Abstract
Background
Persistent congestion with deteriorating renal function is an important cause of adverse outcomes in heart failure. We aimed to characterize new approaches to evaluate renal congestion using Doppler ultrasonography.
Methods and Results
We enrolled 205 patients with suspected or prediagnosed pulmonary hypertension (PH) undergoing right heart catheterization. Patients underwent renal Doppler ultrasonography and assessment of invasive cardiopulmonary hemodynamics, echocardiography, renal function, intra‐abdominal pressure, and neurohormones and hydration status. Four spectral Doppler intrarenal venous flow patterns and a novel renal venous stasis index (RVSI) were defined. We evaluated PH‐related morbidity using the Cox proportional hazards model for the composite end point of PH progression (hospitalization for worsening PH, lung transplantation, or PH‐specific therapy escalation) and all‐cause mortality for 1‐year after discharge. The prognostic utility of RVSI and intrarenal venous flow patterns was compared using receiver operating characteristic curves. RVSI increased in a graded fashion across increasing severity of intrarenal venous flow patterns (P<0.0001) and was significantly associated with right heart and renal function, intra‐abdominal pressure, and neurohormonal and hydration status. During follow‐up, the morbidity/mortality end point occurred in 91 patients and was independently predicted by RVSI (RVSI in the third tertile versus referent: hazard ratio: 4.72 [95% CI, 2.10–10.59; P<0.0001]). Receiver operating characteristic curves suggested superiority of RVSI to individual intrarenal venous flow patterns in predicting outcome (areas under the curve: 0.789 and 0.761, respectively; P=0.038).
Conclusions
We propose RVSI as a conceptually new and integrative Doppler index of renal congestion. RVSI provides additional prognostic information to stratify PH for the propensity to develop right heart failure.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT03039959.
Keywords: cardiorenal syndromes, intrarenal venous flow patterns, pulmonary hypertension, renal Doppler ultrasonography, venous congestion
Subject Categories: Heart Failure, Cardiorenal Syndrome
Clinical Perspective
What Is New?
Doppler‐derived intrarenal venous flow patterns having been shown to predict adverse outcomes in patients with heart failure; however, these approaches do not reflect the continuum of renal congestion because classification of intrarenal venous flow into different categories may miss important changes within those categories.
In patients with suspected pulmonary hypertension undergoing right heart catheterization, we developed a continuous index from intrarenal venous flow patterns, and we propose the renal venous stasis index as a conceptually new and integrative Doppler measure of renal congestion.
Our study suggests that renal venous stasis index may be superior to individual intrarenal venous flow pattern in predicting outcome in patients with pulmonary hypertension.
What Are the Clinical Implications?
Renal venous stasis index provides additional prognostic information to stratify pulmonary hypertension for the propensity to develop right heart failure.
Longitudinal studies are needed to clarify the role of renal venous stasis index in the management of pulmonary hypertension.
Introduction
Heart failure (HF) is a major cause of death worldwide1 and the leading cause of hospitalization in both the United States and Europe.2 In addition to low cardiac output, persistent congestion with deterioration of renal function due to progressive right ventricular (RV) failure has been identified as an important cause of adverse outcomes in HF.3, 4, 5
Congestion may lead to a vicious circle of renal dysfunction, increases in intra‐abdominal pressure, neurohormonal activation, excessive renal tubular sodium reabsorption, fluid overload, and diuretic resistance, leading to further RV stress.6, 7 Elevation of right atrial pressure (RAP) is transmitted to the renal veins, causing increased interstitial and tubular hydrostatic pressure within the encapsulated kidney, which decreases net glomerular filtration rate (GFR) and oxygen delivery.8 Similar pathophysiological mechanisms are expected to occur during increases in intra‐abdominal pressure.6 Congestion may also directly compress vessels in the renal parenchymal regions or reduce vessel compliance.9 Consequently, changes in vessel shape and function may lead to transient and cardiac cycle‐dependent stasis of renal venous flow and to changes in intrarenal venous flow (IRVF) patterns.
Doppler ultrasonography was recently proposed to evaluate renal congestion, with different IRVF patterns and the intrarenal venous impedance index having been shown to predict diuretic response and adverse outcomes in patients with HF or undergoing cardiac surgery.9, 10, 11, 12 However, these approaches do not reflect the continuum of renal congestion: classification of IRVF patterns into different categories may miss important changes within those categories, and the venous impedance index does not distinguish between IRVF patterns with different degrees of venous stasis. We sought to identify and rigorously characterize a new approach to evaluate the continuum of renal congestion based on Doppler renal venous flow.
Methods
Study Design and Participants
We prospectively enrolled consecutive hospital inpatients aged ≥18 years with suspected or prediagnosed pulmonary hypertension (PH) who were undergoing invasive right heart catheterization (RHC) between January 2017 and September 2017 at the Department of Pulmonology, University Hospital Giessen and Marburg, Giessen, Germany. PH is the most common precursor to RV failure13 and thus represents an ideal scenario for studying congestion. Suspicion of PH was determined on clinical grounds including echocardiographic evaluation, in accordance with the most recent guidelines for the diagnosis and treatment of PH.14 Patients with prediagnosed PH had received the diagnosis based on previous RHC. Diagnosis and classification of PH and pulmonary vasoactive treatment were based on current guidelines.14 PH was defined as invasively measured mean pulmonary arterial pressure (PAP) ≥25 mm Hg at rest. Patients were assigned a diagnosis of pulmonary arterial hypertension (group 1), PH due to left heart disease (group 2), PH due to lung diseases and/or hypoxia (group 3), chronic thromboembolic PH (group 4), or PH with unclear and/or multifactorial mechanisms (group 5)14 by a multidisciplinary board. Patients receiving PH therapy could enter the study without restrictions. HF was diagnosed according to current guidelines.15
Patients were excluded if they had chronic kidney disease stage 5, preexisting acute kidney injury, non–end‐stage renal disease with extracorporeal or peritoneal ultrafiltration due to diuretic‐resistant fluid overload, prediagnosed glomerulonephritis, autosomal dominant polycystic kidney disease, or postrenal obstruction; if they were recipients of solid‐organ transplants; or if they had received NSAIDs within 72 hours before RHC. The exclusion criteria acute kidney injury and chronic kidney disease were diagnosed by an adjudication committee of 3 expert nephrologists. Chronic kidney disease was considered as estimated GFR (eGFR; creatinine–cystatin C equation) <60 mL/min per 1.73 m2 or the presence of microalbuminuria independent of eGFR.16 Acute kidney injury was defined as an increase in serum creatinine by ≥0.3 mg/dL within 48 hours or ≥1.5 times baseline within the prior 7 days17 (determined by all available serum creatinine values from hospital and outpatient medical records within the previous 90 days). Diuretic‐resistant fluid overload was defined as the inability to achieve an adequate negative fluid balance when the following 4 therapeutic options had been exploited: (1) restriction of fluid intake to <1.5 L/day and sodium chloride intake to ≤6.0 g/day; (2) (continuous) intravenous infusion of furosemide (minimum 500 mg/day); (3) sequential nephron blockade with the addition of a thiazide diuretic (eg, hydrochlorothiazide minimum 25 mg/day or xipamide minimum 20 mg/day); (4) addition of an aldosterone antagonist if tolerable with serum potassium level (spironolactone minimum 25 mg/day or eplerenone minimum 50 mg/day). RHC data, echocardiography, renal function, IRVF patterns, laboratory measurements, intra‐abdominal pressure, and bioimpedance data were evaluated separately, as described in the next section, by examiners who were blinded to the other data. All patients were included in the Giessen PH registry.18
The study was approved by the local Human Research Ethics Committee (AZ 237/16) and complied with the Declaration of Helsinki. The study was registered at ClinicalTrials.gov (identifier: NCT03039959). All participants gave signed informed consent. The data that support the findings of this study are available from the corresponding author on reasonable request.
Procedures and Measurements
Renal Doppler ultrasonography
Ultrasound and spectral Doppler analyses were performed in duplicate by 2 independent nephrologists with experience in Doppler ultrasound, using an EPIQ 5 system (Philips Healthcare) with a sector transducer frequency range of 2.5–5.0 MHz. The analyses were performed after a Swan‐Ganz catheter had been inserted for RHC assessment (described in the next section) and the patient had rested in a supine position for ≥10 minutes. Color Doppler images were used to identify interlobar vessels. Pulsed Doppler waveforms of the interlobar arteries and veins were recorded simultaneously with the patient in a resting decubitus position. Except for renal resistive index (RRI), which was assessed in both kidneys, all renal Doppler ultrasonography studies were performed in the right kidney (except in cases of unsatisfactory image quality) because left renal vein phasicity may be attenuated because of entrapment in the fork between the abdominal aorta and the superior mesenteric artery. In addition, the left ovarian or testicular veins drain into the left renal vein, which, in the rare event of ovarian or testicular varicosis, may affect renal venous flow.
All values were recorded as means of at least 3 measurements obtained in different interlobar vessels over 3 cardiac cycles during sinus rhythm. If atrial fibrillation was present, an index beat (the beat following 2 preceding cardiac cycles of equal duration) was used for each measurement. Venous impedance index and RRI were calculated as follows: (maximum flow velocity−minimum diastolic flow velocity)/maximum flow velocity.9 A side‐to‐side difference in RRI of >5% between the kidneys was considered indicative for significant renal artery stenosis.19 RRI <0.7 was regarded as normal.20
IRVF patterns were characterized by a blinded adjudication committee comprising a nephrologist, an angiologist, and a pulmonologist blinded to clinical, laboratory, and RHC data. If 2 reviewers disagreed, a third reviewer provided input and consensus was developed. The IRVF patterns were broadly categorized into continuous (noncongestive) and discontinuous (nadir velocity=0) flow patterns. We further classified the discontinuous IRVF patterns into 3 stages: pulsatile, biphasic (with venous peaks during systole and diastole), and monophasic (with venous peak during diastole; Figure 1).
Figure 1.
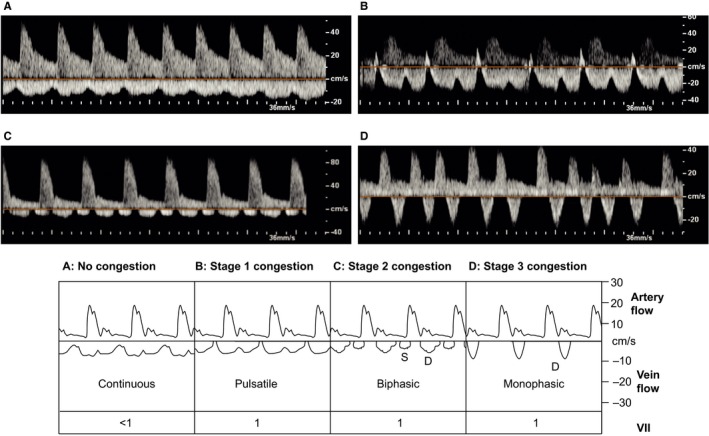
Congestion stages as defined by intrarenal venous flow patterns. Pulsed‐wave Doppler samples of renal congestion patterns in the interlobar renal vessel. The upward Doppler signal shows the intrarenal arterial flow, which is used to measure renal resistive index; the downward Doppler signal shows the venous flow, used to measure venous impedance index or renal venous stasis index. A, No congestion: continuous venous flow. B, Stage 1 congestion: pulsatile venous flow. C, Stage 2 congestion: biphasic venous flow. D, Stage 3 congestion: monophasic venous flow. D indicates diastole; S, systole; VII, venous impedance index.
To reflect the full continuum of renal congestion, we defined and evaluated a new, continuous ratio, the renal venous stasis index (RVSI). RVSI indicates the proportion of the cardiac cycle during which there is no renal venous outlet flow and is calculated as follows: (cardiac cycle time−venous flow time)/cardiac cycle time (Figure 2).
Figure 2.
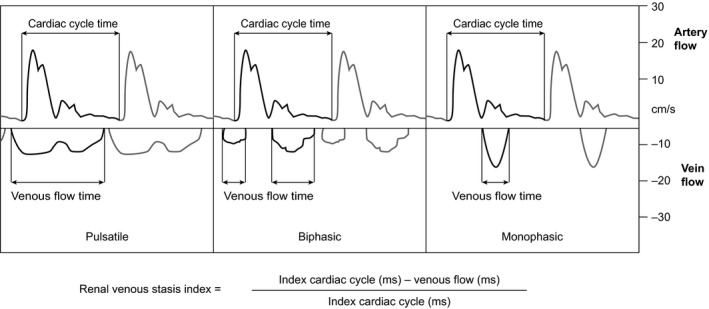
Renal venous stasis index (RVSI). The RVSI is a novel Doppler‐based parameter to estimate severity of renal congestion. Pulsed‐wave Doppler samples of renal congestion patterns in the interlobar renal vessel are shown. The upward Doppler signal shows the intrarenal arterial flow, which is used to measure cardiac cycle time; the downward Doppler signal shows the venous flow, used to measure venous flow time. Under physiological conditions, the index is zero due to the presence of a continuous venous flow, whereas it increases with rising severity of congestion. The figure illustrates the method of measurement of RVSI in different congestion stages. ms indicates milliseconds.
Right heart catheterization
On the day of RHC, each patient took his or her usual dose of medication at 07:00 am except for the maintenance dose of diuretics. In patients with long‐term oxygen treatment, oxygen was applied via nasal cannula at the previously prescribed flow rate. A Swan‐Ganz catheter (7F balloon tipped; Baxter Healthcare) was inserted under local anesthesia in the right internal jugular vein. RHC was performed according to current guidelines,14 with assessment of mean PAP, RAP, pulmonary vascular resistance, pulmonary capillary wedge pressure, cardiac output (thermodilution method), cardiac index, mean arterial pressure, and mixed venous oxygen saturation immediately after renal ultrasonography (for details see Data S1).
Echocardiography
Echocardiography was performed by experienced echocardiographers 1 day before RHC using Vivid E9 and Vivid S5 systems (GE Healthcare). Right heart parameters (RV myocardial performance index [Tei index], tricuspid annular plane systolic excursion, systolic PAP, right atrial size, basal diameter of the right ventricle, inferior vena cava diameter, and systolic free wall myocardial velocity) and left heart parameters (left atrial and ventricular diameters and the ratio of mitral inflow velocity to lateral annular relaxation velocity [E/e’]) were measured as recommended.21
Other measures
Body composition (including hydration status) was analyzed by bioimpedance spectroscopy using the body composition monitor (Fresenius Medical Care; see Data S1) before catheterization. Hydration status was also evaluated by clinical assessment (edema) and ultrasound (ascites and pleural effusion). Intra‐abdominal pressure was measured via an indwelling urinary catheter in all patients using the transvesical method as previously described (Data S1).6 The mean arterial pressure was calculated as follows: [systolic blood pressure+(2×diastolic blood pressure)]/3. The abdominal perfusion pressure was determined as mean arterial pressure minus intra‐abdominal pressure.6 The renal filtration gradient can be estimated as glomerular filtration pressure minus proximal tubular pressure.6 In the presence of elevated intra‐abdominal pressure, proximal tubular pressure may be assumed to equal intra‐abdominal pressure, and thus glomerular filtration pressure can be estimated mean arterial pressure minus intra‐abdominal pressure. The renal filtration gradient was therefore calculated as follows: mean arterial pressure−(2×intra‐abdominal pressure). Intra‐abdominal pressures from 4 to 7 mm Hg were considered as normal range, whereas values ≥12 mm Hg were considered as intra‐abdominal hypertension.22 Six‐minute walk distance and New York Heart Association functional class were assessed 1 day before RHC according to current guidelines.15, 23 Loop diuretic doses were converted to furosemide equivalents with 20 mg torasemide equal to 80 mg furosemide for oral diuretics and 20 mg torasemide equal to 40 mg furosemide for intravenous diuretics.24 Thiazide diuretics included hydrochlorothiazide and xipamide. If triamterene was taken, it was used in a fixed diuretic combination with hydrochlorothiazide. Aldosterone antagonists included spironolactone and eplerenone.
Laboratory methods
Laboratory methods are detailed in Data S1. Briefly, blood samples were collected on the day of RHC from the Swan‐Ganz catheter after the patient had rested in a supine position for ≥60 minutes. Urine samples were collected from first morning‐void specimens. BNP (B‐type natriuretic peptide), copeptin, creatinine, and cystatin C were measured using chemiluminescence, time‐resolved amplified cryptate emission, photometric‐enzymatic, and immunoturbidimetric methods, respectively. We chose BNP and copeptin as biomarkers of neurohormonal activation because both are commonly used for diagnosis and determining prognosis in HF.15 The eGFR was determined using both creatinine25 and creatinine–cystatin C26 Chronic Kidney Disease–Epidemiology Collaboration equations.
Follow‐Up and End Points
Clinical outcomes were evaluated for 1 year after discharge. Patients were closely followed during the observation period by clinical visits or telephone interviews. Changes of medication for clinical reasons were permitted (the primary physician caring for the patient was blinded to the IRVF patterns and RVSI results). Use of diuretics was at the discretion of the treating physician. At 1 year, all patients were recontacted for follow‐up analyses at the nephrology outpatient department. If the primary cause of PH was surgically treated (eg, pulmonary thrombendarterectomy in patients with chronic thromboembolic PH or lung transplantation), the patients were followed until the surgical procedure. If a patient died outside of the hospital, telephone calls to the general practitioner or the family members were performed to confirm the date of death.
We evaluated the first occurrence of a composite end point of PH‐related morbidity (any hospitalization for worsening of PH, lung transplantation, or need for escalation of PH‐specific therapy) and death from any cause. In addition, the following components of the composite end point were each analyzed separately: unscheduled hospitalization due to fluid overload with requirement for an increase in diuretic therapy (eg, due to pulmonary or peripheral edema, pleural effusion, ascites, or recent increase of body weight by ≥10%); need for escalation or change of PH‐specific therapy due to clinical and echocardiographic progress of PH; and death from any cause. Patients who underwent pulmonary thromboendarterectomy were considered as withdrawn alive. All available medical records were collected, and morbidity and mortality data were evaluated according to the predefined end point components in a blinded fashion by a clinical end point adjudication committee including medical experts in nephrology, PH, and cardiology who were unaware of the IRVF patterns and RVSI results and not responsible for the primary care of the patient.
Statistical Analysis
Descriptive statistics were expressed as mean±SD or median (interquartile range [IQR]) for continuous variables, and frequency (percentage) for categorical variables. Patient characteristics were compared between subgroups using ANOVA, Mann‐Whitney U tests, or Kruskal–Wallis tests for continuous variables and χ2 tests for categorical variables. Intra‐ and interobserver reliability was evaluated using the intraclass correlation coefficient. To understand the relationships of renal function and RVSI with other continuous variables, we performed Spearman and Pearson correlation analysis. Correlation coefficient values >0.3 were considered relevant. Kaplan‐Meier analysis was performed to determine the relationship of RVSI and IRVF patterns with clinical end points. Risk factors for clinical end points were determined with Cox proportional hazards models. Univariate factors with P<0.05 were entered into the multiple Cox regression model. A stepwise backward procedure was used in multiple Cox regression analysis (likelihood ratio). RVSI values >0 were divided into tertiles, and then the hazard rates of each tertile for reaching the clinical end points were calculated in relation to the control group (RVSI=0). Receiver operating characteristic curves were used to evaluate RVSI and IRVF as predictors of binary clinical end points. Time‐to‐event information and censoring were ignored when computing the areas under the curves. Receiver operating characteristics were compared with the DeLong test implemented in the R package pROC (1.14.0).27, 28 Overall, the significance level was set at α=0.05 except in multiple Cox regression analysis, where the significance level was α=0.10. The Bonferroni correction was applied to adjust for multiple testing. The size of the RHC cohort was estimated based on feasibility considerations. The power to detect a hazard ratio >2.0 at α=0.05 between the third tertile RVSI group (n=49) versus RVSI=0 (n=59) was 50%. The power was calculated in R (3.5.1) using the function powerCT from the package powerSurvEpi (0.1.0), based on a method proposed by Freedman.27, 29, 30 All other statistical analyses were performed using SPSS 23.0 software (IBM Corp).
Results
Patients
Of 270 eligible patients undergoing RHC, 205 patients were enrolled and included in the analysis (Figure 3). None were lost to follow‐up. In all patients except 2, renal Doppler studies were performed in the right kidney. IRVF pattern classifications were completely consistent, and RVSI measurements showed excellent reliability in both intra‐ and interobserver comparisons (Table S1). Patients’ baseline characteristics are shown in Table 1 and Table S2.
Figure 3.
Study flow chart. The diagram describes the protocol used for the enrollment of patients in this study.
Table 1.
Clinical Characteristics, Invasive Hemodynamics, Echocardiographic Data, Renal Function, and Neurohormonal and Hydration Status According to RVSI Tertile
| All patients(N=205) | RVSI 0(n=59) | RVSI Tertiles | P Valuea | |||
|---|---|---|---|---|---|---|
| First, 0 to ≤0.12 (n=49) | Second, >0.12 to ≤0.32 (n=48) | Third, >0.32(n=49) | ||||
| Demographics | ||||||
| Age, y | 68.0 (57.0–78.0) | 68.0 (55.0–73.0) | 67.0 (51.0–75.5) | 72.5 (61.0–78.0) | 74.0 (65.0–81.0) | 0.0152 |
| Male, n (%) | 87 (42.4) | 24 (40.7) | 17 (34.7) | 17 (35.4) | 29 (59.2) | 0.0488 |
| Body mass index, kg/m2 | 27.82±6.07 | 29.03±6.05 | 26.05±6.37 | 28.30±6.84 | 27.67±4.52 | 0.075 |
| Baseline clinical data | ||||||
| Oxygen supply, n (%) | 118 (57.6) | 28 (47.5) | 28 (57.1) | 32 (66.7) | 30 (61.2) | 0.224 |
| Pao 2 b | 69.32±11.71 | 71.09±10.74 | 68.11±11.21 | 68.00±10.01 | 69.67±14.57 | 0.474 |
| Paco 2 b | 38.57±8.98 | 40.20±11.68 | 37.76±7.30 | 37.38±7.88 | 38.59±7.66 | 0.367 |
| 6MWD, m | 277.23±136.05 | 309.76±118.16 | 285.08±156.43 | 283.77±131.30 | 223.80±127.02 | 0.0098 |
| NYHA classification, n (%) | ||||||
| 1–2 | 44 (21.5) | 15 (25.4) | 12 (24.5) | 13 (27.1) | 4 (8.2) | 0.178 |
| 3–4 | 161 (78.5) | 44 (74.6) | 37 (75.5) | 35 (72.9) | 45 (91.8) | |
| Laboratory data | ||||||
| Leukocytes, ×109/L | 7.43±2.49 | 7.33±2.53 | 7.72±2.29 | 7.14±2.68 | 7.56±2.47 | 0.674 |
| Hemoglobin, g/dL | 13.27±2.09 | 13.95±1.83 | 13.53±2.06 | 13.49±2.18 | 13.02±2.31 | 0.504 |
| Sodium, mmol/L | 139.56±3.07 | 139.32±3.15 | 139.73±2.72 | 140.15±3.14 | 139.10±3.22 | 0.343 |
| Potassium, mmol/L | 3.67±0.42 | 3.65±0.40 | 3.64±0.34 | 3.63±0.44 | 3.73±0.47 | 0.612 |
| Uric acid, mg/dL | 6.77±2.52 | 6.26±2.18 | 5.89±2.07 | 6.65±2.31 | 8.38±2.82 | <0.0001 |
| Albumin, g/dL | 38.20±3.22 | 38.91±3.18 | 37.49±3.25 | 38.09±2.96 | 38.18±2.42 | 0.162 |
| C‐reactive protein, mg/L | 5.22 (1.52–11.44) | 3.13 (1.07–8.60) | 3.70 (0.50–11.58) | 5.22 (2.05–11.48) | 7.20 (3.18–14.51) | 0.0172 |
| Comorbidities, n (%) | ||||||
| Hypertension | 128 (62.4) | 35 (59.3) | 29 (59.2) | 32 (66.7) | 32 (65.3) | 0.800 |
| Diabetes mellitus | 48 (23.4) | 12 (20.3) | 9 (18.4) | 9 (18.8) | 18 (36.7) | 0.092 |
| Atrial fibrillation | 56 (27.3) | 7 (11.9) | 14 (28.6) | 9 (18.8) | 26 (53.1) | <0.0001 |
| Maintenance therapy | ||||||
| ACEI or ARB, n (%) | 83 (40.5) | 23 (39.0) | 18 (36.7) | 20 (41.7) | 22 (44.9) | 0.858 |
| β‐Blocker | 103 (50.2) | 25 (42.4) | 18 (36.7) | 27 (56.3) | 33 (67.3) | 0.0095 |
| Loop diuretic dose, mg/dc | 40 (0.0–60.0) | 20 (0.0–40.0) | 20 (0.0–55.0) | 30 (0.0–55.0) | 80 (40.0–170.0) | <0.0001 |
| PH‐specific therapy (%) | ||||||
| Treatment‐naive | 116 (56.6) | 42 (71.2) | 21 (42.9) | 25 (47.9) | 30 (61.2) | 0.0292 |
| Monotherapy | 49 (23.9) | 8 (13.6) | 18 (36.7) | 11 (22.9) | 12 (24.5) | |
| Dual therapy | 28 (13.7) | 6 (10.2) | 7 (14.3) | 12 (25.0) | 3 (6.1) | |
| Triple therapy | 12 (5.9) | 3 (5.1) | 3 (6.1) | 2 (4.2) | 4 (8.2) | |
| Hemodynamics | ||||||
| Mean PAP, mm Hg | 34.84±14.63 | 24.10±9.62 | 34.78±12.79 | 41.10±16.03 | 41.69±12.39 | <0.0001 |
| PVR, dyne∙s/cm5 | 394 (214–604) | 229 (110–420) | 422 (214–589) | 516 (279–679) | 495 (313–833) | <0.0001 |
| RAP, mm Hg | 5.76±5.63 | 2.46±3.66 | 3.88±3.76 | 5.56±3.72 | 11.80±6.06 | <0.0001 |
| Cardiac index, L/min/m2 | 2.73±0.98 | 2.98±1.01 | 2.88±1.16 | 2.67±0.68 | 2.32±0.89 | 0.0032 |
| PCWP, mm Hg | 9.0 (5.0–13.0) | 7.0 (4.0–10.0) | 8.0 (5.0–12.0) | 9.5 (6.0–14.8) | 12.0 (8.0–18.0) | <0.0001 |
| Mixed venous oxygen saturation, % | 63.76±8.35 | 66.70±6.42 | 65.15±7.17 | 65.00±5.85 | 57.62±10.43 | <0.0001 |
| Heart rate, beats/min | 71.62±13.23 | 72.00±11.32 | 69.84±12.90 | 71.38±11.49 | 73.18±16.95 | 0.65 |
| MAP, mm Hgd | 84.25±11.57 | 85.22±10.28 | 82.76±13.39 | 84.81±10.82 | 84.02±11.99 | 0.72 |
| Echocardiographic parameters | ||||||
| Right heart | ||||||
| TAPSE, mm | 19.89±4.41 | 21.88±3.82 | 21.14±4.44 | 19.50±3.64 | 16.63±3.85 | <0.0001 |
| RV myocardial performance index (Tei index) | 0.49±0.22 | 0.46±0.20 | 0.45±0.21 | 0.54±0.24 | 0.49±0.24 | 0.334 |
| RV S’, cm/s | 11.60±3.52 | 12.95±3.31 | 12.13±3.27 | 11.49±3.29 | 9.54±3.36 | <0.0001 |
| TAPSE/systolic PAP ratio | 0.39±0.21 | 0.55±0.24 | 0.38±0.22 | 0.33±0.12 | 0.30±0.12 | <0.0001 |
| Tricuspid insufficiency, n (%) | <0.0001 | |||||
| Mild | 66 (32.2) | 34 (57.6) | 10 (20.4) | 12 (25.0) | 10 (20.4) | |
| Moderate | 112 (54.6) | 23 (39.0) | 31 (63.3) | 32 (66.7) | 26 (53.1) | |
| Severe | 25 (12.2) | 1 (1.7) | 7 (14.3) | 4 (8.3) | 13 (26.5) | |
| RA area, cm2 | 18.89±6.72 | 15.14±6.30 | 18.48±5.64 | 19.21±5.64 | 23.58±6.44 | <0.0001 |
| RV diameter, mm | 40.78±8.08 | 37.93±7.38 | 41.09±6.97 | 40.52±7.75 | 44.40±9.00 | 0.0005 |
| IVC, cm | 2.27±0.49 | 2.01±0.52 | 2.26±0.42 | 2.34±0.41 | 2.53±0.43 | <0.0001 |
| Left heart | ||||||
| LVEF, % | 60.0 (60.0–65.0) | 60 (60.0–65.0) | 62 (60.0–65.0) | 60 (60.0–65.0) | 60 (55.0–65.0) | 0.0442 |
| LA diameter, mm | 41.98±6.86 | 39.78±6.35 | 40.33±6.98 | 41.42±6.16 | 46.43±5.98 | <0.0001 |
| LVEDD, mm | 46.03±5.59 | 46.28±4.73 | 45.26±5.73 | 45.83±6.23 | 46.67±5.81 | 0.65 |
| E/e’ ratio | 12.98±5.34 | 11.07±3.52 | 12.46±4.50 | 14.09±5.69 | 14.79±6.78 | 0.0023 |
| Renal function | ||||||
| Serum creatinine, mg/dLe | 1.01±0.45 | 0.92±0.40 | 0.79±0.21 | 0.98±0.36 | 1.35±0.55 | <0.0001 |
| Cystatin C, mg/L | 1.10 (0.91–1.52) | 0.98 (0.81–1.24) | 1.01 (0.88–1.18) | 1.27 (0.97–1.62) | 1.53 (1.10–2.09) | <0.0001 |
| Urea, mg/dLf | 47.44±35.85 | 40.97±26.71 | 34.29±13.13 | 44.69±25.25 | 71.10±54.75 | <0.0001 |
| eGFR (CKD‐EPI creatinine equation), mL/min/1.73 m2 g | 74.45±26.12 | 80.07±24.52 | 87.31±18.86 | 72.69±24.01 | 56.57±26.75 | <0.0001 |
| eGFR (CKD‐EPI creatinine–cystatin C equation), mL/min/1.73 m2 h | 68.58±26.86 | 77.68±27.65 | 80.80±20.25 | 64.06±22.56 | 49.84±24.49 | <0.0001 |
| Renal filtration gradient, mm Hgi | 69.30±12.46 | 73.70±10.60 | 69.62±13.32 | 69.81±11.12 | 63.20±12.81 | <0.0001 |
| Urine PCR, mg/g creatinine | 58.8 (40.2–114.2) | 51.5 (36.4–72.6) | 55.3 (37.5–85.4) | 58.0 (39.9–92.9) | 116.2 (49.1–190.8) | <0.0001 |
| Urine ACR, mg/g creatinine | 11.4 (6.3–29.7) | 9.3 (5.2–16.0) | 9.0 (5.7–18.5) | 10.3 (6.7–19.0) | 29.7 (11.7–107.8) | <0.0001 |
| Urine α1MCR, mg/g creatinine | 10.9 (6.0–19.1) | 8.7 (5.1–16.5) | 8.7 (5.7–15.1) | 12.0 (6.5–21.4) | 15.4 (7.6–32.7) | 0.0092 |
| Renal Doppler ultrasonography | ||||||
| Venous impedance index | 0.84±0.26 | 0.44±0.12 | 1.00±0 | 1.00±0 | 1.00±0 | <0.0001 |
| RRI | 0.71±0.07 | 0.69±0.08 | 0.69±0.07 | 0.73±0.07 | 0.74±0.06 | <0.0001 |
| Neurohormonal status | ||||||
| BNP, pg/mL | 138.0 (50.0–321.0) | 46.0 (26.0–113.0) | 98.0 (38.0–264.5) | 198.5 (111.3–322.5) | 468.0 (228.5–820.0) | <0.0001 |
| Copeptin, pmol/L | 11.1 (5.8–23.3) | 9.1 (4.6–16.0) | 7.3 (4.9–14.4) | 14.0 (5.2–23.5) | 23.2 (11.1–39.6) | <0.0001 |
| Urine feNa, % | 0.6 (0.4–1.3) | 0.7 (0.4–1.2) | 0.6 (0.4–1.1) | 0.6 (0.4–1.2) | 1.2 (0.4–2.1) | 0.072 |
| Hydration status | ||||||
| Peripheral edema, n (%) | 60 (29.3) | 13 (22.0) | 11 (22.4) | 16 (33.3) | 20 (40.8) | 0.105 |
| Pleural effusion, n (%) | 17 (8.3) | 3 (5.1) | 3 (6.1) | 3 (6.3) | 8 (16.3) | 0.137 |
| Ascites, n (%) | 7 (3.4) | 0 (0) | 1 (2.0) | 0 (0) | 6 (12.2) | 0.0013 |
| Hydration status (as measured by bioimpedance)j | 0.71±2.12 | −0.14±1.41 | 0.50±1.78 | 1.18±2.41 | 1.50±2.48 | <0.0001 |
| ECW/ICW ratioj | 0.88±0.12 | 0.85±0.11 | 0.85±0.09 | 0.89±0.13 | 0.91±0.13 | 0.0286 |
| Intra‐abdominal pressure measurement | ||||||
| Intra‐abdominal pressure, mm Hg | 7.0 (6.0–9.0) | 6.0 (5.0–6.0) | 6.0 (6.0–7.0) | 7.0 (7.0–8.0) | 10.0 (9.0–12.0) | <0.0001 |
| Abdominal perfusion pressure, mm Hgk | 76.78±11.81 | 79.46±10.38 | 77.31±10.89 | 76.19±13.32 | 73.61±12.21 | 0.078 |
Values are mean±SD or median (interquartile range) except as noted. Additional data are provided in the Data S1. ACEI indicates angiotensin‐converting enzyme inhibitor; ACR, albumin/creatinine ratio; α1MCR, α1‐microglobulin/creatinine ratio; ARB, angiotensin receptor blocker; BNP, B‐type natriuretic peptide; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; ECW, extracellular water; E/e’ ratio, ratio of mitral inflow velocity to lateral annular relaxation velocity; eGFR, estimated glomerular filtration rate; feNa, fractional excretion of sodium; ICW, intracellular water; IVC, inferior vena cava; LA, left atrial; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; NYHA, New York Heart Association; PAP, pulmonary arterial pressure; PCR, protein/creatinine ratio; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RA, right atrial; RAP, right atrial pressure; RRI, renal resistive index; RV, right ventricular; RV S’, systolic annular tissue velocity of the lateral tricuspid annulus; RVSI, renal venous stasis index; 6MWD indicates 6‐min walk distance; TAPSE, tricuspid annular plane systolic excursion.
After application of the Bonferroni correction, P<0.0008 was considered significant.
Blood gas measurements were taken from arterialized capillary ear lobe blood during right heart catheterization. In patients with long‐term oxygen treatment, oxygen was applied by nasal cannula at the previously prescribed flow rate. To convert mm Hg to kPa, multiply by 0.133.
A total of 14 patients received intravenous furosemide.
MAP was calculated as follows: (systolic blood pressure+[2×diastolic pressure])/3.
To convert the values for serum creatinine to μmol/L, multiply by 88.4.
To convert the values for urea to blood urea nitrogen, multiply by 0.467.
eGFR was calculated with the CKD‐EPI equation based on serum creatinine.25
eGFR was calculated with the CKD‐EPI equation based on serum creatinine and cystatin C.26
The renal filtration gradient was calculated as follows: MAP–(2×intra‐abdominal pressure).6
Additional bioimpedance data are provided in Data S1.
Abdominal perfusion pressure was calculated as MAP minus intra‐abdominal pressure.6
Association of RVSI with IRVF patterns and demographic and clinical characteristics
After completion of recruitment, we confirmed the predefined IRVF patterns with invasive hemodynamics and echocardiography and assessed their associations with other parameters (Figure S1 and Table S3). By definition, patients with no renal congestion had RVSI=0 and were assigned as the referent group. As shown, RVSI showed a significant stepwise increase along the predefined IRVF patterns (P<0.0001; Figure 4).
Figure 4.
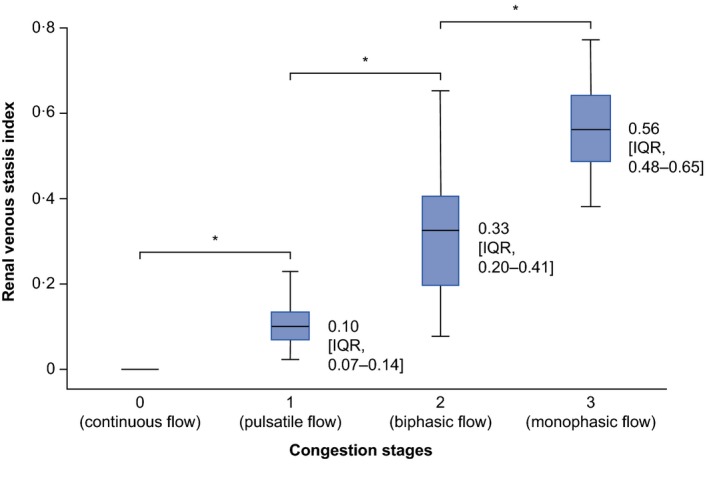
Association of renal venous stasis index with congestion stages. Under physiological conditions, the renal venous stasis index is zero due to the presence of a continuous venous flow, whereas it increases with rising severity of congestion. Horizontal lines indicate median, boxes indicate interquartile range (IQR), and whiskers indicate minimum and maximum values. Data labels show median (IQR).
Table 1 and Figure 5 show the associations of RVSI tertiles with key clinical parameters (additional parameters are shown in Table S4). Cardiopulmonary hemodynamics (evaluated by RHC) worsened with increasing RVSI tertile, with RAP showing the clearest association. RV systolic function (tricuspid annular plane systolic excursion) showed a significant stepwise decrease along the tertiles, with manifest dysfunction at the highest tertile. Right atrial and ventricular diameter, left atrial diameter, and E/e’ ratio significantly increased along the RVSI tertiles.
Figure 5.
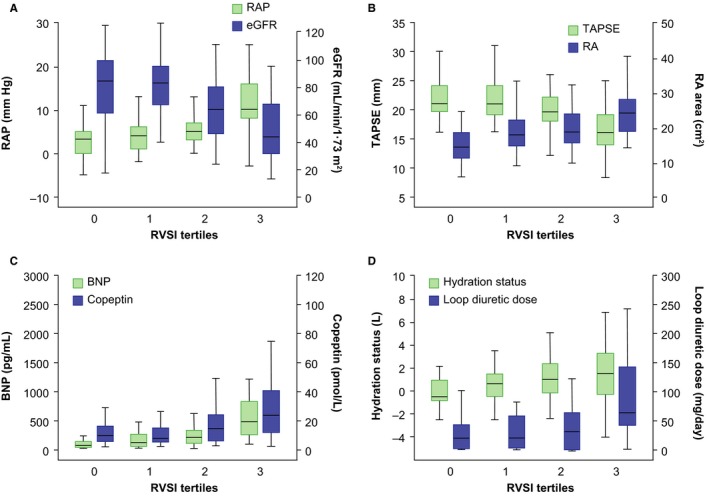
RVSI and associated clinical parameters. Severity of renal congestion can be evaluated by measurement of RVSI using renal Doppler ultrasonography. The figure illustrates the associations of RVSI tertiles with RAP and renal function (A), right ventricular systolic function and RA area (B), neurohormonal status (C), and hydration status (D). Fluid overload as measured by bioimpedance is likely to occur because of hemodynamic alterations and neurohormonal activation leading to a deterioration of renal function and fluid retention. BNP indicates B‐type natriuretic peptide; eGFR, estimated glomerular filtration rate (based on Chronic Kidney Disease Epidemiology Collaboration creatinine–cystatin C equation); RA, right atrial; RAP, right atrial pressure; RVSI, renal venous stasis index; TAPSE, tricuspid annular plane systolic excursion; VII, venous impedance index.
Patients with no congestion had normal mean serum creatinine and eGFR values. From the second RVSI tertile onward, there was gradual lower eGFR and renal filtration gradient across RVSI tertiles, whereas from RVSI=0 (no congestion) to the first RVSI tertile, there was no significant change in serum creatinine (P=0.09), eGFR (P=0.53), and cystatin C (P=0.70). RRI significantly increased with increasing RVSI tertile. Of note, none of the patients exhibited a significant difference in mean RRI values between kidneys (indicative of renal artery stenosis). There was a significant increase in proteinuria, albuminuria, and tubular proteinuria (α1‐microglobulin) with increasing RVSI tertile, but the median values stayed within the physiological range.
RVSI tertiles were associated with levels of BNP and copeptin, as well as hydration status (as measured by bioimpedance), loop diuretic dose, and intra‐abdominal pressure. Fluid overload was detected as an extracellular fluid expansion in relation to intracellular fluid depletion. Of note, all patients with ascites were in the highest RVSI tertile and exhibited a monophasic IRVF pattern except 1 patient who was diagnosed with hepatitis C–associated liver cirrhosis and porto‐PH who was within the first RVSI tertile.
Correlation analyses (Figure S2 and Table S5) showed relevant and statistically significant relationships between RVSI and cardiopulmonary hemodynamics, echocardiographic parameters, renal function, intra‐abdominal pressure, and neurohormonal and hydration status. As expected, tricuspid insufficiency had an impact on RVSI values (median RVSI: 0.00 [IQR: 0.15–0.36] in mild, 0.13 [IQR: 0.17–0.46] in moderate, and 0.33 [IQR: 0.26–0.72] in severe tricuspid insufficiency; P<0.0001). However, in multivariate Cox regression analysis, RVSI was superior to tricuspid insufficiency in predicting the composite end point and all individual components. Furthermore, RVSI values were significantly increased in patients with versus without atrial fibrillation (0.28 versus 0.09; P<0.0001); this large difference was not due to interobserver variability (intraclass correlation coefficient was >0.9 in both groups).
Of the echocardiographic and hemodynamic parameters assessed, right atrial area and RAP showed the strongest correlations with renal function (Table S6). Arterial blood gas measurements showed no correlation with renal function.
Analysis of PH subtypes
Baseline parameters significantly differed across the PH subtype groups, and confirmed the correctness of the classification of each group (Table S7). All 30 patients with PH due to left heart disease had HF with preserved ejection fraction (Table S2). Patients with PH due to left heart disease exhibited the highest RVSI values and were most likely to have a monophasic pattern; they also had the poorest right and left heart function, lowest renal function, and highest BNP levels and intra‐abdominal pressures.
Clinical outcomes
All 205 patients were included in the analysis of outcomes. During the observational period (12 months [range: 11–13 months]), the composite end point of PH‐related morbidity and death from any cause occurred in 91 of 205 patients (Table S8). We observed 64 (31.2%) unscheduled hospitalizations for fluid overload, 71 (34.6%) escalations of PH‐specific therapy, and 21 (10.2%) deaths. Five patients underwent pulmonary thromboendarterectomy, and 1 patient underwent lung transplantation.
Patients in higher RVSI tertiles had increased rates of the composite end point (Figure 6) and 2 of the individual components (Figure S3). Analysis of outcomes by IRVF patterns showed broadly similar trends but with some overlap between the groups with biphasic and monophasic IRVF patterns (Figure S4). In multiple Cox regression analysis, RVSI tertiles remained independent predictors of the composite end point and 2 of the individual components (Table 2; univariate analyses are provided in Tables S9–S12).
Figure 6.
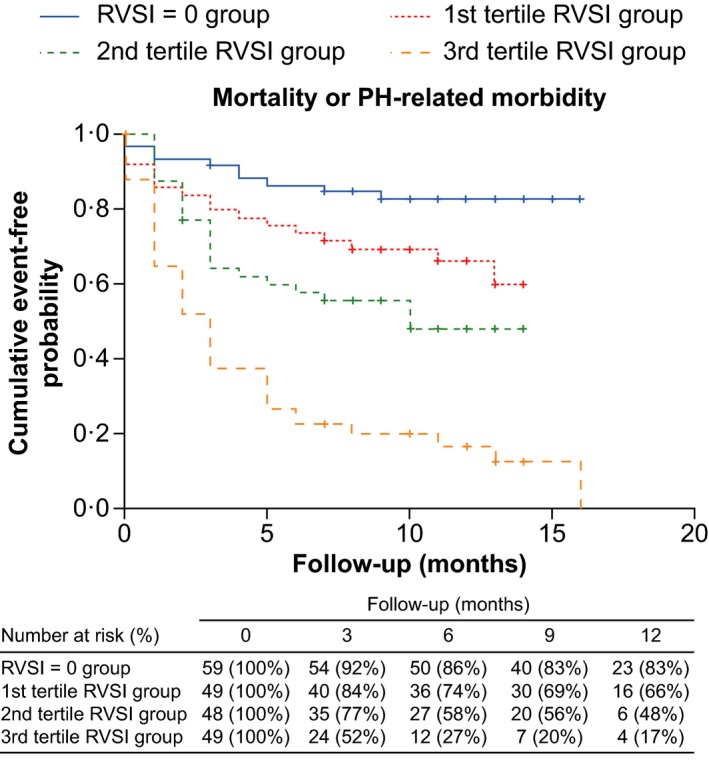
Kaplan‐Meier estimate curve according to RVSI tertiles. Patients in the third tertile group had a significantly higher probability than other patients of the composite end point of PH‐related morbidity or death from any cause (P<0.0001). PH indicates pulmonary hypertension; RVSI, renal venous stasis index.
Table 2.
Predictors of Clinical End Points Identified by the Cox Proportional Hazards Model
| Predictor | Univariate | Multiple | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| PH‐related morbidity and death from any cause | ||||
| RVSI tertiles | 20.57 (9.03–46.87) | <0.0001 | 0.0015 | |
| First tertile group vs RVSI 0 | 2.31 (1.06–5.05) | 0.0363 | 2.30 (0.95–5.53) | 0.064 |
| Second tertile group vs RVSI 0 | 3.63 (1.71–7.65) | 0.0007 | 3.41 (1.49–7.81) | 0.0037 |
| Third tertile group vs RVSI 0 | 8.70 (4.33–17.48) | <0.0001 | 4.72 (2.10–10.59) | <0.0001 |
| Congestion stages | 2.00 (1.63–2.44) | <0.0001 | 0.0012 | |
| Stage 1 vs stage 0 | 2.65 (1.29–5.44) | 0.0078 | 2.61 (1.18–5.80) | 0.0182 |
| Stage 2 vs stage 0 | 6.35 (3.08–13.09) | <0.0001 | 4.90 (2.15–11.18) | <0.0001 |
| Stage 3 vs stage 0 | 8.45 (3.98–17.96) | <0.0001 | 4.07 (1.68–9.85) | 0.0019 |
| Uric acid | 1.25 (1.16–1.34) | <0.0001 | 1.15 (1.05–1.26) | 0.0017 |
| Atrial fibrillation | 2.56 (1.68–3.88) | <0.0001 | 1.94 (1.05–3.56) | 0.0355 |
| 6MWD | 0.997 (0.996–0.999) | 0.0006 | 0.997 (0.995–0.999) | 0.0099 |
| LA diameter | 1.07 (1.04–1.10) | <0.0001 | 1.05 (1.01–1.10) | 0.0301 |
| Age | 1.02 (1.00–1.03) | 0.0439 | 0.98 (0.96–1.00) | 0.079 |
| Unscheduled hospitalization due to fluid overload | ||||
| RVSI tertiles | 1.71 (1.48–1.98) | <0.0001 | 0.0016 | |
| First tertile group vs RVSI 0 | 6.49 (1.42–29.64) | 0.0157 | 5.50 (1.09–27.85) | 0.0395 |
| Second tertile group vs RVSI 0 | 10.98 (2.52–47.76) | 0.0014 | 6.27 (1.36–28.96) | 0.0187 |
| Third tertile group vs RVSI 0 | 35.60 (8.54–148.38) | <0.0001 | 13.01 (2.95–57.34) | 0.0007 |
| Congestion stages | 2.49 (1.94–3.20) | <0.0001 | 0.0193 | |
| Stage 1 vs stage 0 | 7.36 (1.71–31.72) | 0.0074 | 5.01 (1.14–22.07) | 0.0334 |
| Stage 2 vs stage 0 | 25.51 (6.05–107.67) | <0.0001 | 8.84 (1.98–39.46) | 0.0043 |
| Stage 3 vs stage 0 | 32.17 (7.44–139.09) | <0.0001 | 5.06 (1.02–25.20) | 0.0478 |
| Uric acid | 1.29 (1.19–1.41) | <0.0001 | 1.27 (1.13–1.43) | <0.0001 |
| PCWP | 1.08 (1.05–1.11) | <0.0001 | 1.04 (1.01–1.08) | 0.0159 |
| Urine α1MCR | 1.01 (1.01–1.02) | <0.0001 | 1.01 (1.00–1.02) | 0.0262 |
| Atrial fibrillation | 4.05 (2.47–6.63) | <0.0001 | 1.88 (1.00–3.54) | 0.0510 |
| 6MWD | 0.996 (0.994–0.998) | <0.0001 | 0.99 (0.99–1.00) | 0.0006 |
| RA area | 1.11 (1.07–1.14) | <0.0001 | 1.05 (1.00–1.10) | 0.0477 |
| Urine feNa | 1.21 (1.07–1.36) | 0.0017 | 0.82 (0.67–1.00) | 0.0547 |
| NYHA classification | 1.81 (1.24–2.64) | 0.0022 | 0.60 (0.34–1.05) | 0.074 |
| Escalation of PH‐specific therapy | ||||
| Mixed venous oxygen saturation | 0.92 (0.90–0.95) | <0.0001 | 0.96 (0.93–1.00) | 0.0403 |
| Uric acid | 1.26 (1.16–1.36) | <0.0001 | 1.31 (1.02–1.26) | 0.0242 |
| RVSI tertiles | 1.43 (1.26–1.63) | <0.0001 | 0.0186 | |
| First tertile group vs RVSI 0 | 2.16 (0.89–5.24) | 0.087 | 1.89 (0.68–5.27) | 0.2241 |
| Second tertile group vs RVSI 0 | 3.52 (1.53–8.07) | 0.0030 | 2.91 (1.13–7.51) | 0.0271 |
| Third tertile group vs RVSI 0 | 7.03 (3.22–15.35) | <0.0001 | 4.29 (1.63–11.27) | 0.0031 |
| Congestion stages | 1.86 (1.49–2.33) | <0.0001 | 0.0106 | |
| Stage 1 vs stage 0 | 2.37 (1.05–5.35) | 0.0373 | 2.13 (0.84–5.37) | 0.110 |
| Stage 2 vs stage 0 | 6.22 (2.79–13.87) | <0.0001 | 4.44 (1.74–11.32) | 0.0018 |
| Stage 3 vs stage 0 | 6.39 (2.73–14.97) | <0.0001 | 3.31 (1.13–9.70) | 0.0293 |
| E/e’ ratio | 1.07 (1.03–1.12) | 0.0006 | 1.06 (1.01–1.10) | 0.0138 |
| 6MWD | 0.997 (0.995–0.999) | 0.0013 | 0.998 (0.996–1.00) | 0.073 |
| LVEDD | 0.95 (0.91–0.99) | 0.0221 | 0.94 (0.89–0.99) | 0.0179 |
| All‐cause mortality | ||||
| Mixed venous oxygen saturation | 0.92 (0.88–0.96) | <0.0001 | 0.94 (0.89–0.99) | 0.0120 |
| RA area | 1.10 (1.04–1.17) | 0.0018 | 1.07 (1.01–1.15) | 0.0341 |
| NYHA classification | 2.65 (1.30–5.41) | 0.0074 | 1.97 (0.99–3.90) | 0.0536 |
Table includes only variables that remained significant after Cox regression analysis. All available variables were included in the univariate analyses, and variables that were significant in the univariate analyses are provided in Tables S9–S12. α1MCR indicates α1‐microglobulin‐to‐creatinine ratio; E/e’ ratio, ratio of mitral inflow velocity to lateral annular relaxation velocity; feNa, fractional excretion of sodium; HR, hazard ratio; LA, left atrial; LVEDD, left ventricular end‐diastolic diameter; NYHA, New York Heart Association; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension; RA, right atrial; RVSI, renal venous stasis index; 6MWD indicates 6‐min walk distance.
During the observational period, 3 patients developed stage 3 acute kidney injury with diuretic‐resistant fluid overload and required RRT; all 3 exhibited a monophasic IRVF pattern with a median RVSI of 0.64 (IQR: 0.59–0.73) at baseline.
RVSI versus IRVF patterns for prediction of clinical outcomes
Receiver operating characteristic curves suggested that RVSI was a more sensitive and specific predictor of the composite end point than the individual IRVF patterns (areas under the curve: 0.789 and 0.761, respectively; P=0.038; Figure S5). The maximal Youden statistic was obtained for an RVSI cutoff of 0.14, yielding 77% specificity and 63% sensitivity. RVSI ≥0.14 was also an independent predictor of the composite end point when added in multiple Cox regression analysis instead of RVSI tertiles. A model including both RVSI and IRVF patterns as predictor variables indicated superiority of RVSI over IRVF patterns (Wald values: 2.817 and 2.059, respectively). Comparisons of RVSI and IRVF patterns for prediction of the individual component end points are shown in Figure S5 and Table S13.
Discussion
We developed a continuous index from Doppler‐derived IRVF patterns and propose the RVSI as a simple, noninvasive, and integrative Doppler measure of renal congestion. In patients undergoing RHC based on clinical grounds, the RVSI was correlated with invasive hemodynamics. Furthermore, our data suggest that the RVSI may be superior to individual IRVF patterns in predicting outcome.
Elevated RAP has been identified as a main driver of deteriorating renal function in acutely decompensated HF.3, 4, 5 Only 3 HF studies9, 10, 11 have previously investigated the association of Doppler‐derived IRVF patterns and venous impedance index with RAP and their utility in predicting diuretic response and adverse outcomes. Furthermore, we assessed the tricuspid annular plane systolic excursion/systolic PAP ratio as a parameter of right ventricle–pulmonary artery coupling, which was recently demonstrated to be associated with prognosis in patients with pulmonary arterial hypertension and HF with preserved ejection fraction.31, 32 The association of the tricuspid annular plane systolic excursion/systolic PAP ratio and RVSI further emphasizes the meaning of RVSI as a marker of renal congestion, as it mirrors not only RV failure but also right ventricle–pulmonary artery uncoupling when afterload exceeds contractility.
IRVF depends on extrinsic factors (interstitial pressure and intra‐abdominal pressure) and intravenous pressure, which is highly dependent on RAP.33, 34, 35 Under physiological conditions, intrarenal veins exhibit continuous flow independent of renal function,36, 37 with superimposed biphasic forward velocities that peak during systole (reflecting right atrial filling during RV ejection) and diastole (reflecting RAP release after the tricuspid valve opens and RV filling occurs). With increasing RAP, intrarenal veins become less compliant, dampening the continuous flow to a discontinuous (RVSI>0) flow and increasing prominence of the superimposed biphasic forward velocities. Further increases in RAP may ultimately lead to a diastolic‐only (monophasic) flow pattern, in which renal venous outflow may exclusively depend on RV filling. Of note, the increase in RAP during end‐diastole (corresponding to atrial contraction) can be transmitted to the renal veins, potentially causing a reversal of vein flow, as recently described38; this may have been masked by the arterial waveforms in our analysis of interlobar arteries and veins.
Deterioration of renal function in right HF appears to be mainly hemodynamic (congestive), independent of PH subtype, and associated with activation of the neurohormonal system and fluid overload. This type of congestive nephropathy can be described as a gradual decrease of renal function as RVSI worsens, with no proteinuria even at severe congestion. Interestingly, we see no significant changes in creatinine, eGFR, or cystatin C in patients with RVSI in the first tertile compared with normal RVSI (=0). This could be explained by renal lymphatic flow increasing dramatically with early congestion, consequently preventing an increase in renal interstitial pressure until full saturation,39, 40 and suggests that an increase of RVSI from 0 to the first tertile may be a more sensitive marker to identify patients at risk for subsequent renal function decline than established biomarkers such as creatinine and cystatin C. Transmission of venous congestion to the renal veins is thought to impair GFR by increasing pressure in the efferent end of the glomerular capillary, which reduces glomerular–capillary hydrostatic pressure8; this can be reversed by lowering renal venous pressure in experimental models.34 As an additional component, concomitant elevation of renal interstitial pressure is likely to reduce glomerular net ultrafiltration pressure by opposing glomerular–capillary hydrostatic pressure and to reduce renal blood flow, as shown by the significant increases in RRI in our population. Future studies need to determine whether therapies that reduce RAP will improve renal function and particularly which RAP range must be achieved to provide an acceptable balance of RV and renal function.
Our study has all the limitations of retrospective analyses of prospectively collected data. Limitations include its single‐center design, selection bias, and moderate sample size and duration of follow‐up. We assumed that the monophasic IRVF pattern reflects venous pressure release resulting from RV filling, but we did not determine end‐diastolic filling pressures or RV volumes. In addition, entering RVSI and congestion stages to 1 Cox regression model was done for comparison of their prognostic value but may lead to overfitting of the model. All echocardiography data were collected 1 day before RHC. Although PH‐specific therapy was not initiated or changed in the interval between these assessments, some patients received additional diuretics when fluid overload was present, which limits the interpretation of echocardiography relative to RHC data. Renal venous congestion does not necessarily indicate right HF because tricuspid insufficiency with intact RV function may also induce congestion, and discontinuous IRVF patterns have been described in obstructive nephropathy,41 where they are at least partly explained by increased renal interstitial pressure subsequent to ureter obstruction.
Our study confirmed the prognostic relevance of renal venous congestion, and the novel RVSI in particular showed promise as a simple, noninvasive, and objective Doppler measure. RVSI and IRVF patterns may be useful to identify patients who are likely to experience adverse outcomes. Longitudinal studies are needed to clarify their roles in the management of HF.
Conclusions
Our analyses of patients undergoing RHC present RVSI as a novel Doppler measurement of renal congestion that may be superior to IRVF patterns in predicting outcome in patients with PH. Further studies are needed to validate our findings and assess the utility of RVSI in PH management.
Sources of Funding
This study was funded by the German Research Foundation Collaborative Research Centers 1213, projects B08 and B07. The funder of the study had no role in the study design; the collection, analysis, and interpretation of data; writing of the report; and the decision to submit the article for publication.
Disclosures
Seeger disclosed personal fees for consulting from Bayer Pharma AG, from Liquidia Technologies, Inc, and from United Therapeutics Corporation outside the submitted work. Gall discloses personal fees and nonfinancial support from Actelion, AstraZeneca, Bayer, BMS, GlaxoSmithKline, Janssen Cilag, Lilly, MSD, Novartis, Pfizer, and United Therapeutics/OMT outside the submitted work. Ghofrani discloses grants from the German Research Foundation during the conduct of the study and personal fees from Actelion, Bayer, GSK, Novartis, Pfizer, Bellerophon Pulse Technologies, and MSD Merck Sharpe & Dohme outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Data S1. Supplemental Methods.
Table S1. Intraclass Correlation Coefficient for Renal Venous Stasis Index Measured by 2 Independent Nephrologists
Table S2. Classification of the Right Heart Catheterization Cohort According to Pulmonary Hypertension Subcategories
Table S3. Clinical Characteristics, Invasive Hemodynamics, Echocardiographic Data, Renal Function, and Neurohormonal and Hydration Status Stratified According to Congestion Stages as Determined by Intrarenal Venous Flow Patterns
Table S4. Additional Data on Clinical Characteristics, Invasive Hemodynamics, Echocardiographic Data, Renal Function, and Neurohormonal and Hydration Status According to Congestion Stages as Determined by Renal Venous Stasis Index
Table S5. Correlation of Renal Venous Stasis Index With Relevant Parameters*
Table S6. Correlation of Renal Function With Relevant Parameters*
Table S7. Clinical Characteristics, Invasive Hemodynamics, Echocardiographic Data, Renal Function, and Neurohormonal and Hydration Status According to Pulmonary Hypertension Groups
Table S8 . Outcomes in the Right Heart Catheterization Cohort
Table S9. Predictors of Morbidity and Mortality by the Univariate Cox Proportional Hazards Model
Table S10. Predictors of Unscheduled Hospitalization Because of Fluid Overload by the Univariate Cox Proportional Hazards Model
Table S11. Predictors of Escalation of Pulmonary Hypertension–Specific Therapy by the Univariate Cox Proportional Hazards Model
Table S12. Predictors of Death From Any Cause by the Univariate Cox Proportional Hazards Model
Table S13. Performance of Renal Venous Stasis Index Versus Intrarenal Venous Flow Patterns in Models Including Both Variables for Prediction of Secondary End Points
Figure S1. Intrarenal venous flow patterns and associated clinical parameters.
Figure S2. Correlation of renal venous stasis index with right atrial pressure (A), tricuspid annular plane systolic excursion (B), estimated glomerular filtration rate (C), and intra‐abdominal pressure (D).
Figure S3. Kaplan‐Meier estimate curves according to renal venous stasis index tertiles.
Figure S4. Kaplan‐Meier estimate curves according to intrarenal venous flow patterns.
Figure S5. Comparison of renal venous stasis index and intrarenal venous flow patterns as predictors of the primary and secondary clinical end points.
Acknowledgments
The authors thank the nursing staff of the pulmonology and nephrology departments for their hard work and commitment to patient well‐being. Without their support, this work would not have been possible. Special thanks are extended to Susanne Wissgott and Isabell Heineke for their efforts in patient recruitment. Editorial assistance was provided by Claire Mulligan, PhD (Beacon Medical Communications Ltd, Brighton, UK), funded by the University of Giessen. The authors shared study design, data collection, data analysis, and data interpretation, as well as preparation, review, and approval of the article. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for study concept and design: Husain‐Syed, Birk, Ronco, Schörmann, Gall, and Ghofrani. Gall and Ghofrani conceived the study concept and share the senior authorship of the article. Literature research and clinical advice: Birk, Ronco, Bauer, Walmrath, Seeger and McCullough. Acquisition, analysis, or interpretation of data: Husain‐Syed, Birk, Ronco, Schörmann, Tello, Richter, Wilhelm, Sommer, Steyerberg, Bauer, McCullough, Gall, Ghofrani. Drafting of the article: Husain‐Syed, Birk, Ronco, Wilhelm, Seeger, McCullough, Gall, and Ghofrani. Critical revision of the article for important intellectual content: Husain‐Syed, Birk, Ronco, Schörmann, Tello, Richter, Wilhelm, Sommer, Steyerberg, Bauer, Walmrath, Seeger, McCullough, Gall, and Ghofrani. Adjudication of renal function: Husain‐Syed, Birk, Ronco. Adjudication of IRVF patterns: Husain‐Syed, Bauer, Ghofrani. Adjudication of end point: Walmrath, Ronco, Seeger. Statistical analysis: Wilhelm, Steyerberg, Gall. Study supervision: Gall and Ghofrani.
(J Am Heart Assoc. 2019;8:e013584 DOI: 10.1161/JAHA.119.013584.)
References
- 1. Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1:4–25. [DOI] [PubMed] [Google Scholar]
- 2. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. [DOI] [PubMed] [Google Scholar]
- 3. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–8. [DOI] [PubMed] [Google Scholar]
- 5. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS; CHAMPION Trial Study Group . Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–66. [DOI] [PubMed] [Google Scholar]
- 6. Mullens W, Abrahams Z, Skouri HN, Francis GS, Taylor DO, Starling RC, Paganini E, Tang WH. Elevated intra‐abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol. 2008;51:300–6. [DOI] [PubMed] [Google Scholar]
- 7. Husain‐Syed F, McCullough PA, Birk HW, Renker M, Brocca A, Seeger W, Ronco C. Cardio‐Pulmonary‐Renal Interactions: a Multidisciplinary Approach. J Am Coll Cardiol. 2015;65:2433–48. [DOI] [PubMed] [Google Scholar]
- 8. Jessup M, Costanzo MR. The cardiorenal syndrome: do we need a change of strategy or a change of tactics? J Am Coll Cardiol. 2009;53:597–9. [DOI] [PubMed] [Google Scholar]
- 9. Iida N, Seo Y, Sai S, Machino‐Ohtsuka T, Yamamoto M, Ishizu T, Kawakami Y, Aonuma K. Clinical Implications of Intrarenal Hemodynamic Evaluation by Doppler Ultrasonography in Heart Failure. JACC Heart failure. 2016;4:674–82. [DOI] [PubMed] [Google Scholar]
- 10. Nijst P, Martens P, Dupont M, Tang WHW, Mullens W. Intrarenal Flow Alterations During Transition From Euvolemia to Intravascular Volume Expansion in Heart Failure Patients. JACC Heart failure. 2017;5:672–681. [DOI] [PubMed] [Google Scholar]
- 11. Puzzovivo A, Monitillo F, Guida P, Leone M, Rizzo C, Grande D, Ciccone MM, Iacoviello M. Renal venous pattern: a new parameter for predicting prognosis in heart failure outpatients. J Cardiovasc Dev Dis. 2018;5:E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beaubien‐Souligny W, Benkreira A, Robillard P, Bouabdallaoui N, Chasse M, Desjardins G, Lamarche Y, White M, Bouchard J, Denault A. Alterations in Portal Vein Flow and Intrarenal Venous Flow Are Associated With Acute Kidney Injury After Cardiac Surgery: A Prospective Observational Cohort Study. J Am Heart Assoc. 2018;7:e009961. doi: 10.1161/JAHA.118.009961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mehra MR, Park MH, Landzberg MJ, Lala A, Waxman AB; International Right Heart Failure Foundation Scientific Working Group . Right heart failure: toward a common language. J Heart Lung Transplant. 2014;33:123–6. [DOI] [PubMed] [Google Scholar]
- 14. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck‐Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H, Luis Zamorano J. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 15. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 16. Kidney Disease: Improving Global Outcomes (KDIGO) Chronic Kidney Disease Work Group. KDIGO . Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2012;2013:1–150. [Google Scholar]
- 17. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group . KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 18. Gall H, Felix JF, Schneck FK, Milger K, Sommer N, Voswinckel R, Franco OH, Hofman A, Schermuly RT, Weissmann N, Grimminger F, Seeger W, Ghofrani HA. The Giessen Pulmonary Hypertension Registry: survival in pulmonary hypertension subgroups. J Heart Lung Transplant. 2017;36:957–967. [DOI] [PubMed] [Google Scholar]
- 19. Li JC, Jiang YX, Zhang SY, Wang L, Ouyang YS, Qi ZH. Evaluation of renal artery stenosis with hemodynamic parameters of Doppler sonography. J Vasc Surg. 2008;48:323–8. [DOI] [PubMed] [Google Scholar]
- 20. Sugiura T, Wada A. Resistive index predicts renal prognosis in chronic kidney disease. Nephrol Dial Transplant. 2009;24:2780–5. [DOI] [PubMed] [Google Scholar]
- 21. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713;quiz 786–788. [DOI] [PubMed] [Google Scholar]
- 22. Mohmand H, Goldfarb S. Renal dysfunction associated with intra‐abdominal hypertension and the abdominal compartment syndrome. J Am Soc Nephrol. 2011;22:615–21. [DOI] [PubMed] [Google Scholar]
- 23. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 24. Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin Pharmacol Therap. 1995;57:601–9. [DOI] [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD‐EPI Investigators . Estimating glomerular filtration rate from serum creatinine and cystatin C. New Engl J Med. 2012;367:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; Version 3.5.1. 2018. Available at: https://www.R-project.org/. Accessed November 20, 2018. [Google Scholar]
- 28. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Muller M. pROC: an open‐source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qiu JC W, Lazarus R, Rosner B, Ma J. powerSurvEpi: power and sample size calculation for survival analysis of epidemiological studies. R package Version 0.1.0. 2018. Available at: https://CRAN.R-project.org/package=powerSurvEpi. Accessed November 20, 2018.
- 30. Freedman LS. Tables of the numbers of patients required in clinical trials using the log‐rank test. Stat Med. 1982;1:121–129. [DOI] [PubMed] [Google Scholar]
- 31. Tello K, Axmann J, Ghofrani HA, Naeije R, Narcin N, Rieth A, Seeger W, Gall H, Richter MJ. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int J Cardiol. 2018;266:229–235. [DOI] [PubMed] [Google Scholar]
- 32. Guazzi M, Dixon D, Labate V, Beussink‐Nelson L, Bandera F, Cuttica MJ, Shah SJ. RV Contractile Function and its Coupling to Pulmonary Circulation in Heart Failure With Preserved Ejection Fraction: stratification of Clinical Phenotypes and Outcomes. JACC Cardiovasc Imaging. 2017;10:1211–1221. [DOI] [PubMed] [Google Scholar]
- 33. Burnett JC Jr, Knox FG. Renal interstitial pressure and sodium excretion during renal vein constriction. Am J Physiol. 1980;238:F279–82. [DOI] [PubMed] [Google Scholar]
- 34. Winton FR. The influence of venous pressure on the isolated mammalian kidney. J Physiol. 1931;72:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Winton FR. Arterial, Venous, Intrarenal, and Extrarenal Pressure Effects on Renal Blood Flow. Circ Res. 1964;15(SUPPL):103–9. [PubMed] [Google Scholar]
- 36. Avasthi PS, Greene ER, Scholler C, Fowler CR. Noninvasive diagnosis of renal vein thrombosis by ultrasonic echo‐Doppler flowmetry. Kidney Int. 1983;23:882–7. [DOI] [PubMed] [Google Scholar]
- 37. Jeong SH, Jung DC, Kim SH, Kim SH. Renal venous doppler ultrasonography in normal subjects and patients with diabetic nephropathy: value of venous impedance index measurements. J Clin Ultrasound. 2011;39:512–8. [DOI] [PubMed] [Google Scholar]
- 38. Meier M, Johannes Jabs W, Guthmann M, Geppert G, Aydin A, Nitschke M. Sonographic Venous Velocity Index Identifies Patients with Chronic Kidney Disease and Severe Diastolic Dysfunction. Ultrasound Int Open. 2018;4:E142–E148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haddy FJ, Scott J, Fleishman M, Emanuel D. Effect of change in renal venous pressure upon renal vascular resistance, urine and lymph flow rates. Am J Physiol. 1958;195:97–110. [DOI] [PubMed] [Google Scholar]
- 40. Lebrie SJ, Mayerson HS. Influence of elevated venous pressure on flow and composition of renal lymph. Am J Physiol. 1960;198:1037–40. [DOI] [PubMed] [Google Scholar]
- 41. Bateman GA, Cuganesan R. Renal vein Doppler sonography of obstructive uropathy. AJR Am J Roentgenol. 2002;178:921–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods.
Table S1. Intraclass Correlation Coefficient for Renal Venous Stasis Index Measured by 2 Independent Nephrologists
Table S2. Classification of the Right Heart Catheterization Cohort According to Pulmonary Hypertension Subcategories
Table S3. Clinical Characteristics, Invasive Hemodynamics, Echocardiographic Data, Renal Function, and Neurohormonal and Hydration Status Stratified According to Congestion Stages as Determined by Intrarenal Venous Flow Patterns
Table S4. Additional Data on Clinical Characteristics, Invasive Hemodynamics, Echocardiographic Data, Renal Function, and Neurohormonal and Hydration Status According to Congestion Stages as Determined by Renal Venous Stasis Index
Table S5. Correlation of Renal Venous Stasis Index With Relevant Parameters*
Table S6. Correlation of Renal Function With Relevant Parameters*
Table S7. Clinical Characteristics, Invasive Hemodynamics, Echocardiographic Data, Renal Function, and Neurohormonal and Hydration Status According to Pulmonary Hypertension Groups
Table S8 . Outcomes in the Right Heart Catheterization Cohort
Table S9. Predictors of Morbidity and Mortality by the Univariate Cox Proportional Hazards Model
Table S10. Predictors of Unscheduled Hospitalization Because of Fluid Overload by the Univariate Cox Proportional Hazards Model
Table S11. Predictors of Escalation of Pulmonary Hypertension–Specific Therapy by the Univariate Cox Proportional Hazards Model
Table S12. Predictors of Death From Any Cause by the Univariate Cox Proportional Hazards Model
Table S13. Performance of Renal Venous Stasis Index Versus Intrarenal Venous Flow Patterns in Models Including Both Variables for Prediction of Secondary End Points
Figure S1. Intrarenal venous flow patterns and associated clinical parameters.
Figure S2. Correlation of renal venous stasis index with right atrial pressure (A), tricuspid annular plane systolic excursion (B), estimated glomerular filtration rate (C), and intra‐abdominal pressure (D).
Figure S3. Kaplan‐Meier estimate curves according to renal venous stasis index tertiles.
Figure S4. Kaplan‐Meier estimate curves according to intrarenal venous flow patterns.
Figure S5. Comparison of renal venous stasis index and intrarenal venous flow patterns as predictors of the primary and secondary clinical end points.


