Abstract
Background
Information is scarce regarding effects of antihypertensive medication on blood pressure variability (BPV) and associated clinical outcomes. We examined whether antihypertensive treatment changes BPV over time and whether such change (decline or increase) has any association with long‐term mortality in an elderly hypertensive population.
Methods and Results
We used data from a subset of participants in the Second Australian National Blood Pressure study (n=496) aged ≥65 years who had 24‐hour ambulatory blood pressure recordings at study entry (baseline) and then after a median of 2 years while on treatment (follow‐up). Weighted day‐night systolic BPV was calculated for both baseline and follow‐up as a weighted mean of daytime and nighttime blood pressure standard deviations. The annual rate of change in BPV over time was calculated from these BPV estimates. Furthermore, we classified both BPV estimates as high and low based on the baseline median BPV value and then classified BPV changes into stable: low BPV, stable: high BPV, decline: high to low, and increase: low to high. We observed an annual decline (mean±SD: −0.37±1.95; 95% CI, −0.54 to −0.19; P<0.001) in weighted day‐night systolic BPV between baseline and follow‐up. Having constant stable: high BPV was associated with an increase in all‐cause mortality (hazard ratio: 3.03; 95% CI, 1.67–5.52) and cardiovascular mortality (hazard ratio: 3.70; 95% CI, 1.62–8.47) in relation to the stable: low BPV group over a median 8.6 years after the follow‐up ambulatory blood pressure monitoring. Similarly, higher risk was observed in the decline: high to low group.
Conclusions
Our results demonstrate that in elderly hypertensive patients, average BPV declined over 2 years of follow‐up after initiation of antihypertensive therapy, and having higher BPV (regardless of any change) was associated with increased long‐term mortality.
Keywords: ambulatory blood pressure, cardiovascular events, change in blood pressure variability, elderly, hypertension
Subject Categories: Epidemiology, Risk Factors, High Blood Pressure
Clinical Perspective
What Is New?
This study examines whether blood pressure variability (BPV) changes following initiation of antihypertensive treatment in an elderly hypertensive population.
The study examines the association of changes (increase or decrease) in BPV with long‐term survival.
What Are the Clinical Implications?
Higher ambulatory systolic BPV estimated from ambulatory blood pressure recordings (either pretreatment or while on treatment) is a strong predictor of cardiovascular and all‐cause mortality.
Treating elderly hypertensive patients with antihypertensive therapy could reduce blood pressure as well as ambulatory systolic BPV.
Presence of high systolic BPV, which is defined as having systolic BPV above the baseline median level, regardless of whether levels remain high or decline during the follow‐up period was associated with increased mortality during long‐term follow‐up.
Introduction
Blood pressure variability (BPV) has been identified as a potential target for management of hypertension because BPV appears to be associated with hypertension‐associated organ damage and is a predictor of future cardiovascular events regardless of age.1, 2, 3, 4 However, findings regarding the relationship between BPV and clinical outcomes have not been consistent.5, 6, 7 BPV is defined as the variation between multiple blood pressure (BP) records obtained from either ambulatory or visit‐to‐visit (ie, same clinic visit or between different clinic visits) BP records for an individual. BPV estimated from either visit‐to‐visit or ambulatory BP (ABP) records has been associated with increased cardiovascular risk.3, 8, 9
We recently reported that BPV estimated using ABP monitoring (ABPM) performs better in future cardiovascular risk prediction compared with BPV estimated from clinic visit‐to‐visit BP records (from the same participants).10 Because ABPM estimates BPV over a short period of time, it overcomes the lack of consensus on the requirements for the number of clinic visits for BPV measurement and the time interval between clinic visits to estimate visit‐to‐visit BPV.11 We also reported that among the different ABPM estimates of BPV, weighted day‐night and daytime systolic BP (SBP) variability perform better for predicting future all‐cause and cardiovascular mortality compared with 24‐hour and nighttime BPV.10
Evidence from preclinical and clinical studies suggests that use of antihypertensive therapy could reduce BPV, and this treatment‐related reduction of BPV may contribute to cardiovascular protection.12, 13, 14 To our knowledge, no information is available on the relationship between reducing BPV by using BP‐lowering medication and long‐term clinical outcomes.
In this study, we conducted a post hoc analysis with the aim of evaluating the effect of BP‐lowering treatment on systolic BPV (SBPV) estimated from ABPM recordings in an elderly hypertensive Australian population. We also explored whether any change in ambulatory SBPV (ASBPV) had an association with all‐cause and cardiovascular mortality outcomes over long‐term follow‐up.
Methods
The data that support the findings of this study are available from the ANBP2 (Second Australian National Blood Pressure Study) Management Committee on reasonable request (contact C.M.R., christopher.reid@curtin.edu.au).
Study Settings
We used data from a subgroup of participants in ANBP2 who were aged ≥65 years (at enrollment) and who participated in the ABP substudy (n=735). ANBP2 was a prospective randomized open‐label trial with blinded assessment of end points. The protocols for both the main study and the ABP substudy were approved by the ethics committee of the Royal Australian College of General Practitioners and conducted in accordance with the Helsinki Declaration of the World Medical Association. All participants gave written informed consent. ANBP2 was conducted with 6083 elderly hypertensive participants during 1995–2001 in 1594 family medical practices from 5 Australian states. The primary aim of the study was to compare the outcome of treatment using a BP‐lowering drug regimen based on either a diuretic or an angiotensin‐converting enzyme inhibitor in these elderly hypertensive participants. Details about the study design, treatments, results, and recruitment processes were published previously.15, 16 After the end of the ANBP2 clinical trial (median: 4.1 years), a further follow‐up for a total of ≈11 years was conducted to determine participants’ survival status. In this current analysis, we included only the ABP substudy participants who had completed a “successful” 24‐hour ABP recording at study entry (baseline, pretreatment) and then again after a median of 2 years while on treatment (follow‐up).
BP Measurement and BPV
ABP was measured at baseline before the commencement of randomized antihypertensive treatment and then again during the clinical trial period after a median of ≈2 years after randomization into the study. ABP recordings were made with a Spacelabs 90207 portable noninvasive device (Spacelabs Healthcare) using an appropriately sized cuff. During ABP measurement, SBP and diastolic BP were recorded at 30‐minute intervals throughout a 26‐hour recording period. Only the BP measurements made from 2 to 26 hours on each ambulatory record were considered for this analysis. A successful recording was defined as having ≥80% technically satisfactory readings (ie, ≥38 readings) over the 2‐ to 26‐hour recording period.
We previously demonstrated that weighted day‐night SBPV avoids the confounding effect of nocturnal BP reduction and the morning rise in BP and has been shown to correlate better with survival (all‐cause and cardiovascular mortality)10 and end‐organ damage17 in relation to 24‐hour BPV; therefore, we used this approach as the measure of SBPV in the current analysis. To estimate SBPV, we applied the same procedure for the baseline (pretreatment) and follow‐up (on treatment) BP records. Briefly, SBPV for an individual participant was initially measured as the standard deviation of SBP between readings for the separate daytime and nighttime BP records. Time definitions have been used to define the day period (10 am to 8 pm) and the night period (12 am to 6 am).17 Then we calculated weighted day‐night ASBPV from the mean of day and night SBPV corrected for the time interval in each observed period (day‐night) using the following equation: [{(daytime SD×10)+(nighttime SD×6)}/16].18
Change in SBPV
The annual rate of change in weighted day‐night ASBPV (between baseline and follow‐up) was calculated from these SBPV estimates. Furthermore, we classified both ASBPV estimates as high or low based on the baseline median ASBPV value because there is no agreed cutoff point to define high SBPV; then we classified ASBPV changes in relation to baseline into stable: low BPV, stable: high BPV, decline: high to low, or increase: low to high.
Follow‐Up and End Points
The association of weighted day‐night SBPV changes with mortality was assessed over a median of 8.6 years (interquartile range: 8.0–9.2 years) after the follow‐up ABPM during the clinical trial period until data were censored at October 31, 2009. This follow‐up period includes part of the ANBP2 clinical trial period (median: 2.1 years) together with a posttrial follow‐up period (median: 6.9 years; Figure 1). During the clinical trial period, a blinded independent end point committee adjudicated all study end points, including causes of death. Posttrial survival information was determined by linkage to the Australian Institute of Health and Welfare National Death Index (death registry). International Classification of Diseases, Tenth Revision (ICD‐10) coding was used to identify cause of death. For the purpose of the current study, we used all‐cause mortality and cardiovascular mortality (composed of sudden cardiac death, fatal stroke, fatal myocardial infarction, and “other” cardiovascular causes of death) as end points.
Figure 1.
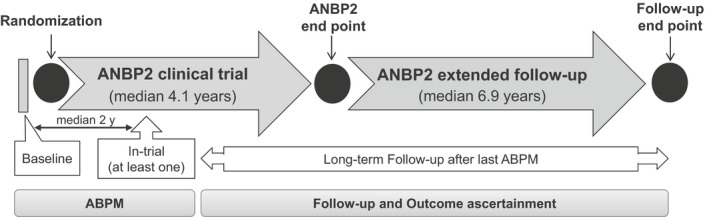
Overview of ANBP2 (Second Australian National Blood Pressure Study) and long‐term follow‐up, ABPM (ambulatory blood pressure monitoring), clinical outcome ascertainment, and analysis time periods.
Statistical Analyses
Statistical analyses were performed based on the intention‐to‐treat principle using Stata v15.1 for Windows (StataCorp). We summarized the baseline and on‐treatment demographic and clinical characteristics of the participants overall and compared them across the classifications based on ASBPV changes in relation to baseline (stable: low BPV, stable: high BPV, decline: high to low and increase: low to high). Continuous variables were compared using ANOVA, and categorical variables were compared using χ2 tests in relation to those who experienced stable: low BPV over the follow‐up time. Thereafter, we used Cox proportional hazards models to explore the effects of in‐trial changes in ASBPV (weighted day‐night) on all‐cause and cardiovascular mortality using the subgroup observed as stable: low BPV as the comparator. For this analysis, we used each participant's follow‐up ABPM measurement date as his or her observation start time and that participant's data were censored at October 31, 2009, or earlier if he or she died. The Cox proportional hazards model was adjusted for each participant's baseline characteristics such as age, sex, smoking and alcohol drinking status, previous history of cardiovascular disease, renal function (based on estimated glomerular filtration rate calculated using the CKD‐EPI [Chronic Kidney Disease Epidemiology Collaboration] equation), diabetes mellitus, plasma total and HDL (high‐density lipoprotein) cholesterol concentrations, physical activity, and mean ASBP at baseline. In addition, in‐trial randomized treatment group (angiotensin‐converting enzyme inhibitor/diuretics) and number of antihypertensive drugs used by each participant were included because these variables showed strong associations with ≥1 end point in the current analysis or in earlier published papers.19 Furthermore, we conducted regression analysis to identify the factors associated with changes in ASBPV. We used simple linear regression analyses to identify the baseline and in‐trial characteristics (Table 1) that showed an association with change in ASBPV. Those variables that showed an association (P<0.10) with change in ASBPV were included in a multinomial linear regression model to identify the determinants of change in ASBPV.
Table 1.
BP at Baseline and During Follow‐Up Period Along With Corresponding Changes and Distribution of Baseline Characteristics of the ANBP2 ABPM Substudy Participants Overall and Categorized by Weighted Day‐Night SBPV Change During in‐trial Follow‐Up
| Overall (N=496) | Stable: Low BPV (n=185, 37.3%) | Stable: High BPV (n=131, 26.4%) | Decline: High to Low (n=117, 23.6%) | Increase: Low to High (n=63, 12.7%) | |
|---|---|---|---|---|---|
| ASBP and variability | |||||
| At baseline | |||||
| Daytime SBP | 156±15 | 150±13 | 162±16* | 157±14* | 159±16* |
| Nighttime SBP | 137±16 | 132±13 | 143±19* | 137±15* | 138±15* |
| 24‐h SBP | 149±14 | 144±12 | 155±15* | 149±13* | 151±14* |
| Pulse pressure, 24 h | 66±12 | 61±10 | 71±13* | 67±11* | 65±11* |
| Weighted day‐night BPV | 11.84±2.91 | 9.36±1.44 | 14.58±2.44* | 13.66±1.54* | 10.06±1.00* |
| At follow‐up | |||||
| Daytime SBP | 142±15 | 138±13 | 147±16* | 138±14 | 147±15* |
| Nighttime SBP | 126±15 | 122±13 | 131±16* | 124±14 | 129±17* |
| 24‐h SBP | 136±14 | 132±12 | 142±14* | 134±13 | 140±15* |
| Pulse pressure, 24 h | 61±11 | 57±10 | 65±12* | 61±11* | 62±10* |
| Weighted day‐night BPV | 11.24±3.16 | 9.02±1.47 | 14.71±2.65* | 9.50±1.20* | 13.74±2.01* |
| Change in SBP and variability | |||||
| Daytime SBP | −14±15 | −13±12 | −15±17 | −18±16* | −11±13 |
| Nighttime SBP | −11±14 | −10±12 | −12±17 | −13±13 | −9±13 |
| 24‐h SBP | −13±13 | −11±11 | −13±15 | −16±13* | −11±11 |
| Pulse pressure, 24 h | −5±8 | −5±6 | −6±10 | −7±8* | −3±6 |
| Weighted day‐night BPV | −0.60±3.28 | −0.34±1.89 | 0.13±3.11 | −4.16±1.87* | 3.65±2.20* |
| Annual change in ASBPV, mean±SD (95% CI) | −0.37±1.95 (−0.54 to −0.19) | −0.21±1.08 (−0.5 to −0.37) | 0.09±1.88 (−0.24 to 0.41) | −2.42±1.28* (−2.65 to −2.18) | 2.04±1.41* (1.68–2.39) |
| Baseline characteristics | |||||
| Age, y, mean±SD | 71.4±4.6 | 70.5±4.4 | 73.0±4.9* | 71.3±4.6 | 71.0±4.4 |
| ≥75 y, %) | 26.4 | 20.5 | 36.6* | 26.5 | 22.2 |
| Male, % | 56.9 | 62.7 | 54.2 | 44.4* | 68.3 |
| Education, % | |||||
| Primary | 9.7 | 6.5 | 9.2 | 15.4* | 9.5 |
| Some high school | 44.0 | 48.1 | 39.7 | 41.9* | 44.4 |
| Completed high school/university | 46.4 | 45.4 | 51.2 | 42.7* | 46.0 |
| Regional location, % | 0.8 | 1.1 | 0.0 | 0.9 | 1.6 |
| BMI, kg/m2, mean±SD | 27.0±3.7 | 27.0±3.2 | 26.4±3.7 | 27.1±3.9 | 27.7±4.3 |
| Obese (BMI ≥30), % | 19.8 | 15.7 | 17.6 | 27.4* | 22.2 |
| Current smoker, % | 5.0 | 2.7 | 8.4* | 1.7 | 11.1* |
| Current drinker, % | 79.2 | 74.6 | 84.0* | 74.4 | 92.1* |
| Previous antihypertensive therapy, % | 68.5 | 65.9 | 66.4 | 77.8* | 63.5 |
| Previous CVD, % | 11.7 | 9.7 | 17.6* | 10.3 | 7.9 |
| Diabetes mellitus | 6.0 | 4.3 | 8.4 | 6.0 | 6.3 |
| Depression | 4.8 | 3.2 | 9.2* | 4.3 | 1.6 |
| Raised cholesterol (>6.5 mmol/L), % | 20.6 | 22.7 | 21.5 | 19.7 | 14.5 |
| Low HDL (<1 mmol/L), % | 12.5 | 14.8 | 9.3 | 9.6 | 17.7 |
| eGFR, mL/min/1.73 m2, mean±SD | 68.9±13.5 | 70.7±14.0 | 67.6±14.0 | 67.2±12.1* | 69.6±12.9 |
| Physical activities in previous 2 wk, % | |||||
| No exercise | 26.6 | 31.3 | 25.9* | 22.2* | 22.2 |
| 1–6 h | 28.4 | 33.0 | 19.9* | 26.5* | 36.5 |
| ≥7 h | 45.0 | 35.7 | 54.2* | 51.3* | 41.3 |
| In‐trial characteristics | |||||
| Randomized to receive ACEI, % | 49.2 | 51.9 | 48.9 | 42.7 | 54.0 |
| No. of antihypertensives | |||||
| 1 | 50.0 | 54.6 | 49.6 | 43.6* | 49.2 |
| ≥2 | 46.0 | 39.5 | 48.1 | 53.8* | 46.0 |
| No drug | 4.0 | 5.9 | 2.3 | 2.6* | 4.8 |
ABPM indicates ambulatory blood pressure monitoring; ACEI, angiotensin‐converting enzyme inhibitor; ANBP2, Second Australian National Blood Pressure Study; ASBP, ambulatory systolic blood pressure; ASBPV, ambulatory systolic blood pressure variability; BMI, body mass index; BP, blood pressure; BPV, blood pressure variability; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; SBP, systolic blood pressure; SBPV, systolic blood pressure variability.
Significant differences (P<0.05) with those observed as stable: low BPV.
Results
Of those who participated in the ANBP2 ambulatory substudy, 702 (of 735) had successful baseline (prerandomization) ABP records and 515 (of 538 with follow‐up ABP records) had successful follow‐up (on treatment) ABP records. In total, 496 participants had successful ABP records for both baseline and follow‐up. The average 24‐hour ABP (systolic/diastolic) for participants at baseline was 149/83 mm Hg and during follow‐up was 136/76 mm Hg. Average ASBP over 24 hours, during day and nighttime; estimated weighted day‐night ASBPV at baseline and follow‐up; and changes between follow‐up and baseline values are summarized in Table 1.
Change in ASBPV
Among the study participants we observed a decline in weighted day‐night ASBPV (mean±SD: −0.60±3.28 mm Hg; 95% CI, −0.89 to −0.31; P<0.001) over a median of 2 years of follow‐up (interquartile range: 1.9–2.1 years). We observed that the change in ASBPV was positively associated with change in SBP between baseline and follow‐up ABPM (Figure 2).The observed annual rate of decline of weighted day‐night ASBPV was −0.37±1.95 mm Hg (95% CI, −0.54 to −0.19). We used the baseline median weighted SBPV (11.59 mm Hg) to classify baseline and follow‐up ASBPV as low or high and then changes in ASBPV as stable: low BPV, stable: high BPV, decline: high to low, or increase: low to high. Overall, 63% retained a similar level of ASBPV (stable, high or low) at baseline and during in‐trail follow‐up (37% had stable: low BPV and 26% had stable: high BPV), whereas 24% had decline: high to low and 13% had increase: low to high ASBPV while on antihypertensive medication.
Figure 2.
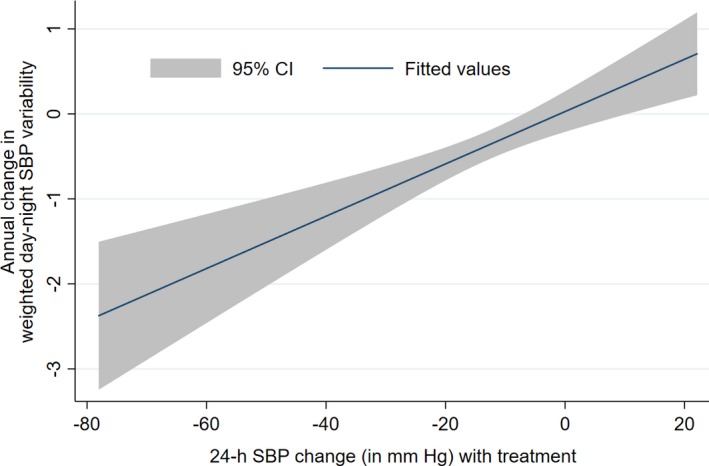
Association between weighted day‐night ASBP change and ASBP variability change. ASBP indicates ambulatory systolic blood pressure; SBP, systolic blood pressure.
Table 1 summarizes the weighted day‐night ASBPV, changes in ASBPV and the corresponding baseline and in‐trial demographic and clinical characteristics of the participants according to classified groups based on changes in ASBPV. Compared with the stable: low BPV group, we observed higher ASBP and weighted 24‐hour ASBPV at baseline and during the follow‐up period in the other 3 groups (stable: high BPV, decline: high to low and increase: low to high), except for follow‐up ASBP of the decline: high to low group (Table 1). We observed that the participants who experienced a decline in ASBPV from baseline to follow‐up (decline: high to low group), compared with the stable: low BPV group, were mostly female, were obese, had previous history of treatment with an antihypertensive, were physically active at baseline, and had been using multiple antihypertensive medications (independent of the randomization treatment group) during the follow‐up period. In addition at baseline, compared with the stable: low BPV group, those who had stable: high BPV were mostly older, had previous history of cardiovascular disease, were more likely to be active smokers and alcohol drinkers, and those who had increase: low to high ASBPV were more likely to be active smokers and alcohol drinkers.
Relationship Between Change in ASBPV and Mortality
Among the study participants there were 96 deaths (23.8 per 1000 person‐years) from any cause over a median of 8.6 years (interquartile range: 8.0–9.2 years) following the last ABP measurement. Of these 96 deaths, 49 deaths (12.2 per 1000 person‐years) were of cardiovascular origin. We observed that increases per millimeters of mercury in weighted day‐night ASBPV at both baseline and follow‐up were significant predictors of all‐cause and cardiovascular mortality independent of baseline SBP (Table 2). However, we did not observe any significant association of mortality (hazard ratio, all‐cause: 0.98 [95% CI, 0.88–1.09]; P=0.74; cardiovascular: 0.95 [95% CI, 0.82–1.10]; P=0.46) with change in weighed day‐night SBPV (per mm Hg) over time.
Table 2.
Association of Baseline and Follow‐Up Weighted Day‐Night SBPV (per mm Hg Increase) With Mortality
| SBPV | All‐Cause Mortality, HR (95% CI) | Cardiovascular Mortality, HR (95% CI) |
|---|---|---|
| At baseline | ||
| Model 1 | 1.20 (1.12–1.28), P<0.001 | 1.26 (1.15–1.39), P<0.001 |
| Model 2 | 1.19 (1.10–1.28), P<0.001 | 1.24 (1.12–1.36), P<0.001 |
| At follow‐up | ||
| Model 1 | 1.12 (1.05–1.19), P<0.001 | 1.15 (1.05–1.25), P=0.002 |
| Model 2 | 1.11 (1.04–1.18), P=0.002 | 1.11 (1.02–1.22), P=0.017 |
Model 1: HR adjusted for age, sex, smoking, drinking, history of previous heart disease, diabetes, raised cholesterol, low HDL (high‐density lipoprotein) cholesterol, and estimated glomerular filtration rate at baseline and in‐trial use of either angiotensin‐converting enzyme inhibitors or diuretics and number of antihypertensive drugs. Model 2: model 1 adjusted for corresponding mean ambulatory systolic blood pressure (baseline/follow‐up). HR indicates hazard ratio; SBPV, systolic blood pressure variability.
We observed significantly higher risk of all‐cause and cardiovascular mortality in those who had high ASBPV at baseline (either stable: high BPV or decline: high to low groups) compared with those who had constant low weighed day‐night ASBPV (stable: low BPV; Table 3). There was no significant difference in mortality among participants who had low ASBPV at baseline but had an increase in ASBPV later during the follow‐up period (increase: low to high group) compared with those who had stable low ASBPV (stable: low BPV group; Table 3). The mortality incidence curves for all‐cause and cardiovascular mortality by ASBPV change status (stable: low BPV, stable: high BPV, decline: high to low and increase: low to high) over the follow‐up period are shown in Figure 3. A restricted analysis was conducted among the participants who had higher ASBPV at baseline to assess any beneficial effect of ASBPV lowering in the decline: high to low group in relation to those with stable: high BPV. We did not observe any significant difference in mortality risk between these 2 groups (Figure 4).
Table 3.
Effect of Change in SBPV on All‐Cause and Cardiovascular Mortality in Relation to Those With Stable: Low BPV
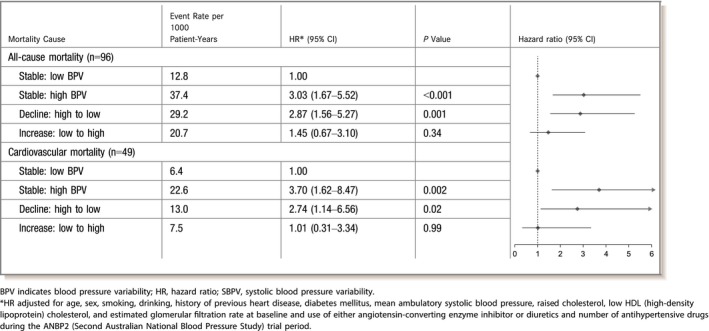
Figure 3.
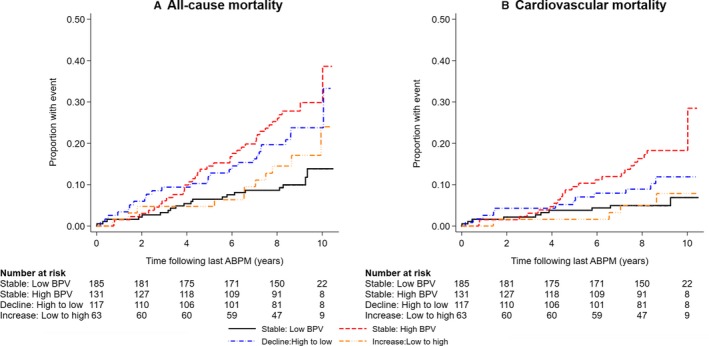
Incidence of all‐cause mortality (A) and cardiovascular mortality (B) following last ABPM by ambulatory systolic BPV change status among treated hypertensive patients. ABPM indicates ambulatory blood pressure monitoring; BPV, blood pressure variability.
Figure 4.
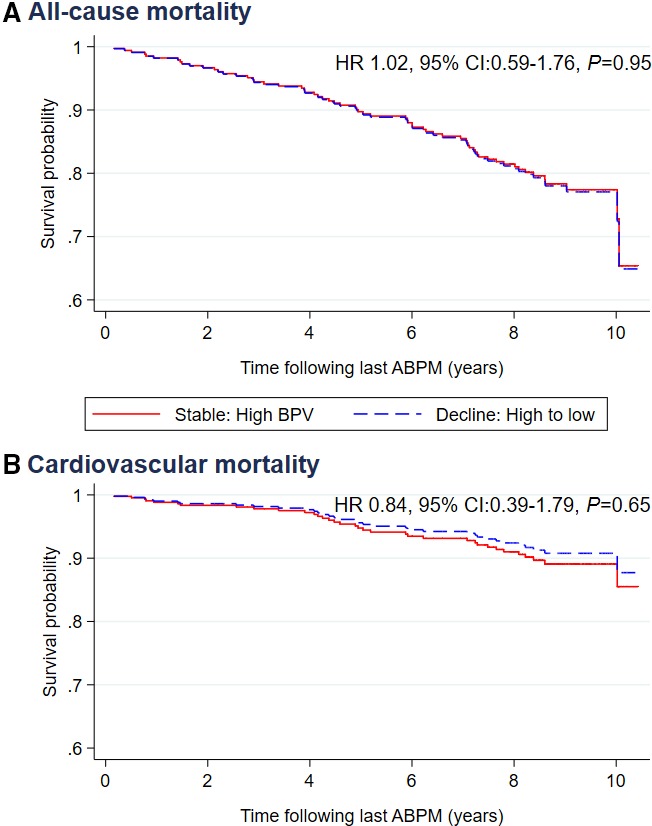
Survival by ambulatory systolic BPV change among treated hypertensive patients with high BPV at baseline for all‐cause mortality (A) and cardiovascular mortality (B) following last ABPM. ABPM indicates ambulatory blood pressure monitoring; BPV, blood pressure variability; HR, hazard ratio.
Determinants of ASBPV Change During the Trial Period
The results of the simple linear regression analysis of variables from the baseline and follow‐up periods that could have an association with change in ASBPV are presented in Table 4. In the multinomial linear regression model (which included only variables that showed an association of P<0.10 in Table 4), we observed that change in weighted day‐night ASBPV over the follow‐up period was related positively to change in 24‐hour ASBP, to male sex, and to being an active smoker at baseline (Table 5).
Table 4.
Association of Different Baseline and In‐Trial Factors With Change in ASBPV in Simple Linear Regression Analysis
| Coefficient | 95% Confidence Interval | P Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Change in 24‐h SBP (per mm Hg/y) | 0.06 | 0.03 | 0.08 | <0.001 |
| Age 75 y or more (vs <75 y) | −0.08 | −0.47 | 0.31 | 0.67 |
| Male (vs female) | 0.53 | 0.19 | 0.88 | 0.003 |
| Previous history of heart disease | −0.18 | −0.72 | 0.35 | 0.50 |
| Previous history of antihypertensive use | −0.25 | −0.62 | 0.12 | 0.19 |
| eGFR (per mL/min/1.73 m2) | 0.01 | −0.005 | 0.02 | 0.23 |
| Low HDL | 0.30 | −0.22 | 0.83 | 0.26 |
| Raised cholesterol | −0.10 | −0.53 | 0.32 | 0.64 |
| Active smoker | 1.01 | 0.23 | 1.79 | 0.01 |
| Active drinker | 0.53 | 0.11 | 0.95 | 0.01 |
| Diabetes mellitus | 0.05 | −0.67 | 0.77 | 0.90 |
| Depression | −0.42 | −1.23 | 0.38 | 0.30 |
| Exercise in previous 2 wk | ||||
| None | Ref | |||
| 1–6 h | −0.14 | −0.60 | 0.33 | 0.56 |
| ≥7 h | −0.09 | −0.51 | 0.33 | 0.68 |
| ACEI (vs diuretic) | 0.30 | −0.04 | 0.65 | 0.08 |
| No. of BP‐lowering medications used | ||||
| 1 | Ref | |||
| ≥2 | −0.13 | −0.49 | 0.22 | 0.45 |
| None | −0.06 | −0.95 | 0.83 | 0.90 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ASBPV, ambulatory systolic blood pressure variability; BP, blood pressure; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; Ref, referent; SBP, systolic blood pressure.
Table 5.
Determinants of Change in ASBPV in a Multinomial Linear Regression Model
| Coefficient (95% CI) | P Value | |
|---|---|---|
| Change in 24‐h SBP (per mm Hg/y) | 0.06 (0.03–0.08) | <0.001 |
| Male (vs female) | 0.44 (0.10–0.78) | 0.01 |
| Active smoker | 1.06 (0.29–1.81) | 0.01 |
| Active drinker | 0.39 (−0.03 to 0.80) | 0.07 |
| ACEI (vs diuretic) | 0.17 (−0.16 to 0.50) | 0.32 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ASBPV, ambulatory systolic blood pressure variability; SBP, systolic blood pressure.
Discussion
We demonstrated in our elderly hypertensive cohort that treatment with BP‐lowering medication was associated with reduction in ASBPV, expressed as weighted day‐night ASBPV. We also showed that having baseline ASBPV above the median regardless of any change over the follow‐up period had an increased risk of all‐cause and cardiovascular mortality compared with those who had below‐median ASBPV at baseline, independent of other risk factors for higher mortality. The latter is a novel finding.
Use of antihypertensive medications has been observed to be associated with decreased ASBPV based on 24‐hour ABPM in earlier studies,14, 20 and the reduction of BPV has been shown to be positively correlated to reduction in BP.14 During the in‐trial period of our study, over a median 2 years following the baseline ABP measurement, we also observed a small but statistically significant annual decline in weighted ASBPV (0.37 mm Hg) among the treated hypertensive study participants, and the reduction in ASBPV was positively correlated with lowering of BP. These findings suggest that changing SBPV is largely dependent on the change in SBP. Although use of multiple antihypertensive drugs was previously reported to be associated with changes in BPV,20 we did not observe any such association. We also did not observe any difference in the role of the different randomized drug‐treatment regimens (based on angiotensin‐converting enzyme inhibitor or thiazide diuretic) on ASBPV. Our relatively small sample size and the small number of people taking multiple antihypertensive medications in our study may be the underlying reasons for not confirming the previous observation. We also observed that male participants, active smokers, and active alcohol drinkers had higher SBPV.
In our study, the changes we observed in BPV over follow‐up after introduction of BP‐lowering treatment were reduction in ASBPV (decline: high to low) in 24% of the participants and rise in ASBPV (increase: low to high) in 13% of the participants compared with ASBPV status at baseline. The remaining 63% of participants had no change in ASBPV despite also using BP‐lowering medication. During the follow‐up period, higher all‐cause and cardiovascular mortality was observed among those who had constant higher ASBPV (stable: high BPV) or who experienced reduction in ASBPV from high baseline to low (decline: high to low) compared with those who had constant low ASBPV (stable: low BPV). Consequently, in the current analysis, lowering higher ASBPV did not have any significant beneficial effect on lowering of mortality risk. In addition, those who were observed to have an increase in ASBPV from low ASBPV at baseline did not experience any significant increase in mortality risk. To our knowledge, no other study to date has reported the associations we have described.
Our study has several limitations. First, for each individual participant, we used only one 24‐hour ABP recording at baseline and one during follow‐up. However, we observed that higher SBPV measured both at baseline and follow‐up could predict mortality outcome. Second, we did not have data on participants’ BP and BPV status and antihypertensive medication use during the extended follow‐up time after the formal clinical trial had been completed, which might have affected the association of change in BPV with the long‐term outcome of interest, namely, mortality. Furthermore, we could not explore the effect of the use of additional individual drug classes (eg, β‐blockers or calcium channel blockers) on BPV because, for the majority of participants, information on the duration (ie, start and/or end date) of additional drug use was inadequate. Third, the number of participants included in the current analysis is relatively small, possibly leading to a type II error. However, this study is one of the first to assess the relationship of survival and BPV change over time, in our case in elderly hypertensive participants. Fourth, we did not consider participants’ chronic pain, anxiety, or other factors that might have affected change in BPV. Fifth, we used standard deviations to estimate BPV instead of other measures. In an earlier study conducted in the same population, we found that BPV estimated using standard deviation performed similarly to or better than other BPV estimation techniques.1 Furthermore, in various population‐based studies, standard deviation of 24‐hour BP has frequently been reported to demonstrate an association with cardiovascular events and mortality.8, 21, 22 Last, ANBP2 was conducted in elderly hypertensive people treated in the context of a clinical trial; therefore, the findings from this present study may not be applicable to other age groups or settings.
Conclusions
This study presents the factors associated with changes in ASBPV among elderly hypertensive people over time and the relationship of changes in ASBPV to mortality in these people. In this study we observed that treating elderly hypertensive people with BP‐lowering drugs reduced BP and ASBPV. We observed that the presence of high BPV (ie, BPV above the baseline median level regardless of whether levels remain high or decline during follow‐up) was associated with increased mortality over long‐term follow‐up. A reduction in ASBPV while on BP‐lowering treatment did not lower the risk of mortality. Those who were most at risk of higher mortality associated with higher baseline ASBPV were typically older, had previous history of cardiovascular disease, and were more likely to be active smokers and alcohol drinkers. Our results fill the information gap regarding the relationship of BPV and changes associated with BP‐lowering treatment to subsequent mortality. Our results suggest that further research is needed with larger sample sizes and other population groups to confirm or refute our findings that reducing BPV with BP‐lowering treatment does not alter subsequent outcome.
Appendix
The following people participated in ANBP2 (Second Australian National Blood Pressure Study): Regional Coordinating Centers: M. Nelson, A. Bruce, P. Beckinsale, J. Thompson, M. McMurchie, G. Fraser, D. Gleave, V. Cope, F. DeLooze, S. Moore, C. Dibben, J. Newbury; Data Management and National Coordinating Centers: H. Miles, B. McDermott, K. Willson, C. Bear; Genetic Subcommittee: M. West, S. Harrap, C. Johnston, L. Beilin, P. Ryan, L. Wing, C. Reid; Ambulatory Blood Pressure Monitoring Subcommittee: L. Beilin, M. Brown, P. Ryan, L. Wing, C. Reid; Left Ventricular Hypertrophy Subcommittee: G. Jennings, P. Fletcher, M. Feneley, E. Dewar, L. Wing, C. Reid; Data Audit Subcommittee: J. McNeil, L. Wing, J. Marley, C. Reid; Finance Subcommittee: C. Johnston, G. Jennings, L. Wing, C. Reid; Health Economic–Quality‐of‐Life Subcommittee: J. Marley, J. Moss, P. Webb, P. Glasziou, F. Boyle, J. Primrose, L. Wing, C. Reid; Family Practitioner Advisory Committee: I. Steven, L. Piterman, F. De Looze, J. Dickinson, J. Gambrill, P. Joseph, C. Reid; End‐Point Committee: D. Hunt, G. Donnan, L. Wing, T. Morgan; Independent Data Audit Subcommittee: J. Chalmers, J. Whitworth, S. MacMahon, C. Silagy (deceased).
Sources of Funding
ANBP2 (Second Australian National Blood Pressure Study) was supported by the Australian Commonwealth Department of Health and Aging, the National Health and Medical Research Council (NHMRC) of Australia (grant no. 546272), and Merck Sharp & Dohme Pty. Ltd. The long‐term ANBP2 cohort follow‐up study was supported by an NHMRC program grant (no. 546272) awarded to Christopher M. Reid, who is supported by an NHMRC research fellowship (no. 1045862).
Disclosures
None.
Acknowledgments
The authors thank the financial supporters of ANBP2 (Second Australian National Blood Pressure Study) and the participants, study staff, data management centers, and ANBP2 Management Committee.
(J Am Heart Assoc. 2019;8:e012630 DOI: 10.1161/JAHA.119.012630.)
Contributor Information
Enayet K. Chowdhury, Email: enayet.chowdhury@curtin.edu.au.
the Second Australian National Blood Pressure Study Group:
A. Bruce, P. Beckinsale, J. Thompson, M. McMurchie, G. Fraser, D. Gleave, V. Cope, F. DeLooze, S. Moore, C. Dibben, J. Newbury, B. McDermott, K. Willson, C. Bear, S. Harrap, C. Johnston, P. Ryan, M. Brown, P. Ryan, P. Fletcher, M. Feneley, E. Dewar, J. Marley, J. Marley, J. Moss, P. Webb, P. Glasziou, F. Boyle, J. Primrose, L. Piterman, F. De Looze, J. Dickinson, J. Gambrill, P. Joseph, G. Donnan, T. Morgan, J. Whitworth, S. MacMahon, and C. Silagy
References
- 1. Chowdhury EK, Owen A, Krum H, Wing LMH, Nelson MR, Reid CM. Systolic blood pressure variability is an important predictor of cardiovascular outcomes in elderly hypertensive patients. J Hypertens. 2014;32:525–533. [DOI] [PubMed] [Google Scholar]
- 2. Shimbo D, Newman JD, Aragaki AK, LaMonte MJ, Bavry AA, Allison M, Manson JE, Wassertheil‐Smoller S. Association between annual visit‐to‐visit blood pressure variability and stroke in postmenopausal women/novelty and significance. Hypertension. 2012;60:625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlof B, Sever PS, Poulter NR. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. [DOI] [PubMed] [Google Scholar]
- 4. Tatasciore A, Renda G, Zimarino M, Soccio M, Bilo G, Parati G, Schillaci G, De Caterina R. Awake systolic blood pressure variability correlates with target‐organ damage in hypertensive subjects. Hypertension. 2007;50:325–332. [DOI] [PubMed] [Google Scholar]
- 5. Asayama K, Wei F‐F, Hara A, Hansen Tine W, Li Y, Staessen JA. Prognosis in relation to blood pressure variability. Hypertension. 2015;65:1170–1179. [DOI] [PubMed] [Google Scholar]
- 6. Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Bjorklund‐Bodegard K, Richart T, Ohkubo T, Jeppesen J, Torp‐Pedersen C, Dolan E, Kuznetsova T, Stolarz‐Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka‐Jaszcz K, Imai Y, Wang JG, Ibsen H, O'Brien E, Staessen JA; Int Database Ambulatory Blood P . Prognostic value of reading‐to‐reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55:1049–1057. [DOI] [PubMed] [Google Scholar]
- 7. Schutte R, Thijs L, Liu Y‐P, Asayama K, Jin Y, Odili A, Gu Y‐M, Kuznetsova T, Jacobs L, Staessen JA. Within‐subject blood pressure level–not variability–predicts fatal and nonfatal outcomes in a general population. Hypertension. 2012;60:1138–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, Ota M, Nagai K, Araki T, Satoh H, Ito S, Hisamichi S, Imai Y. Prognostic significance of blood pressure and heart rate variabilities: the OHASAMA study. Hypertension. 2000;36:901–906. [DOI] [PubMed] [Google Scholar]
- 9. Eguchi K, Hoshide S, Schwartz JE, Shimada K, Kario K. Visit‐to‐visit and ambulatory blood pressure variability as predictors of incident cardiovascular events in patients with hypertension. Am J Hypertens. 2012;25:962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chowdhury EK, Wing LMH, Jennings GLR, Beilin LJ, Reid CM; Committee AM . Visit‐to‐visit (long‐term) and ambulatory (short‐term) blood pressure variability to predict mortality in an elderly hypertensive population. J Hypertens. 2018;36:1059–1067. [DOI] [PubMed] [Google Scholar]
- 11. Diaz KM, Tanner RM, Falzon L, Levitan EB, Reynolds K, Shimbo D, Muntner P. Visit‐to‐visit variability of blood pressure and cardiovascular disease and all‐cause mortality a systematic review and meta‐analysis. Hypertension. 2014;64:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlof B, Poulter NR, Sever PS. Effects of beta blockers and calcium‐channel blockers on within‐individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–480. [DOI] [PubMed] [Google Scholar]
- 13. Xie HH, Shen FM, Xu LP, Han P, Miao CY, Su DF. Reduction of blood pressure variability by combination therapy in spontaneously hypertensive rats. J Hypertens. 2007;25:2334–2344. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Agnoletti D, Safar ME, Blacher J. Effect of antihypertensive agents on blood pressure variability: the Natrilix SR versus candesartan and amlodipine in the reduction of systolic blood pressure in hypertensive patients (X‐CELLENT) study. Hypertension. 2011;58:155–160. [DOI] [PubMed] [Google Scholar]
- 15. Reid CM. Australian comparative outcome trial of angiotensin‐converting enzyme inhibitor‐ and diuretic‐based treatment of hypertension in the elderly (ANBP2): objectives and protocol. Clin Exp Pharmacol Physiol. 1997;24:188–192. [DOI] [PubMed] [Google Scholar]
- 16. Wing LMH, Reid CM, Ryan P, Beilin LJ, Brown MA, Jennings GLR, Johnston CI, McNeil JJ, Macdonald GJ, Marley JE, Morgan TO, West MJ. A comparison of outcomes with angiotensin‐converting‐enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003;348:583–592. [DOI] [PubMed] [Google Scholar]
- 17. Staessen JA, Thijs L, Fagard R, O'Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA. 1999;282:539–546. [DOI] [PubMed] [Google Scholar]
- 18. Bilo G, Giglio A, Styczkiewicz K, Caldara G, Maronati A, Kawecka‐Jaszcz K, Mancia G, Parati G. A new method for assessing 24‐h blood pressure variability after excluding the contribution of nocturnal blood pressure fall. J Hypertens. 2007;25:2058–2066. [DOI] [PubMed] [Google Scholar]
- 19. Wing LMH, Brown MA, Beilin LJ, Ryan P, Reid CM. Reverse white‐coat hypertension’ in older hypertensives. J Hypertens. 2002;20:639–644. [DOI] [PubMed] [Google Scholar]
- 20. Omboni S, Kario K, Bakris G, Parati G. Effect of antihypertensive treatment on 24‐h blood pressure variability: pooled individual data analysis of ambulatory blood pressure monitoring studies based on olmesartan mono or combination treatment. J Hypertens. 2018;36:720–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mancia G, Parati G, Hennig M, Flatau B, Omboni S, Glavina F, Costa B, Scherz R, Bond G, Zanchetti A. Relation between blood pressure variability and carotid artery damage in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis (ELSA). J Hypertens. 2001;19:1981–1989. [DOI] [PubMed] [Google Scholar]
- 22. Sega R, Corrao G, Bombelli M, Beltrame L, Facchetti R, Grassi G, Ferrario M, Mancia G. Blood pressure variability and organ damage in a general population: results from the PAMELA study (Pressioni Arteriose Monitorate E Loro Associazioni). Hypertension. 2002;39:710–714. [DOI] [PubMed] [Google Scholar]


