Abstract
Background
Stroke and thromboembolic events may still occur in “clinically low‐risk” atrial fibrillation (AF) patients as categorized by CHA 2 DS 2‐VASc score. Our aim was to assess the proportion of “clinically low‐risk” patients using a nongender CHA 2 DS 2‐VASc (ie, CHA 2 DS 2‐VA) score of 0 to 1 among patients who experienced AF‐associated stroke and to identify markers associated with stroke in “clinically low‐risk” patients.
Methods and Results
We retrospectively recruited nonvalvular AF patients who experienced embolic stroke between 2013 and 2016 from 9 institutes in Korea. AF patients with CHA 2 DS 2‐VA score of 0 to 1 at the time of stroke were analyzed and compared with “clinically low‐risk” AF patients without stroke. A total of 3033 subjects with AF‐associated stroke were recruited. Of these, 583 patients (19.2%) had CHA 2 DS 2‐VA score of 0 to 1. On multivariate analysis, age (≥60 years), N‐terminal pro B‐type natriuretic peptide (≥300 pg/mL), creatinine clearance (<50 mL/min), and left atrial dimension (≥45 mm) were independently associated with stroke. With the combined application of these 4 factors (collectively, ABCD score) to the “clinically low‐risk” patients, the c‐index was 0.858 (95% CI 0.838–0.877; P<0.001).
Conclusions
The present study suggests a new insight into how additional use of markers can further refine stroke risk differentiation among AF patients initially classified as “clinically low‐risk.”
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT03147911.
Keywords: ABCD score; atrial fibrillation; risk score; risk stratification; stroke, ischemic
Subject Categories: Atrial Fibrillation, Ischemic Stroke
Clinical Perspective
What Is New?
Among patients who had been classified as low risk by the CHA2DS2‐VASc scheme, the characteristics of patients who experienced ischemic stroke were investigated.
What Are the Clinical Implications?
Considering the improved risk–benefit ratio of non‐vitamin K oral anticoagulants, an updated or adjuvant scheme should be required in order to identify patients who are at a truly low risk for stroke.
Although the ABCD (age [≥60 years], NT‐proBNP (N‐terminal pro B‐type natriuretic peptide) [≥300 pg/mL], creatinine clearance [<50 mL/min], and left atrial dimension [≥45 mm]) scheme was derived from the retrospective, cross‐sectional assessment, it is worth examining in a prospective way.
Introduction
Patients with atrial fibrillation (AF) have a 5‐fold increased risk of stroke compared with matched individuals without AF, and strokes associated with AF are more likely to be fatal and disabling.1 Effective stroke prevention requires oral anticoagulation (OAC),2 but this should be counterbalanced by the potential risk of OAC‐related bleeding events.1 As a consequence, decision‐making on whether OAC therapy should be prescribed requires careful risk stratification.3
The CHA2DS2‐VASc score is now used in many guidelines for risk stratification and has a good performance in identifying nonvalvular AF patients at low stroke risk.4 Nevertheless, stroke and thromboembolic events still occur in such “low‐risk” AF patients as categorized by the CHA2DS2‐VASc score.3, 5 Recently, the introduction of the non‐vitamin K oral anticoagulants (NOACs) has changed the landscape for stroke prevention in AF, although regional differences are apparent.6 The availability of NOAC has shifted the stroke treatment threshold down to ≈0.9 event per 100‐person years; indeed, NOACs can be considered even for AF patients with a nongender CHA2DS2‐VASc score of 1.7, 8
The incidence of stroke among AF patients, especially in a CHA2DS2‐VASc score of 0 or 1, varies among different cohort populations.5 Previous studies have suggested that the application of a modified CHA2DS2‐VASc score might be more appropriate for stroke prevention in Asian populations.9, 10 In addition, the influence of female sex (the Sc criterion in CHA2DS2‐VASc) on stroke risk in “low‐risk” AF patients is debatable because female sex may be a risk modifier rather than a risk factor.11, 12 In such low‐risk patients, use of the CHA2DS2‐VA score (ie, female sex is excluded, or nongender CHA2DS2‐VASc score) would suffice for risk stratification of such AF patients.12
Given that stroke events still occur in nonvalvular AF patients who are considered clinically “low risk” with a CHA2DS2‐VA score of 0 or 1, further refinement of stroke risk stratification would be helpful in identifying those AF patients who may or may not get benefit from OAC therapy. Biomarkers have been proposed to be useful for this purpose.13
Our aim was to assess the proportion of embolic stroke in “low‐risk” AF patients using a nongender CHA2DS2‐VASc score (ie, CHA2DS2‐VA) of 0 to 1 and to identify markers associated with stroke. Second, we used these data to derive a novel risk stratification schema that includes biomarkers, for refining stroke risk stratification among this “low‐risk” cohort.
Methods
Study Population and Design
This study is composed of 2 separate cohorts. First, we retrospectively reviewed data of nonvalvular AF patients with cardioembolic stroke between January 2013 and December 2016 from the nationwide stroke registry including 9 institutes in Korea. Of these, consecutive patients with a CHA2DS2‐VA score of 0 or 1 at the time of stroke event were enrolled. Second, we defined a control group as subjects who were matched by the nearest‐neighbor method with type of AF and CHA2DS2‐VASc score among nonvalvular AF patients without stroke from a consortium AF registry from 3 Korea University Hospitals from 2015 to 2016.14
The CHA2DS2‐VA score was calculated by adding 2 points for age ≥75 years old and prior stroke or transient ischemic attack, and 1 point for congestive heart failure (or left ventricular ejection fraction ≤40%), hypertension, age 65 to 74 years, diabetes mellitus, and vascular disease (prior myocardial infarction, peripheral artery disease or aortic plaque) with a maximum 8 points.12, 15
Medical records of all subjects were comprehensively reviewed for demographic data, cardiovascular risk factors, parameters of transthoracic echocardiography, and laboratory data (complete blood counts, red blood cell distribution width, NT‐proBNP [N‐terminal pro B‐type natriuretic peptide], blood urea nitrogen, creatinine, uric acid, and low‐density cholesterol). Left ventricular ejection fraction was calculated using Simpson's biplane method and dimension of the left atrium (LA) was measured using M‐mode of transthoracic echocardiography.
This study was conducted with approval of the Institutional Review Board at each institution. The authors declare that all supporting data are available within the article. The board waived the need for patient consent because of the retrospective, cross‐sectional analysis design of the present study. All procedures performed in the present study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee. This study was registered with ClinicalTrials.gov, unique identifier NCT03147911.
AF Diagnosis and Cardioembolic Stroke
Diagnosis of AF was made on ECG showing the typical pattern of AF with >30 s AF episode duration. AF was defined as paroxysmal AF if its duration was <7 days and persistent AF if its duration was ≥7 days. Nonvalvular AF was defined as the absence of mitral stenosis (>mild) and prosthetic mechanical mitral valve. Diagnosis of cardioembolic stroke was confirmed by the neurologists of each institute according to TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria.16
Statistical Analysis
Normally distributed continuous variables were expressed as mean and SD, and categorical data were expressed as numbers and percentages. Nonparametrically distributed data were reported as median values with interquartile ranges. For comparison across groups, continuous variables were compared using the Student t test or analysis of variance, as appropriate, and categorical variables were analyzed using the χ2 test or Fisher exact test, as appropriate. Univariate logistic regression analysis was performed to identify the variables that were significantly related to stroke event. A multivariate logistic regression model was used to investigate the independent risk factors of stroke events. Variables that showed P value of <0.1 from the univariate logistic regression model were entered into the multivariate regression models. To assess the performance of the proposed risk differentiation scheme, receiver operating characteristic analysis was performed and the result of the receiver operating characteristic analysis was internally validated and calibrated using the bootstrapping technique to correct bias of the model. To compare the performance of different models, 2 receiver operating characteristic curves were compared according to the method as described by DeLong et al.17 In order to quantitatively compare the ability to differentiate patients at risk according to each scheme, the integrated discrimination improvement and the net reclassification index (NRI) with a category‐free option were calculated.18 A P value of <0.05 was considered statistically significant. All statistical analyses were performed by R version 3.2.1 (Foundation for Statistical Computing, Vienna, Austria) and STATA version 13.0 (Stata Corp, College Station, TX).
Results
A total of 3033 nonvalvular AF subjects with cardioembolic stroke were recruited. In Table 1, 583 patients (19.2%) had a CHA2DS2‐VA score of 0 or 1 (198 patients [6.5%] in CHA2DS2‐VA score of 0, 385 patients [12.7%] in CHA2DS2‐VA score of 1). As a control group, 598 subjects were extracted from the nonvalvular AF registry consortium by nearest‐neighbor propensity matching. Stroke patients were significantly older, with a female predominance, and had significantly lower estimated creatinine clearance rate (CCr), larger LA dimension, and higher uric acid and NT‐proBNP levels, as compared with controls. When compared with the stroke group, control subjects had a significantly higher prevalence of hypertension and diabetes mellitus. There was no significant difference in mean CHA2DS2‐VA score between the 2 groups.
Table 1.
Baseline Characteristics of Subjects
| Stroke (N=583) | Control (N=598) | P Value | |
|---|---|---|---|
| Age, y | 60.9±8.4 | 52.3±9.5 | <0.001 |
| Age ≥65 y | 176 (30.2%) | 41 (6.9%) | <0.001 |
| Female sex | 183 (31.4%) | 146 (24.4%) | 0.009 |
| Persistent AF | 318 (55.1%) | 306 (51.2%) | 0.195 |
| CHF | 27 (4.6%) | 41 (6.9%) | 0.129 |
| HTN | 158 (27.1%) | 228 (38.1%) | <0.001 |
| DM | 20 (3.4%) | 41 (6.9%) | 0.011 |
| Vascular disease | 4 (0.7%) | 12 (2.0%) | 0.087 |
| CHA2DS2‐VA score | 0.065 | ||
| 0 | 198 (34.0%) | 235 (39.3%) | |
| 1 | 385 (66.0%) | 363 (60.7%) | |
| Sex with CHA2DS2‐VA score | |||
| Male with 0 | 136 (23.3%) | 179 (29.9%) | 0.012 |
| Male with 1 | 264 (45.3%) | 273 (45.7%) | 0.945 |
| Female with 0 | 62 (10.6%) | 56 (9.4%) | 0.528 |
| Female with 1 | 121 (20.8%) | 90 (15.1%) | 0.013 |
| Male with 0 or 1 and female with 0 | 462 (79.2%) | 508 (84.9%) | 0.013 |
| LVEF, % | 60.0 (10.2) | 62.5 (5.0) | 0.144 |
| LA dimension, mL | 43.1 (9.0) | 39.5 (7.5) | <0.001 |
| Hb, g/dL | 14.2 (2.2) | 14.1 (2.6) | 0.703 |
| RDW, % | 13.2 (1.2) | 13.1 (0.9) | 0.142 |
| LDL, mg/dL | 101.7±33.4 | 104.6±28.7 | 0.149 |
| CCr, mL/min | 78.2 (31.6) | 98.9 (32.6) | <0.001 |
| Uric acid, mg/mL | 5.3 (2.2) | 4.8 (2.2) | <0.001 |
| NT‐proBNP pg/mL | 524.5 (608.5) | 80.3 (172.9) | <0.001 |
Data are presented as mean±SD or median (interquartile range). AF indicates atrial fibrillation; CCr, creatinine clearance rate; CHA2DS2‐VA, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, age 65 to 74 years; CHF, congestive heart failure; DM, diabetes mellitus; vascular disease, peripheral artery disease, myocardial infarction or aortic plaque; Hb, hemoglobin; HTN, hypertension; LA, left atrium; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; RDW, red blood cell distribution width.
Based on the CHA2DS2‐VASc score, 462 patients with stroke (15.2%) had a CHA2DS2‐VASc score of 0 or 1 (136 patients [4.5%] in CHA2DS2‐VASc score of 0, 326 patients [10.7%] in a CHA2DS2‐VASc score of 1). Baseline characteristics of subjects with CHA2DS2‐VASc score of 0 or 1 are described in Table 2.
Table 2.
Baseline Characteristics of Subjects With CHA2DS2‐VASc Score of 0 or 1
| Stroke (N=462) | Control (N=508) | P Value | |
|---|---|---|---|
| Age, y | 59.8±8.3 | 51.3±9.6 | <0.001 |
| Age ≥65 y | 114 (24.7%) | 28 (5.5%) | <0.001 |
| Female sex | 62 (13.4%) | 56 (11.0%) | 0.297 |
| Persistent AF | 246 (53.7%) | 264 (52.0%) | 0.633 |
| CHF | 18 (3.9%) | 30 (5.9%) | 0.196 |
| HTN | 112 (24.2%) | 173 (34.1%) | 0.001 |
| DM | 17 (3.7%) | 30 (5.9%) | 0.144 |
| Vascular disease | 3 (0.6%) | 12 (2.4%) | 0.058 |
| CHA2DS2‐VASc score | 0.317 | ||
| 0 | 136 (29.4%) | 179 (35.2%) | |
| 1 | 326 (70.6%) | 329 (64.8%) | |
| Sex with CHA2DS2‐VASc score | |||
| Male with 0 | 136 (29.4%) | 179 (35.2%) | 0.063 |
| Male with 1 | 264 (57.1%) | 273 (53.7%) | 0.317 |
| Female with 0 | 62 (13.4%) | 56 (11.0%) | 0.297 |
| LVEF, % | 59.0 (11.0) | 62.5 (7.5) | 0.123 |
| LA dimension, mL | 43.1 (9.0) | 39.5 (7.5) | <0.001 |
| Hb, g/dL | 14.4 (2.2) | 14.4 (2.6) | 0.864 |
| RDW, % | 13.2 (1.2) | 13.1 (0.9) | 0.096 |
| LDL, mg/dL | 102.0 (44.5) | 100.0 (39.0) | 0.239 |
| CCr, mL/min | 80.8 (31.9) | 100.0 (32.3) | <0.001 |
| Uric acid, mg/mL | 5.6 (2.1) | 5.1 (2.2) | <0.001 |
| NT‐proBNP pg/mL | 480.0 (574.0) | 76.8 (171.6) | <0.001 |
Data are presented as mean±SD or median (interquartile range). AF indicates atrial fibrillation; CCr, creatinine clearance rate; CHA2DS2‐VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, age 65 to 74 years; CHF, congestive heart failure; DM, diabetes mellitus; vascular disease, peripheral artery disease, myocardial infarction or aortic plaque; Hb, hemoglobin; HTN, hypertension; LA, left atrium; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; RDW, red blood cell distribution width.
Biomarkers Associated With Stroke
On univariate analysis, older age (≥60 years), being female, hypertension, diabetes mellitus, LA dimension (≥45 mm), red blood cell distribution width, CCr (<50 mL/min), uric acid (>7 mg/dL), and NT‐proBNP (≥300 pg/mL) were significantly associated with stroke events (Table 3). Type of AF and left ventricular ejection fraction were not significantly related with stroke events. Eleven variables (older age, being female, hypertension, diabetes mellitus, vascular disease, CHA2DS2‐VA score, LA dimension [≥45 mm], red blood cell distribution width, CCr [<50 mL/min], uric acid [>7 mg/dL], and NT‐proBNP [≥300 pg/mL]) were included in the multivariate logistic regression analysis.
Table 3.
Univariate and Multivariate Logistic Regression Analysis for Factors Associated With Stroke in Patients With AF
| CHA2DS2‐VA Score of 0 or 1 | |||||||
|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Pt. | |||||
| ß | OR (95% CI) | P Value | ß | OR (95% CI) | P Value | ||
| Age ≥60 y | 1.75 | 5.78 (4.5–7.45) | <0.001 | 1.623 | 5.06 (3.38–7.56) | <0.001 | 1 |
| Female sex | 0.348 | 1.42 (1.10–1.83) | 0.008 | 0.047 | 1.05 (0.69–1.59) | 0.823 | |
| Persist AF | 0.158 | 1.17 (0.93–1.47) | 0.176 | ||||
| CHF | −0.416 | 0.66 (0.40–1.09) | 0.103 | ||||
| HTN | −0.505 | 0.60 (0.47–0.77) | <0.001 | −0.197 | 0.82 (0.49–1.37) | 0.448 | |
| DM | −0.729 | 0.48 (0.28–0.83) | 0.009 | −0.360 | 0.70 (0.28–1.75) | 0.442 | |
| Vascular disease | −1.087 | 0.34 (0.11–1.05) | 0.061 | −0.564 | 0.57 (0.11–2.89) | 0.496 | |
| CHA2DS2‐VA score | 0.230 | 1.26 (0.98–1.60) | 0.057 | −0.375 | 0.69 (0.41–1.16) | 0.163 | |
| LVEF, % | −0.010 | 0.99 (0.98–1.0) | 0.138 | ||||
| LA diameter ≥45 mm | 1.312 | 3.71 (2.83–4.87) | <0.001 | 1.39 | 4.01 (2.70–5.97) | <0.001 | 1 |
| RDW | 0.172 | 1.19 (1.07–1.32) | 0.001 | −0.035 | 0.97 (0.86–1.09) | 0.561 | |
| LDL, mg/dL | −0.003 | 1.00 (0.99–1.00) | 0.143 | ||||
| CCr (<50 mL/min) | 2.483 | 11.98 (5.13–27.97) | <0.001 | 1.933 | 6.91 (2.06–23.22) | 0.002 | 1 |
| Uric acid (>7 mg/dL) | 0.658 | 1.93 (1.32–2.83) | 0.008 | 0.186 | 1.20 (0.68–2.14) | 0.527 | |
| NT‐proBNP (≥300 pg/mL) | 2.759 | 15.78 (11.81–21.08) | <0.001 | 2.005 | 7.42 (5.15–10.70) | <0.001 | 1 |
AF indicates atrial fibrillation; CCr, creatinine clearance rate; CHA2DS2‐VA, congestive heart failure, hypertension, age ≥75, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, age 65 to 74; CHF, congestive heart failure; DM, diabetes mellitus; vascular disease, peripheral artery disease, myocardial infarction or aortic plaque; HTN, hypertension; LA, left atrium; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; OR, odds ratio; Pt, point; RDW, red blood cell distribution width.
On the multivariate regression model, age (≥60), NT‐proBNP (≥300 pg/mL), CCr (<50 mL/min), and LA dimension (≥45 mm) were independently associated with stroke events. These were used to derive a simple score, ABCD (Age, NT‐proBNP, CCr, and Dimension of the LA) score, based on the independent associated factors for stroke events. The β coefficient of each independent risk factor was assigned to a score number and each selected factor was assigned 1 point.
Performance of ABCD Score in Differentiating in “Clinically Low‐Risk” Patients
The distribution of the CHA2DS2‐VA score of 0 to 1 patients by the ABCD score is shown in Figure 1. There was a significant increase in stroke events according to increase in the ABCD score points (P<0.001) in patients with a CHA2DS2‐VA score of 0 to 1 and those with a CHA2DS2‐VASc score of 0 to 1.
Figure 1.
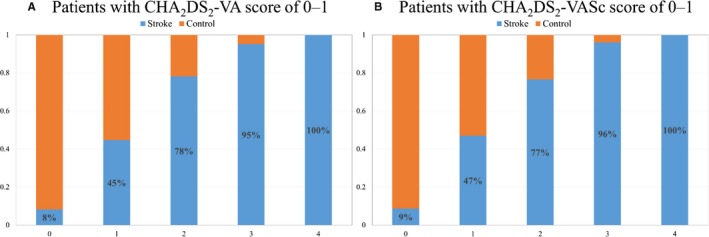
Distribution of AF patients with stroke event according to the ABCD score. A, Distribution of ABCD score in patients with CHA 2 DS 2‐VA score 0 or 1 is shown. B, Distribution of ABCD score in patients with CHA 2 DS 2‐VASc score 0 or 1 is shown. ABCD indicates age, NT‐proBNP, CCr, and dimension of the LA; AF, atrial fibrillation; CHA 2 DS 2‐VA, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, and age 65 to 74; and CHA 2 DS 2‐VASc, congestive heart failure, hypertension, age ≥75, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, age 65 to 74 years, and sex category; CCr, creatinine clearance rate; LA, left atrium; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide.
Figure 2 shows the area under the resulting c‐indexes (based on receiver operating characteristic curves) of the ABCD score. The c‐index of the ABCD score was 0.858 (95% CI 0.838–0.877; P<0.001) in patients with a CHA2DS2‐VA score of 0 to 1, and 0.850 (95% CI 0.827–0.873; P<0.001) in those with a CHA2DS2‐VASc score of 0 to 1. The internally validated c‐index of ABCD score was 0.780 (95% CI 0.752–0.808) using a bootstrapping procedure. With Hosmer–Lemeshow test, model calibration of ABCD score showed good agreements between observed stroke event and expected stroke event (P=0.077, Figure 3).
Figure 2.
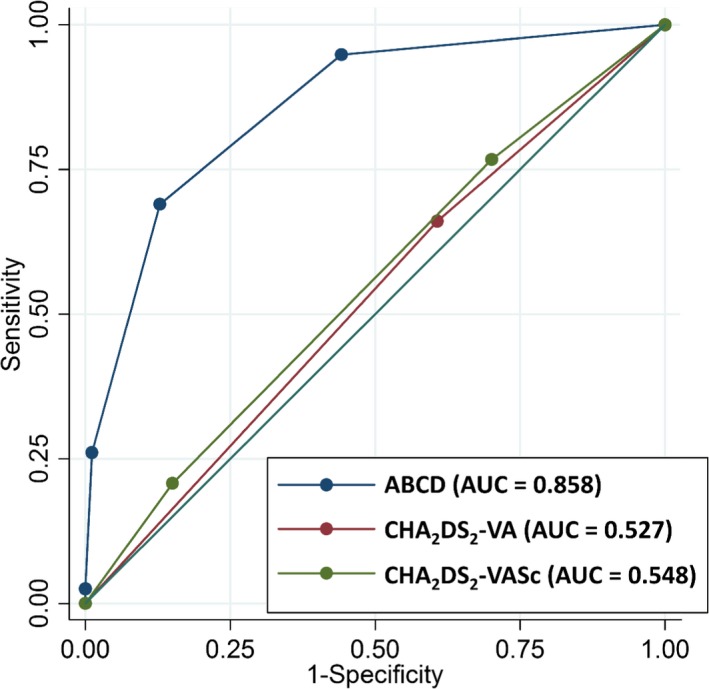
Receiver operating characteristic curve of the ABCD score for identifying a population with a truly low risk of stroke in AF patients. C‐index of ABCD score was 0.858 (95% CI 0.838–0.877) and its risk stratification in low‐risk group was superior to that of CHA 2 DS 2‐VA or CHA 2 DS 2‐VASc score. ABCD indicates age, NT‐proBNP, CCr, and dimension of the LA; AF, atrial fibrillation; CHA 2 DS 2‐VA, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, and age 65 to 74 years; AUC, area under the curve; CHA 2 DS 2‐VASc, congestive heart failure, hypertension, age ≥75, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, age 65 to 74 years, and sex category.
Figure 3.
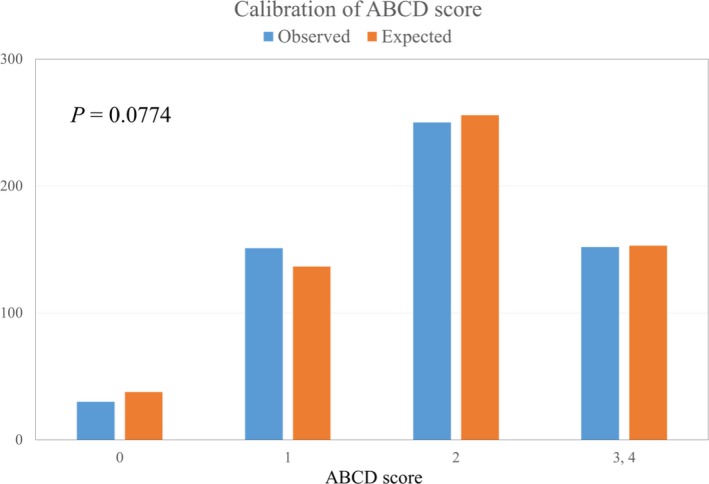
Calibration of ABCD score. ABCD score categories were defined as low risk (score=0), moderate risk (score 1, 2), and high risk (score 3, 4). There were no significant differences between observed stroke event number (blue) and expected stroke event number (red). ABCD indicates age, NT‐proBNP, CCr, and dimension of the LA; AF, atrial fibrillation; CHA 2 DS 2‐VA, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, and age 65 to 74 years; CCr, creatinine clearance rate; LA, left atrium; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide.
ABCD Score Versus CHA2DS2‐VA or CHA2DS2‐VASc Score
The c‐indexes of the CHA2DS2‐VA and CHA2DS2‐VASc score were 0.527 (95% CI 0.499–0.554; P<0.001) and 0.548 (0.519–0.577; P<0.001), respectively (Figure 2). Corrected c‐indexes from 1000 bootstrap samples for ABCD, CHA2DS2‐VA, and CHA2DS2‐VASc score were 0.870 (95% CI 0.848–0.891), 0.632 (95% CI 0.606–0.656), and 0.649 (95% CI 0.621–0.677), respectively. The c‐index of the ABCD score was higher than those of the CHA2DS2‐VA or CHA2DS2‐VASc score (Table 4; DeLong test z=21.53; P<0.001, z=19.08; P<0.001, respectively).
Table 4.
C‐Indexes, IDI, and NRI of the ABCD Score in Comparison With CHA2DS2‐VA and CHA2DS2‐VASc Score
| C‐Index | 95% CI | P Value | z Statistics* | P Value* | IDI* | P Value* | NRI* | P Value* | |
|---|---|---|---|---|---|---|---|---|---|
| ABCD | 0.858 | 0.838–0.877 | <0.001 | ||||||
| CHA2DS2‐VA | 0.527 | 0.499–0.554 | <0.001 | 21.53 | <0.001 | 0.339 | <0.001 | 0.769 | <0.001 |
| CHA2DS2‐VASc | 0.548 | 0.519–0.577 | <0.001 | 19.08 | <0.001 | 0.334 | <0.001 | 0.787 | <0.001 |
ABCD indicates age ≥60 years, NT‐proBNP ≥300 pg/mL, CCr <50 mL/min, and dimension of LA ≥45 mm; CHA2DS2‐VA, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, age 65 to 74 years; CHA2DS2‐VASc, congestive heart failure, hypertension, age ≥75, diabetes mellitus, prior stroke or transient ischemic attack, vascular disease, age 65 to 74 years, sex category; CCr, creatinine clearance rate; IDI, integrated discriminatory improvement; NRI, net reclassification index; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide.
For comparison with ABCD score.
The integrated discrimination improvement of the ABCD score was significantly improved as compared with CHA2DS2‐VA and CHA2DS2‐VASc score (Table 4; integrated discrimination improvement=0.339; P<0.001, integrated discrimination improvement=0.334; P<0.001, respectively). Significant NRIs were present with the ABCD score as compared with CHA2DS2‐VA and CHA2DS2‐VASc score, respectively (Table 4).
Discussion
The present study has several noteworthy findings: (1) among AF patients who developed a stroke, 19.2% had a CHA2DS2‐VA score of 0 or 1; (2) clinical, biomarker, and imaging factors (ie, age [≥60], NT‐proBNP [≥300 pg/mL], CCr [<50 mL/min], dimension of LA [≥45 mm], incorporated in the ABCD score) were independently associated with stroke events even in AF patients with a CHA2DS2‐VA score of 0 to 1 and CHA2DS2‐VASc score of 0 to 1; and (3) the ABCD score was significantly superior to those of the CHA2DS2‐VA or CHA2DS2‐VASc score in differentiating “truly low‐risk” patients.
The present study provides new insights into how addition of biomarkers can further refine stroke risk stratification among AF patients initially defined as “clinically low risk” based on the CHA2DS2‐VA criteria. Major guidelines have recommended OAC treatment for prevention of thromboembolic events in patients with AF and CHA2DS2‐VA and CHA2DS2‐VASc score of ≥2.19, 20 Nevertheless, AF patients with 1 nongender CHA2DS2‐VASc risk factor still have an elevated risk of stroke compared with patients with a CHA2DS2‐VASc score of 0 and the clinical benefit of OAC may be positive in these patients.5, 7 However, the risk contribution of each component of the CHA2DS2‐VASc score is not homogeneous.21 As a consequence, decision‐making for OAC in patients with AF and a CHA2DS2‐VASc score of 1 requires consideration of bleeding risk and an individual weighing of stroke risk.19, 21 In this regard, adjuvant risk stratification using biomarkers may provide additional information to aid OAC treatment decision‐making in these patients.19
The incidence of stroke in risk stratification scheme according to the CHA2DS2‐VASc score also varies among different cohorts because of the characteristics of subjects, such as the proportions of different ethnicity, prevalence of chronic kidney disease, degree of LA remodeling, etc.10 Furthermore, the impact of female sex on stroke among low‐risk patients with AF is controversial,11, 12, 22, 23 and 1 recent study revealed that female sex was a “risk modifier” for stroke rather than a risk factor.12 Of note, the incidence of stroke in Asian people may be higher compared with the white population, especially in patients with AF and a CHA2DS2‐VASc score of 0 in whom OAC therapy is not indicated.10, 24
Therefore, identifying “truly low‐risk” patients using an adjuvant risk stratification scheme would be of clinical value in patients with AF and a low‐risk CHA2DS2‐VA or CHA2DS2‐VASc profile (score of 0 or 1). Indeed, the approach to stroke prevention in AF has moved towards the default being OAC use unless the patient was deemed low risk, so relying on clinical risk stratification alone has limitations. As shown in this study, one fifth of patients with cardioembolic stroke were defined as clinically “low risk” based on the CHA2DS2‐VA criteria, showing that further improvement in refining risk stratification is still needed in this group. Biomarkers have been proposed to have such a role.
Biomarkers (“biological markers”), whether blood, urine, or imaging based, will always improve on stroke risk stratification based on clinical factors.25 Nevertheless, the use of multiple biomarkers has to be balanced against simplicity and practicality (and costs) of decision‐making for OAC use, for stroke prevention in AF. In the present study, the ABCD score, which includes 2 blood biomarkers (NT‐proBNP, creatinine clearance) and 1 imaging marker (LA dimension), showed good discriminatory value in both AF patients with a CHA2DS2‐VA score of 0 to 1 or a CHA2DS2‐VASc score of 0 to 1. Our results would suggest that the ABCD score can help physicians discriminate patients who have a truly low risk among AF patients with a CHA2DS2‐VA or CHA2DS2‐VASc score of 0 to 1 and would not require OAC treatment.
Consistent with our study, previous reports have demonstrated that elevated biomarkers such as NT‐proBNP level, red blood cell distribution width, and uric acid level were significantly associated with stroke in patients with AF.26, 27, 28 In the present study, red blood cell distribution width, uric acid levels (>7 mg/dL), and NT‐proBNP level (≥300 pg/mL) were positively associated with stroke events on univariate analysis; however, only NT‐proBNP level (≥300 pg/mL) was an independent predictor for stroke on multivariate analysis. Indeed, NT‐proBNP levels have been positively associated with the incidence of stroke.26, 29
In the present study, CCr (<50 mL/min) was another independent risk factor of stroke and this corresponds with prior studies.30, 31 Age is also a powerful driver of stroke risk in AF,32 and also contributes 1 point to the ABCD score. Finally, LA enlargement contributes to blood stasis into the LA, and endothelial dysfunction of the LA may lead to thrombus formation.33 LA enlargement may be a marker of atrial cardiomyopathy, which may cause thromboembolic events given atrial tissue abnormalities, such as fibrosis, endothelial cell dysfunction, and myocyte apoptosis.33, 34, 35 Some studies have also shown that a dilated LA (≥45 mm) is associated with increased risk of stroke, consistent with our results.36, 37
Limitations
Several limitations should be acknowledged. First, we were unable to estimate the annual stroke rate based on the ABCD score because of the retrospective nature and cross‐sectional design of the present study. Second, since our study included only a Korean population, these results cannot be extrapolated to subjects of other ethnicities. Third, although the internal bootstrapping approach was conducted to supplement the absence of an external validation cohort, further studies on cohorts with different ethnicity will be needed to validate the ABCD score.
Conclusions
The present study provides new insights into how addition of biomarkers can further refine stroke risk stratification among AF patients initially defined as clinically “low risk.” Almost one fifth of AF patients initially defined as “low risk” based on nongender clinical CHA2DS2‐VASc criteria experienced embolic stroke. The ABCD score that applies biomarkers (NT‐proBNP, creatinine clearance) and imaging (LA dimension) can further refine stroke risk stratification in this “clinically low‐risk” patient group, and can help discriminate the AF population who are a “truly low‐risk” group.
Sources of Funding
This study was sponsored by a grant from Samjin Pharmaceutical Co. Ltd.
Disclosures
Lip reports consulting for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi‐Sankyo. Lip also reports being a speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi‐Sankyo. No fees are directly received personally. The remaining authors have no disclosures to report.
Acknowledgments
The authors thank Soon‐Young Hwang, Yanguang Li, and Alena Shantsilla for their advice on statistical analyses.
(J Am Heart Assoc. 2019;8:e012697 DOI: 10.1161/JAHA.119.012697.)
References
- 1. Lip G, Freedman B, De Caterina R, Potpara TS. Stroke prevention in atrial fibrillation: past, present and future. Comparing the guidelines and practical decision‐making. Thromb Haemost. 2017;117:1230–1239. [DOI] [PubMed] [Google Scholar]
- 2. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 3. Overvad TF, Nielsen PB, Lip GY. Treatment thresholds for stroke prevention in atrial fibrillation: observations on the CHA2DS2‐VASc score. Eur Heart J Cardiovasc Pharmacother. 2017;3:37–41. [DOI] [PubMed] [Google Scholar]
- 4. Kim TH, Yang PS, Kim D, Yu HT, Uhm JS, Kim JY, Pak HN, Lee MH, Joung B, Lip GYH. CHA2DS2‐VASc score for identifying truly low‐risk atrial fibrillation for stroke: a Korean nationwide cohort study. Stroke. 2017;48:2984–2990. [DOI] [PubMed] [Google Scholar]
- 5. Nielsen PB, Chao TF. The risks of risk scores for stroke risk assessment in atrial fibrillation. Thromb Haemost. 2015;113:1170–1173. [DOI] [PubMed] [Google Scholar]
- 6. Mazurek M, Huisman MV, Rothman KJ, Paquette M, Teutsch C, Diener HC, Dubner SJ, Halperin JL, Ma CS, Zint K, Elsaesser A, Lu S, Lip GYH; GLORIA‐AF Investigators . Regional differences in antithrombotic treatment for atrial fibrillation: insights from the GLORIA‐AF Phase II Registry. Thromb Haemost. 2017;117:2376–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lip GY, Skjoth F, Nielsen PB, Larsen TB. Non‐valvular atrial fibrillation patients with none or one additional risk factor of the CHA2DS2‐VASc score. A comprehensive net clinical benefit analysis for warfarin, aspirin, or no therapy. Thromb Haemost. 2015;114:826–834. [DOI] [PubMed] [Google Scholar]
- 8. Lip GYH, Skjoth F, Nielsen PB, Kjaeldgaard JN, Larsen TB. Effectiveness and safety of standard‐dose nonvitamin K antagonist oral anticoagulants and warfarin among patients with atrial fibrillation with a single stroke risk factor: a nationwide cohort study. JAMA Cardiol. 2017;2:872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chao TF, Wang KL, Liu CJ, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chung FP, Liao JN, Chen TJ, Chiang CE, Lip GY, Chen SA. Age threshold for increased stroke risk among patients with atrial fibrillation: a nationwide cohort study from Taiwan. J Am Coll Cardiol. 2015;66:1339–1347. [DOI] [PubMed] [Google Scholar]
- 10. Chao TF, Lip GY, Liu CJ, Tuan TC, Chen SJ, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Chen TJ, Chiang CE, Chen SA. Validation of a modified CHA2DS2‐VASc score for stroke risk stratification in Asian patients with atrial fibrillation: a nationwide cohort study. Stroke. 2016;47:2462–2469. [DOI] [PubMed] [Google Scholar]
- 11. Wagstaff AJ, Overvad TF, Lip GY, Lane DA. Is female sex a risk factor for stroke and thromboembolism in patients with atrial fibrillation? A systematic review and meta‐analysis. QJM. 2014;107:955–967. [DOI] [PubMed] [Google Scholar]
- 12. Nielsen PB, Skjoth F, Overvad TF, Larsen TB, Lip GYH. Female sex is a risk modifier rather than a risk factor for stroke in atrial fibrillation: should we use a CHA2DS2‐VA score rather than CHA2DS2‐VASc? Circulation. 2018;137:832–840. [DOI] [PubMed] [Google Scholar]
- 13. Thomas MR, Lip GY. Novel risk markers and risk assessments for cardiovascular disease. Circ Res. 2017;120:133–149. [DOI] [PubMed] [Google Scholar]
- 14. Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 16. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 17. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 18. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207‐112. [DOI] [PubMed] [Google Scholar]
- 19. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; Group ESCSD . 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 20. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC/AHA Task Force Members . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 21. Savino JA III, Halperin JL. Should patients with atrial fibrillation and 1 stroke risk factor (CHA2DS2‐VASc score 1 in men, 2 in women) be anticoagulated? The CHA2 DS2‐VASc 1 conundrum: decision making at the lower end of the risk spectrum. Circulation. 2016;133:1504–1511; discussion 1511. [DOI] [PubMed] [Google Scholar]
- 22. Hughes M, Lip GY; Guideline Development Group, National Clinical Guideline for Management of Atrial Fibrillation in Primary and Secondary Care, National Institute for Health and Clinical Excellence . Stroke and thromboembolism in atrial fibrillation: a systematic review of stroke risk factors, risk stratification schema and cost effectiveness data. Thromb Haemost. 2008;99:295–304. [DOI] [PubMed] [Google Scholar]
- 23. Tomita H, Okumura K, Inoue H, Atarashi H, Yamashita T, Origasa H, Tsushima E; J‐RHYTHM Registry Investigators . Validation of risk scoring system excluding female sex from CHA2DS2‐VASc in Japanese patients with nonvalvular atrial fibrillation—subanalysis of the J‐RHYTHM registry. Circ J. 2015;79:1719–1726. [DOI] [PubMed] [Google Scholar]
- 24. Chang KC, Wang YC, Ko PY, Wu HP, Chen YW, Muo CH, Sung FC, Li TC, Hsu CY. Increased risk of first‐ever stroke in younger patients with atrial fibrillation not recommended for antithrombotic therapy by current guidelines: a population‐based study in an East Asian cohort of 22 million people. Mayo Clin Proc. 2014;89:1487–1497. [DOI] [PubMed] [Google Scholar]
- 25. Lip GY. Stroke and bleeding risk assessment in atrial fibrillation: when, how, and why? Eur Heart J. 2013;34:1041–1049. [DOI] [PubMed] [Google Scholar]
- 26. Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, Hohnloser SH, Reilly PA, Vinereanu D, Siegbahn A, Yusuf S, Wallentin L. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a randomized evaluation of long‐term anticoagulation therapy (RE‐LY) substudy. Circulation. 2012;125:1605–1616. [DOI] [PubMed] [Google Scholar]
- 27. Lee KH, Park HW, Cho JG, Yoon NS, Kim SS, Kim MR, Kim MC, Cho KH, Kim HK, Kim CH, Kim KH, Jun SJ, Kim WJ, Lee KJ, Jeong HC, Cho JY, Park KH, Sim D, Yoon HJ, Kim KH, Hong YJ, Kim JH, Ahn Y, Jeong MH, Park JC. Red cell distribution width as a novel predictor for clinical outcomes in patients with paroxysmal atrial fibrillation. Europace. 2015;17(suppl 2):ii83–ii88. [DOI] [PubMed] [Google Scholar]
- 28. Yang XL, Kim Y, Kim TJ, Jung S, Kim CK, Lee SH. Association of serum uric acid and cardioembolic stroke in patients with acute ischemic stroke. J Neurol Sci. 2016;370:57–62. [DOI] [PubMed] [Google Scholar]
- 29. Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Christersson C, Ezekowitz J, Gersh BJ, Hanna M, Hohnloser S, Horowitz J, Huber K, Hylek EM, Lopes RD, McMurray JJ, Granger CB. N‐terminal pro‐B‐type natriuretic peptide for risk assessment in patients with atrial fibrillation: insights from the ARISTOTLE trial (apixaban for the prevention of stroke in subjects with atrial fibrillation). J Am Coll Cardiol. 2013;61:2274–2284. [DOI] [PubMed] [Google Scholar]
- 30. Marinigh R, Lane DA, Lip GY. Severe renal impairment and stroke prevention in atrial fibrillation: implications for thromboprophylaxis and bleeding risk. J Am Coll Cardiol. 2011;57:1339–1348. [DOI] [PubMed] [Google Scholar]
- 31. Szymanski FM, Lip GY, Filipiak KJ, Platek AE, Hrynkiewicz‐Szymanska A, Opolski G. Stroke risk factors beyond the CHA(2)DS(2)‐VASc score: can we improve our identification of “high stroke risk” patients with atrial fibrillation? Am J Cardiol. 2015;116:1781–1788. [DOI] [PubMed] [Google Scholar]
- 32. Marinigh R, Lip GY, Fiotti N, Giansante C, Lane DA. Age as a risk factor for stroke in atrial fibrillation patients: implications for thromboprophylaxis. J Am Coll Cardiol. 2010;56:827–837. [DOI] [PubMed] [Google Scholar]
- 33. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D'Avila A, Dobrev D, Fenelon G, Gonzalez M, Hatem SN, Helm R, Hindricks G, Ho SY, Hoit B, Jalife J, Kim YH, Lip GY, Ma CS, Marcus GM, Murray K, Nogami A, Sanders P, Uribe W, Van Wagoner DR, Nattel S; Document R . EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. 2016;18:1455–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hirsh BJ, Copeland‐Halperin RS, Halperin JL. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism: mechanistic links and clinical inferences. J Am Coll Cardiol. 2015;65:2239–2251. [DOI] [PubMed] [Google Scholar]
- 35. Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016;47:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hamatani Y, Ogawa H, Takabayashi K, Yamashita Y, Takagi D, Esato M, Chun YH, Tsuji H, Wada H, Hasegawa K, Abe M, Lip GY, Akao M. Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non‐valvular atrial fibrillation. Sci Rep. 2016;6:31042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yaghi S, Moon YP, Mora‐McLaughlin C, Willey JZ, Cheung K, Di Tullio MR, Homma S, Kamel H, Sacco RL, Elkind MS. Left atrial enlargement and stroke recurrence: the Northern Manhattan Stroke Study. Stroke. 2015;46:1488–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]


