Abstract
Background
Plaque erosion is responsible for 25% to 40% of patients with acute coronary syndromes (ACS). Recent studies suggest that anti‐thrombotic therapy without stenting may be an option for this subset of patients. Currently, however, an invasive procedure is required to make a diagnosis of plaque erosion. The aim of this study was to identify clinical or laboratory predictors of plaque erosion in patients with ACS to enable a diagnosis of erosion without additional invasive procedures.
Methods and Results
Patients with ACS who underwent optical coherence tomography imaging were selected from 11 institutions in 6 countries. The patients were classified into plaque rupture, plaque erosion, or calcified plaque, and predictors were identified using multivariable logistic modeling. Among 1241 patients with ACS, 477 (38.4%) patients were found to have plaque erosion. Plaque erosion was more frequent in non–ST‐segment elevation‐ACS than in ST‐segment–elevation myocardial infarction (47.9% versus 29.8%, P=0.0002). Multivariable logistic regression models showed 5 independent parameters associated with plaque erosion: age <68 years, anterior ischemia, no diabetes mellitus, hemoglobin >15.0 g/dL, and normal renal function. When all 5 parameters are present in a patient with non–ST‐segment elevation‐ACS, the probability of plaque erosion increased to 73.1%.
Conclusions
Clinical and laboratory parameters associated with plaque erosion are explored in this retrospective registry study. These parameters may be useful to identify the subset of ACS patients with plaque erosion and guide them to conservative management without invasive procedures. The results of this exploratory analysis need to be confirmed in large scale prospective clinical studies.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT03479723.
Keywords: acute coronary syndrome, optical coherence tomography, plaque erosion
Subject Categories: Acute Coronary Syndromes, Optical Coherence Tomography (OCT)
Clinical Perspective
What Is New?
Five parameters associated with plaque erosion have been identified: age <68 years, anterior ischemia, no diabetes mellitus, hemoglobin >15.0 g/dL, and normal renal function.
These parameters indicate that plaque erosion may have different pathophysiology: “non‐traditional” factors including abnormal local fluid dynamics, rather than the traditional vascular inflammation, may play an important role.
What Are the Clinical Implications?
If these parameters are proven to be predictive of erosion in prospective studies, a subset of acute coronary syndromes patients with high probability of plaque erosion may be easily identified.
This subset of patients may be managed conservatively with anti‐thrombotic therapy without invasive procedures.
Plaque erosion is reported to be responsible for about 25% to 40% of patients with acute coronary syndromes (ACS).1, 2, 3 Current guidelines recommend early invasive strategy for all ACS patients except for the low‐risk subgroups4, 5 and coronary stents are implanted in the majority of the cases. Recent studies reported that ACS patients with plaque erosion might be treated conservatively without stenting.6, 7, 8 In these studies, a diagnosis of plaque erosion was made by intracoronary optical coherence tomography (OCT). If the patients with plaque erosion are identified by demographic information or by simple laboratory tests, invasive procedures and procedure‐related complications may be avoided, and healthcare costs may be significantly reduced. However, demographic characteristics specific for the patients with plaque erosion are unknown. Although several groups published reports on plaque erosion,9, 10, 11 the study population in each report was too small or unbalanced to identify comprehensive characteristics associated with plaque erosion. The aim of the current study was to identify the predictors for plaque erosion in patients with ACS.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Participants
A new multi‐center longitudinal international registry was created for this study from 11 institutions in 6 countries (Table S1) (Identification of Predictors for Coronary Plaque Erosion in Patients with Acute Coronary Syndrome study, http://www.clinicaltrials.gov: NCT03479723). Consecutive patients with ACS who had OCT imaging of the culprit lesion were eligible for the study. We included only patients who had undergone an OCT procedure, therefore this study was not “all‐comer” registry. ACS includes ST‐segment–elevation myocardial infarction (STEMI) and non–ST‐segment–elevation ACS (NSTE‐ACS) which included non–ST‐segment–elevation myocardial infarction (NSTEMI) and unstable angina pectoris. The data set consists of previously published1, 2, 9, 10 (n=695) and unpublished (n=1004) patients. Among 1699 patients, 458 patients were excluded and 1241 patients were included in the final cross‐sectional analysis (Figure S1). The protocol was approved by the institutional review board at each site and written informed consent was obtained from all patients before enrollment. The definitions are described in Data S1. Demographic data, medical history, laboratory data at admission, percutaneous coronary intervention procedure data, post‐percutaneous coronary intervention biomarkers, and in‐hospital death were collected. OCT imaging and angiographic imaging at index procedure were also collected. All images were de‐identified, digitally stored, and sent to Massachusetts General Hospital (Boston, MA, USA), where analysis was performed.
OCT Image Acquisition
A frequency‐domain OCT system (C7/C8 ILUMIEN OCT Intravascular Imaging Systems, St. Jude Medical, St. Paul, Minnesota) or a time‐domain OCT system (M2/M3 Cardiology Imaging System, St. Jude Medical, Westford, Massachusetts) was used in this study. The detailed technique of intracoronary OCT imaging was described in the previous report.12
OCT Analysis
The methods of OCT analysis are summarized in Data S1. Underlying plaque type in the culprit lesion was categorized into 3 groups using the previously established OCT criteria; plaque rupture, plaque erosion, calcified plaque (Figure 1A through 1C). Plaque rupture was defined by the presence of fibrous cap discontinuity with a communication between the lumen and the inner core of plaque or with a cavity formation within the plaque (Figure 1A).13, 14 Plaque erosion was identified by the presence of the attached thrombus overlying an intact and visualized plaque, luminal surface irregularity at the culprit lesion in the absence of thrombus, or attenuation of the underlying plaque by thrombus without superficial lipid or calcification immediately proximal or distal to the site of thrombus (Figure 1B).1, 2, 11 Calcified plaque was defined by the presence of superficial substantive calcium at the culprit site without evidence of ruptured lipid plaque (Figure 1C).1, 2 Culprit lesions that did not satisfy these criteria were classified as others. Tissue characterization of underlying plaque was performed using the previously established criteria.15, 16 Plaques were classified into 2 categories: (1) Fibrous plaque (homogeneous and high‐backscattering region) or (2) Lipid plaque (low signal region with a diffuse border). Lipid‐rich plaque was defined as a plaque with lipid arc >90°. For each lipid plaque, the thinnest fibrous cap thickness and the maximal lipid arc were measured. A thin‐cap fibroatheroma was defined as plaque with a lipid arc >90° and the thinnest part of the fibrous cap <65 μm. Macrophage accumulations were defined as signal‐rich, distinct, or confluent punctuate regions with heterogeneous backward shadows. Calcification was recorded as well‐delineated, low backscattering heterogeneous regions. Minimal flow area was also measured for each lesion.
Figure 1.
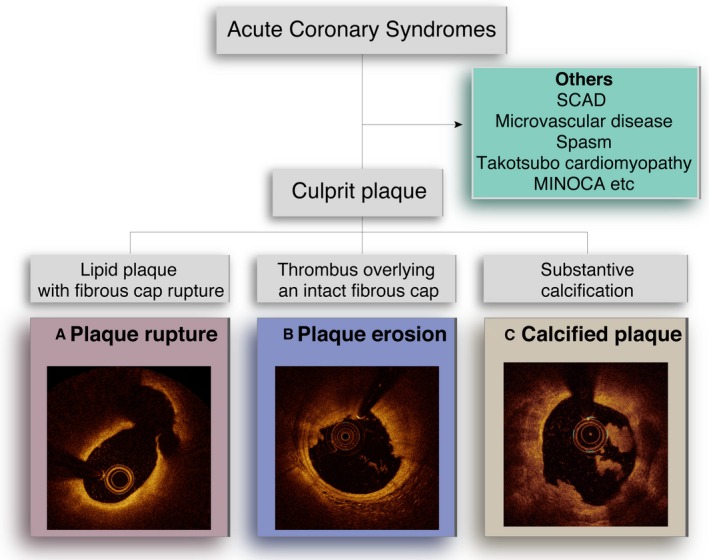
Optical coherence tomography images of 3 plaque pathologies. A, Plaque rupture was defined by the presence of fibrous cap discontinuity with a communication between the lumen and the inner core of a plaque or with a cavity formation within the plaque. B, Plaque erosion was defined as a culprit plaque with an intact fibrous cap with or without attached thrombus. C, Calcified plaque was defined by the presence of superficial substantive calcium at the culprit site without evidence of ruptured lipid plaque. Others include spontaneous coronary artery dissection, microvascular disease, spasm, Takotsubo cardiomyopathy, myocardial infarction with non‐obstructive coronary arteries, etc. MINOCA indicates myocardial infarction with non‐obstructive coronary arteries; SCAD, spontaneous coronary artery dissection.
Angiographic Analysis
Coronary angiograms were analyzed with the Cardiovascular Angiography Analysis System (Pie Medical Imaging B.V., Maastricht, The Netherlands). The reference diameter, minimum lumen diameter, diameter stenosis, area stenosis, and lesion length were measured. Thrombolysis in Myocardial Infarction flow grade was also evaluated for the culprit vessel.
Statistical Analysis
Categorical outcomes were presented as counts and proportions (%). Continuous outcomes were expressed as mean±SD. For overall between‐group comparisons, 1‐way analysis of variance or Kruskal–Wallis test was applied for continuous outcomes and Chi‐square or Fisher exact test for categorical outcomes was applied. Then post‐hoc comparisons with controlling type‐1 error by using Bonferroni correction were performed if the overall test was significant with P<0.05. To identify the parameters associated with plaque erosion, univariable and multivariable logistic regression models were applied using variables that can be readily obtained in the emergency department (age, sex, medical history, medication at admission, location of ischemia, simple laboratory test (white blood cell count, hemoglobin, creatine phosphokinase) and renal function (normal renal function was defined as no history of chronic kidney disease and estimated glomerular filtration rate (eGFR) >60 mL/min at admission). Age and hemoglobin cut‐off were determined using Youden index. Variables with a P<0.10 in the univariate test were entered into the multivariable modeling. All statistical analyses were performed using JMP PRO 13.0 (SAS Institute Inc, Cary, NC).
Results
Among 1241 patients, 607 patients (48.9%) were classified as plaque rupture, 477 patients (38.4%) as plaque erosion, and 157 patients (12.7%) as calcified plaque. In 648 STEMI patients, 385 (59.4%) patients had plaque rupture and 193 (29.8%) patients had plaque erosion. In 593 NSTE‐ACS patients, 222 (37.4%) patients had plaque rupture and 284 (47.9%) patients had plaque erosion. Inter‐observer and intra‐observer variability were assessed by the evaluation of all images by 2 independent observers and by the same observer at 2 separate time points, respectively. The intra‐observer Kappa coefficients for plaque rupture, plaque erosion, and calcified plaque were 0.902, 0.922, 0.934, respectively. The inter‐observer Kappa coefficients for plaque rupture, plaque erosion, and calcified plaque were 0.878, 0.895, 0.935, respectively. Plaque erosion was significantly more frequent in NSTE‐ACS than in STEMI (47.9% versus 29.8%, P<0.0002) (Figure 2).
Figure 2.
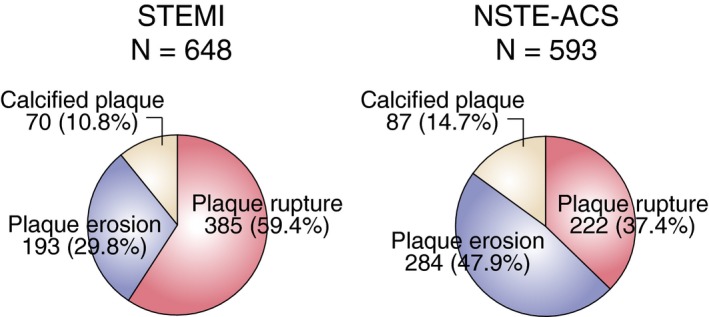
Prevalence of plaque rupture, erosion, and calcified plaque in ST‐segment–elevation myocardial infarction and non–ST‐segment–elevation acute coronary syndrome. Among 1241 patients, 648 presented with ST‐segment–elevation myocardial infarction and 593 with non–ST‐segment–elevation acute coronary syndrome. The prevalence of plaque rupture, plaque erosion, and calcified plaque was 59.4%, 29.8%, and 10.8% in ST‐segment–elevation myocardial infarction; 37.4%, 47.9%, and 14.7% in non–ST‐segment–elevation acute coronary syndrome. The prevalence of plaque erosion was significantly higher in non–ST‐segment–elevation acute coronary syndrome than in ST‐segment–elevation myocardial infarction patients (47.9% vs 29.8%, P=0.0002). NSTE‐ACS indicates non–ST‐segment–elevation acute coronary syndrome; STEMI, ST‐segment–elevation myocardial infarction.
Patients Characteristics
Baseline characteristics of each group are summarized in Table 1 and Table S2. Compared with the non‐erosion group, the plaque erosion group was younger and more frequently presented with NSTE‐ACS. Hypertension, diabetes mellitus, and chronic kidney disease were significantly less frequent in the plaque erosion group. The plaque erosion group also had higher levels of hemoglobin, lower levels of inflammatory markers (high‐sensitivity C‐reactive protein and white blood cell count), and a better lipid profile. The calcified plaque group was older and had a higher prevalence of hypertension, diabetes mellitus, and chronic kidney disease, compared with the other groups. The patients in the calcified plaque group were on more medications on admission. Plaque erosion was located more frequently in the left anterior descending artery than in the right coronary artery (56.6% versus 28.5%), whereas plaque rupture was located equally in the left anterior descending and the right coronary artery (45.8% versus 40.5%). Angiographic and OCT analysis consistently showed plaque erosion patients had less complex and less vulnerable lesions, compared with patients with non‐erosion.
Table 1.
Patient Characteristics
| Plaque Rupture (n=607) | Plaque Erosion (n=477) | Calcified Plaque (n=157) | P Value | P Valuea | |||
|---|---|---|---|---|---|---|---|
| PR vs PE | PE vs CP | PR vs CP | |||||
| Age, y | 65.3±11.9 | 62.8±12.3 | 69.9±9.2 | <0.0001 | 0.0009 | <0.001 | <0.0001 |
| Men | 478 (78.8) | 380 (79.7) | 121 (77.1) | 0.78 | |||
| Presentation | <0.0001 | <0.0001 | 0.36 | <0.0001 | |||
| STEMI | 385 (63.4) | 193 (40.5) | 70 (44.6) | ||||
| NSTE‐ACS | 222 (36.6) | 284 (59.5) | 87 (55.4) | ||||
| Hypertension | 401 (66.1) | 283 (59.3) | 123 (78.3) | <0.0001 | 0.03 | <0.0001 | 0.003 |
| Dyslipidemia | 442 (72.8) | 329 (69.0) | 114 (72.6) | 0.35 | |||
| Diabetes mellitus | 201 (33.1) | 130 (27.3) | 64 (40.8) | 0.004 | 0.04 | 0.001 | 0.07 |
| Current smoker | 254 (42.0) | 196 (41.4) | 39 (25.0) | 0.0003 | 0.86 | 0.0002 | 0.0001 |
| Past smoker | 106 (17.5) | 106 (22.4) | 52 (33.3) | <0.0001 | 0.05 | 0.006 | <0.0001 |
| Current+past smoker | 360 (59.5) | 302 (63.9) | 91 (58.3) | 0.27 | ··· | ··· | ··· |
| Previous MI | 45 (7.4) | 30 (6.3) | 19 (12.1) | 0.06 | |||
| Previous PCI | 56 (9.2) | 40 (8.4) | 23 (14.7) | 0.06 | |||
| Family history | 88 (14.5) | 85 (17.8) | 32 (20.4) | 0.13 | |||
| CKD | 113 (18.6) | 52 (10.9) | 51 (32.5) | <0.0001 | 0.0004 | <0.0001 | 0.0002 |
| Laboratory data | |||||||
| WBC, /μL | 9675±3386 | 9076±3270 | 8717±3500 | 0.001 | 0.004 | 0.26 | 0.001 |
| Hemoglobin, g/dL | 14.0±1.8 | 14.2±1.7 | 13.3±2.2 | <0.0001 | 0.03 | <0.0001 | 0.003 |
| LDL‐C, mg/dL | 128±42 | 121±41 | 108±41 | <0.0001 | 0.005 | 0.001 | <0.0001 |
| Creatinine, mg/dL | 0.97±0.79 | 0.90±0.72 | 1.65±2.41 | <0.0001 | 0.16 | 0.0003 | 0.0008 |
| hs‐CRP, mg/dL | 0.84±2.25 | 0.45±0.95 | 0.92±1.95 | 0.003 | 0.0004 | 0.003 | 0.05 |
| CPK, IU/L | 518±928 | 371±652 | 438±935 | 0.03 | 0.004 | 0.47 | 0.13 |
| Medication at admission | |||||||
| Aspirin | 92 (19.7) | 75 (20.3) | 54 (37.2) | <0.0001 | 0.84 | <0.0001 | <0.0001 |
| P2Y12 inhibitor | 38 (8.1) | 41 (11.1) | 27 (18.5) | 0.002 | 0.14 | 0.03 | 0.0003 |
| Statin | 115 (24.6) | 82 (22.2) | 59 (40.7) | <0.0001 | 0.41 | <0.0001 | 0.0002 |
| ACE‐I/ARB | 134 (28.6) | 105 (28.3) | 70 (48.0) | <0.0001 | 0.92 | <0.0001 | <0.0001 |
| β‐blocker | 63 (13.5) | 60 (16.2) | 39 (26.7) | 0.0008 | 0.27 | 0.006 | 0.0002 |
| Angiographic data | |||||||
| Lesion location | 0.0002 | 0.0002 | 0.77 | 0.007 | |||
| LAD | 278 (45.8) | 270 (56.6) | 93 (59.2) | ||||
| LCX | 83 (13.7) | 71 (14.9) | 20 (12.7) | ||||
| RCA | 246 (40.5) | 136 (28.5) | 44 (28.0) | ||||
| QCA | |||||||
| RVD, mm | 2.97±0.70 | 2.86±0.70 | 2.87±0.74 | 0.04 | 0.02 | 0.70 | 0.02 |
| MLD, mm | 0.51±0.59 | 0.68±0.66 | 0.73±0.68 | <0.0001 | <0.0001 | 0.48 | 0.0005 |
| Diameter stenosis (%) | 83.2±18.4 | 77.0±20.2 | 75.4±20.8 | <0.0001 | <0.0001 | 0.43 | <0.0001 |
| Lesion length, mm | 16.1±7.8 | 15.1±6.5 | 17.8±8.5 | 0.0004 | 0.02 | 0.0005 | 0.02 |
| TIMI flow grade 0 to 1 | 242 (39.9) | 119 (25.0) | 37 (23.6) | <0.0001 | <0.0001 | 0.73 | 0.0002 |
| Multivessel disease | 232 (39.1) | 149 (32.5) | 78 (52.0) | <0.0001 | 0.03 | <0.0001 | 0.004 |
| Type B2/C lesion | 483 (79.6) | 286 (60.0) | 122 (77.7) | <0.0001 | <0.0001 | <0.0001 | 0.61 |
| OCT findings | |||||||
| Lipid‐rich plaque | 554 (91.3) | 178 (37.3) | 24 (15.3) | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| TCFA | 373 (61.5) | 33 (6.9) | 5 (3.2) | <0.0001 | <0.0001 | 0.09 | <0.0001 |
| Macrophage | 486 (80.1) | 263 (55.1) | 43 (27.4) | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Calcification | 231 (38.1) | 160 (33.5) | 158 (100) | <0.0001 | 0.12 | <0.0001 | <0.0001 |
| Minimum flow area, mm2 | 1.38±1.01 | 1.43±1.34 | 1.78±1.43 | <0.0001 | 0.51 | <0.0001 | <0.0001 |
| Minimum FCT, μm | 69±33 | 121±65 | 115±81 | <0.0001 | <0.0001 | 0.18 | 0.0007 |
| Max lipid arc, degree | 308±64 | 276±80 | 273±78 | <0.0001 | <0.0001 | 0.84 | 0.03 |
ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CAD, coronary artery disease; CKD, chronic kidney disease; CP, calcified plaque; CPK, creatine phosphokinase; FCT, fibrous cap thickness; hs‐CRP, high‐sensitivity C‐reactive protein; LAD, left anterior descending artery; LCX, left circumflex artery; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction; MLD, minimum lumen diameter; NSTE‐ACS, non–ST‐segment–elevation acute coronary syndrome; PCI, percutaneous coronary intervention; PE, plaque erosion; QCA, quantitative coronary angiography; PR, plaque rupture; RCA, right coronary artery; RVD, reference vessel diameter; STEMI, ST‐segment–elevation myocardial infarction; TCFA, thin‐cap fibroatheroma; TIMI, Thrombolysis in Myocardial Infarction; WBC, white blood cell count.
P<0.017 was considered significant.
Procedural detail, clinical course, and in‐hospital outcome are shown in Table S3. Thrombectomy and stenting were more frequently performed in the plaque rupture group than in the plaque erosion group. There was no significant difference in in‐hospital outcome between the plaque erosion and the non‐erosion group except lower level of post‐procedure peak creatine phosphokinase in the erosion group (1264±1901 versus 1973±2332, P<0.001).
STEMI and NSTE‐ACS in Plaque Erosion
When the erosion patients were divided based on clinical presentation, those with STEMI were more frequently smokers, had higher levels of serum low‐density lipoproteins, and were on fewer medications (aspirin, P2Y12 inhibitors, statin, angiotensin‐converting enzyme inhibitor/ARB, β‐blocker) on admission than those with NSTE‐ACS (Table S4).
Predictors of Plaque Erosion
Age cut‐off <68 years, hemoglobin cut‐off <15.0 g/dL were determined to maximize the area under the receiver operating characteristic (ROC) curve. As shown in Table 2, age <68 years (odds ratio [OR]: 1.56, 95% CI; 1.16–2.09, P=0.003), anterior ischemia (OR: 1.41, 95% CI: 1.06–1.86, P=0.02), no diabetes mellitus (OR: 1.47, 95% CI: 1.08–2.01, P=0.01), hemoglobin >15.0 g/dL (OR: 1.48, 95% CI: 1.09–2.01, P=0.01), and normal renal function (OR: 1.97, 95% CI: 1.32–2.95, P=0.0009) were found to be the independent parameters associated with plaque erosion. When all 5 parameters are present in a patient with NSTE‐ACS, the probability of plaque erosion increased to 73.1% (Figure 3). When all 5 conditions were present in NSTE‐ACS patients, the odds ratio of plaque erosion was 3.40 (95% CI: 1.39–8.29, P=0.007). A 1000 bootstrap‐samples based estimation for the probability of plaque erosion was conducted. The estimated probabilities are within the initial estimates (Table S5).
Table 2.
Clinical and Laboratory Predictors of Plaque Erosion
| Variables | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age <68 y | 1.65 | 1.31 to 2.08 | <0.0001 | 1.56 | 1.16 to 2.09 | 0.003 |
| Anterior ischemia | 1.38 | 1.10 to 1.74 | 0.006 | 1.41 | 1.06 to 1.86 | 0.02 |
| No DM | 1.42 | 1.10 to 1.82 | 0.006 | 1.47 | 1.08 to 2.01 | 0.01 |
| Hemoglobin >15.0 g/dL | 1.67 | 1.25 to 2.34 | 0.0006 | 1.48 | 1.09 to 2.01 | 0.01 |
| Normal renal function | 2.23 | 1.60 to 3.13 | <0.0001 | 1.97 | 1.32 to 2.95 | 0.0009 |
| No hypertension | 1.50 | 1.18 to 1.90 | 0.0009 | 1.26 | 0.94 to 1.68 | 0.13 |
| Statin | 0.72 | 0.53 to 0.97 | 0.03 | 1.21 | 0.88 to 1.69 | 0.24 |
DM indicates diabetes mellitus; OR, odds ratio.
Figure 3.
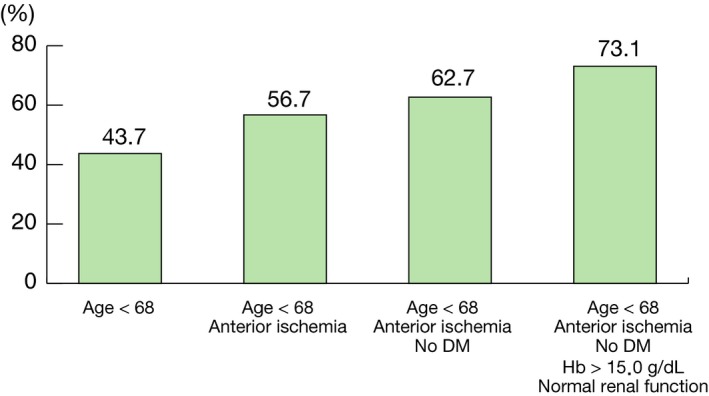
Probability of plaque erosion. When all 5 parameters are present in a patient with non–ST‐segment–elevation acute coronary syndrome, the probability of plaque erosion increased to 73.1%. When a patient with non–ST‐segment–elevation acute coronary syndrome has all 5 parameters, the odd ratio increases to 3.40. DM indicates diabetes mellitus.
Discussion
This is an international collaborative work that includes >1200 patients with both STEMI and NSTE‐ACS from 11 institutions in 6 countries to identify parameters associated with plaque erosion. Plaque erosion was more frequent in NSTE‐ACS than in STEMI. Five parameters associated with plaque erosion are identified: (1) Age <68 years, (2) anterior ischemia, (3) no diabetes mellitus, (4) hemoglobin >15.0 g/dL, and (5) normal renal function. When all these 5 conditions are present in a patient with NSTE‐ACS, the probability of plaque erosion increases up to 73%.
Traditional Coronary Risk Factors in Plaque Erosion
Some of the conventional risk factors for coronary artery disease (older age, diabetes mellitus, dyslipidemia, chronic kidney disease, hypertension) were less frequent in plaque erosion, while younger age, no diabetes mellitus, and normal renal function were associated with plaque erosion in the current study. In addition, high‐sensitivity C‐reactive protein and white blood cell count were also significantly lower in the erosion patients. OCT analysis also demonstrated significantly lower levels of plaque vulnerability (lipid‐rich plaque, thin‐cap fibroatheroma, and macrophage). Taken together, all these data suggest that plaque erosion has a distinctly different pathobiology compared with plaque rupture. Previous pathology studies reported that the characteristics of plaque erosion, which include thrombus over less atherosclerotic and less vulnerable lesions without disruption of plaque structure and absence of endothelial cell layer.3, 17, 18 However, the underlying mechanism of local thrombosis remains unclear. A “2‐hit scheme” was proposed for the pathogenesis of plaque erosion.19, 20 The “first‐hit” is chronic endothelial activation, propensity to slough, and impaired ability to repair the damaged endothelium. Disturbed flow was suspected to be the etiology, leading to activation of endothelial Toll‐like receptor 2. The “second‐hit” is recruitment of neutrophils via Toll‐like receptor 2 and subsequent formation of neutrophil extracellular traps, as well as local production of chemo‐attractants, thrombin, and fibrin, which further entrap platelets and lead to thrombus formation. This process is independent from pan‐vascular chronic inflammation that lead to atherosclerosis, therefore plaque erosion can occur in younger patients with less atherosclerosis.
Local Fluid Dynamics in Plaque Erosion
Interestingly, high hemoglobin level was found to be a strong factor associated with plaque erosion. The changes of hemoglobin level over time were not collected. Therefore, a causal relationship cannot be established. However, several studies reported hemoconcentration was the risk factor for myocardial infarction.21 Hemoconcentration can increase blood viscosity, resulting in elevation of local endothelial shear stress. High shear condition activates both platelets and coagulation factors.22, 23 It was recently reported that high endothelial shear stress was associated with the thrombus in plaque erosion patients.24 Therefore, high hemoglobin levels attributable to several systemic condition may contribute to formation of occlusive thrombus in plaque erosion. In addition, plaque erosion was more frequently observed in the left anterior descending artery with anterior ischemia than in the right coronary artery or circumflex. The left anterior descending artery has more side branches than other vessels. The presence of side branches affects the conditions of local flow dynamics and distribution of endothelial shear stress,25 which may play a key role in pathogenesis of erosion.
STEMI and NSTE‐ACS in Plaque Erosion
In the current study, 41% of plaque erosion patients presented with STEMI and the remaining 59% of patients presented with NSTE‐ACS. Because of relatively preserved vascular structure, plaque erosion may be prone to develop non‐occlusive thrombus or occlusive thrombus that may be easily embolized distally. Several differences were noted between these 2 groups: the erosion patients with STEMI were more frequently smokers, had higher levels of serum low‐density lipoproteins, and took fewer medications on admission than those with NSTE‐ACS (Table S4). The medications might have protected these patients from persistent occlusive thrombus formation. In addition, the smoking rate was significantly higher in plaque erosion patients with STEMI. Several previous studies reported that plaque erosion is associated with smoking.11, 17, 18 Smoking promotes activation of both platelets and clotting factors.26, 27 In addition, smoking causes endothelial damage28 as well as activation of Rho‐kinase,29 which leads to vascular hyperconstriction or vasospasm.
Clinical Significance of Predictors of Plaque Erosion
This study included only patients who had OCT imaging, therefore the result might not represent a general ACS population. Taken together, however, the current study suggests that, contrary to atherosclerotic lesions with underlying chronic inflammatory processes, plaque erosion might be the result of a combination of several “non‐traditional” factors including endothelial, vasomotion, fluid dynamic, and systemic effects (Figure 4). Thus, the optimal management for plaque erosion might be different from the traditional treatment of plaque rupture. In the EROSION (Effective Anti‐Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography‐Based Management in Plaque Erosion) study, we demonstrated the feasibility and safety of anti‐thrombotic therapy without stenting in patients with ACS caused by plaque erosion.6 We also reported a further decrease in thrombus volume between 1 month and 1 year, and a majority of patients with plaque erosion who were managed with aspirin and ticagrelor without stenting remained free of major adverse cardiac events up to 1 year.30 These results suggest that a tailored approach based on the underlying pathobiology of ACS may be warranted. Ultimately, whereas emergency invasive procedure with primary percutaneous coronary intervention is the standard of care for the patients with STEMI, those with NSTE‐ACS can often be stabilized with anti‐thrombotic therapy. Identification of a subset of NSTE‐ACS patients with high probability of plaque erosion may facilitate an early triage of the patients and provide an opportunity for tailored therapy.
Figure 4.
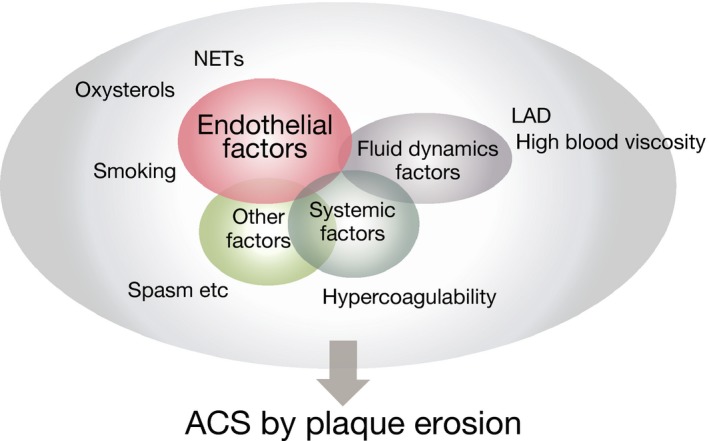
Pathogenesis of plaque erosion. Plaque erosion might be the result of a combination of several “non‐traditional” factors; endothelial factors, vasomotion factors, fluid dynamics factors, and systemic factors. ACS indicates acute coronary syndromes; LAD, left anterior descending artery; NETs, neutrophil extracellular traps.
A different approach will be needed not only for the management after ACS, but also for prevention. In the EROSION study, all patients were treated with dual anti‐platelet therapy for 1 year.30, 31 Anti‐thrombotic therapy should be the mainstay for plaque erosion patients without stenting. However, optimal duration/choice of medications remains unknown. The restoration of endothelial function should also be considered. Cessation of smoking could be particularly important.
Study Limitations
This study has several limitations. First, only patients who had undergone an OCT procedure were included, and the decision to perform OCT was left at the discretion of each operator. Therefore, true denominator is unknown and the inherent selection bias cannot be excluded. However, investigators in participating institutions have extensive experience with OCT and OCT is routinely being performed in almost all comers. Second, since this was not a prospective study, there is the possibility of unmeasured confounders. Third, although data were collected from 11 institutions including 3 in Europe and 1 in the United States, the majority of patients were Asians. Fourth, no biomarkers were measured. If biomarkers specific for plaque erosion are discovered, it may significantly increase the accuracy of non‐invasive diagnosis of plaque erosion. Fifth, it can indeed be difficult to make a diagnosis of plaque erosion in the presence of large thrombus burden. Aspiration thrombectomy was allowed, but the cases with balloon angioplasty were excluded to avoid iatrogenic damage to the vessel wall including plaque rupture. The majority of plaque ruptures are associated with superficial lipid and an emptied cavity. Those cases with large residual thrombus after thrombectomy or the cases with unclear diagnosis were excluded.
Conclusions
Plaque erosion was more frequent in NSTE‐ACS than in STEMI. Five parameters associated with plaque erosion have been identified; younger age, anterior ischemia, no diabetes mellitus, normal renal function, and higher hemoglobin levels. These parameters might be useful for identification of patients with plaque erosion for possible tailored therapy based on underlying pathobiology of ACS. The results of this exploratory analysis need to be confirmed in large scale prospective clinical studies.
Disclosures
Dr. Jang has received an educational grant from Abbott Vascular and Medicure. Dr. Adriaenssens has received grants and consulting fees from Abbott Vascular. The remaining authors have no disclosures to report.
Supporting information
Data S1. Supplemental methods.
Table S1. Participating Sites
Table S2. Patient Characteristics (Erosion vs Non‐Erosion)
Table S3. Procedure Detail, Post Procedure Biomarker, In‐Hospital Outcome
Table S4. Patient Characteristics (STEMI vs NSTE‐ACS in Erosion Patients)
Table S5. Estimation for the Probability of Plaque Erosion
Figure S1. Study flowchart.
Acknowledgments
We are grateful to Greg Gheewalla (Massachusetts General Hospital), Lally Lay Yee Chan, PhD (The Chinese University of Hong Kong), Sarah Reniers (University Hospitals Leuven), Masahiro Imura (Nippon Medical School Chiba Hokusoh Hospital) for data collection and management work at each site. Dr. Jang's research was supported by Mr. Michael and Mrs. Kathryn Park and by Mrs. Gill and Mr. Allan Gray.
(J Am Heart Assoc. 2019;8:e012322 DOI: 10.1161/JAHA.119.012322.)
Contributor Information
Taishi Yonetsu, Email: t-yonetsu.cvm@tmd.ac.jp.
Ik‐Kyung Jang, Email: ijang@mgh.harvard.edu.
References
- 1. Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, Kato K, Yonetsu T, Vergallo R, Hu S, Tian J, Lee H, Park S‐J, Jang YS, Raffel OC, Mizuno K, Uemura S, Itoh T, Kakuta T, Choi S‐Y, Dauerman HL, Prasad A, Toma C, McNulty I, Zhang S, Yu B, Fuster V, Narula J, Virmani R, Jang I‐K. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62:1748–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Higuma T, Soeda T, Abe N, Yamada M, Yokoyama H, Shibutani S, Vergallo R, Minami Y, Ong DS, Lee H, Okumura K, Jang IK. A combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patients with ST‐segment elevation myocardial infarction. JACC Cardiovasc Interv. 2015;8:1166–1176. [DOI] [PubMed] [Google Scholar]
- 3. Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J. 2013;34:719–728. [DOI] [PubMed] [Google Scholar]
- 4. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S; ESC Scientific Document Group . 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 5. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ; ACC/AHA Task Force Members . 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e344–e426. [DOI] [PubMed] [Google Scholar]
- 6. Jia H, Dai J, Hou J, Xing L, Ma L, Liu H, Xu M, Yao Y, Hu S, Yamamoto E, Lee H, Zhang S, Yu B, Jang I‐K. Effective anti‐thrombotic therapy without stenting: intravascular optical coherence tomography‐based management in plaque erosion (the EROSION study). Eur Heart J. 2017;38:792–800. [DOI] [PubMed] [Google Scholar]
- 7. Prati F, Uemura S, Souteyrand G, Virmani R, Motreff P, Di Vito L, Biondi‐Zoccai G, Halperin J, Fuster V, Ozaki Y, Narula J. OCT‐based diagnosis and management of STEMI associated with intact fibrous cap. JACC Cardiovasc Imaging. 2013;6:283–287. [DOI] [PubMed] [Google Scholar]
- 8. Souteyrand G, Viallard L, Combaret N, Pereira B, Clerfond G, Malcles G, Barber‐Chamoux N, Prati F, Motreff P. Innovative invasive management without stent implantation guided by optical coherence tomography in acute coronary syndrome. Arch Cardiovasc Dis. 2018;111:666–677. [DOI] [PubMed] [Google Scholar]
- 9. Niccoli G, Montone RA, Di Vito L, Gramegna M, Refaat H, Scalone G, Leone AM, Trani C, Burzotta F, Porto I, Aurigemma C, Prati F, Crea F. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur Heart J. 2015;36:1377–1384. [DOI] [PubMed] [Google Scholar]
- 10. Yonetsu T, Lee T, Murai T, Suzuki M, Matsumura A, Hashimoto Y, Kakuta T. Plaque morphologies and the clinical prognosis of acute coronary syndrome caused by lesions with intact fibrous cap diagnosed by optical coherence tomography. Int J Cardiol. 2016;203:766–774. [DOI] [PubMed] [Google Scholar]
- 11. Dai J, Xing L, Jia H, Zhu Y, Zhang S, Hu S, Lin L, Ma L, Liu H, Xu M, Ren X, Yu H, Li L, Zou Y, Zhang S, Mintz GS, Hou J, Yu B. In vivo predictors of plaque erosion in patients with ST‐segment elevation myocardial infarction: a clinical, angiographical, and intravascular optical coherence tomography study. Eur Heart J. 2018;39:2077–2085. [DOI] [PubMed] [Google Scholar]
- 12. Kato K, Yonetsu T, Kim SJ, Xing L, Lee H, McNulty I, Yeh RW, Sakhuja R, Zhang S, Uemura S, Yu B, Mizuno K, Jang IK. Nonculprit plaques in patients with acute coronary syndromes have more vulnerable features compared with those with non‐acute coronary syndromes a 3‐vessel optical coherence tomography study. Circ Cardiovasc Imaging. 2012;5:433–440. [DOI] [PubMed] [Google Scholar]
- 13. Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, Tanimoto T, Matsuo Y, Masho T, Kitabata H, Tsuda K, Tomobuchi Y, Akasaka T. Assessment of culprit lesion morphology in acute myocardial infarction. ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50:933–939. [DOI] [PubMed] [Google Scholar]
- 14. Jang IK, Tearney GJ, MacNeill B, Takano M, Moselewski F, Iftima N, Shishkov M, Houser S, Aretz HT, Halpern EF, Bouma BE. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111:1551–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Kang D‐H, Halpern EF, Tearney GJ. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106:1640–1645. [DOI] [PubMed] [Google Scholar]
- 16. Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho J, Chowdhary S, Costa MA, de Silva R, Dijkstra J, Di Mario C, Dudeck D, Falk E, Feldman MD, Fitzgerald P, Garcia H, Gonzalo N, Granada JF, Guagliumi G, Holm NR, Honda Y, Ikeno F, Kawasaki M, Kochman J, Koltowski L, Kubo T, Kume T, Kyono H, Lam CCS, Lamouche G, Lee DP, Leon MB, Maehara A, Manfrini O, Mintz GS, Mizuno K, Morel M, Nadkarni S, Okura H, Otake H, Pietrasik A, Prati F, Räber L, Radu MD, Rieber J, Riga M, Rollins A, Rosenberg M, Sirbu V, Serruys PWJC, Shimada K, Shinke T, Shite J, Siegel E, Sonada S, Suter M, Takarada S, Tanaka A, Terashima M, Troels T, Uemura S, Ughi GJ, van Beusekom HMM, van der Steen AFW, van Es G‐A, van Soest G, Virmani R, Waxman S, Weissman NJ, Weisz G. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies. J Am Coll Cardiol. 2012;59:1058–1072. [DOI] [PubMed] [Google Scholar]
- 17. White SJ, Newby AC, Johnson TW. Endothelial erosion of plaques as a substrate for coronary thrombosis. Thromb Haemost. 2016;115:509–519. [DOI] [PubMed] [Google Scholar]
- 18. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. [DOI] [PubMed] [Google Scholar]
- 19. Quillard T, Araújo HA, Franck G, Shvartz E, Sukhova G, Libby P. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur Heart J. 2015;36:1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Franck G, Mawson T, Sausen G, Salinas M, Masson GS, Cole A, Beltrami‐Moreira M, Chatzizisis Y, Quillard T, Tesmenitsky Y, Shvartz E, Sukhova GK, Swirski FK, Nahrendorf M, Aikawa E, Croce KJ, Libby P. Flow perturbation mediates neutrophil recruitment and potentiates endothelial injury via TLR2 in mice: implications for superficial erosion. Circ Res. 2017;121:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cecchi E, Liotta AA, Gori AM, Valente S, Giglioli C, Lazzeri C, Sofi F, Gensini GF, Abbate R, Mannini L. Comparison of hemorheological variables in ST‐elevation myocardial infarction versus those in non‐ST‐elevation myocardial infarction or unstable angina pectoris. Am J Cardiol. 2008;102:125–128. [DOI] [PubMed] [Google Scholar]
- 22. Bark DL, Ku DN. Platelet transport rates and binding kinetics at high shear over a thrombus. Biophys J. 2013;105:502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siedlecki CA, Lestini BJ, Kottke‐Marchant KK, Eppell SJ, Wilson DL, Marchant RE. Shear‐dependent changes in the three‐dimensional structure of human von Willebrand factor. Blood. 1996;88:2939–2950. [PubMed] [Google Scholar]
- 24. Yamamoto E, Thondapu V, Poon E, Sugiyama T, Fracassi F, Dijkstra J, Lee H, Ooi A, Barlis P, Jang IK. Endothelial shear stress and plaque erosion: a computational fluid dynamics and optical coherence tomography study. JACC Cardiovasc Imaging. 2019;12:374–375. DOI: 10.1016/j.jcmg.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 25. Barlis P, Poon EKW, Thondapu V, Grundeken MJ, Tu S, Hayat U, Ooi A, Moore S, Tenekecioglu E, Wykrzykowska JJ, Serruys PW. Reversal of flow between serial bifurcation lesions: insights from computational fluid dynamic analysis in a population‐based phantom model. EuroIntervention. 2015;11:e1–e3. [DOI] [PubMed] [Google Scholar]
- 26. Caponnetto P, Russo C, Di Maria A, Morjaria JB, Barton S, Guarino F, Basile E, Proiti M, Bertino G, Cacciola RR, Polosa R. Circulating endothelial‐coagulative activation markers after smoking cessation: a 12‐month observational study. Eur J Clin Invest. 2011;41:616–626. [DOI] [PubMed] [Google Scholar]
- 27. Nowak J, Murray JJ, Oates JA, FitzGerald GA. Biochemical evidence of a chronic abnormality in platelet and vascular function in healthy individuals who smoke cigarettes. Circulation. 1987;76:6–14. [DOI] [PubMed] [Google Scholar]
- 28. Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–1203. [DOI] [PubMed] [Google Scholar]
- 29. Hidaka T, Hata T, Soga J, Fujii Y, Idei N, Fujimura N, Kihara Y, Noma K, Liao JK, Higashi Y. Increased leukocyte rho kinase (ROCK) activity and endothelial dysfunction in cigarette smokers. Hypertens Res. 2010;33:354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xing L, Yamamoto E, Sugiyama T, Jia H, Ma L, Hu S, Wang C, Zhu Y, Li L, Xu M, Liu H, Bryniarski K, Hou J, Zhang S, Lee H, Yu B, Jang I‐K. EROSION Study (Effective Anti‐Thrombotic Therapy Without Stenting: intravascular Optical Coherence Tomography‐Based Management in Plaque Erosion): a 1‐year follow‐up report. Circ Cardiovasc Interv. 2017;10:e005860. [DOI] [PubMed] [Google Scholar]
- 31. Sugiyama T, Xing L, Yamamoto E, Fracassi F, Lee H, Yu B, Jang IK. Thrombus resolution with tirofiban in the conservative management of patients presenting with plaque erosion. Coron Artery Dis. 2018;29:301–308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.
Table S1. Participating Sites
Table S2. Patient Characteristics (Erosion vs Non‐Erosion)
Table S3. Procedure Detail, Post Procedure Biomarker, In‐Hospital Outcome
Table S4. Patient Characteristics (STEMI vs NSTE‐ACS in Erosion Patients)
Table S5. Estimation for the Probability of Plaque Erosion
Figure S1. Study flowchart.


