Abstract
Background
More than 4 million cardiac noninvasive diagnostic tests are performed annually in the United States. However, questions remain regarding their effectiveness in improving clinical outcomes. We sought to evaluate whether noninvasive diagnostic tests were associated with lower rates of myocardial infarction or cardiovascular death when compared with no testing.
Methods and Results
We performed a retrospective, population‐based cohort study of adults evaluated for chest pain and discharged home from an emergency department in Ontario, Canada. Propensity score matching was employed to reduce confounding between the testing and nontesting groups. There were 370 863 patients evaluated in our cohort. Rates of the composite outcome were low for both groups after propensity‐score matching (0.29% and 0.78% for the nontesting group at 90 days and 1 year, respectively, and 0.34% and 0.68% for the noninvasive diagnostic test group at 90 days and 1 year respectively). Over 1 year, patients undergoing noninvasive diagnostic testing had a small but statistically significant lower hazard of developing the composite outcome of myocardial infarction or cardiovascular mortality (hazard ratio, 0.87; 95% CI, 0.78–0.96 [P<0.01]), which appears to be driven by the high‐risk subgroup (hazard ratio, 0.75; 95% CI, 0.61–0.92 [P<0.01]).
Conclusions
We report a lower observed rate of the composite outcome of cardiovascular death or myocardial infarction associated with noninvasive diagnostic testing following evaluation for chest pain in the emergency department. This lower rate was driven by the high‐risk subgroup. These results suggest that risk‐based testing should be considered for patients discharged from the emergency department for chest pain.
Keywords: chest pain, coronary artery disease, emergency department
Subject Categories: Health Services, Diagnostic Testing, Cardiovascular Disease
Clinical Perspective
What Is New?
Given the low event rates in contemporary cohorts being worked up for chest pain, it is unclear whether noninvasive testing is associated with incremental downstream improvements in outcomes when compared with no testing.
In our real‐world, population‐based cohort of adults discharged from the emergency department after evaluation for chest pain, noninvasive testing was associated with a small reduction in rates of downstream myocardial infarction or cardiovascular death.
This reduced rate was driven by the high‐risk patient subgroup, and noninvasive testing did not confer benefit in intermediate‐ and low‐risk patient subgroups.
What Are the Clinical Implications?
Noninvasive testing after emergency department discharge may be overutilized in low‐ and intermediate‐risk patients.
Risk‐based testing should be considered for patients discharged from the emergency department for chest pain.
Introduction
Chest pain is one of the most common clinical manifestations of cardiovascular disease and results in the performance of more than 4 million cardiac noninvasive diagnostic tests annually in the United States, with accelerating utilization rates in recent years.1, 2, 3 However, despite widespread use, questions remain regarding the effectiveness of noninvasive tests in improving downstream clinical outcomes.3, 4, 5, 6, 7, 8 PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) and SCOT‐HEART (Scottish Computed Tomography of the HEART Trial) reported low rates of major adverse cardiovascular events (MACE) in patients undergoing different types of noninvasive tests.2, 9, 10 Given the low event rates in contemporary cohorts being worked up for chest pain, it is unclear whether noninvasive testing (NIT) can lead to incremental downstream improvements in outcomes when compared with no testing. Unfortunately, neither the PROMISE nor the SCOT‐HEART trial included no‐testing arms to compare outcomes between strategies of any NIT versus no testing in order to help shed light onto this question.
In order to further explore the utility of NIT in patients evaluated for chest pain we sought to evaluate whether any such testing is associated with lower rates of MACE when compared with no testing. In order to address this objective, we used a well‐defined cohort of patients who were recently discharged from the emergency department (ED) after assessment for chest pain in which a potential cardiac etiology was considered and an acute coronary syndrome was ruled out. We hypothesized that there would be no significant difference in outcomes between those patients who underwent NIT and those who were not tested.
Methods
Availability of Data
The data set from this study is held securely in coded form at ICES. While data‐sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at www.ices.on.ca/DAS. The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the programs may rely on coding templates or macros that are unique to ICES.
Design and Derivation of the Cohort
We employed a retrospective cohort study design. Patients entered the cohort if they were evaluated for chest pain in an ED in Ontario, Canada, between April 1, 2010, and November 30, 2015, and discharged home after evaluation. Moreover, to be included in the cohort, patients must have undergone an ECG within 1 day of the ED visit. Patients were followed for 30 days after the index chest pain visit to determine whether they received 1 of 4 NITs currently available in Ontario: Graded exercise stress test, stress echocardiography, myocardial perfusion imaging, or coronary computed tomography angiography. Those undergoing testing were classified in the NIT arm of the study and those who did not were classified in the nontesting arm. We excluded patients who underwent an ED visit for chest pain or an NIT during the preceding 12 months. We also excluded patients older than 80 years. In elderly patients, comorbid conditions such as frailty are highly influential in physicians’ decisions not to pursue testing. Thus, we believed that including this group of elderly patients in our cohort would introduce unacceptable bias into our study.
Data Sources
The National Ambulatory Care Reporting System (NACRS) was used to determine patient ED visits. NACRS contains data for all hospital‐ and community‐based ambulatory care in Canada including information on ED discharge diagnosis.11, 12 Information to identify patient receipt of NIT and ECGs was obtained from the Ontario Health Insurance Plan (OHIP) physician claims database using billing codes that were used in previous studies.13, 14 Cardiovascular death was determined from the Office of the Registrar General‐Deaths database (International Classification of Diseases, Ninth Revision [ICD‐9] codes I00–I78). This is a data set containing information on all registered deaths in Ontario including cause of death. Physician specialty was determined by linking the OHIP database with the ICES physician database. The RPDB (Registered Persons Database), a registry of Ontario residents who are registered for Ontario health insurance coverage, was used to obtain demographic information. Median neighborhood income was obtained by linking the Census Area Profile with patients’ postal codes of residence from RPDB using the Postal Code Conversion File. Hospitalizations, including those for acute myocardial infarction (MI), were determined using the Canadian Institutes for Health Information (CIHI) Discharge Abstract Database (CIHI‐DAD). This database was also used to determine receipt of invasive angiography, percutaneous coronary intervention (PCI), and coronary artery bypass grafting (CABG). The Ontario Hypertension Database and Ontario Diabetes Databases were used to obtain hypertension and diabetes mellitus status, respectively. The Ontario Drug Benefit database was used to determine receipt of prescription medication in patients 65 years and older.
These data sets were linked using unique encoded identifiers and analyzed at ICES. The use of data in this project was authorized under section 45 of Ontario's Personal Health Information Protection Act, which does not require review by a research ethics board or informed consent of study participants. Given Canada's single‐payer government‐funded healthcare system, we were able to extract patient information with virtually 100% coverage of the population of Ontario.
Processes of Care
Patients were followed for a maximum of 1 year after presentation for chest pain in order to ascertain receipt of angiography, PCI, CABG, and physician follow‐up by both general practitioners and cardiologists.
Outcomes
The main outcome was a composite of time to hospitalization for acute MI or cardiovascular death. This outcome was ascertained for a maximum of 1 year after the date of NIT or the assigned pseudo‐test date. Since the last person entering our cohort could have entered it on November 30, 2015, the follow‐up period to evaluate for outcomes was extended to December 31, 2016. Definitions that we used to determine these outcomes have been previously validated using administrative data from CIHI‐DAD and have been extensively utilized in the literature.15, 16, 17, 18, 19
Statistical Analysis
Propensity score matching was employed to account for potential imbalances in measured baseline covariates between the testing and no‐testing groups. The propensity score was estimated using a random effects logistic regression model that incorporated physician‐specific random effects that accounted for the clustering of patients within the most‐responsible ED physician. Using this model, we regressed receipt of testing within 30 days of the index ED visit (versus no testing within 30 days) on the following baseline covariates: age, Charlson comorbidity score, tertile of hospital ED volume, sex, rural location, income quintile, diabetes mellitus, dyslipidemia, hypertension, chronic obstructive pulmonary disease, active cancer, metastatic cancer, type of hospital (teaching versus community and hospitals capable of performing PCI versus those that are not), history of heart failure, unstable angina, MI, peripheral vascular disease, revascularization (PCI/CABG), cerebrovascular disease, and whether the patient was assessed by a cardiologist in the ED. The index date for those undergoing NIT was the date of testing. For patients not undergoing testing, pseudo‐test dates were then created in the following manner. We estimated the empirical distribution of lag times (time between ED discharge and testing) for patients who underwent testing within 30 days. For each nontest patient, a random lag time was drawn from the empirical distribution determined above. This lag time was then added to each nontest patient to derive their pseudo‐test date. Patients who experienced cardiovascular death before their test or pseudo‐test date were excluded. This method was employed to both minimize the potential for immortal time bias while maintaining the maximum number of outcome events in our analyses and has been previously utilized by our group when evaluating the impact of electrophysiology visits on outcomes in patients with atrial fibrillation.20 The propensity score was estimated using variables measured at the index date. Propensity score matching was used to match test and nontest patients. Participants were matched on the logit of the propensity score using calipers with a width of 0.2 of the SD of the logit of the propensity score.21 Standardized differences were used in the unmatched and matched samples to assess for balance of baseline covariates between the testing and nontesting groups. A standardized difference of <0.10 was deemed to be acceptable. Patients were then followed from the test/pseudo‐test date to determine outcomes.
After matching was complete, processes of care and outcomes at 90 days and 1 year after the test/pseudo‐test date were determined and compared between the 2 groups. Given the presence of competing risks (eg, noncardiovascular death), a cause‐specific hazards model was used to compare the rate of our composite outcome of time to MI and/or cardiovascular death between the testing and no‐testing groups.22 A robust variance estimator was used to account for the matched nature of the sample.21 This model allows for a comparison of the cause‐specific hazard of cardiovascular death or MI between the 2 groups. It is equivalent to the Cox proportional hazards model in the setting without competing risks.
Subgroup Analyses
To ascertain whether cardiovascular risk influences the relationship between NIT and outcomes, we stratified our analyses according to the baseline cardiovascular risk of the patient. We did this by matching on the propensity score and subgroup variable. We defined patients as high risk if he/she had a prior MI, unstable angina, or a revascularization procedure (PCI or CABG). Patients with ≥1 cardiovascular risk factor (eg, dyslipidemia, diabetes mellitus, or hypertension) were categorized as intermediate risk. Patients with no cardiovascular risk factors were categorized as low risk. Comorbidities or cardiac procedures within 5 years of the index event were identified. The risk‐stratification tool that we used has been previously shown by our group to be able to classify patients discharged from the ED into different strata of adverse outcomes.11, 23
Cumulative Incidence Frequency Curves
Cumulative incidence frequency curves for the incidence of the main outcome were constructed for the testing and nontesting arms both for the overall cohort and for each risk strata. These curves accounted for the competing risk of noncardiovascular death. Cumulative incidence frequency curves were compared between groups using the Wald test from a Fine‐Gray model that accounted for clustering within matched pairs in a manner previously described.24
Results
Derivation of the Study Cohort
A total of 497 013 patients were discharged home after ED assessment for chest pain from hospitals across Ontario between April 1, 2010, and November 30, 2015. Of these, 125 957 patients were excluded for having a visit to the ED for chest pain or an NIT in the prior year or for not having an ECG performed within 1 day of the ED visit, and 193 patients were excluded before their pseudo‐test date. A total of 370 863 patients remained in our final cohort (Figure 1).
Figure 1.
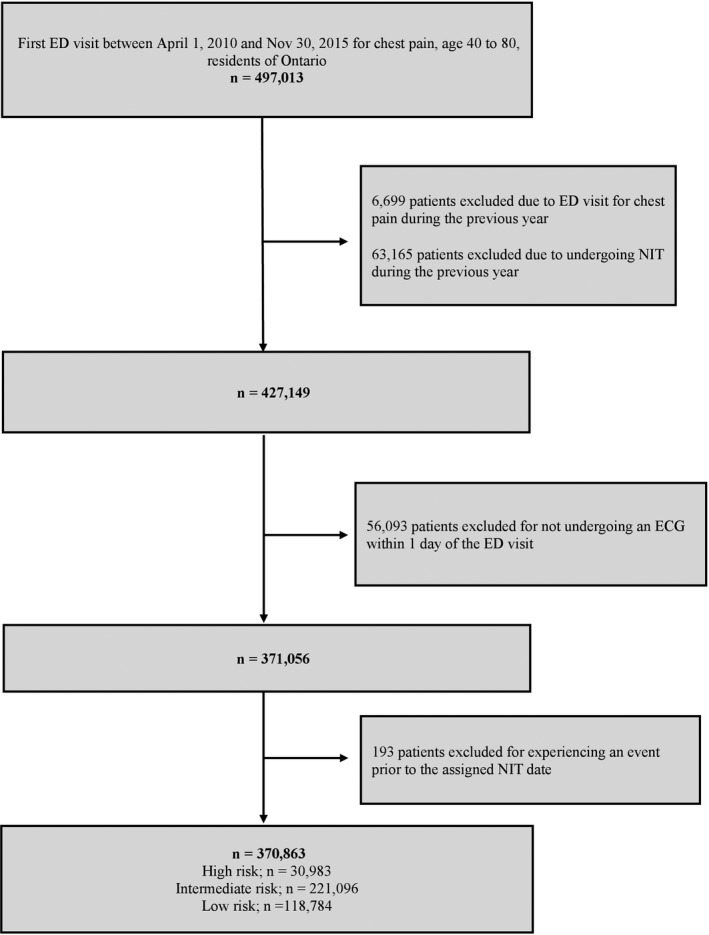
Derivation of the study cohort. ED indicates emergency department; NIT, noninvasive testing.
Baseline Characteristics
Baseline characteristics of patients before propensity score matching are summarized in Table S1. Patient baseline characteristics after matching are summarized in Table 1. We were able to identify 96 457 propensity score–matched pairs. A total of 95% of patients with NIT were successfully matched. The testing and nontesting groups were well balanced, with no meaningful differences (presence of a standardized difference >0.10) between the groups in terms of age, sex, other cardiovascular risk factors, income distribution, or noncardiac comorbidities. Further, there were no significant differences in the matched sample between the testing and the nontesting groups with regards to the types of hospitals in which the patients were assessed. Approximately 15% of patients were assessed in the EDs of teaching hospitals and 35% of patients were assessed in hospitals with cardiac catheterization capabilities.
Table 1.
Baseline Patient Characteristics After the Propensity Score Match
| Baseline Characteristic | No Testing (n=96 457) | Testing (n=96 457) | Standardized Difference |
|---|---|---|---|
| Age, mean±SD, y | 56.8±10.5 | 56.8±10.4 | <0.01 |
| Women | 47 594 (49.3) | 48 296 (50.1) | 0.01 |
| Income quintile | |||
| 1 | 17 352 (18.0) | 18 013 (18.7) | 0.02 |
| 2 | 18 658 (19.3) | 18 841 (19.5) | <0.01 |
| 3 | 19 707 (20.4) | 19 669 (20.4) | <0.01 |
| 4 | 20 752 (21.5) | 20 442 (21.2) | 0.01 |
| 5 | 19 770 (20.5) | 19 264 (20.0) | 0.01 |
| Rural location of the test | 10 128 (10.5) | 10 683 (11.1) | 0.02 |
| Median time to test or pseudo‐test (IQR), d | 9.0 (4.0–18.0) | 9.0 (4.0–17.0) | 0.04 |
| Assessment in a teaching hospital | 14 082 (14.6) | 14 854 (15.4) | 0.02 |
| Assessment in a hospital with cardiac catheterization capabilities | 34 425 (35.7) | 33 846 (35.1) | 0.01 |
| Evaluated by a cardiologist while in the ED | 1218 (1.3) | 1233 (1.3) | <0.01 |
| Risk group | |||
| High | 6178 (6.4) | 6178 (6.4) | <0.01 |
| Intermediate | 62 065 (64.3) | 62 065 (64.3) | <0.01 |
| Low | 28 214 (29.3) | 28 214 (29.3) | <0.01 |
| Hospital volume | |||
| High | 26 044 (27.0) | 27 045 (28.0) | 0.02 |
| Intermediate | 33 714 (35.0) | 33 818 (35.1) | <0.01 |
| Low | 36 699 (38.1) | 35 594 (36.9) | 0.02 |
| Comorbidities in the past 5 y | |||
| Unstable angina | 1962 (2.0) | 1975 (2.1) | <0.01 |
| Congestive heart failure | 633 (0.7) | 600 (0.6) | <0.01 |
| MI | 2544 (2.6) | 2512 (2.6) | 0.01 |
| Peripheral vascular disease | 2093 (2.2) | 2087 (2.2) | <0.01 |
| Cerebrovascular disease | 656 (0.7) | 678 (0.7) | <0.01 |
| Cancer | 2457 (2.6) | 2599 (2.7) | 0.01 |
| Renal disease | 455 (0.5) | 439 (0.5) | <0.01 |
| Diabetes mellitus | 18 210 (18.9) | 18 266 (18.9) | 0.01 |
| Dyslipidemia | 48 745 (50.5) | 48 676 (50.5) | <0.01 |
| Hypertension | 43 741 (45.4) | 44 103 (45.7) | 0.01 |
| Chronic obstructive pulmonary disease | 814 (0.8) | 902 (0.9) | 0.01 |
| Charlson score, mean (SD) | 0.3±0.8 | 0.3±0.8 | 0.01 |
| Medication use in the previous 90 d (in patients 65 y and older) | |||
| ACEIs/ARBs | 10 925 (45.5) | 10 886 (45.5) | <0.01 |
| Statins | 10 611 (44.2) | 10 551 (44.1) | <0.01 |
| β‐Blockers | 5979 (24.9) | 5394 (22.6) | 0.06 |
Values are expressed as number (percentage) unless otherwise indicated. ACEIs indicates angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; ED, emergency department; IQR, interquartile range.
Risk stratification: high risk: prior myocardial infarction (MI), unstable angina, or a revascularization procedure (percutaneous coronary intervention or coronary artery bypass grafting); intermediate risk: ≥1 cardiovascular risk factor (eg, dyslipidemia, diabetes mellitus, or hypertension); low risk: no cardiovascular risk factors.
Processes of Care
Patients who underwent testing were 11% more likely to see a family physician within 1 year after discharge (hazard ratio [HR], 1.11; 95% CI, 1.10–1.12). They were also 74% more likely to consult with a cardiologist during the same time frame (HR, 1.74; 95% CI, 1.72–1.77).
At both 90 days and 1‐year postdischarge, patients who received NIT were more likely to undergo invasive evaluation and revascularization. At 1 year, they were almost 3‐fold more likely to undergo invasive angiography (HR, 2.83; 95% CI, 2.72–2.95), ≈2.5‐fold more likely to undergo PCI (HR, 2.54; 95% CI, 2.36–2.73), and ≈2.5‐fold more likely to undergo CABG (HR, 2.53; 95% CI, 2.25–2.85). Patients undergoing NIT and 65 years and older were more likely to be prescribed cardiac medications, namely angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers (≈10% more likely), statins (≈16% more likely), or β‐blockers (≈11% more likely), when compared with patients not undergoing testing (see Table 2).
Table 2.
Processes of Care at 90 d and 1 y, Comparing the No‐Testing and Testing Groups
| No Testing | Testing | HR (95% CI) | |
|---|---|---|---|
| 90 d | |||
| Invasive angiography | 1517 (1.57) | 6593 (6.84) | 4.48 (4.23–4.73) |
| PCI | 479 (0.50) | 1889 (1.96) | 3.97 (3.59–4.39) |
| CABG | 187 (0.19) | 633 (0.66) | 3.39 (2.88–3.99) |
| Cardiologist visit | 13 975 (14.49) | 26 530 (27.50) | 2.08 (2.03–2.12) |
| Primary care physician visit | 65 861 (68.28) | 70 163 (72.74) | 1.14 (1.13–1.16) |
| ACEIs/ARBs | 11 225 (46.78) | 11 982 (50.10) | 1.11 (1.08–1.14) |
| Statins | 11 052 (46.05) | 12 306 (51.46) | 1.17 (1.14–1.20) |
| β‐Blockers | 6458 (26.91) | 7168 (29.97) | 1.15 (1.12–1.19) |
| 1 y | |||
| Invasive angiography | 3107 (3.22) | 8475 (8.79) | 2.83 (2.72–2.95) |
| PCI | 970 (1.01) | 2442 (2.53) | 2.54 (2.36–2.73) |
| CABG | 376 (0.39) | 950 (0.96) | 2.53 (2.25–2.85) |
| Cardiologist visit | 21 794 (22.60) | 33 967 (35.22) | 1.74 (1.72–1.77) |
| Primary care physician visit | 87 593 (90.81) | 88 950 (92.22) | 1.11 (1.10–1.12) |
| ACEIs/ARBs | 13 061 (54.43) | 13 784 (57.64) | 1.10 (1.07–1.12) |
| Statins | 13 372 (55.72) | 14 626 (61.16) | 1.16 (1.14–1.19) |
| β‐Blockers | 7791 (32.47) | 8345 (34.89) | 1.11 (1.08–1.15) |
Values are expressed as number (percentage) unless otherwise indicated. ACEIs indicates angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention.
Medication use is reported in patients 65 years and older.
Outcomes
Event rates were low for both groups, with only ≈0.3% of patients in either group having an MI or experiencing cardiovascular death within 90 days of the ED visit (Table 3). These trends persisted for the duration of our follow‐up, with only 0.68% of patients in the testing and 0.78% of patients in the nontesting group experiencing the composite outcome within 1 year after presentation to the ED (Table 4). Patients undergoing testing did not have significantly different hazards of experiencing the composite outcome over 90 days after the test date/pseudo‐test date when compared with those who were not tested (HR, 1.17; 95% CI, 1.00–1.37 [P=0.05]) (Figure 2A). However, over 1 year, patients who underwent testing had a small but statistically significant lower hazard of developing the composite outcome when compared with those who were not tested (HR, 0.87; 95% CI, 0.78–0.96 [P<0.01]) (Figure 2B). The number needed to treat to prevent 1 event of MI or cardiovascular death was 974 at this 1‐year time frame.
Table 3.
Outcomes at 90 d, Comparing the No‐Testing and Testing Groups
| No Testing | Testing | Adjusted HR (95% CI) | P Value | |
|---|---|---|---|---|
| Overall | ||||
| Cardiovascular death, MI | 281 (0.29) | 329 (0.34) | 1.17 (1.00–1.37) | 0.05 |
| Cardiovascular death | 87 (0.09) | 37 (0.04) | 0.43 (0.29–0.63) | <0.01 |
| MI | 205 (0.21) | 296 (0.31) | 1.45 (1.21–1.73) | <0.01 |
| High risk | ||||
| Cardiovascular death, MI | 73 (1.18) | 69 (1.12) | 0.95 (0.68–1.31) | 0.74 |
| Cardiovascular death | 30 (0.49) | 13 (0.21) | 0.43 (0.23–0.83) | <0.01 |
| MI | 45 (0.73) | 57 (0.92) | 1.27 (0.86–1.88) | 0.23 |
| Intermediate risk | ||||
| Cardiovascular death, MI | 172 (0.28) | 203 (0.33) | 1.18 (0.96–1.45) | 0.11 |
| Cardiovascular death | 48 (0.08) | 19 (0.03) | 0.40 (0.23–0.67) | <0.01 |
| MI | 132 (0.21) | 187 (0.30) | 1.42 (1.13–1.77) | <0.01 |
| Low risk | ||||
| Cardiovascular death, MI | 36 (0.13) | 57 (0.20) | 1.58 (1.04–2.41) | 0.03 |
| Cardiovascular death | 9 (0.03) | ≤5* | 0.56 (0.19–1.66) | 0.29 |
| MI | 28 (0.10) | ≥50* | 1.86 (1.17–2.94) | <0.01 |
Values are expressed as number (percentage) unless otherwise indicated. HR indicates hazard ratio.
Due to reidentification risk, ICES prohibits publication of cell sizes of <6.
Risk stratification: high risk: prior myocardial infarction (MI), unstable angina, or a revascularization procedure (percutaneous coronary intervention or coronary artery bypass grafting); intermediate risk: ≥1 cardiovascular risk factors (eg, dyslipidemia, diabetes mellitus, or hypertension); low risk: no cardiovascular risk factors.
Table 4.
Outcomes at 1 y, Comparing the No‐Testing and Testing Groups
| No Testing | Testing | Adjusted HR (95% CI) | P Value | |
|---|---|---|---|---|
| Overall | ||||
| Cardiovascular death, MI | 754 (0.78) | 655 (0.68) | 0.87 (0.78–0.96) | <0.01 |
| Cardiovascular death | 241 (0.25) | 124 (0.13) | 0.51 (0.41–0.64) | <0.01 |
| MI | 544 (0.56) | 548 (0.57) | 1.00 (0.90–1.13) | 0.90 |
| High risk | ||||
| Cardiovascular death, MI | 222 (3.59) | 167 (2.70) | 0.75 (0.61–0.92) | <0.01 |
| Cardiovascular death | 88 (1.42) | 43 (0.70) | 0.49 (0.39–0.70) | <0.01 |
| MI | 142 (2.30) | 133 (2.15) | 0.94 (0.74–1.19) | 0.58 |
| Intermediate risk | ||||
| Cardiovascular death, MI | 436 (0.70) | 385 (0.62) | 0.88 (0.77–1.01) | 0.07 |
| Cardiovascular death | 125 (0.20) | 63 (0.10) | 0.50 (0.37–0.68) | <0.01 |
| MI | 330 (0.53) | 328 (0.53) | 0.99 (0.85–1.16) | 0.94 |
| Low risk | ||||
| Cardiovascular death, MI | 96 (0.34) | 103 (0.37) | 1.07 (0.81–1.42) | 0.62 |
| Cardiovascular death | 28 (0.10) | 18 (0.06) | 0.64 (0.36–1.16) | 0.14 |
| MI | 72 (0.26) | 87 (0.31) | 1.21 (0.88–1.65) | 0.23 |
Values are expressed as number (percentage) unless otherwise indicated. HR indicates hazard ratio.
Risk stratification: high risk: prior myocardial infarction (MI), unstable angina, or a revascularization procedure (percutaneous coronary intervention or coronary artery bypass grafting); intermediate risk: ≥1 cardiovascular risk factors (eg, dyslipidemia, diabetes mellitus, or hypertension); low risk: no cardiovascular risk factors.
Figure 2.
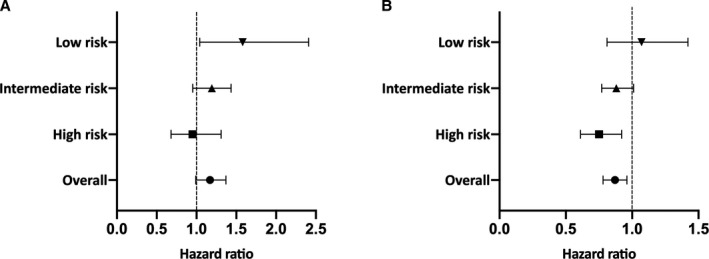
A, Cardiovascular death or myocardial infarction (MI) at 90 days: testing vs nontesting groups. B, Cardiovascular death or MI at 1 year: testing vs nontesting groups.
Subgroup Analyses According to Cardiovascular Risk
A total of 30 983 patients in our cohort were categorized as high risk, while 221 096 were categorized as intermediate risk and 118 784 as low risk. High‐risk patients in our cohort who underwent testing had significantly lower hazards of MI or death when compared with similar‐risk patients who were not tested. At 1 year, they experienced an ≈25% reduction in cardiovascular death and MI when compared with high‐risk patients who were not tested (HR, 0.75; 95% CI, 0.61–0.92 [P<0.01]) (Table 4). The number needed to treat to prevent 1 event of MI or cardiovascular death was 112 in the high‐risk subgroup. In contrast, for the intermediate‐ and low‐risk groups, there were no significant reductions in the composite outcome attributed to testing. Cumulative incidence frequency curves highlight the discrepancy between the high‐ and the intermediate‐ and low‐risk groups with respect to the impact of NIT (Figure 3).
Figure 3.
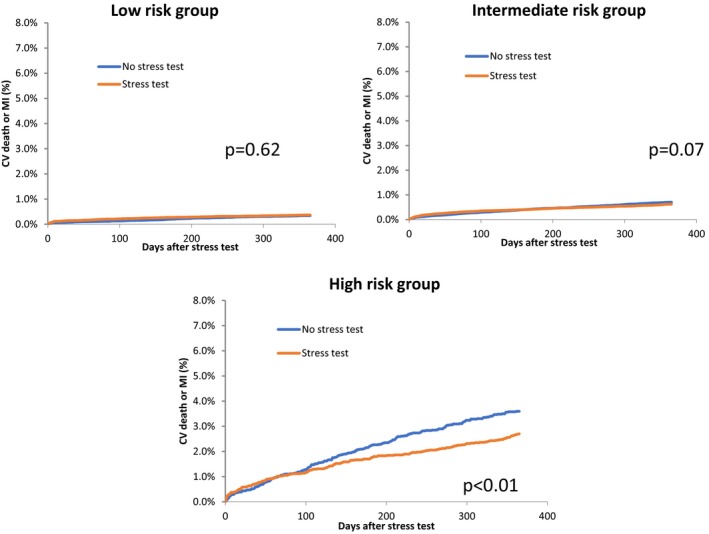
Cumulative incidence frequency curves for the low‐, intermediate‐, and high‐risk groups comparing cardiovascular (CV) death or myocardial infarction (MI) between the testing and the nontesting groups.
Discussion
In our large, population‐based study we report that there were low rates of MI and cardiovascular death in patients discharged from the ED after assessment for chest pain. Further, those tested were more likely to pursue follow‐up with both family physicians and cardiologists and undergo invasive angiography and revascularization procedures. At 1 year, patients who were tested experienced a significant reduction in the hazards for cardiovascular death or acute MI. This result was driven by the high‐risk subgroup. There were no significant improvements in outcomes in either the low‐ or intermediate‐risk patient groups that were attributable to NIT.
It is currently unclear whether more NIT leads to better outcomes in patients undergoing evaluation for CAD. The question of whether any NIT results in improved clinical outcomes was reinforced when 2 large trials, the PROMISE and SCOT‐HEART trials, as well as large observational population‐based studies, reported low rates of MI and death.2, 9, 10, 25 Importantly, neither the PROMISE nor the SCOT‐HEART trial had “no‐testing” arms. In this study, we gained insights into the utility of NIT for coronary artery disease by evaluating the subset of patients who presented to the ED with chest pain and who were subsequently discharged home.
Currently, the American Heart Association strongly advocates for performing NIT in patients who present for chest pain to the ED in whom an ACS has been excluded.26 This recommendation is largely based on older data reporting relatively high rates of MI and death in patients discharged from the ED after presentation with chest pain.27 However, in contemporary practice, there is little evidence to support the fact that NIT in this setting leads to improvements in clinical outcomes. One recent study using administrative data of privately insured individuals in the United States reported low 1‐year rates of MI in patients discharged from the ED after being assessed for chest pain.28 Similar to this study, our population‐based data reported low rates of MI and cardiovascular death in patients discharged from the ED after presentation with chest pain. In addition, our results demonstrated a small overall reduction in MACE, driven by reductions in cardiovascular mortality, for patients undergoing NIT. This reduction in MACE was driven by the high‐risk patient group.
It is important to note that we observed higher rates of processes of care in the NIT arm of our study. For example, we found that patients undergoing NIT also underwent more downstream revascularization procedures and were more likely to be started on medications such as statins. They were also more likely to undergo evaluation by a cardiologist. We speculate that NIT may have initiated a cascade of events leading to improved downstream processes of care that ultimately may have led to the lower rates of MACE that we observed. While this cascade of events is speculative, it is consistent with the overall rationale of evaluating outcomes related to diagnostic testing strategies. This rationale argues that evaluation of outcomes in this context is not simply a reflection of receipt or nonreceipt of the diagnostic test itself but rather is also indicative of a chain of real‐world events and decisions triggered by the diagnostic test that ultimately lead to potential observed differences in cardiovascular outcomes.2, 29
Clinical Importance and Health Policy Implications
To the best of our knowledge, our study is the first to compare mortality between testing and no‐testing strategies in patients being evaluated for CAD following ED discharge after presentation for chest pain. Our results argue against the guideline recommendations for broad‐based testing and suggest that a risk‐based decision to defer testing or a “watchful waiting” strategy of careful management of cardiovascular risk factors in low‐ and intermediate‐risk patients may be appropriate and sufficient in many situations. Limiting diagnostic testing to patients most likely to benefit is important given its proliferation over the past few decades, its associated costs, and the resultant emphasis that researchers and policy makers are currently placing on the importance of choosing diagnostic testing wisely.
Study Limitations
This study must be interpreted in the context of its limitations. First, as this is an observational study, there is the potential for selection bias in terms of who did and did not get tested. To mitigate against this limitation, we used a well‐defined patient population and propensity score matching to account for observed differences between patients tested and patients who were not–ensuring balance in terms of clinically important covariates. While this technique accounted for measured covariates, it is incapable of addressing the potential impact of unmeasured covariates, such as chest pain characteristics and ECG findings, which are often important in the physician decision‐making process when ordering noninvasive testing. Second, these results reflect patterns of care and outcomes in Ontario and may not necessarily be generalizable to other jurisdictions. However, Ontario is a large and diverse province consisting of ≈14 million people, similar in nature to many diverse populations around the world. The population‐level analyses that we performed (ie, having virtually 100% coverage of the population) further enhances the generalizability of our findings. Third, we did not use a validated risk score such as the HEART (History, ECG, Age, Risk factors and Troponin) or TIMI (thrombolysis in myocardial infarction) score to risk stratify our patients into high‐, intermediate‐, and low‐risk strata. It was not feasible to calculate these scores with our available data. Instead, we chose to use a simple and practical risk‐stratification tool that we could feasibly derive from our data. Similar risk‐stratification techniques utilizing administrative data have been shown to be able to classify patients discharged from the ED into different strata of adverse outcomes.11, 23 In addition, we recognize that use of our risk‐stratification tool is likely to be less accurate when compared with validated tools.
Conclusions
Our population‐based study reported a lower observed rate of the composite outcome of cardiovascular death or MI associated with NIT following evaluation for chest pain in the ED. This lower event rate was driven by the high‐risk subgroup with no significant reductions noted in the intermediate‐ and low‐risk patient groups. These results suggest that NIT for CAD after ED discharge may currently be overutilized and that risk‐based testing should be considered for patients discharged from the ED for chest pain.
Sources of Funding
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by the CIHI. However, the analyses, conclusions, opinions, and statements expressed in the material are those of the authors, not necessarily of CIHI. This study was also funded by operating funds from a Foundation grant (FDN‐154333) from the Canadian Institutes for Health Research (Dr Ko) and a Grant‐in‐Aid (grant number G‐19‐0026297) from the Heart and Stroke Foundation of Canada (Dr Roifman). Dr Roifman is also supported by National New Investigator and Ontario Clinician Scientist Phase 1 Awards from the Heart and Stroke Foundation of Canada. Drs Ko and Austin are supported by Mid‐Career Investigator Awards from the Heart and Stroke Foundation of Canada. Dr Wijeysundera is supported by the Ontario Clinician Scientist Phase 2 Award from the Heart and Stroke Foundation of Canada. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; or decision to submit the article for publication.
Disclosures
None.
Supporting information
Table S1. Baseline Patient Characteristics Before the Propensity Score Match
(J Am Heart Assoc. 2019;8:e013824 DOI: 10.1161/JAHA.119.013824.)
References
- 1. Douglas PS, Taylor A, Bild D, Bonow R, Greenland P, Lauer M, Peacock F, Udelson J. Outcomes research in cardiovascular imaging: report of a workshop sponsored by the National Heart, Lung, and Blood Institute. Circ Cardiovasc Imaging. 2009;2:339–348. [DOI] [PubMed] [Google Scholar]
- 2. Fordyce CB, Newby DE, Douglas PS. Diagnostic strategies for the evaluation of chest pain: clinical implications from SCOT‐HEART and PROMISE. J Am Coll Cardiol. 2016;67:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levin DC, Rao VM. Turf wars in radiology: the overutilization of imaging resulting from self‐referral. J Am Coll Radiol. 2004;1:169–172. [DOI] [PubMed] [Google Scholar]
- 4. Levin DC, Rao VM, Parker L, Frangos AJ, Sunshine JH. Recent trends in utilization rates of noncardiac thoracic imaging: an example of how imaging growth might be controlled. J Am Coll Radiol. 2007;4:886–889. [DOI] [PubMed] [Google Scholar]
- 5. Levin DC, Rao VM, Parker L, Frangos AJ, Sunshine JH. Recent trends in utilization rates of abdominal imaging: the relative roles of radiologists and nonradiologist physicians. J Am Coll Radiol. 2008;5:744–747. [DOI] [PubMed] [Google Scholar]
- 6. Lucas FL, DeLorenzo MA, Siewers AE, Wennberg DE. Temporal trends in the utilization of diagnostic testing and treatments for cardiovascular disease in the United States, 1993–2001. Circulation. 2006;113:374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maitino AJ, Levin DC, Parker L, Rao VM, Sunshine JH. Practice patterns of radiologists and nonradiologists in utilization of noninvasive diagnostic imaging among the Medicare population 1993–1999. Radiology. 2003;228:795–801. [DOI] [PubMed] [Google Scholar]
- 8. Maitino AJ, Levin DC, Parker L, Rao VM, Sunshine JH. Nationwide trends in rates of utilization of noninvasive diagnostic imaging among the Medicare population between 1993 and 1999. Radiology. 2003;227:113–117. [DOI] [PubMed] [Google Scholar]
- 9. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al‐Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. SCOT‐HEART investigators . CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT‐HEART): an open‐label, parallel‐group, multicentre trial. Lancet. 2015;385:2383–2391. [DOI] [PubMed] [Google Scholar]
- 11. Czarnecki A, Chong A, Lee DS, Schull MJ, Tu JV, Lau C, Farkouh ME, Ko DT. Association between physician follow‐up and outcomes of care after chest pain assessment in high‐risk patients. Circulation. 2013;127:1386–1394. [DOI] [PubMed] [Google Scholar]
- 12. Czarnecki A, Wang JT, Tu JV, Lee DS, Schull MJ, Lau C, Farkouh ME, Wijeysundera HC, Ko DT. The role of primary care physician and cardiologist follow‐up for low‐risk patients with chest pain after emergency department assessment. Am Heart J. 2014;168:289–295. [DOI] [PubMed] [Google Scholar]
- 13. Roifman I, Rezai MR, Wijeysundera HC, Chow BJ, Wright GA, Tu JV. Utilization of cardiac computed tomography angiography and outpatient invasive coronary angiography in Ontario, Canada. J Cardiovasc Comput Tomogr. 2015;9:567–571. [DOI] [PubMed] [Google Scholar]
- 14. Roifman I, Wijeysundera HC, Austin PC, Maclagan LC, Rezai MR, Wright GA, Tu JV. Temporal trends in the utilization of noninvasive diagnostic tests for coronary artery disease in Ontario between 2008 and 2014: a population‐based study. Can J Cardiol. 2017;33:279–282. [DOI] [PubMed] [Google Scholar]
- 15. Filate WA, Johansen HL, Kennedy CC, Tu JV. Regional variations in cardiovascular mortality in Canada. Can J Cardiol. 2003;19:1241–1248. [PubMed] [Google Scholar]
- 16. Tu JV, Chu A, Maclagan L, Austin PC, Johnston S, Ko DT, Cheung I, Atzema CL, Booth GL, Bhatia RS, Lee DS, Jackevicius CA, Kapral MK, Tu K, Wijeysundera HC, Alter DA, Udell JA, Manuel DG, Mondal P, Hogg W; Cardiovascular Health in Ambulatory Care Research Team (CANHEART) . Regional variations in ambulatory care and incidence of cardiovascular events. CMAJ. 2017;189:E494–E501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tu JV, Chu A, Donovan LR, Ko DT, Booth GL, Tu K, Maclagan LC, Guo H, Austin PC, Hogg W, Kapral MK, Wijeysundera HC, Atzema CL, Gershon AS, Alter DA, Lee DS, Jackevicius CA, Bhatia RS, Udell JA, Rezai MR, Stukel TA. The Cardiovascular Health in Ambulatory Care Research Team (CANHEART): using big data to measure and improve cardiovascular health and healthcare services. Circ Cardiovasc Qual Outcomes. 2015;8:204–212. [DOI] [PubMed] [Google Scholar]
- 18. Lee DS, Stitt A, Wang X, Yu JS, Gurevich Y, Kingsbury KJ, Austin PC, Tu JV. Administrative hospitalization database validation of cardiac procedure codes. Med Care. 2013;51:e22–e26. [DOI] [PubMed] [Google Scholar]
- 19. Yan AT, Koh M, Chan KK, Guo H, Alter DA, Austin PC, Tu JV, Wijeysundera HC, Ko DT. Association between cardiovascular risk factors and aortic stenosis: the CANHEART aortic stenosis study. J Am Coll Cardiol. 2017;69:1523–1532. [DOI] [PubMed] [Google Scholar]
- 20. Singh SM, Webster L, Qiu F, Austin PC, Ko DT, Tu JV, Wijeysundera HC. Effect of electrophysiology assessment on mortality and hospitalizations in patients with new‐onset atrial fibrillation. Am J Cardiol. 2018;121:830–835. [DOI] [PubMed] [Google Scholar]
- 21. Austin PC. The use of propensity score methods with survival or time‐to‐event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ko DT, Dattani ND, Austin PC, Schull MJ, Ross JS, Wijeysundera HC, Tu JV, Eberg M, Koh M, Krumholz HM. Emergency department volume and outcomes for patients after chest pain assessment. Circ Cardiovasc Qual Outcomes. 2018;11:e004683. [DOI] [PubMed] [Google Scholar]
- 24. Austin PC, Fine JP. Propensity‐score matching with competing risks in survival analysis. Stat Med. 2019;38:751–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roifman I, Wijeysundera HC, Austin PC, Rezai MR, Wright GA, Tu JV. Comparison of anatomic and clinical outcomes in patients undergoing alternative initial non‐invasive testing strategies for the diagnosis of stable coronary artery disease. J Am Heart Assoc. 2017;6:e005462 DOI: 10.1161/JAHA.116.005462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amsterdam EA, Kirk JD, Bluemke DA, Diercks D, Farkouh ME, Garvey JL, Kontos MC, McCord J, Miller TD, Morise A, Newby LK, Ruberg FL, Scordo KA, Thompson PD; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research . Testing of low‐risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122:1756–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, Griffith JL, Selker HP. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342:1163–1170. [DOI] [PubMed] [Google Scholar]
- 28. Foy AJ, Liu G, Davidson WR Jr, Sciamanna C, Leslie DL. Comparative effectiveness of diagnostic testing strategies in emergency department patients with chest pain: an analysis of downstream testing, interventions, and outcomes. JAMA Intern Med. 2015;175:428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Douglas PS, Taylor A, Bild D, Bonow R, Greenland P, Lauer M, Peacock F, Udelson J. Outcomes research in cardiovascular imaging: report of a workshop sponsored by the National Heart, Lung, and Blood Institute. JACC Cardiovasc Imaging. 2009;2:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Patient Characteristics Before the Propensity Score Match
Data Availability Statement
The data set from this study is held securely in coded form at ICES. While data‐sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at www.ices.on.ca/DAS. The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the programs may rely on coding templates or macros that are unique to ICES.


