Abstract
Background:
Studies have demonstrated that exposure to fine particulate matter (PM2.5) is linked to cardiovascular disease (CVD), which is exacerbated in patients with pre-existing conditions such as obesity. In the present study, we examined cardiac function of obese mice exposed to PM2.5 and determined if mild exercise affected cardiac function.
Methods:
Obese mice (ob/ob) (leptin deficient, C57BL/6J background) were exposed to either filtered air (FA) or PM2.5 at an average concentration of 32μg/m3 for 6 h/day, 5 days/week for 9 months. Following exposure, mice were divided into four groups: (1) FA sedentary, (2) FA treadmill exercise, (3) PM2.5 sedentary, and (4) PM2.5 treadmill exercise and analyzed after 8 weeks of exercise training.
Results:
Echocardiography showed increased left ventricular end systolic (LVESd) and diastolic (LVEDd) diameters in PM2.5 sedentary mice compared to FA sedentary mice. There was increased expression of ICAM1, VCAM and CRP markers in sedentary PM2.5 mice compared to FA mice. Both FA and PM2.5 exercised mice showed decreased posterior wall thickness in systole compared to FA sedentary mice, coupled with altered expression of inflammatory markers following exercise.
Conclusion:
Obese mice exposed to PM2.5 for 9 months showed cardiac dysfunction, which was not improved following mild exercise training.
Keywords: particulate matter, obesity, cardiovascular function, exercise, treadmill
Introduction
Airborne particulates have gained attention regarding their significance on human health as reports claim the environmental stressor ranks 9th in overall cause of mortality worldwide(l). Notably, exposure to particulate matter <2.5μm in diameter (PM2.5) has been shown to increase cardiovascular disease occurrence by penetrating deeply into the lungs and diffusing into the bloodstream to produce a host of deleterious effects(2). The above mechanisms ultimately lead to an inflammatory response by the heart and lungs, a factor contributing to abnormal cardiac function(3).
According to the Center for Disease Control and Prevention, nearly 93.3 million people in the United States are affected by obesity, contributed in part by a poor diet and sedentary lifestyle. Obese individuals are predisposed to developing cardiovascular disease (CVD), and obesity and air pollution exposure further act as comorbid factors leading to increased CVD incidence. One of the major contributors to CVD is the persistent chronic systemic inflammation which induces cardiac dysfunction(4, 5, 6).In particular, left ventricular (LV) filling pressure, an index of LV function, is found to be impaired in obese individuals(7, 8).
Exercise is an important therapeutic tool used as a preventative measure against obesity and the resultant cardiac dysfunction. Varying cardiac responses occur with respect to differing exercise intensities. Studies examining these differences typically note greater cardiac remodeling in high intensity regimens(9) compared to little or no cardiac remodeling in low intensity exercise regimens(10). Emerging evidence suggests that exercise reverses adverse cardiac remodeling, ultimately leading to a lower occurrence of CVD(11). In a mouse model of diet-induced obesity, exercise training has a beneficial effect on cardiac health by enhancing cardiac function and improving mechanical efficiency(12). In contrast, another study showed that exercise was not able to reverse abnormalities in expression of L-type calcium channels in diet-induced obesity in rats, leading to continued cardiac dysfunction(13).Similar studies have shown that endurance exercise in obese mice aggravated cardiac abnormalities through increased fibrosis and impaired mitochondrial biogenesis, although improved skeletal muscle metabolism was still observed(14). Studies utilizing the ob/ob obese mouse model indicated varying results following exercise, including one by Sennott et al. that found improved blood glucose levels in mice assigned to voluntary running compared to both treadmill and sedentary groups(15).
Studies conducted on ob/ob mice reported cardiac hypertrophy and altered metabolism in response to defective leptin signaling(16, 17). Previous work in our lab has demonstrated adverse cardiac function due to PM2.5 exposure in both adult and adolescent mice through LV remodeling and impaired sarcomere function(18, 19). Introducing additional stressors, such as PM2.5 exposure, has not yet been explored in the ob/ob model. The aim of the current study was to examine cardiac effects in PM2.5 - exposed obese mice following mild treadmill exercise training.
Materials and Methods
Animals and Exposure
All animal experiments were performed in alignment with NIH guidelines approved by the Institutional Animal Care and Use Committee (IACUC) at The Ohio State University, Columbus, Ohio. Twelve week old male ob/ob mice (C57BL/6J background strain) were obtained from Jackson Laboratories (Bar Harbor, ME). The ob/ob mouse is leptin deficient as a result of a homozygous mutation in the ob gene. All mice were housed in our AAALAC-approved facility for one week before beginning the 9-month exposure period. Animals were exposed to concentrated PM2.5 from the Columbus, OH region using the system “Ohio’s Air Pollution Exposure System for the Interrogation of Systemic Effects” (OASIS-1) aerosol concentration system located at the Ohio State University(18). Animals were exposed either to PM2.5 or filtered air (FA), which included an identical system with the exception of a HEPA filtered inserted at the inlet to remove all ambient particulates. The average PM2.5 concentration that the animals were exposed to in this study was 32μg/m3 over the exposure period, similar to concentrations seen in our previous studies(20, 21). Following exposure, all mice were housed in room air for the duration of treadmill exercise.
Treadmill exercise protocol
Following 9 months of exposure (12 months of age), the mice were separated into the following groups: 1) FA sedentary (FA Sed; n=5), 2) PM2.5 sedentary (PM2.5 Sed; n=5), 3) FA exercise (FA Ex; n=3), and 4) PM2.5 exercise (PM2.5 Ex; n=4). Mice were not exposed to PM2.5 during the exercise period. All animals were group housed under similar conditions. Initially, mice were exposed to adaptive training at 2.5 m/min for 10 min per session for the first three days. Exercise involved forced treadmill training 5 days/wk, consisting of 30 minutes of daily training at a speed of 2.5 m/min at 0% incline for 8 weeks. Mice were monitored throughout the exercise periods and electrical stimulus used to motivate animals during exercise.
Echocardiography
Mice in all four groups underwent echocardiographic assessment using a 40 MHz transducer using the Vevo 2100 (Visualsonics, Toronto, ON, Canada). Isoflurane was administered at 1.5–2% (delivered with 100% O2) as anesthesia through a nose cone and body temperature was maintained at 37°C. Following fur removal, pre-warmed ultrasound gel (Aquasonic; Parker Laboratories, Fairfield, NJ) was applied onto the chest. A 15 MHz probe was placed in the parasternal, short axis orientation. M mode data was collected and analyzed with the average of three cine loops for the following parameters: LV end-systolic and end-diastolic internal dimensions (LVESd and LVEDd), as well as systolic and diastolic posterior wall thickness (PWTs and PWTd). Percent fractional shortening (%FS) was calculated using the equation: %FS= [(LVEDd-LVESd)/LVEDd* 100]. All analyses were performed in accordance with the American Heart Association defined technique by an investigator blinded to group assignments.
Quantitative real-time PCR
Total RNA was extracted from snap frozen left ventricular cardiac tissue via the RNeasy kit (Qiagen, Hilden, Germany). Concentrations were determined using a NanoDrop 2000c (Thermo-Scientific, Wilmington, DE). iScript Supermix kit (Bio-Rad, Hercules, CA) was used to reverse transcribe 1 ng of RNA to generate cDNA. This process used the CFX96 Thermocycler (Bio-Rad, Hercules, CA). Primers were used at a final concentration of 0.25–0.5 μM for target genes. Gene-specific primer sequences listed in Table 1 were used and normalized to Gapdh expression. The formula 2-ΔΔCt was used to quantify relative gene expression(where Ct is threshold cycle)(22). The three-step amplification protocol was as follows: denaturation at 95°Cfor 10 min followed by 39 cycles of denaturation (95°C, Isecond), annealing (65°C, 10seconds), and extension (72°C, 20seconds).
Table 1 :
Primer sequences used for PCR amplification
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Gapdh | ATGGTGAAGGTCGGTGTGAACGG | AGGGGTCGTTGATGGCAACAATCT |
| CRP | GTCTGCTACGGGGATTGTAGA | CACCGCCATACGAGTCCTG |
| ICAM-1 | TGCCTCTGAAGCTCGGATATAC | TCTGTCGAACTCCTCAGTCAC |
| VCAM-1 | GTTCCAGCGAGGGTCTACC | AACTCTTGGCAAACATTAGGTGT |
Statistical analyses
Data were assessed using Prism 6.0 (Graphpad Software, San Diego, CA) and differences were considered statistically significant when P ≤ 0.05 via Student’s t-test or one-way ANOVA followed by Tukey’s post hoc analyses.
Results
PM2.5 exposure does not affect body weight or heart weight in obese sedentary or exercised mice.
To study the effects of mild exercise on biometric parameters, we compared the exercised animals to not only age-matched sedentary controls but also evaluated the difference due to FA or PM2.5 exposure. Body weight (BW), heart weight (HW), HW/BW ratio remain unaltered sedentary as well as exercised groups (Table 2).
Table 2:
Biometric data of FA and PM2.5 exposed sedentary and exercise mice
| FA Sedentary (n=5) |
PM2.5 Sedentary (n=5) |
p- value |
FA Exercise (n=3) |
PM2.5 Exercise (n=4) |
p- value |
|
|---|---|---|---|---|---|---|
| Body Weight, g | 70.40±2.52 | 64.91±8.74 | 0.56 | 65.87±2.97 | 67.85±2.41 | 0.62 |
| Heart Weight, mg | 180±10 | 200±10 | 0.35 | 180±20 | 180±20 | 0.99 |
| HW/BW, mg/g | 2.61±0.12 | 3.32±0.39 | 0.11 | 2.70±0.19 | 2.79±0.19 | 0.77 |
FA, filtered air; PM2.5, particulate matter (<2.5 μm diameter)
PM2.5 exposure leads to increased LV dimensions of obese sedentary mice.
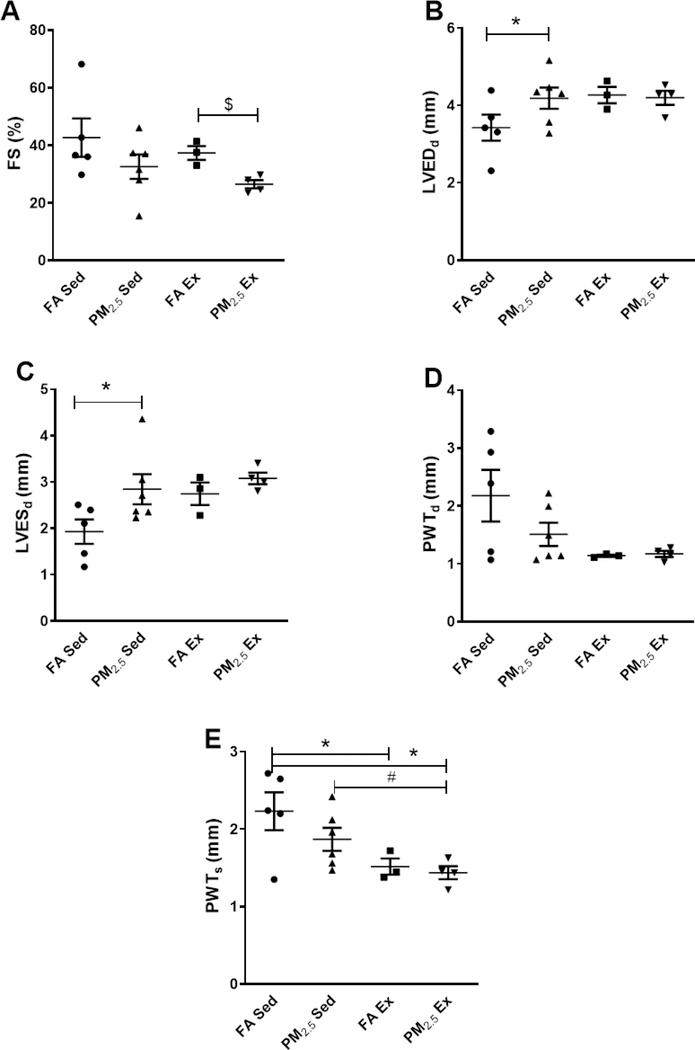
Echocardiographic analyses of sedentary mice exposed to PM2.5 demonstrated increased LVESd (1.93 ± 0.26 mm FA Sed; 2.84 ± 0.32 mm PM2.5 Sed; p=0.03) and LVEDd (3.42 ± 0.33 mm FA Sed; 4.18 ± 0.27 mm PM2.5 Sed; p=0.05) (Figure 1B & 1C). Posterior wall thickness was not different during systole (PWTs) (2.23 ± 0.24 mm FA Sed; 1.86 ± 0.14 mm PM2.5 Sed; p=0.10) or diastole (PWTd) (2.17 ± 0.44 mm FA Sed; 1.50 ± 0.20 mm PM2.5 Sed; p=0.90) between both groups (Figure 1D & 1E). A slight decrease in %FS (42.70±6.70 FA Sed; 32.60±4.24 PM2.5 Sed; p=0.10) was observed in PM2.5 sedentary mice compared to FA exposed control mice (Figure 1A).
Figure 1:
Echocardiographic parameters comparing cardiac functional changes among FA (Sed: n=5; Ex: n=3) or PM2.5 (Sed: n=5; Ex: n=4) exposed sedentary and exercise mouse groups. A. Fractional shortening (%FS), B. Left ventricular end-diastolic diameter (LVEDd), C. Left ventricular end-systolic diameter (LVESd), D. Posterior wall thickness during diastole (PWTd), E. Posterior wall thickness during systole (PWTs). Five beat cycles were captured and three loops averaged per assessment. Data are expressed as ± S.E.M. *p ≤0.05 vs FA sedentary, #p<0.05 vs PM2.5 sedentary,$ $p<0.01 vs FA exercise.
PM2.5 leads to LVcontractile dysfunction in obese exercise mice.
Following PM2.5 exposure, 8 weeks of treadmill exercise resulted in a significant reduction in %FS (37.37 ± 2.41 FA Ex; 26.54 ± 1.42 PM2.5 Ex; p=0.009) in PM2.5EX compared to FA Ex mice (Figure 1A). While this decreased %FS is consistent with ventricular systolic dysfunction, other measurements such as LVESd (2.74 ± 0.24 mm FA Ex; 3.08 ± 0.12 mm PM2.5 Ex; p=0.12), LVEDd (4.26 ± 0.21 mm FA Ex; 4.19 ± 0.18 mm PM2.5 Ex; p=0.81), PWTs (1.51 ± 0.10 mm FA Ex; 1.43 ± 0.08 mm PM2.5 Ex; p=0.57) and PWTd (1.14 ± 0.01 mm FA Ex; 1.17 ± 0.05 mm PM2.5 Ex; p=0.66) for ventricular remodeling were not different (Figure 1B-E).
When comparing sedentary groups (both FA and PM2.5) with exercised groups (both FA and PM2.5), we observed a significant difference only in PWTs (Figure 1E), while the remaining parameters were unchanged.
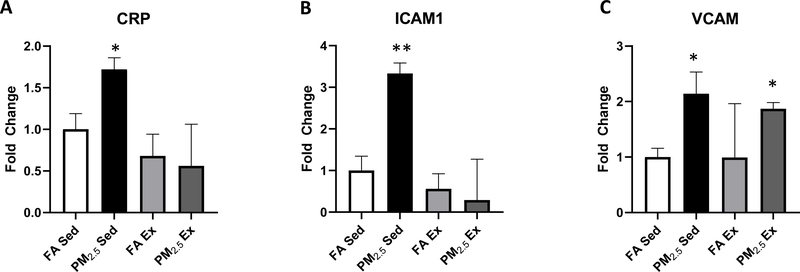
PM2.5 exposure increases inflammatory mRNA expression in obese sedentary mice with no effect in exercised mice.
We analyzed markers of cardiac inflammation in sedentary mice exposed either to FA or PM2.5. Measurements of gene expression demonstrated significantly increased C-reactive protein (CRP), an inflammatory protein associated with long term diseases and immune activation after PM2.5 exposure. Additionally, markers for intracellular adhesion molecule 1 (ICAM1) and vascular cell adhesion protein (VCAM), both important for leukocyte signaling, were increased in PM2.5 exposed mice compared to FA controls (Figure 2). There was no difference observed in the expression of CRP, ICAM and VCAM between FA Ex and PM2.5 Ex mice (Figure 2). Additionally, comparison of sedentary to exercised mice yielded no significant difference within either exposure group for each marker analyzed (Figure 2).
Figure 2:
Quantitative polymerase chain reaction (qPCR) analysis of RNA samples comparing changes among FA or PM2.5 exposed sedentary and exercise mouse groups. A. CRP, B. ICAM1, C. VCAM. Results are mean ± S.E.M. Data are expressed as ± S.E.M. *p<0.05, **p<0.01 vs FA sedentary.
Discussion
It is well known that a sedentary lifestyle and obesity coexist and that both are associated with CVD(23, 24). As obesity positively correlates with heart failure, atrial fibrillation, coronary artery disease, and hypertension(25, 26), it is considered as one of the leading risk factors for CVD. Several studies have also indicated that aged obese mice have increased cardiomyocyte apoptosis and decreased survival(27), coupled with LV diastolic functional abnormalities(28, 29, 30). PM2.5 exposure, on the other hand, has recently been determined to be a leading causative agents for the development of CVD(18, 20, 21). Exercise is an effective treatment for improving cardiometabolic function in obese individuals; however, the effects of exercise on cardiac function after PM2.5 exposure in obese individuals has not been fully defined. The goal of the present study was to examine the effects of forced treadmill exercise on cardiac function of leptin deficient obese (ob/ob) mice exposed to PM2.5. Following 8 weeks of mild treadmill exercise, we did not observe changes in biometric parameters between PM2.5 exposed sedentary and exercised mice. In PM2.5 exposed sedentary mice, there was evidence of contractile dysfunction manifested as altered LV internal dimensions both during systole and diastole, suggestive of contractile dysfunction potentially leading to LV volume overload. Our findings are similar to a cardiac phenotype observed in previous studies(28, 29, 30).
While molecular mechanisms remain unclear, activation of an inflammatory response (CRP, ICAM1, and VCAM) could be one of the mechanisms behind cardiac dysfunction observed in PM2.5 exposed sedentary mice as it is regarded as a stimulus to various cardiac pathophysiological events. Upregulation of inflammatory markers identifies additional physiological stress as inflammation has been identified as a response to myocardial damage(31). Several studies demonstrated a link between pro-inflammatory cytokines and left ventricular hypertrophy and dysfunction(32). This pro-inflammatory response is significant as chronic levels of inflammatory proteins have been observed to contribute to and exacerbate insulin resistance in cardiomyocytes, leading to impaired metabolic function(33). Further, obese subjects exposed to particulate matter demonstrated changes in the DNA methylation of CD14 and TLR4, pathways heavily involved in an inflammatory response(34). Increased CRP levels have been observed in those with obesity and pre-clinical diabetes(35), demonstrating a link between obesity and CRP regulation as seen in our study.
Data suggest that regular exercise has beneficial effects on cardiovascular function partly by eliciting anti-inflammatory properties, resulting in decreased CVD development and favorable cardiac remodeling(36). Therefore, we evaluated the cardiac effects of exercise in PM2.5 exposed obese mice. A recent study found that exercise later in life benefitted LV function in middle-aged people who lived a sedentary lifestyle(37), suggesting exercise during later periods of life may produce cardiac benefits. In our study, however, we found no improvement in cardiac function when mice underwent mild exercise training. Exercised ob/ob mice exposed to PM2.5 showed even worse cardiac function with significantly decreased %FS consistent with that observed in heart failure. There was also no change in the expression of inflammatory markers following exercise training in FA and PM2.5 exposed ob/ob mice. Therefore, contrary to previous evidence, mild exercise may have introduced an additional stressor to cause a further decline in cardiac function.
Conclusion and future directions
The present study aimed at identifying the potential CVD burden in PM2.5 exposed obese mice. The national ambient air quality standard (NAQS) set by the U.S. Environmental Protection Agency (U.S. EPA) for PM2.5 are 35 μg/m3/per day and 12μg/m3/year. The levels achieved in 9 months of exposure in this study are significantly more than the annual limit. This suggests that highly polluted areas which are exceeding EPA standards pose serious risks to those who have risk factors for CVD such as obesity. ob/ob sedentary mice demonstrated contractile dysfunction following PM2.5exposure. Mild exercise intervention for 8 weeks failed to improve cardiac function following 9 months of exposure to ob/ob PM2.5 mice. Exercise training has been shown to exert beneficial effects on cardiovascular function, and the positive outcomes have been substantiated in various experimental models of exercise training (38, 39). However, our results indicated that mild forced exercise does not result in cardioprotection in the PM2.5 exposed ob/ob mouse model, exacerbating rather than attenuating cardiac dysfunction. We acknowledge factors that may contribute to disease exacerbation due to exercise: (1) Long-term PM2.5 exposure resulted in significant aging of the mice which is considered as the largest risk factor for CVD(40). In this study, animals were aged by the time they were subjected to mild treadmill training, which could have served as an additional deleterious factor along with PM2.5 exposures compared to young mice used in other positive outcome studies; (2) type of exercise could also have beneficial or adverse effects on the cardiac outcomes. In our study, we used a mild exercise training protocol that did not exert beneficial cardiac effects. (3) We observed impaired exercise tolerance in ob/ob mice and consider it as the limiting factor in exercise associated beneficial effect. Leptin selectively stimulates phosphorylation and activation of the α2 catalytic subunit of AMPK (α2-AMPK), an enzyme that appears to have a significant role in the promotion of exercise tolerance in skeletal muscle(41). Our present study utilized leptin deficient ob/ob mice and hence impaired α2-AMPK activity that may directly contribute to reduced exercise capacity resulting in non-beneficial effects in both FA and PM2.5 exposed exercised groups. We additionally acknowledge three limitations of the study design: (1) A single echocardiographic time point did not permit analysis of cardiac changes during PM2.5 exposure or exercise; (2) Low animal numbers, especially in the FA Ex group, may have contributed to a large error bar in VCAM data; and (3) qPCR data were not confirmed with protein or histological experiments. Thus, on the basis of these findings, further studies are required to determine whether leptin deficiency is contributing to the aberrant response to exercise. Future studies should also examine the effect of early interventional strategies in CVD animal models exposed long term to high levels of ambient particulates.
-
–
Particulate Matter (PM) exposure and obesity are risk factors for the development of cardiovascular disease (CVD).
-
–
Exercise has been shown to induce cardioprotective responses and is used as a prevention measure against weight gain.
-
–
Our study adds data to the literature suggesting PM exposure on cardiac function is especially dangerous in those who are obese as exercise introduces an additional stressor instead of cardioprotective effects.
-
–
The current data demonstrates that the effects of PM2.5 exposure on cardiac function may be mediated through upregulation of inflammatory pathways.
Funding
This work was supported in part by National Institutes of Health grants HL138738 and AG060542 to KIS and AG057046, HL139348, NR012618 and ES019923 to LEW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380: 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanwar V, Katapadi A, Adelstein JM, Grimmer JA, Wold LE. Cardiac pathophysiology in response to environmental stress: a current review. Curr Opin Physiol 2018; 1: 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorr MW, Youtz DJ, Eichenseer CM, Smith KE, Nelin TD, Cormet-Boyaka E, et al. In vitro particulate matter exposure causes direct and lung-mediated indirect effects on cardiomyocyte function. Am J Physiol Heart Circ Physiol 2015;309: H53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oktay AA, Lavie CJ, Kokkinos PF, Parto P, Pandey A, Ventura HO. The Interaction of Cardiorespiratory Fitness With Obesity and the Obesity Paradox in Cardiovascular Disease. Prog Cardiovasc Dis 2017;60: 30–44. [DOI] [PubMed] [Google Scholar]
- 5.Jing L, Nevius CD, Friday CM, Suever JD, Pulenthiran A, Mejia-Spiegeler A, et al. Ambulatory systolic blood pressure and obesity are independently associated with left ventricular hypertrophic remodeling in children. J Cardiovasc Magn Reson 2017;19: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sung J, Ho CT, Wang Y. Preventive mechanism of bioactive dietary foods on obesity-related inflammation and diseases. Food Funct 2018;9: 6081–6095. [DOI] [PubMed] [Google Scholar]
- 7.Zarich SW, Kowalchuk GJ, McGuire MP, Benotti PN, Mascioli EA, Nesto RW. Left ventricular filling abnormalities in asymptomatic morbid obesity. Am J Cardiol 1991;68: 377–381. [DOI] [PubMed] [Google Scholar]
- 8.Lavie CJ, Cahalin LP, Chase P, Myers J, Bensimhon D, Peberdy MA, et al. Impact of cardiorespiratory fitness on the obesity paradox in patients with heart failure. Mayo Clin Proc 2013;88: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novoa U, Arauna D, Moran M, Nunez M, Zagmutt S, Saldivia S, et al. High-Intensity Exercise Reduces Cardiac Fibrosis and Hypertrophy but Does Not Restore the Nitroso-Redox Imbalance in Diabetic Cardiomyopathy. Oxid Med Cell Longev 2017;2017: 7921363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanter M, Aksu F, Takir M, Kostek O, Kanter B, Oymagil A. Effects of Low Intensity Exercise Against Apoptosis and Oxidative Stress in Streptozotocin-induced Diabetic Rat Heart. Exp Clin Endocrinol Diabetes 2017;125: 583–591. [DOI] [PubMed] [Google Scholar]
- 11.Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and Atrial Fibrillation Prevalence, Pathogenesis, and Prognosis: Effects of Weight Loss and Exercise. J Am Coll Cardiol 2017;70: 2022–2035. [DOI] [PubMed] [Google Scholar]
- 12.Boardman NT, Hafstad AD, Lund J, Rossvoll L, Aasum E. Exercise of obese mice induces cardioprotection and oxygen sparing in hearts exposed to high-fat load. Am J Physiol Heart Circ Physiol 2017;313: H1054-H1062. [DOI] [PubMed] [Google Scholar]
- 13.da Silva VL, Lima-Leopoldo AP, Ferron AJT, Cordeiro JP, Freire PP, de Campos DHS, et al. Moderate exercise training does not prevent the reduction in myocardial L-type Ca. Physiol Rep 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan Z, Kronemberger A, Blomme J, Call JA, Caster HM, Pereira RO, et al. Exercise leads to unfavourable cardiac remodelling and enhanced metabolic homeostasis in obese mice with cardiac and skeletal muscle autophagy deficiency. Sci Rep 2017;7: 7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sennott J, Morrissey J, Standley PR, Broderick TL. Treadmill exercise training fails to reverse defects in glucose, insulin and muscle GLUT4 content in the db/db mouse model of diabetes. Pathophysiology 2008; 15: 173–179. [DOI] [PubMed] [Google Scholar]
- 16.Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 2005; 146: 5341–5349. [DOI] [PubMed] [Google Scholar]
- 17.Dong F, Zhang X, Yang X, Esberg LB, Yang H, Zhang Z, et al. Impaired cardiac contractile function in ventricular myocytes from leptin-deficient ob/ob obese mice. J Endocrinol 2006;188: 25–36. [DOI] [PubMed] [Google Scholar]
- 18.Wold LE, Ying Z, Hutchinson KR, Velten M, Gorr MW, Velten C, et al. Cardiovascular remodeling in response to long-term exposure to fine particulate matter air pollution. Circ Heart Fail 2012;5: 452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorr MW, Velten M, Nelin TD, Youtz DJ, Sun Q, Wold LE. Early life exposure to air pollution induces adult cardiac dysfunction. Am J Physiol Heart Circ Physiol 2014;307: H1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanwar V, Adelstein JM, Grimmer JA, Youtz DJ, Sugar BP, Wold LE. PM2.5 exposure in utero contributes to neonatal cardiac dysfunction in mice. Environ Pollut 2017;230: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanwar V, Adelstein JM, Grimmer JA, Youtz DJ, Katapadi A, Sugar BP, et al. Preconception Exposure to Fine Particulate Matter Leads to Cardiac Dysfunction in Adult Male Offspring. J Am Heart Assoc 2018;7: e010797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25: 402–408. [DOI] [PubMed] [Google Scholar]
- 23.Barnes AS. Obesity and sedentary lifestyles: risk for cardiovascular disease in women. Tex Heart Inst J 2012;39: 224–227. [PMC free article] [PubMed] [Google Scholar]
- 24.Kesaniemi YK, Danforth E Jr., Jensen MD, Kopelman PG, Lefebvre P, Reeder BA. Dose-response issues concerning physical activity and health: an evidence- based symposium. Med Sci Sports Exerc 2001;33: S351–358. [DOI] [PubMed] [Google Scholar]
- 25.Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med 2002;162: 1867–1872. [DOI] [PubMed] [Google Scholar]
- 26.Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med 2001; 161: 1581–1586. [DOI] [PubMed] [Google Scholar]
- 27.Barouch LA, Gao D, Chen L, Miller KL, Xu W, Phan AC, et al. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res 2006;98: 119–124. [DOI] [PubMed] [Google Scholar]
- 28.Hall ME, Maready MW, Hall JE, Stec DE. Rescue of cardiac leptin receptors in db/db mice prevents myocardial triglyceride accumulation. Am J Physiol Endocrinol Metab 2014;307: E316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christoffersen C, Bollano E, Lindegaard ML, Bartels ED, Goetze JP, Andersen CB, et al. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology 2003;144: 3483–3490. [DOI] [PubMed] [Google Scholar]
- 30.Miki T, Yuda S, Kouzu H, Miura T. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev 2013; 18: 149–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandavia CH, Aroor AR, Demarco VG, Sowers JR. Molecular and metabolic mechanisms of cardiac dysfunction in diabetes. Life Sci 2013;92: 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tham YK, Bernardo BC, Ooi JY, Weeks KL, McMullen JR. Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch Toxicol 2015;89: 1401–1438. [DOI] [PubMed] [Google Scholar]
- 33.Fuentes-Antras J, Ioan AM, Tunon J, Egido J, Lorenzo O. Activation of toll-like receptors and inflammasome complexes in the diabetic cardiomyopathy- associated inflammation. Int J Endocrinol 2014;2014: 847827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantone L, Iodice S, Tarantini L, Albetti B, Restelli I, Vigna L, et al. Particulate matter exposure is associated with inflammatory gene methylation in obese subjects. Environ Res 2017;152: 478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sardu C, Pieretti G, D’Onofrio N, Ciccarelli F, Paolisso P, Passavanti MB, et al. Inflammatory Cytokines and SIRT1 Levels in Subcutaneous Abdominal Fat: Relationship With Cardiac Performance in Overweight Pre-diabetics Patients. Front Physiol 2018;9: 1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nystoriak MA, Bhatnagar A. Cardiovascular Effects and Benefits of Exercise. Front Cardiovasc Med 2018;5: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howden EJ, Sarma S, Lawley JS, Opondo M, Cornwell W, Stoller D, et al. Reversing the Cardiac Effects of Sedentary Aging in Middle Age-A Randomized Controlled Trial: Implications For Heart Failure Prevention. Circulation 2018;137: 1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musman J, Pons S, Barau C, Caccia C, Leoni V, Berdeaux A, et al. Regular treadmill exercise inhibits mitochondrial accumulation of cholesterol and oxysterols during myocardial ischemia-reperfusion in wild-type and ob/ob mice. Free Radic Biol Med 2016;101: 317–324. [DOI] [PubMed] [Google Scholar]
- 39.Pons S, Martin V, Portal L, Zini R, Morin D, Berdeaux A, et al. Regular treadmill exercise restores cardioprotective signaling pathways in obese mice independently from improvement in associated co-morbidities. J Mol Cell Cardiol 2013;54: 82–89. [DOI] [PubMed] [Google Scholar]
- 40.Steenman M, Lande G. Cardiac aging and heart disease in humans. Biophys Rev 2017;9: 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev 2009;89: 1025–1078. [DOI] [PubMed] [Google Scholar]