 24, 2010; 1–117. Available at: http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf. Accessed August 28, 2018.
- 9.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000;12:255–266. [DOI] [PubMed] [Google Scholar]
- 10.Lundgren JD, Babiker AG, Gordin F, et al. ; INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV Infection. N Engl J Med. 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngarina M, Kilewo C, Karlsson K, et al. Virologic and immunologic failure, drug resistance and mortality during the first 24 months postpartum among HIV-infected women initiated on antiretroviral therapy for life in the Mitra plus Study, Dar es Salaam, Tanzania. BMC Infect Dis. 2015;15:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okawa S, Chirwa M, Ishikawa N, et al. Longitudinal adherence to antiretroviral drugs for preventing mother-to-child transmission of HIV in Zambia. BMC Pregnancy Childbirth. 2015;15:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myer L, Dunning L, Lesosky M, et al. Frequency of viremic episodes in HIV-infected women initiating antiretroviral therapy during pregnancy: a cohort study. Clin Infect Dis. 2017;64:422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosseinipour M, Nelson JAE, Trapence C, et al. Viral suppression and HIV drug resistance at 6 months among women in Malawi's option B+ program: results from the PURE Malawi study. J Acquir Immune Defic Syndr. 2017;75(suppl 2):S149–S155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erlwanger AS, Joseph J, Gotora T, et al. Patterns of HIV care clinic attendance and adherence to antiretroviral therapy among pregnant and breastfeeding women living with HIV in the context of option B+ in Zimbabwe. J Acquir Immune Defic Syndr. 2017;75(suppl 2):S198–S206. [DOI] [PubMed] [Google Scholar]
- 16.Nance N, Pendo P, Masanja J, et al. Short-term effectiveness of a community health worker intervention for HIV-infected pregnant women in Tanzania to improve treatment adherence and retention in care: a cluster-randomized trial. PLoS One. 2017;12:e0181919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myer L, Phillips TK, Zerbe A, et al. Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: a randomised controlled trial. PLoS Med. 2018;15:e1002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myer L, Iyun V, Zerbe A, et al. Differentiated models of care for postpartum women on antiretroviral therapy in Cape Town, South Africa: a cohort study. J Int AIDS Soc. 2017;20(suppl 4):21636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLean E, Renju J, Wamoyi J, et al. “I wanted to safeguard the baby”: a qualitative study to understand the experiences of Option B+ for pregnant women and the potential implications for 'test-and-treat' in four sub-Saharan African settings. Sex Transm Infect. 2017;93(suppl 3):pii: e052972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy JM. Women's expectations of treatment and care after an antenatal HIV diagnosis in Lilongwe, Malawi. Reprod Health Matters. 2009;17:152–161. [DOI] [PubMed] [Google Scholar]
- 21.Kim MH, Zhou A, Mazenga A, et al. Why did I stop? Barriers and facilitators to uptake and adherence to ART in option B+ HIV care in Lilongwe, Malawi. PLoS One. 2016;11:e0149527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert RF, Orrell C, Bangsberg DR, et al. Factors that motivated otherwise healthy HIV-positive young adults to access HIV testing and treatment in South Africa. AIDS Behav. 2018;22:733–741. [DOI] [PubMed] [Google Scholar]
- 23.Katz IT, Dietrich J, Tshabalala G, et al. Understanding treatment refusal among adults presenting for HIV-testing in Soweto, South Africa: a qualitative study. AIDS Behav. 2015;19:704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musheke M, Bond V, Merten S. Deterrents to HIV-patient initiation of antiretroviral therapy in urban Lusaka, Zambia: a qualitative study. AIDS Patient Care STDS. 2013;27:231–241. [DOI] [PubMed] [Google Scholar]
- 25.Mancinelli S, Galluzzo CM, Andreotti M, et al. Virological response and drug resistance 1 and 2 Years post-partum in HIV-infected women initiated on life-long antiretroviral therapy in Malawi. AIDS Res Hum Retroviruses. 2016;32:737–742. [DOI] [PubMed] [Google Scholar]
- 26.Kreitchmann R, Coelho DF, Kakehasi FM, et al. Long-term postpartum adherence to antiretroviral drugs among women in Latin America. Int J STD AIDS. 2016;27:377–386. [DOI] [PubMed] [Google Scholar]
- 27.Kreitchmann R, Harris DR, Kakehasi F, et al. Antiretroviral adherence during pregnancy and postpartum in Latin America. AIDS Patient Care STDS. 2012;26:486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azale T, Fekadu A, Hanlon C. Treatment gap and help-seeking for postpartum depression in a rural African setting. BMC Psychiatry. 2016;16:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yator O, Mathai M, Vander Stoep A, et al. Risk factors for postpartum depression in women living with HIV attending prevention of mother-to-child transmission clinic at Kenyatta National Hospital, Nairobi. AIDS Care. 2016;28:884–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villegas L, McKay K, Dennis CL, et al. Postpartum depression among rural women from developed and developing countries: a systematic review. J Rural Health. 2011;27:278–288. [DOI] [PubMed] [Google Scholar]
- 31.Barber TJ, Harrison L, Asboe D, et al. Frequency and patterns of protease gene resistance mutations in HIV-infected patients treated with lopinavir/ritonavir as their first protease inhibitor. J Antimicrob Chemother. 2012;67:995–1000. [DOI] [PubMed] [Google Scholar]
- 32.Lathouwers E, De Meyer S, Dierynck I, et al. Virological characterization of patients failing darunavir/ritonavir or lopinavir/ritonavir treatment in the ARTEMIS study: 96-week analysis. Antivir Ther. 2011;16:99–108. [DOI] [PubMed] [Google Scholar]
- 33.Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J Acquir Immune Defic Syndr. 2010;53:323–332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.