Abstract
C57BL6/J (B6) mice lacking Se-dependent GSH peroxidase 1 and 2 (GPx1/2-DKO) develop mild to moderate ileocolitis around weaning. These DKO mice have a disease resembling human very-early-onset inflammatory bowel disease (VEOIBD), which is associated with mutations in NADPH oxidase genes. Drugs including dexamethasone (Dex), Tofacitinib (Tofa; a Janus kinase/JAK inhibitor) and anti-TNF antibody are effective to treat adult, but not pediatric IBD.
Aims:
To test the efficacy of hydrophobic Dex and hydrophilic Dex phosphate (Dex phos), Tofa, anti-Tnf Ab, Noxa1ds-TAT and gp91ds-TAT peptides (inhibiting NOX1 and NOX2 assembly respectively), antioxidant MJ33 and ML090, and pifithrin-α (p53 inhibitor) on alleviation of gut inflammation in DKO weanlings.
Main Methods:
All treatments began on 22-day-old GPx1/2-DKO mice. The mouse intestine pathology was compared between the drug- and vehicle-treated groups after six or thirteen days of treatment.
Key findings:
Among all drugs tested, Dex, Dex phos and Tofa were the strongest to suppress ileocolitis in the DKO weanlings. Dex, Dex phos and Tofa inhibited crypt apoptosis and increased crypt density. Dex or Dex phos alone also inhibited cell proliferation, exfoliation and crypt abscess in the ileum. Dex, but not Tofa, retarded mouse growth. Both Dex and Tofa inhibited ileum Nox1, Nox4 and Duox2, but not Nox2 gene expression. Noxa1ds-TAT and gp91ds-TAT peptides as well as MJ33 had subtle effect on suppressing pathology, while others had negligible effect.
Significance:
These findings suggest that NADPH oxidases can be novel drug targets for pediatric IBD therapy, and Tofa may be considered for treating VEOIBD.
Keywords: Dexamethasone, glutathione peroxidase, NADPH oxidase, Tofacitinib, very-early-onset inflammatory bowel disease
1. Introduction
Imbalance in epithelial reactive oxygen species (ROS) is suspected to contribute to very-early-onset inflammatory bowel disease (VEOIBD, diagnosed before 6 years of age) (1). Up to one-half of children with chronic granulomatous disease (CGD) have IBD, and CGD patients often have a mutated NOX2/CYBB/gp91phox gene (2). Defects in NOX1 and DUOX2 are linked to VEOIBD (3–6). Furthermore, IBD and VEOIBD patients have elevated NOX1 and DUOX2 gene expression (7–10). These results suggest that NOX1 and DUOX2 are involved in IBD pathogenesis.
Mice deficient in GSH peroxidase-1 and −2 (GPx1/2-DKO) have colitis before weaning and ileitis soon after weaning (10–12). The penetrance of ileitis was >95% in 35-day-old C57BL6 (B6) GPx1/2-DKO mice, while B6 non-DKO littermates (GPx1+/−GPx2−/− or GPx1−/−GPx2+/−) are virtually disease-free (10, 11). Gut microbes are essential for disease onset in the GPx1/2-DKO mice (13, 14). Conventionally raised GPx1/2-DKO weanlings have increased expression of Nox1 in the intestinal epithelium (10, 11). Both Nox1 and Duox2 gene expression are induced by bacterial components and inflammatory cytokines (15–17). We have demonstrated that NOX1 and DUOX2 are major contributors to the ileocolitis in GPx1/2-DKO mice by studying Nox1-GPx1/2 and Duoxa2-GPx1/2 triple-knockout mice (10, 11). These observations show parallels between VEOIBD and the GPx1/2-DKO ileocolitis.
We previously tested several inhibitors of NADPH oxidases (NOXs) and antioxidants to treat ileocolitis in the GPx1/2-DKO mice with limited success. Two NOX inhibitors, di-2-thienyliodonium and thioridazine, decreased gut pathology, but with significant side effects (10). Here, we studied four more specific NOX inhibitors, including a NOX2-specific gp91ds-TAT, three NOX1-specific Noxa1ds-TAT, ML090 and MJ33 (via a novel PLA2 dependent pathway), as well as anti-apoptotic pifithrin-α (a p53 inhibitor) on GPx1/2-DKO mice (18–24)
Anti-inflammatory drugs, including Dex, Tofa and anti-TNF Ab, are used to treat adult IBD, but not VEOIBD (25–29). Glucocorticoids are anti-inflammatory mediators used for short-term IBD therapy (30). However, glucocorticoids have both protective and deleterious effects in mouse colitis (31). Glucocorticoids induce annexin A1 (ANXA1 or lipocortin 1) expression, which induces resolution of inflammation and wound healing via NOX1 activation (32, 33). Both NOX1 and DUOX2 gene expression can be induced by TNF-α and interferon-γ in the intestinal epithelium (34–36). NOX1 is also induced by pro-inflammatory IL-4 and IL-13 in colon cells (37). Here, we investigated if Dex alleviates ileocolitis in the GPx1/2-DKO mice. If so, does Dex impact Nox1 and Duox2 gene expression?
Tofa, an oral JAK inhibitor targeting mainly JAK1 and JAK3, is effective for adult human IBD and oxazolone-induced colitis (25, 38). Tofa inhibits inflammation via different pathways from Dex (39). Many pro-inflammatory cytokines in IBD use the JAK family of tyrosine kinases for signal transduction. For instance, transcriptional activation of NOX1 by IL-4 and IL-13 pro-inflammatory cytokines is mediated through JAK1/STAT6 signaling in cancer cells (37). Therefore, we also evaluated the efficacy of Tofa on the very-early-onset ileocolitis in the GPx1/2-DKO mice, and whether Tofa inhibits Nox1 or Duox2 gene expression. This work may be useful to develop NOX1-and DUOX2-specific inhibitors for VEOIBD therapy.
2. Materials and Methods
2.1. Mice
Generation and breeding of B6 GPx1/2-DKO (Gpx1tm1Ysh/Gpx2tm2Coh, MGI: 2651587) colony in the Animal Resources Center at City of Hope (COH) has been reported previously (10, 11). Briefly, male GPx1/2-DKO mice were bred with female non-GPx1/2-DKO mice with a wild-type (WT) GPx1 or GPx2 allele (GPx1+/−GPx2−/− or GPx1−/−GPx2+/−) to produce GPx1/2-DKO and non-GPx1/2-DKO littermates. The B6 non-DKO mice rarely have pathology and are virtually indistinguishable from WT. The non-DKO littermates were better controls than WT mice since they shared the same bedding, being exposed to the same microflora. Breeders were fed LabDiet 5062 (9% fat, St. Louis, MO 63144), and 21-day-old pups were weaned onto LabDiet 5061 (5% fat). Both male and females were studied. All mice had free access to food and water.
2.2. Drug treatment
All treatments began on 22-day-old mice, since the ileum pathology of GPx1/2-DKO mice increases from 24 to 35 days of age (10). Mice were administered drugs commencing around 6 A.M. after weighing and inspection for disease signs. The regimen for drug administration is shown in Supplementary Table 1. Effects of drugs were analyzed on 35-day-old mice, except for some mice treated a hydrophilic dexamethasone phosphate (Dex phos), which were analyzed on day 28 along with respective controls. Dex was dissolved in DMSO, Dex phos in PBS; both were obtained from Sigma-Aldrich, St. Louis, MO 63178. The vehicle controls were DMSO (Sigma-Aldrich) and PBS (I.P.; Mediatech, Inc. Manassas, VA, 20109), respectively.
Other drugs used here are: Anti-TNFα Ab (XT22, rat anti-mouse monoclonal neutralizing antibody; Thermo Scientific, Waltham, MA, 02451); gp91ds-TAT (blocks p67phox recruitment to NOX2) and Noxa1ds-TAT (YGRKKRRQRRREPMDALGKAKV-[CONH2]; modified to mouse Noxa1 sequence with a TAT motif; blocks NOX1 enzyme assembly) peptides were manufactured by Synthetic and Biopolymer Chemistry Core Facility COH (19, 24); MJ33 (inhibits NOX1 activity indirectly by inhibiting the PLA2 activity of peroxiredoxin-6)(21), ML090 (SID-26535836; 5,11-Dihydroquinoxalino [2,3-b] quinoxaline; a putative NOX1–4 inhibitor)(22) and pifithrin-α (p53 inhibitor)(23) were obtained from Cayman Chemical (Ann Arbor, Michigan, 48108). Tofacitinib citrate (Tofa) was obtained from LC laboratories (Woburn, MA 01801). No morbidity was observed for all drugs. The vehicle for anti-TNF, gp91ds-TAT and Noxa1ds-TAT was PBS and all were administered I.P. The vehicle for MJ33 and pifithrin-α was DMSO, which were administered I.P. ML90 and Tofa were suspended in 1.2% methyl cellulose, 0.1% polysorbate 80 in water (oral vehicle; Sigma-Aldrich) and administered by gavage (10). Their control mice received vehicle only by gavage.
Doses for most compounds were selected based on the literature for rodent testing, which are referred in Supplementary Table 1. We used 1 mg/Kg Dex based on a mouse study by Ho et al. (40). A higher dose, 2 mg/Kg was used for Dex phos since Ren at al. treated mice with 5 mg/Kg Dex and did not observe adverse effects (41). The dose of 30 mg/Kg Tofa was used following Beattie’s report that oral dosing of 10 and 15 mg/Kg three times a day decreased disease activity index in oxazolone-induced colitis (25). For Noxa1ds-TAT, 5 mg/kg was used initially, and was repeated at 8.5 mg/kg without any sign of morbidity (24). For ML090 since no published data on rodents were available, we used the middle dose after testing 3 doses (6 mg/kg, 12 mg/kg and 30 mg/kg) and did not observe morbidity.
The number of mice studied for each treatment and its control is listed below: 21 Dex phos-treated and 23 PBS controls were analyzed on 28-day-old mice. Analyzed on 35-day-old mice included 20 Dex-treated, 21 DMSO controls, IP; 18 Tofa-treated, 12 vehicle controls; 18 anti-TNF Ab-treated, 21 gp91ds-TAT-treated, 20 low-dose Noxa1ds-TAT-treated, 15 high-dose Noxa1ds-TAT-treated, 21 PBS controls, IP; 21 MJ33-treated, 14 ML090-treated, 15 pifithrin-α-treated, and 12 oral-vehicle controls. All treatments were approved by the COH IACUC.
2.3. Data and tissue collection
Mice were weighed daily. At necropsy the length of the small and large intestine (excluding cecum) was recorded. One cm each of the distal ileum at the ileocecal junction and rectum were harvested and immersed in RNALater (Qiagen, Valencia, CA, 91355) for 24 hours at 4°C then stored at −80°C for RNA isolation. A section of distal ileum (1–5 cm above the cecum), cecum and mid-to distal colon were processed for histological analysis.
2.4. Histopathology
The histopathological features of mouse ileum and distal colon were evaluated on sections stained with hematoxylin and eosin (H&E) by the Solid Tumor Core at COH. Quantification of ileal and colonic pathology was described previously (10). For colon, we counted apoptotic figures, exfoliation and gland abscesses. Since only 2 gland abscesses were seen among all of the mice studied, no analysis is presented. Histopathological scoring was performed on slides with concealed identification and mixing of experimental groups with the respective control groups to ensure the blind scoring. Scoring was performed on all samples listed in the Drug Treatments section.
Immunohistochemistry (IHC) was performed with anti-Ki-67 antibody (Ventana 30–9; no dilution; Roche diagnostics, Fishers, IN, 46037) to assess proliferation. Scoring of Ki-67 positive cells was done by counting the most normal portions of each section. Nine to 24 crypts were counted for each slide depending on the availability of normal-appearing tissue. Six DKO mice were tested in each of Dex/DMSO-, DMSO-, Tofa- and oral-vehicle-treated groups, and 4 non-DKO mice were tested as normal control. TUNEL (ApopTag Peroxidase In Situ Apoptosis Detection Kit; EMD Millipore, Burlington, MA 1803) and lysozyme Ab (1:1,000, ab108508, Abcam, Cambridge, MA 02139–1517) were performed as described previously (10).
2.5. Real-time quantitative PCR (rt-qPCR)
RNA was isolated using a Qiagen RNeasy kit. The cDNA was prepared using 2 μg total RNA with Promega reagents (Madison, WI, 53711). The rt-qPCR was performed using Taqman primer sets (Thermo Fisher Scientific, Waltham, MA) on a BioRad CFX96 (Irvine, CA, 92618) for 40 cycles (Supplementary Table 2). The Ct values were determined using the ΔΔCT method and quantification was normalized against β-actin.
2.6. Statistical analysis
GraphPad Prism 7.01 was used for statistical analysis. Each data set was checked for a parametric distribution except for the pathology score, which is not a continuous distribution. For the main figures parametric sets were analyzed with ANOVA and Tukey’s multiple corrections and nonparametric sets with the Kruskal-Wallis test and Dunn’s multiple corrections. Multiples testing results are indicated by letters where a>b>c>d; groups with shared letters indicate no difference. Results from pairwise testing are mentioned to indicate suggestive results not found after correction for multiple groups. Data testing analysis for the remaining drugs involved pairwise comparisons vs. respective controls and Bonferroni post-test correction for multiple samples. Since the post-test correction left only a few significant differences, we indicate those few with an asterisk and bracket. Supplementary Table 3 contains results of complete pairwise testing for Figures 2 and 3.
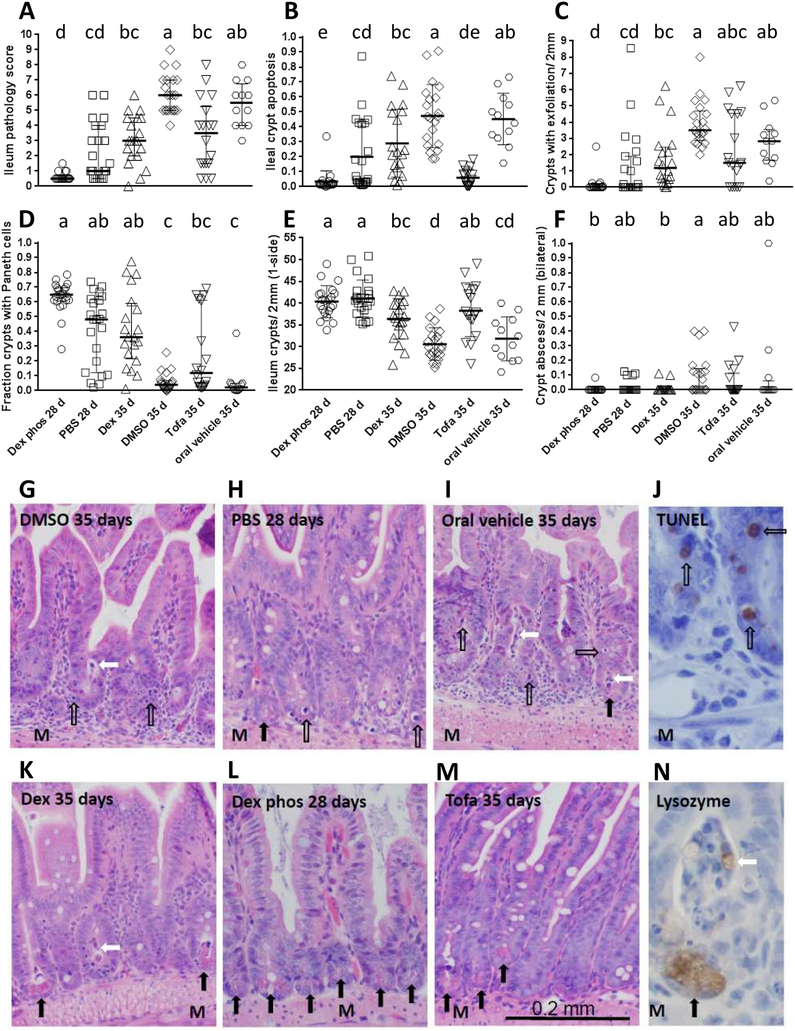
Figure 2: Comparison of total and sub-pathology scores of GPx1/2-DKO ilea treated with immune suppressants.
Panel A shows the total pathology scores compiled from the sub-scores obtained from Panel B and D-F. All scores were done on H&E sections. Each symbol represents one mouse. Panel A, C, D and F were plotted to show median and interquartile range, and Panel B and E for apoptosis and crypt density count were shown as mean and Stdev. Analysis of parametric data is by ANOVA with Tukey’s multiple correction test and non-parametric data by Kruskal-Wallis test with Dunn’s multiple correction test. The letters assigned to each group according to the mean or medium, where a>b>c>d. The groups are significantly different only when they do not share the same letters; i.e. group bc is not different from b or c. The complete results of pairwise comparisons to show suggestive effects are shown in Supplementary Table 3. Panels G-I show H&E histology of GPx1/2-DKO ileum without drug treatment and Panels K-M shows with Dex or Tofa treatment. Panel J is IHC of control DKO ileum stained by TUNEL to verify crypt apoptosis, and Panel N is IHC of ileum labeled with anti-lysozyme antibody to illustrate Paneth cell exfoliation. The open arrows point to apoptotic cells, black arrows point to Paneth cells and white arrows point to an exfoliated Paneth cell. Panels are labeled as vehicle or drug treated and the age of mice analyzed. Original images were 20× for H&E and 40× for IHC. M (muscle layers) indicates the orientation.
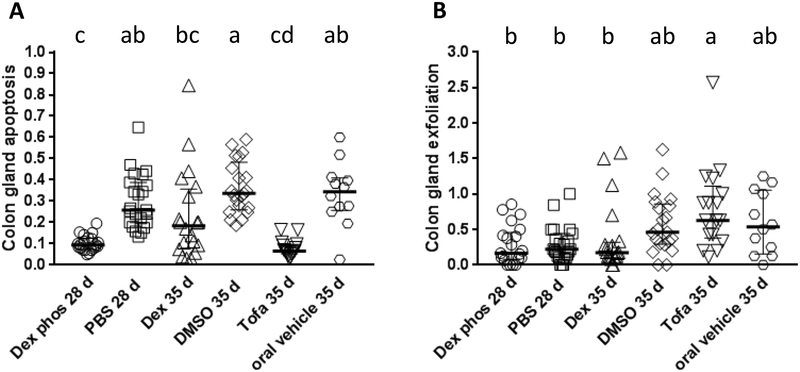
Figure 3: Comparison of apoptosis and gland exfoliation in the GPx1/2-DKO colon treated with immune suppressants.
Each symbol represents one mouse. Panel A and B show the median and interquartile range of number of apoptosis in each gland and exfoliated cells per 10× field, respectively. When groups do not share letters, they are significantly different. The complete results of pairwise comparisons to show suggestive effects are shown in Supplementary Table 3.
3. Results
3.1. Dexamethasone (Dex) and Dex phos alleviated ileocolitis in the GPx1/2-DKO ileum
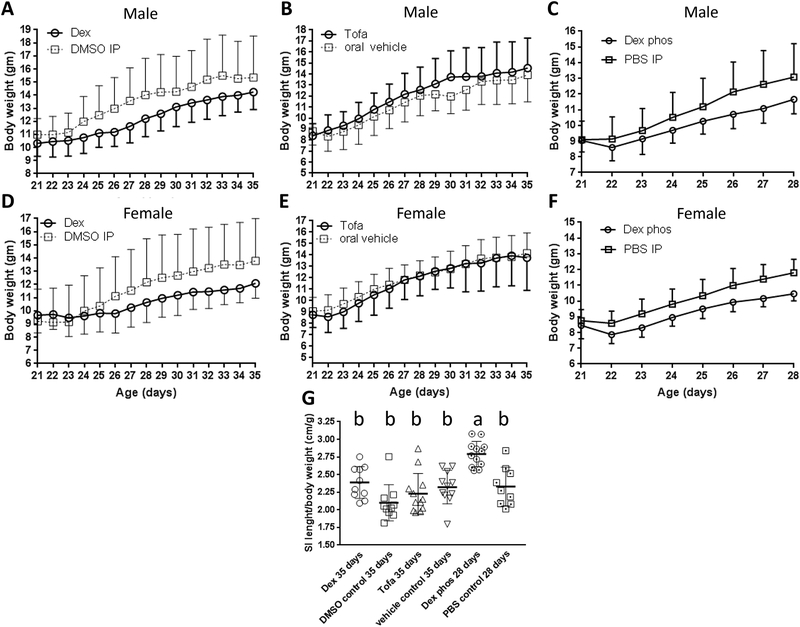
Glucocorticoids have both protective and deleterious effects on experimental colitis in mice (31). It was shown that glucocorticoids inhibited inflammation and induced wound healing via induction of annexin A1 (ANXA1 or lipocortin 1) expression and activation of NOX1 (32, 33). Therefore, we tested whether Dex, a synthetic hydrophobic glucocorticoid, would prevent inflammation analyzed in 35-day-old GPx1/2-DKO mice. The Dex-treated GPx1/2-DKO mice had apparently slower weight gain (even though not statistically significant) than the DMSO-treated mice (Figure 1A, males; 1D, females). The ratio of small intestine length over body weight (to adjust the slower weight gain by Dex treatment) was significantly greater in the Dex mice vs. the DMSO control analyzed by pairwise comparison (p= 0.02; Mann-Whitney, although not different when adjusted for multiple comparisons; Figure 1G).
Figure 1: Comparison of body weight and length of small intestine of GPx1/2-DKO mice from 21 to 35 days of age treated with immune suppressants.
Male mice treated with Dex (Panel A) or Tofa (Panel B) daily and analyzed at 35 days of age, or treated with Dex phos (Panel C) analyzed at 28 days of age vs. the corresponding control set. Differences in weight at the endpoints were not significant. Females showed similar trends (Panels D, E and F). Panel G: Comparison of the lengths of small intestine (SI) adjusted for body weight at either 28 or 35 days of age.
Ileum histology was analyzed to quantify the extent of pathology and inflammation (Figure 2A–2F). DMSO- and Dex-treated DKO Ileum sections stained by H&E are shown in Figure 2G, 2K. The GPx1/2-DKO ileal histopathology shows rampant apoptosis, exfoliation and loss of Paneth cells in the crypt. The pathology score is obtained by combining four sub-scores excluding exfoliation as shown in Figure 2B, 2D–2F. IHC of TUNEL assay and anti-Lysozyme were performed on some slides to confirm the identity of apoptotic cells and Paneth cells, respectively (Figure 2J, 2N). The Dex-treated 35-day-old DKO mice had lower pathology scores in all subcategories compared to DMSO-treated DKO ileum. DMSO did not affect pathology analyzed on 35-day-old DKO mice.
The pathology parameters of untreated DKO and non-DKO mice are shown in Supplementary Figure 1. We have noted that the GPx1/2-DKO mice have peak ileal pathology by 30–35 days of age having increased TNFα mRNA levels, crypt abscesses and submucosal MPO-positive cell infiltration (Supplementary Figure 1A–1F) (10). Duox2 mRNA levels are relatively high in 24-day-old B6 GPx1/2-DKO mice and remain so throughout the study interval (Supplementary Figure 1G)(11).
To examine the short-term effect of glucocorticoid before clear infiltration of inflammatory cells, we used Dex phos to treat GPx1/2-DKO mice and analyzed at 28 days of age along with a PBS control. Dex phos was used since we detected a possible beneficial DMSO effect at 28 days, which was not evident at 35 days (not shown). The short-term Dex phos treatment also showed growth retardation (albeit not significant; Figure 1C, 1F). The ratio of small intestine length over body weight was significantly greater in the Dex phos-treated mice than PBS control that supported the anti-inflammatory effect of Dex Phos (Figure 1G). Even with the lower pathology in the 28-day-old than the 35-day-old DKO mice, Dex phos had likely decreased total pathology score (p<0.0001; pairwise, Mann-Whitney test), clearly inhibited apoptosis, and probably better preserved Paneth cells (p=0.0008; pairwise, Mann-Whitney test) (Figure 2A–2D).
B6 GPx1/2-DKO colon has milder pathology than ileum. The colon apoptotic pathology starts early (<17 days of age) and occurs at about half the levels of the ileum, while colon exfoliation is at one-sixth the levels of ileum. Similar to the ileum, Dex and Dex phos significantly suppressed colon gland apoptosis (Figure 3A). Dex also moderately decreased the levels of exfoliation in the colon (p=0.012; pairwise, Mann-Whitney test; Figure 3B). No gland abscesses were found in either the control or Dex-treated colon.
3.2. Tofacitinib (Tofa) had a significant impact on ileocolitis in the GPx1/2-DKO mice
Tofacitinib (Tofa, a Janus kinase/JAK inhibitor) has anti-inflammatory activity and has shown robust efficacy in treating adult human IBD and oxazolone-induced colitis in mice (25). Here, we tested its effect in very-early-onset ileocolitis in the GPx1/2-DKO mice. Tofa did not affect mouse growth (Figure 1B, male; 1E, female), nor affect the length of small intestine compared to the vehicle-treated GPx1/2-DKO mice (Figure 1G). Tofa significantly inhibited crypt apoptosis and increased crypt density compared to the vehicle-treated GPx1/2-DKO ileum (Figure 2B, 2E). Although Tofa-treated ileum did not lower total pathology score when analyzed with a post-test correction for multiple samples, pairwise comparison showed that Tofa-treated ileum had lower pathology scores than the vehicle control (p=0.015 pairwise, Mann-Whitney test). Similar to Dex, Tofa significantly inhibited colon gland apoptosis (Figure 3A).
3.3. Dex, but not Tofa, suppressed cell proliferation in ileum crypt epithelial cells
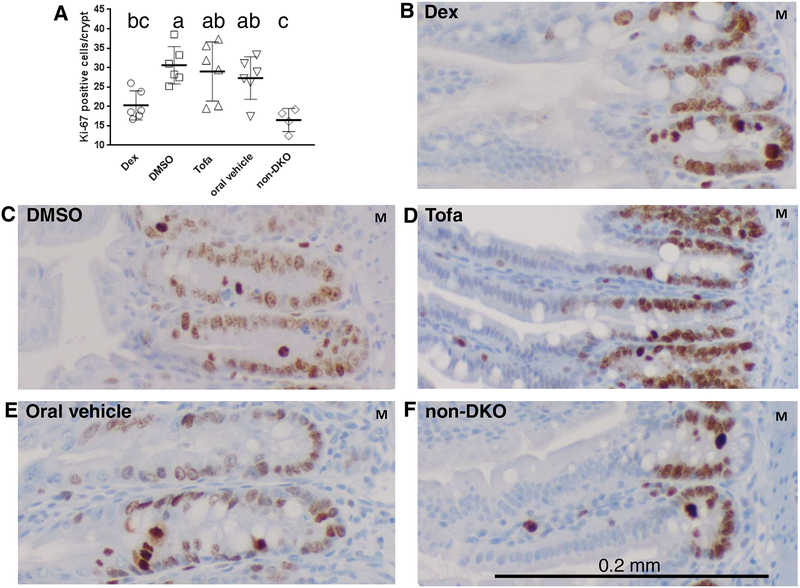
We have reported that GPx1/2-DKO mice have high levels of proliferation in the crypt epithelium compared to the non-DKO mice, which could counter the high level of apoptosis and exfoliation (10, 13). Glucocorticoid inhibition of cell proliferation was reported in DSS-treated colon (31). Tofa regulates lymphocyte activation and proliferation (42), but it is unclear if it affects intestinal epithelial cell proliferation. We analyzed the effect of Dex and Tofa on cell proliferation in the 35-day-old GPx1/2-DKO ileum on sections stained with anti-Ki-67 Ab (Figure 4). Counting the Ki-67+ cells in the relatively undistorted areas, we found Dex, but not Tofa, suppressed cell proliferation in the crypt epithelium.
Figure 4: Comparison of cell proliferation with IHC of anti-Ki-67 Ab treated with Dex or Tofa in 35-day-old GPx1/2-DKO ilea.
Panel A shows the number of Ki-67 positive cells in the crypt of GPx1/2-DKO mice treated with Dex, DMSO, Tofa or Tofa vehicle and untreated non-GPx1/2-DKO mice; the non-GPx1/2-DKO ileum has a background level of proliferating cells. Each symbol represents one mouse, six mice in each group except 4 in non-DKO group. The letters above each groups indicate their values in the order of a>b>c. Groups are different when they don’t share a same letter; i.e. ab is not different from a or bc; analyzed by ANOVA with Tukey’s multiple correction test. Panel F shows a non-DKO control and Panels B-E show GPx1/2-DKO ileum. The images show the representative areas in each group with the original magnification at 20X. The positively stained cells (brown color) are abundant in the crypt, and the section was counterstained with hematoxylin (blue color). M indicates the muscle layer.
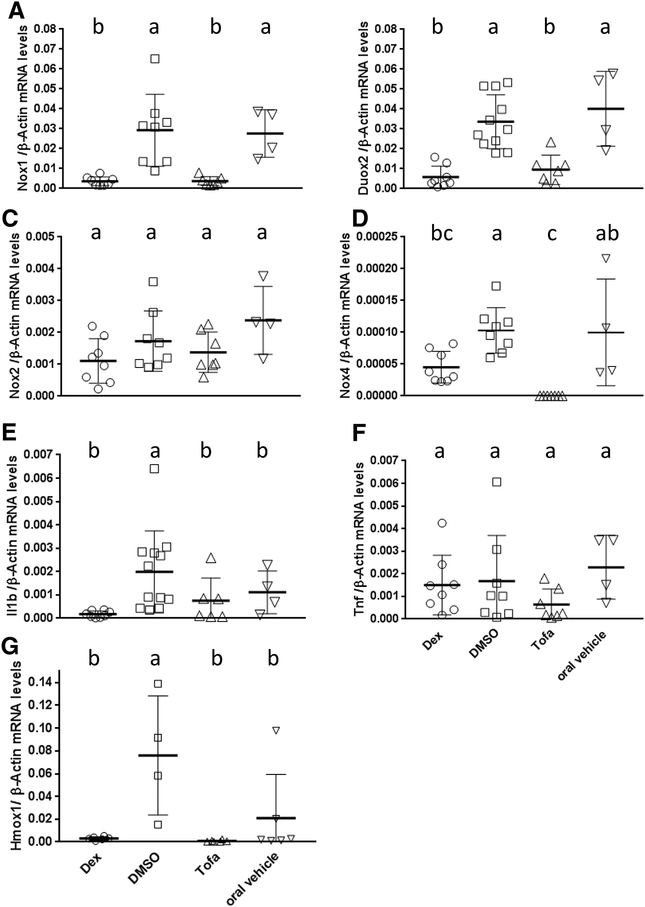
3.4. Both Dex- and Tofa-treated GPx1/2-DKO mice inhibited Nox1, Duox2 and Nox4 but not Nox2 gene expression in the GPx1/2-DKO ileum
We have shown that the very-early-onset ileocolitis in the GPx1/2-DKO mice is driven by NOX1 or DUOX2, since GPx1/2-DKO mice with inactivated NOX1 or DUOX2 enzyme have no or mild inflammation (10, 11). Therefore, we analyzed if Dex and Tofa inhibited Nox1 and Duox2 gene expression in 35-day-old ileum. Dex-treated ileum has dramatically decreased Nox1 and Duox2 mRNA levels, 8-fold and 5.7-fold, respectively compared to the DMSO-treated GPx1/2-DKO mice (Figure 5A, 5B). Similar to Dex, Tofa also inhibited Nox1 and Duox2 gene induction in the GPx1/2-DKO ileum (7.4- and 4.5- fold lower than the vehicle-treated, respectively). Although it is desirable to have IHC to confirm the suppression of NOX1 and DUOX protein expression, our monoclonal Abs against human NOX1 and DUOX (it cannot distinguish DUOX1 and DUOX2) did not work on mouse proteins (43, 44).
Figure 5: Comparison of the mRNA levels in GPx1/2-DKO ileum affected by Dex and Tofa analyzed at 35 days of age.
Panels A-G show the mRNA levels of Nox1, Duox2, Nox2, Nox4, Il1b, Tnf and Hmox1 altered by Dex or Tofa. All mRNA levels were normalized by β-actin mRNA levels. Each symbol represents one mouse (the number of mice in each group is 8, 8–11, 8 and 4 for Dex, DMSO, Tofa and oral vehicle, respectively; except Hmox1:6, 4, 6, 6) and the group is shown as mean ± Stdev. The letters above each groups indicate the value of mRNA levels, where a>b>c. When groups share a same letter, they are not different; i.e. ab is not different from a or bc; analyzed by ANOVA with Tukey’s multiple correction test.
We also analyzed Nox2 and Nox4 gene expression in the GPx1/2-DKO ileum. Nox2 is considered as a marker for inflammation since it is highly expressed in the leukocytes and also expressed in lymphocytes and non-hematopoietic cells (45). Apparently, the slightly lower Nox2 mRNA levels in Dex- or Tofa-treated GPx1/2-DKO mice were not significantly different from the vehicle-treated controls (Figure 5C).
The Nox4 gene is widely expressed in various tissues and cell types including the intestinal epithelium (46, 47). We reported that B6 GPx1/2-DKO ileum had elevated Nox4 gene expression compared with the WT control (11). Here, we found that Nox4 gene expression level was also significantly decreased in Dex- or Tofa-treated GPx1/2-DKO mice, and the Tofa-treated ileum had significantly lower Nox4 mRNA levels than Dex-treated by pairwise comparison (Figure 5D). We noted that the relative Nox4 mRNA levels were much lower than Nox1 or Duox2 mRNA levels.
Heme oxygenase-1 (Hmox1) gene expression is regulated by Nrf2, a redox-sensitive transcription factor which is activated by oxidative stress (48). We analyzed the levels of Hmox1 mRNA levels as an indicator for oxidative stress. Both Dex- and Tofa-treated groups had lower levels of Hmox1 mRNA than in the respective controls, although only the Dex-treated reached statistical significance (Figure 5G).
3.5. Dex but not Tofa inhibited Il1b mRNA induction in the GPx1/2-DKO ileum
We have reported that GPx1/2-DKO ileum had elevated Tnf(α) cytokine protein levels and elevated Il1b mRNA levels compared to non-GPx1/2-DKO mice, whereas GPx1/2-DKO ileum had the same Ifng (interferon-γ) mRNA level as in non-GPx1/2-DKO controls (11). Since Nox1 and Duox2 gene expression can be induced by TNF (and Ifng) in the intestinal epithelium (34–36), here we analyzed Tnf and Il1b mRNA levels mouse ileum. Dex-treated, but not Tofa-treated, GPx1/2-DKO ileum had significantly reduced Il1b mRNA levels than DMSO-treated ileum (Figure 5E). Tofa, but not Dex, decreased Tnf mRNA levels in GPx1/2-DKO ileum compared to vehicle-treated mice when analyzed by pairwise comparison (Figure 5F). We also analyzed mRNA levels of other cytokines Il4 and Il13, which were shown to induce Nox1 mRNA levels in colon cell lines (37). Both mRNAs were undetectable in the GPx1/2-DKO ileum. These results suggest that Dex suppresses Il1b mRNA levels while Tofa has a greater impact on Tnf mRNA levels.
3.6. Nox inhibitors had a mild effect on suppressing ileal pathology in the GPx1/2-DKO mice
Because NOX1 and DUOX2 are major contributors to ileocolitis in the GPx1/2-DKO mice, inhibition of these NOX enzymes should alleviate gut pathology. Although two NOX inhibitors, di-2-thienyliodonium and thioridazine, decreased gut pathology (10), they have significant side effects. Here, we tested four more NOX inhibitors, namely gp91ds-TAT (Nox2ds-TAT), Noxa1ds-TAT, ML090 and MJ33 on the GPx1/2-DKO mice. TAT (trans-activating transcriptional activator), a cell-penetrating peptide found in HIV-1, was added to facilitate cell entry. None of the drugs lowered the total ileal pathology scores (Supplementary Figure 2A). However, peptide Noxa1ds-TAT significantly decreased crypt apoptosis (at 5 mg/kg dose) and increased the crypt density in the ilea (at both 5 and 8.5 mg/kg doses) (Supplementary Figure 2B, 2E). Peptide gp91ds-TAT and MJ33 also significantly increased ileal crypt density in the DKO mice compared to vehicle-treated DKO mice (Supplementary Figure 2E). Other drugs tested including anti-Tnf Ab and pifithrin-α (p53 inhibitor) did not have any effect. High-dose NOXa1ds-TAT may have aggravated colon apoptosis, but none of the drugs tested had other effects on colon (Supplementary Figure 3A, 3B).
4. Discussion
Elevated NOX1 and DUOX2 gene expression is observed in both pediatric and adult ulcerative colitis and Crohn’s disease subjects with crypt or gland expression of DUOX2 in some cases (7–10). Defects in NOX1 and DUOX2 genes are linked to VEOIBD (3–6). In GPx1/2-DKO mice, the Nox1 and Duox2 genes are major contributors to gut inflammation (10, 11). Here, we evaluated the effect of anti-inflammatory drugs to determine their effect on Nox gene expression.
Among the anti-inflammatory drugs tested, we found Dex has the strongest impact on the GPx1/2-DKO mice. Dex, having immunosuppressive effects, has been used for decades in the treatment of IBD flares (30). However, Dex has the undesirable side effect of inhibition of weight gain (49), thus may limit its application to VEOIBD patients. An aspect of the anti-inflammatory effect of glucocorticoids was reported to be activation of NOX1 via annexin A1 induction during wound healing (32, 33). In this study, we found Dex suppressed Nox1 and Duox2 gene expression. Absence of Nox1 expression (Nox1-TKO mice) is associated with a decrease in apoptosis and proliferation of crypt epithelium (11). Unlike the DSS-induced colitis with erosion of epithelium, the ileocolitis in the B6 GPx1/2-DKO mice does not have erosion and so may involve less extensive wound healing. This may explain why Dex alleviated mild inflammation in the DKO mice but aggravated DSS-induced colitis (29, 50).
Tofacitinib (Tofa, a Janus kinase/JAK inhibitor) is recently approved for adults with moderate to severe ulcerative colitis and marketed as Xeljanz®. Many inflammatory cytokines, including IL6, IL13, IL15, IL22–24, IL27 and IFNγ are elevated in either ulcerative colitis or Crohn’s disease, rely on the JAK family of tyrosine kinases for signal transduction (51, 52). Tofa administered orally appeared to modulate intestinal inflammation without systemic immunosuppression (25). Here, we found that Tofa (at 30 mg/kg orally) is effective to suppress the crypt apoptosis in the post-weaning GPx1/2-DKO mice without any impact on growth.
Consistent with our hypothesis that ROS-generating NOX1 and DUOX2 are contributors of ileocolitis in the GPx1/2-DKO mice, both Dex and Tofa inhibited Nox1 and Duox2 gene expression in the ileum. Interestingly, both Dex and Tofa also suppressed Nox4, but not Nox2 (a marker for inflammatory cells) gene expression. It suggests that NOX4 also plays a role in ileocolitis in the GPx1/2-DKO mice. Since very little is known about Nox4 function in the intestine, the role of NOX4 in the GPx1/2-DKO mice should be evaluated in the future. While Dex and Tofa had a similar effect on Nox gene expression, the reason that Dex was more effective than Tofa might be that Dex, but not Tofa, significantly inhibited Il1b gene expression. Other inflammatory responses besides ROS also play an important role in ileocolitis in the GPx1/2-DKO mice. These additional actions of Dex and Tofa complicate any interpretation of action through suppression of enzyme levels.
We suspect that Nox1, which is expressed in the crypt epithelium, is a major contributor to crypt apoptosis (37, 53). Crypt apoptosis is a hallmark of human IBD (54). GPx1/2-DKO ilea deficient in Nox1 gene expression do not have rampant crypt apoptosis as observed in the GPx1/2-DKO mice (11). In contrast, GPx1/2-DKO ilea deficient in Duox2 expression still have similarly high level of apoptosis as the GPx1/2-DKO mice (10). The timing of increasing crypt apoptosis corresponds with the increase of Nox1 gene expression in the GPx1/2-DKO mice (10). Here, both Dex and Tofa decreased Nox1 gene expression and had lowered the crypt apoptosis in the GPx1/2-DKO mice. This is consistent with the notion that NOX1 strongly impacts crypt apoptosis.
Although NOX1 also can promote cell proliferation in colon cell lines, it is puzzling that Dex, but not Tofa, inhibited cell proliferation while both drugs had decreased Nox1 gene expression in the epithelium of the GPx1/2-DKO mice (53). It is possible that the amount of ROS required for proliferation is much lower than that for apoptosis; the residual NOX1 activity is sufficient to induce proliferation. Furthermore, regeneration of inflamed intestine is regulated by multiple pathways, including JAK/STAT, Wnt/Wingless and EGFR pathways. The interplay of the ligands in different pathways may outweigh the effect of NOX1-produced ROS (55).
In this study, we found three more NOX inhibitors have small but significant impact, suppressing one or more aspects of ileal pathology in the GPx1/2-DKO mice. Two are peptides, Noxa1ds-TAT and gp91ds-TAT as well as MJ33. Although Nox2 gene expression was not significantly affected by Dex or Tofa, the ROS generated by NOX2 could still contribute to the gut pathology exhibited in the GPx1/2-DKO mice, which is highly sensitive to ROS. A possible reason for the limited drug effect is that unlike genetically modified mice which are completely devoid of NOX1 or DUOX2 activities, drugs can only partially inhibit enzymes due to limited access and constant enzyme turnover. It is also possible that the gut pathology in the DKO mice once initiated from the ROS produced by NOX1 and DUOX2 it is amplified by inflammatory responses, thus inhibitors of NOX/DUOX alone become less effective with age. Since we observed no side-effects, dose escalation is possible and might yield a better outcome.
Loss of redox balance is involved in the pathogenesis of many diseases including IBD (56). Increase of oxidative stress markers are often detected in the intestinal mucosa of IBD patients, such as overproduction of ROS, increase in 4-hydroxynonenal or malondialdehyde (markers for protein and lipid oxidation) levels, and oxidative DNA damage, etc. Increased NOX1 and DUOX2 gene expression was reported in the gut of IBD patients (5, 9). However, recent studies have shown that partial inactivation of NOX1 and DUOX2 activities may link to VEOIBD (3, 4). Since NOX1 and DUOX2 activation clearly promotes ileocolitis in the DKO mice, which are deficient in antioxidant activity, it is possible that the gut microbes modulated by NOX1 and DUOX2 are the link to IBD. Both NOX1 and DUOX2 gene expression is induced by bacterial colonization as a part of host responses to bacterial signal transduction, which may lead to dysbiosis in humans (57). DKO mice (having elevated Nox1 gene expression) may also have dysbiosis with Escherichia coli overgrowth (10, 58). Thus, gut microbes should be monitored when using NOX/DUOX inhibitors for IBD therapy.
5. Conclusion
Dex and Tofa, two anti-inflammatory drugs used in adult IBD, suppressed markers of pathology in the post-weaning GPx1/2-DKO mice. Tofa did not impact on the growth of weaning mice, and thus may be safe for VEOIBD clinical trials. Dex and Tofa also have an antioxidant effect by suppressing Nox1, Duox2 and Nox4 gene expression in the ileum, suggesting that ROS are a contributing factor to ileocolitis in the GPx1/2-DKO mice. The lowered oxidative stress in the ileum of Dex-treated mice is supported by the low levels of Hmox1 mRNA, which is regulated by the redox-sensitive Nrf2 (48). Although antioxidants and anti-NOX drugs tested thus far had modest effects in suppressing gut pathology in the GPx1/2-DKO mice, it is possible that combining such drugs with conventional therapies will enhance the efficacy of therapy for IBD or VEOIBD.
Supplementary Material
The pathology score criteria include: i) crypt apoptosis (counts of apoptotic figures per crypt), ii) anoikis (scored as crypt exfoliation; i.e. number of crypts with at least 1 exfoliated cell per 10× field), iii) crypt density (number of crypts per 2.2mm), iv) Paneth cell density (fraction crypts with visible Paneth granules), and v) crypt abscesses (counts of abscessed crypts per 10× field of all available tissue on slides). All treatments were done daily from 22 to 34 days of age analyzed at 35 days except for anti-Tnf Ab and pifithrin-α as noted in Supplementary Table 1. Panel A is plotted in box-and-whisker format; it shows the total ileum scores in the order of treated with PBS IP, anti-mouse Tnf Ab, gp91ds-TAT, low- and high-dose of Noxa1ds-TAT, DMSO IP, MJ33, pifithrin-α, oral vehicle and ML090. Comparisons are made against the respective vehicle/route-treated controls (shown in gray boxes) with Bonferroni correction for multiple comparisons (α/n; 0.05/7= 0.0071; *). The individual pathology parameters are shown in Panel B-F from the same groups in the same order, each symbol represents one mouse. Because of the limited numbers of significant differences, they are shown by brackets and asterisks.
Panel A shows counts for apoptosis and Panel B shows exfoliation counts. Each symbol represents one mouse. The pairwise comparison is made against the same vehicle/route controls with Bonferroni correction for multiple samples. *, p < 0.0071. The lone significant difference is shown by bracket and asterisk.
Supplementary Table 1: Drugs evaluated for treatment of ileocolitis in the DKO mice
Panel A shows the increase of apoptotic figures in the DKO mice from 21–35 days of age. The last column was analyzed from 35-day-old non-DKO and wild-type mice with mean crypt apoptosis levels of 0–0.1/crypt. Panels B and E show increased in exfoliation and crypt abscess from 21-to 35-day-old GPx1/2-DKO ileum. Non-DKO and wild-type mice don’t have exfoliated cells in the crypt nor any crypt abscesses and thus are not included. Panels C and D show Paneth cells and crypt density of both GPx1/2-DKO and non-DKO ileum. Panels F and G show temporal changes of TNFα and Duox2 mRNA levels. These are updates from our previous report with new samples obtained for this study (10).
Supplementary Table 2: Primers for TaqMan® Real-time PCR assays
Acknowledgements & Funding Sources
This work was funded by the National Cancer Institute (NCI, Contract No. HHSN261200800001E), National Institutes of Health. COH core facilities supported this study including Histology and Immunohistochemistry performed by Anatomic Pathology Core; housing and breeding mice by Animal Resource Center; gp91ds-TAT and Noxa1ds-TAT peptides were synthesized by Synthetic and Biopolymer Chemistry Core. All COH Cores used here are supported by the NIH P30CA33572. The findings and conclusions of this paper do not necessarily reflect the views of NIH.
Abbreviations:
- B6
C57BL6/J
- COH
City of Hope
- Dex Phos
dexamethasone phosphate
- DKO
double knockout
- DMSO
dimethyl sulfoxide
- Duox2
dual oxidase-2
- GPx
glutathione peroxidase
- Hmox1
heme oxygenase-1
- IBD
inflammatory bowel disease
- Ifng
gamma-interferon
- non-GPX1/2-DKO
mice with a wild-type allele of GPx1 or GPx2
- Nox
NADPH oxidase
- ROS
reactive oxygen species
- TAT
a cell-penetrating peptide named trans-activating transcriptional activator from HIV-1
- Tnf
tumor necrotic factor-alpha
- Tofa
Tofacitinib, or Xeljanz®
- VEO
very-early-onset
- WT
wild-type
Footnotes
Declaration of competing interests
All authors declare no competing interests.
References
- 1.Denson LA. Epithelial Reactive Oxygen Species and Risk for Very Early Onset Inflammatory Bowel Disease. Cellular and molecular gastroenterology and hepatology. 2015. September;1(5):456–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pircalabioru G, Aviello G, Kubica M, Zhdanov A, Paclet MH, Brennan L, et al. Defensive Mutualism Rescues NADPH Oxidase Inactivation in Gut Infection. Cell host & microbe. 2016. May 11;19(5):651–63. [DOI] [PubMed] [Google Scholar]
- 3.Hayes P, Dhillon S, O’Neill K, Thoeni C, Hui KY, Elkadri A, et al. Defects in NADPH Oxidase Genes NOX1 and DUOX2 in Very Early Onset Inflammatory Bowel Disease. Cellular and molecular gastroenterology and hepatology. 2015. September 1;1(5):489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasberger H, Noureldin M, Kao TD, Adler J, Lee JM, Bishu S, et al. Increased risk for inflammatory bowel disease in congenital hypothyroidism supports the existence of a shared susceptibility factor. Scientific reports. 2018. July 5;8(1):10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mouzaoui S, Djerdjouri B, Makhezer N, Kroviarski Y, El-Benna J, Dang PM. Tumor necrosis factor-alpha-induced colitis increases NADPH oxidase 1 expression, oxidative stress, and neutrophil recruitment in the colon: preventive effect of apocynin. Mediators of inflammation. 2014;2014:312484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki TA, Nachman MW. Spatial Heterogeneity of Gut Microbial Composition along the Gastrointestinal Tract in Natural Populations of House Mice. PloS one. 2016; 11(9):e0163720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamm CM, Reimers MA, McCullough CK, Gorbe EB, Lu J, Gu CC, et al. NOD2 status and human ileal gene expression. Inflammatory bowel diseases. 2010. October;16(10): 1649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haberman Y, Tickle TL, Dexheimer PJ, Kim MO, Tang D, Karns R, et al. Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. The Journal of clinical investigation. 2014. August;124(8):3617–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacFie TS, Poulsom R, Parker A, Warnes G, Boitsova T, Nijhuis A, et al. DUOX2 and DUOXA2 form the predominant enzyme system capable of producing the reactive oxygen species H2O2 in active ulcerative colitis and are modulated by 5-aminosalicylic acid. Inflammatory bowel diseases. 2014. March;20(3):514–24. [DOI] [PubMed] [Google Scholar]
- 10.Chu FF, Esworthy RS, Doroshow JH, Grasberger H, Donko A, Leto TL, et al. Deficiency in Duox2 activity alleviates ileitis in GPx1- and GPx2-knockout mice without affecting apoptosis incidence in the crypt epithelium. Redox biology. 2017. April;11:144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esworthy RS, Kim BW, Chow J, Shen B, Doroshow JH, Chu FF. Nox1 causes ileocolitis in mice deficient in glutathione peroxidase-1 and −2. Free radical biology & medicine. 2014. March;68:315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esworthy RS, Aranda R, Martin MG, Doroshow JH, Binder SW, Chu FF. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. American journal of physiology Gastrointestinal and liver physiology. 2001. September;281(3):G848–55. [DOI] [PubMed] [Google Scholar]
- 13.Chu FF, Esworthy RS, Chu PG, Longmate JA, Huycke MM, Wilczynski S, et al. Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer research. 2004. February 1;64(3):962–8. [DOI] [PubMed] [Google Scholar]
- 14.Esworthy RS, Binder SW, Doroshow JH, Chu FF. Microflora trigger colitis in mice deficient in selenium-dependent glutathione peroxidase and induce Gpx2 gene expression. Biological chemistry. 2003. April;384(4):597–607. [DOI] [PubMed] [Google Scholar]
- 15.Jones RM, Neish AS. Redox signaling mediated by the gut microbiota. Free radical biology & medicine. 2017. April;105:41–7. [DOI] [PubMed] [Google Scholar]
- 16.Sommer F, Backhed F. The gut microbiota engages different signaling pathways to induce Duox2 expression in the ileum and colon epithelium. Mucosal immunology. 2015. March;8(2):372–9. [DOI] [PubMed] [Google Scholar]
- 17.Lipinski S, Till A, Sina C, Arlt A, Grasberger H, Schreiber S, et al. DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. Journal of cell science. 2009. October 1; 122(Pt 19):3522–30. [DOI] [PubMed] [Google Scholar]
- 18.Ma MW, Wang J, Dhandapani KM, Wang R, Brann DW. NADPH oxidases in traumatic brain injury - Promising therapeutic targets? Redox biology. 2018. June;16:285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranayhossaini DJ, Rodriguez AI, Sahoo S, Chen BB, Mallampalli RK, Kelley EE, et al. Selective recapitulation of conserved and nonconserved regions of putative NOXA1 protein activation domain confers isoform-specific inhibition of Nox1 oxidase and attenuation of endothelial cell migration. The Journal of biological chemistry. 2013. December 20;288(51):36437–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon J, Wang A, Burke DJ, Boudreau HE, Lekstrom KJ, Korzeniowska A, et al. Peroxiredoxin 6 (Prdx6) supports NADPH oxidase1 (Nox1)-based superoxide generation and cell migration. Free radical biology & medicine. 2016. July;96:99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher AB, Dodia C, Chander A, Jain M. A competitive inhibitor of phospholipase A2 decreases surfactant phosphatidylcholine degradation by the rat lung. The Biochemical journal. 1992. December 1;288 (Pt 2):407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown SJ, Gianni D, Bokoch G, Mercer BA, Hodder P, Rosen HR. Probe Report for NOX1 Inhibitors Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD)2010. [PubMed] [Google Scholar]
- 23.Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999. September 10;285(5434):1733–7. [DOI] [PubMed] [Google Scholar]
- 24.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(−) and systolic blood pressure in mice. Circulation research. 2001. August 31;89(5):408–14. [DOI] [PubMed] [Google Scholar]
- 25.Beattie DT, Pulido-Rios MT, Shen F, Ho M, Situ E, Tsuruda PR, et al. Intestinally-restricted Janus Kinase inhibition: a potential approach to maximize the therapeutic index in inflammatory bowel disease therapy. Journal of inflammation. 2017;14:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onizawa M, Nagaishi T, Kanai T, Nagano K, Oshima S, Nemoto Y, et al. Signaling pathway via TNF-alpha/NF-kappaB in intestinal epithelial cells may be directly involved in colitis-associated carcinogenesis. American journal of physiology Gastrointestinal and liver physiology. 2009. April;296(4):G850–9. [DOI] [PubMed] [Google Scholar]
- 27.Filler SG, Solis NV, Guo J, Doellgast G, Ruiz-Garcia A, Pan WJ. Pharmacokinetics of murine p75-Fc fusion protein and MP6-XT22 anti-murine TNF-alpha mAb in mice. The journal of investigative dermatology Symposium proceedings. 2007. May;12(1):52–6. [DOI] [PubMed] [Google Scholar]
- 28.Mohan VP, Scanga CA, Yu K, Scott HM, Tanaka KE, Tsang E, et al. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infection and immunity. 2001. March;69(3): 1847–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kojouharoff G, Hans W, Obermeier F, Mannel DN, Andus T, Scholmerich J, et al. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clinical and experimental immunology. 1997. February;107(2):353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puhl NJ, Uwiera RR, Yanke LJ, Selinger LB, Inglis GD. Antibiotics conspicuously affect community profiles and richness, but not the density of bacterial cells associated with mucosa in the large and small intestines of mice. Anaerobe. 2012. February;18(1):67–75. [DOI] [PubMed] [Google Scholar]
- 31.Ocon B, Aranda CJ, Gamez-Belmonte R, Suarez MD, Zarzuelo A, Martinez-Augustin O, et al. The glucocorticoid budesonide has protective and deleterious effects in experimental colitis in mice. Biochemical pharmacology. 2016. September 15;116:73–88. [DOI] [PubMed] [Google Scholar]
- 32.Perretti M, D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nature reviews Immunology. 2009. January;9(1):62–70. [DOI] [PubMed] [Google Scholar]
- 33.Leoni G, Alam A, Neumann PA, Lambeth JD, Cheng G, McCoy J, et al. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. The Journal of clinical investigation. 2013. January;123(1):443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuwano Y, Tominaga K, Kawahara T, Sasaki H, Takeo K, Nishida K, et al. Tumor necrosis factor alpha activates transcription of the NADPH oxidase organizer 1 (NOXO1) gene and upregulates superoxide production in colon epithelial cells. Free radical biology & medicine. 2008. December 15;45(12):1642–52. [DOI] [PubMed] [Google Scholar]
- 35.Kuwano Y, Kawahara T, Yamamoto H, Teshima-Kondo S, Tominaga K, Masuda K, et al. Interferon-gamma activates transcription of NADPH oxidase 1 gene and upregulates production of superoxide anion by human large intestinal epithelial cells. American journal of physiology Cell physiology. 2006. February;290(2):C433–43. [DOI] [PubMed] [Google Scholar]
- 36.Gu S, Chen D, Zhang JN, Lv X, Wang K, Duan LP, et al. Bacterial community mapping of the mouse gastrointestinal tract. PloS one. 2013;8(10):e74957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu H, Antony S, Roy K, Juhasz A, Wu Y, Lu J, et al. Interleukin-4 and interleukin-13 increase NADPH oxidase 1-related proliferation of human colon cancer cells. Oncotarget. 2017. June 13;8(24):38113–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collinge M, Ball DJ, Bowman CJ, Nilson AL, Radi ZA, Vogel WM. Immunologic effects of chronic administration of tofacitinib, a Janus kinase inhibitor, in cynomolgus monkeys and rats - Comparison of juvenile and adult responses. Regulatory toxicology and pharmacology : RTP. 2018. April;94:306–22. [DOI] [PubMed] [Google Scholar]
- 39.Hodge JA, Kawabata TT, Krishnaswami S, Clark JD, Telliez JB, Dowty ME, et al. The mechanism of action of tofacitinib - an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clinical and experimental rheumatology. 2016. Mar-Apr;34(2):318–28. [PubMed] [Google Scholar]
- 40.Ho WE, Cheng C, Peh HY, Xu F, Tannenbaum SR, Ong CN, et al. Anti-malarial drug artesunate ameliorates oxidative lung damage in experimental allergic asthma. Free radical biology & medicine. 2012. August 1;53(3):498–507. [DOI] [PubMed] [Google Scholar]
- 41.Ren K, Yuan H, Zhang Y, Wei X, Wang D. Macromolecular glucocorticoid prodrug improves the treatment of dextran sulfate sodium-induced mice ulcerative colitis. Clinical immunology. 2015. September;160(1):71–81. [DOI] [PubMed] [Google Scholar]
- 42.Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. The New England journal of medicine. 2012. August 16;367(7):616–24. [DOI] [PubMed] [Google Scholar]
- 43.Juhasz A, Markel S, Gaur S, Liu H, Lu J, Jiang G, et al. NADPH oxidase 1 supports proliferation of colon cancer cells by modulating reactive oxygen species-dependent signal transduction. The Journal of biological chemistry. 2017. May 12;292(19):7866–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Y, Antony S, Hewitt SM, Jiang G, Yang SX, Meitzler JL, et al. Functional activity and tumor-specific expression of dual oxidase 2 in pancreatic cancer cells and human malignancies characterized with a novel monoclonal antibody. International journal of oncology. 2013. April;42(4):1229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emmerson A, Trevelin SC, Mongue-Din H, Becker PD, Ortiz C, Smyth LA, et al. Nox2 in regulatory T cells promotes angiotensin II-induced cardiovascular remodeling. The Journal of clinical investigation. 2018. July 2;128(7):3088–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dolowschiak T, Chassin C, Ben Mkaddem S, Fuchs TM, Weiss S, Vandewalle A, et al. Potentiation of epithelial innate host responses by intercellular communication. PLoS pathogens. 2010. November 18;6(11):e1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murley JS, Arbiser JL, Weichselbaum RR, Grdina DJ. ROS modifiers and NOX4 affect the expression of the survivin-associated radio-adaptive response. Free radical biology & medicine. 2018. August 1;123:39–52. [DOI] [PubMed] [Google Scholar]
- 48.Kang KW, Lee SJ, Kim SG. Molecular mechanism of nrf2 activation by oxidative stress. Antioxidants & redox signaling. 2005. Nov-Dec;7(11–12):1664–73. [DOI] [PubMed] [Google Scholar]
- 49.Allen DB. Growth suppression by glucocorticoid therapy. Endocrinology and metabolism clinics of North America. 1996. September;25(3):699–717. [DOI] [PubMed] [Google Scholar]
- 50.van Meeteren ME, Meijssen MA, Zijlstra FJ. The effect of dexamethasone treatment on murine colitis. Scandinavian journal of gastroenterology. 2000. May;35(5):517–21. [DOI] [PubMed] [Google Scholar]
- 51.Coskun M, Salem M, Pedersen J, Nielsen OH. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacological research. 2013. October;76:1–8. [DOI] [PubMed] [Google Scholar]
- 52.Hebenstreit D, Horejs-Hoeck J, Duschl A. JAK/STAT-dependent gene regulation by cytokines. Drug news & perspectives. 2005. May;18(4):243–9. [DOI] [PubMed] [Google Scholar]
- 53.Jones RM, Luo L, Ardita CS, Richardson AN, Kwon YM, Mercante JW, et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. The EmBo journal. 2013. November 27;32(23):3017–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goretsky T, Dirisina R, Sinh P, Mittal N, Managlia E, Williams DB, et al. p53 mediates TNF-induced epithelial cell apoptosis in IBD. The American journal of pathology. 2012. October;181(4):1306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Q, Liu M, Hou J, Jiang C, Li S, Wang T. The prevalence of Keshan disease in China. International journal of cardiology. 2013. September 30;168(2):1121–6. [DOI] [PubMed] [Google Scholar]
- 56.Perez S, Talens-Visconti R, Rius-Perez S, Finamor I, Sastre J. Redox signaling in the gastrointestinal tract. Free radical biology & medicine. 2017. March;104:75–103. [DOI] [PubMed] [Google Scholar]
- 57.Aviello G, Knaus UG. ROS in gastrointestinal inflammation: Rescue Or Sabotage? British journal of pharmacology. 2017. June;174(12):1704–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Esworthy RS, Smith DD, Chu FF. A Strong Impact of Genetic Background on Gut Microflora in Mice. International journal of inflammation. 2010. June 1;2010(2010):986046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The pathology score criteria include: i) crypt apoptosis (counts of apoptotic figures per crypt), ii) anoikis (scored as crypt exfoliation; i.e. number of crypts with at least 1 exfoliated cell per 10× field), iii) crypt density (number of crypts per 2.2mm), iv) Paneth cell density (fraction crypts with visible Paneth granules), and v) crypt abscesses (counts of abscessed crypts per 10× field of all available tissue on slides). All treatments were done daily from 22 to 34 days of age analyzed at 35 days except for anti-Tnf Ab and pifithrin-α as noted in Supplementary Table 1. Panel A is plotted in box-and-whisker format; it shows the total ileum scores in the order of treated with PBS IP, anti-mouse Tnf Ab, gp91ds-TAT, low- and high-dose of Noxa1ds-TAT, DMSO IP, MJ33, pifithrin-α, oral vehicle and ML090. Comparisons are made against the respective vehicle/route-treated controls (shown in gray boxes) with Bonferroni correction for multiple comparisons (α/n; 0.05/7= 0.0071; *). The individual pathology parameters are shown in Panel B-F from the same groups in the same order, each symbol represents one mouse. Because of the limited numbers of significant differences, they are shown by brackets and asterisks.
Panel A shows counts for apoptosis and Panel B shows exfoliation counts. Each symbol represents one mouse. The pairwise comparison is made against the same vehicle/route controls with Bonferroni correction for multiple samples. *, p < 0.0071. The lone significant difference is shown by bracket and asterisk.
Supplementary Table 1: Drugs evaluated for treatment of ileocolitis in the DKO mice
Panel A shows the increase of apoptotic figures in the DKO mice from 21–35 days of age. The last column was analyzed from 35-day-old non-DKO and wild-type mice with mean crypt apoptosis levels of 0–0.1/crypt. Panels B and E show increased in exfoliation and crypt abscess from 21-to 35-day-old GPx1/2-DKO ileum. Non-DKO and wild-type mice don’t have exfoliated cells in the crypt nor any crypt abscesses and thus are not included. Panels C and D show Paneth cells and crypt density of both GPx1/2-DKO and non-DKO ileum. Panels F and G show temporal changes of TNFα and Duox2 mRNA levels. These are updates from our previous report with new samples obtained for this study (10).
Supplementary Table 2: Primers for TaqMan® Real-time PCR assays