Abstract
Background
Predicting clinical outcomes after cardiac resynchronization therapy (CRT) and its optimization remain a challenge. We sought to determine whether pre‐ and postimplantation QRS area (QRS area) predict clinical outcomes after CRT.
Methods and Results
In this retrospective study, QRS area, derived from pre‐ and postimplantation vectorcardiography, were assessed in relation to the primary end point of cardiac mortality after CRT with or without defibrillation. Other end points included total mortality, total mortality or heart failure (HF) hospitalization, total mortality or major adverse cardiac events, and the arrhythmic end point of sudden cardiac death or ventricular arrhythmias with or without a shock. In patients (n=380, age 72.0±12.4 years, 68.7% male) undergoing CRT over 7.7 years (median follow‐up: 3.8 years [interquartile range 2.3–5.3]), preimplantation QRS area ≥102 μVs predicted cardiac mortality (HR: 0.36; P<0.001), independent of QRS duration (QRSd) and morphology (P<0.001). A QRS area reduction ≥45 μVs after CRT predicted cardiac mortality (HR: 0.19), total mortality (HR: 0.50), total mortality or heart failure hospitalization (HR: 0.44), total mortality or major adverse cardiac events (HR: 0.43) (all P<0.001) and the arrhythmic end point (HR: 0.26; P<0.001). A concomitant reduction in QRS area and QRSd was associated with the lowest risk of cardiac mortality and the arrhythmic end point (both HR: 0.12, P<0.001).
Conclusions
Pre‐implantation QRS area, derived from vectorcardiography, was superior to QRSd and QRS morphology in predicting cardiac mortality after CRT. A postimplant reduction in both QRS area and QRSd was associated with the best outcomes, including the arrhythmic end point.
Keywords: cardiac resynchronization therapy, left bundle branch block, QRS area, QRS duration, vectorcardiography
Subject Categories: Arrhythmias, Catheter Ablation and Implantable Cardioverter-Defibrillator, Heart Failure
Clinical Perspective
What Is New?
Pre‐implantation QRSarea was superior to QRSd and QRS morphology in predicting cardiac and total mortality after cardiac resynchronization therapy.
Concomitant reductions in QRSarea and QRSd after cardiac resynchronization therapy were associated with the best survival and the lowest risk of heart failure hospitalization, major adverse cardiac events as well as ventricular arrhythmias.
What Are the Clinical Implications?
Reductions in QRSarea and QRSd could be a focus for optimization after cardiac resynchronization therapy implantation.
Introduction
Cardiac resynchronization therapy (CRT) is an established treatment for patients with heart failure (HF), impaired left ventricular (LV) function, and a wide QRS complex.1 As with any medical therapy, its treatment effect is variable. “Nonresponder” rates range from 9% to 68%, depending on the criteria used to define response.2 Although no medical therapy can be expected to be 100% effective, there is a consensus view that response to CRT can be improved.3
Manifold imaging studies explored mechanical dyssynchrony in relation to patient selection and optimization but, ultimately, no single measure of mechanical dyssynchrony has been adopted by clinical guidelines.4 In this context, we should consider CRT is an electrical treatment and that its substrate should be electrical rather than mechanical. In this respect, QRS duration (QRSd) has been adopted as a surrogate of electrical dyssynchrony in randomized, controlled trials,1 and a reduction in QRSd has been shown to predict better long‐term outcomes after CRT.5, 6
Evidence has recently emerged in support of vectorcardiography in the field of CRT. In this respect, QRS area (QRSarea) has been shown to correlate with LV lateral wall activation time,7 the maximum rate of rise of LV pressure (ΔLV dP/dtmax),8, 9 and LV reverse remodeling10 after CRT. Crucially, pre‐implantation QRSarea has also been shown to be superior to pre‐implantation QRSd and QRS morphology in predicting total mortality after CRT.11, 12
Although QRSarea and QRSd duration relate to depolarization in a global sense, QRSarea also yields the dominant axis of the activation sequence.13 Given that the objective of CRT is to make depolarization more synchronous, both the pacing location and timing between LV and right ventricular pacing can be used to manipulate activation sequence. In this study, we explored pre‐ and postimplantation QRSarea and QRSd in relation to long‐term cardiac mortality, HF hospitalization, and major adverse cardiac events (MACEs) after CRT.
Methods
Patients referred for CRT implantation at the University Hospitals Birmingham, Queen Elizabeth, United Kingdom, were retrospectively evaluated. The study was approved by the local Ethics Committee and local Clinical Audit Department, both of which waived patient consent on the basis that all study tests and interventions had already been undertaken. The study conforms with the Declaration of Helsinki.
Study Population
Patients undergoing CRT implantation from November 2011 to June 2018 were identified. Implantation practice adhered to the United Kingdom's National Institute of Clinical Excellence guidelines, which before 2007 recommended CRT with defibrillation (CRFT‐D) only in the context of secondary prevention. After 2014, National Institute of Clinical Excellence recommended cardiac resynchronization therapy with defibrillation rather than CRT‐pacing in nonischemic cardiomyopathy.14
Inclusion criteria were the following: indications for CRT according to National Institute of Clinical Excellence guidance and availability of a digitizable 12‐lead ECG before and after implantation. Exclusion criteria were the following: subjects with technically unsuitable ECGs and patients with congenital heart disease.
Device Therapy
Device implantation was undertaken using standard transvenous techniques with patients under local anesthesia and intravenous sedation. Following implantation, patients were followed up in combined cardiac device therapy/HF clinics. Devices were programmed according to physician discretion. Generally, backup atrial pacing was set at 60 beats/min, and the pacing mode was set to DDDR. The atrioventricular delay was set at 90 ms and the interventricular delay to between 0 and −20 ms (LV first). In patients in permanent atrial fibrillation, right ventricular and LV leads were deployed, a CRT generator was implanted, and devices were programmed to a ventricular triggered mode. Atrioventricular junction ablation was undertaken according to physicians’ discretion. Targeted echocardiographic optimization was only undertaken in symptomatic nonresponders.
Lead positions
The anteroposterior, as well as the left anterior and right anterior oblique fluoroscopic views from coronary sinus venography taken at the time of implantation were used retrospectively to assess the LV lead tip position, as previously described.15 All LV lead positions were assessed retrospectively by an experienced implanter (F.L.) who was blinded to clinical outcome data.
ECG
Pre‐implantation, standard supine 12‐lead ECGs (25 mm/s, 10 mm/mV) were used for analysis. A left bundle branch block (LBBB) was defined as a QRSd >120 ms, rS or QS in lead V1, notched or slurred R‐waves in leads I, aVL, V5 or V6, with absent q waves in leads V5 and V6.16 We used this definition rather than “strict” Strauss criteria, as the latter is not predictive of clinical outcomes after CRT.12 Right bundle branch block was defined as a QRS ≥120 ms, with a wide, positive R‐wave deflection in lead V1 and a slurred S wave in leads I and V6. A nonspecific intraventricular conduction delay was defined as nonpaced QRS >120 ms not fitting these criteria. Postimplantation ECGs were undertaken within 3 months after implantation.
Vectorcardiography
Standard 12‐lead ECGs were first converted to an Extensible Markup Language (XML) format using ECGScan (AMPS LLC, New York, USA), a commercially available program approved by the US Food and Drug Administration. A custom‐made program was used for generation of 2 vectorcardiographies according to Frank's orthogonal lead system using the Kors transformation. The latter was used given previous evidence that it is superior to other vectorcardiography transformations in predicting clinical outcomes after CRT.12 The start and end of the QRS complex were defined semi‐automatically using digital calipers at 200% magnification. For paced rhythms, the onset and end of the QRS complex was measured manually, excluding the pacing spike. Digitization of ECGs and generation of vectorcardiographies were undertaken by a single investigator (O.O.) who was blinded to clinical outcomes collected by another investigator (A.Z.). The QRSarea was calculated as the integral between the ventricular deflection curve and the isoelectric line in each the 3 orthogonal leads (X, Y, and Z), according to the formula: (Figure 1).
Figure 1.
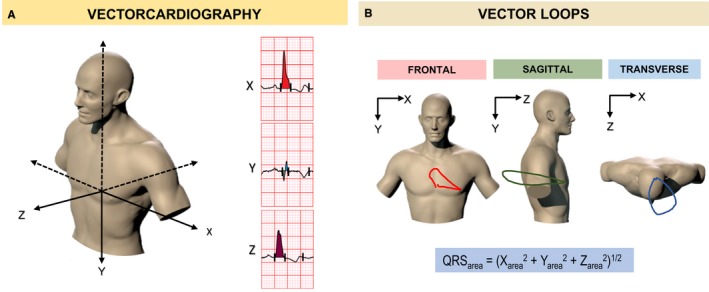
Vectorcardiography in cardiac resynchronization therapy. The vectorcardiogram displays the various features of the ECG, such as the QRS complex, in the form of “loops,” which are determined from vectors representing successive, instantaneous mean electrical forces throughout the cardiac cycle. A, A representation of the 3 vectorcardiogram leads (X, Y, and Z), according to Frank's orthogonal lead system. B, Two‐dimensional vector loops in the frontal (X‐Y leads), sagittal (Y‐Z leads), and transverse (X‐Z leads) planes from a patient with a left bundle branch block. The QRS area is calculated as the integral sum of the area bound by the QRS complex and the isoelectric baseline in each vectorcardiogram lead (X, Y, and Z).
End points
The primary end point was cardiac mortality, which included cardiac transplantation or implantation of a ventricular assist device. The secondary end point was total mortality. Ancillary end points included total mortality or unplanned HF hospitalization; total mortality or unplanned hospitalization for MACE; and the combined end point of sudden cardiac death, ventricular tachycardia, ventricular fibrillation, or shock. MACE included unplanned hospitalization for HF, myocardial infarction, acute coronary syndrome, ventricular arrhythmias, and atrial fibrillation. A HF hospitalization was defined as an unplanned admission related to worsening dyspnea, in association with peripheral edema, pulmonary edema on chest radiography, and requirement for intravenous diuretic therapy. Device‐treated arrhythmias (appropriately treated with shocks or antitachycardia pacing) not leading to an unplanned hospitalization were not regarded as a hospitalization for MACE. Stroke and pulmonary embolism were not regarded as MACE. In composite end points, the first event was included in statistical analyses. Mortality data were collected through medical record and cross‐checked with a national mortality database. Data were collected retrospectively from medical records and entered into an electronic database every 6 months by investigators who were blinded to clinical and imaging data. Events were adjudicated by blinded investigators on a 6‐monthly basis.
Mode of death
Sudden cardiac death was defined as a natural, unexpected death because of cardiac causes, heralded by an abrupt loss of consciousness within 1 hour of the onset of acute symptoms. Death from pump failure was defined as “death after a period of clinical deterioration in signs and symptoms of HF despite medical treatment”.17
Statistical Analysis
Continuous variables are expressed as mean± SD. Normality was tested using the Shapiro–Wilk test. Comparisons between normally distributed continuous variables were made using the Student t test. Categorical variables were analyzed using χ2 tests. Receiver operating characteristic curves were created to assess the predicted probabilities of ECG and vectorcardiography variables in relation to cardiac mortality. A 10‐fold cross‐validation was used as the model validation technique for assessing performance, and the average was calculated over 10 repetitions. The measure with largest area under the receiver operating characteristic (area under the curve [AUC]) was used for subsequent analyses. The Liu method was also applied to estimate nonparametrically the optimal cutoffs for ECG and vectorcardiography measures.18 Kaplan–Meier curves and the log‐rank test were used to assess cumulative survival and Cox proportional hazard models were used to assess relative risks. Proportionality hypotheses were verified by visual examination of log (survival) and Schoenfeld residuals. Variables reaching P<0.10 as univariate predictors of cardiac mortality were entered in multivariate models. Statistical analyses were undertaken by a biostatistician (T.Q.) who did not partake in data collection. The Stata15 (StataCorp, TX) statistical package was used. The package “cvauroc” was used for cross‐validation of the areas under the receiver operating characteristic curve, and package “cutpt” was used for empirical estimation of optimal cutoffs. A 2‐sided P<0.05 was considered statistically significant.
Results
Baseline Characteristics
The analytic sample consisted of 380 patients. As shown in Table 1, baseline characteristics were typical of a CRT population (age 72.0±12.4 years [mean±SD], 68.7% male) with a left ventricular ejection fraction of 25.8±9.9% and a QRSd of 153.5±22.7 ms. The cutoff of pre‐CRT QRSarea derived from the Liu method was 102 μVs (86–119 μVs). The QRSarea groups were well matched for age, New York Heart Association class, diabetes mellitus and hypertension status, upgrade status, left ventricular ejection fraction, and medication. In the QRSarea <102 μVs group, patients were more likely to be male, to have ischemic cardiomyopathy or a previous coronary artery bypass operation, and a greater proportion received CRT with defibrillation rather than CRT‐pacing.
Table 1.
Characteristics of the Study Group According to Pre‐Implantation QRS Area
| All | QRSarea ≥102 μVs | QRSarea <102 μVs | P Value | |
|---|---|---|---|---|
| N | 380 | 197 | 183 | |
| Age, y | 72±12.4 | 72.2±12.9 | 71.9±11.7 | 0.832 |
| Sex (male), n (%) | 261 (68.68) | 119 (60.41) | 142 (77.6) | <0.001 |
| NYHA class, n (%) | ||||
| I | 26 (7.34) | 17 (9.34) | 9 (5.23) | 0.190 |
| II | 87 (24.58) | 46 (25.27) | 41 (23.84) | |
| III | 225 (63.56) | 114 (62.64) | 111 (64.53) | |
| IV | 16 (4.52) | 5 (2.75) | 11 (6.4) | |
| Cause, n (%) | ||||
| Ischemic | 182 (47.89) | 74 (37.56) | 108 (59.02) | <0.001 |
| Nonischemic | 198 (52.11) | 123 (62.44) | 75 (40.98) | |
| Comorbidities, n (%) | ||||
| Diabetes mellitus | 89 (23.42) | 41 (20.81) | 48 (26.23) | 0.213 |
| Hypertension | 106 (27.89) | 57 (28.93) | 49 (26.78) | 0.639 |
| CABG | 64 (16.84) | 23 (11.68) | 41 (22.4) | 0.005 |
| Device type, n (%) | ||||
| CRT‐D | 209 (55.15) | 94 (47.96) | 115 (62.84) | 0.004 |
| CRT‐P | 170 (44.85) | 102 (52.04) | 68 (37.16) | |
| Upgrades, n (%) | ||||
| Pacemaker to CRT‐D | 39 (47.56) | 23 (43.40) | 16 (55.17) | 0.307 |
| Pacemaker to CRT‐P | 43 (52.44) | 30 (56.60) | 13 (44.83) | |
| CRT‐D indicationa | ||||
| Primary prevention | 166 (79.4) | 76 (80.9) | 90 (78.3) | 0.645 |
| Secondary prevention | 43 (20.6) | 18 (19.1) | 25 (21.7) | |
| LVEF, % | 25.8±9.9 | 25.5±9.7 | 26.0±10.3 | 0.633 |
| Medication, n (%) | ||||
| ACEI/ARA | 337 (89.63) | 173 (88.72) | 164 (90.61) | 0.548 |
| β‐Blocker | 277 (73.67) | 140 (71.79) | 137 (75.69) | 0.391 |
| MRA | 167 (44.41) | 80 (41.03) | 87 (48.07) | 0.170 |
| ECG variables | ||||
| Sinus rhythm, n (%) | 269 (70.79) | 152 (77.16) | 117 (63.93) | 0.005 |
| AF/flutter, n (%) | 111 (29.21) | 45 (22.84) | 66 (36.07) | |
| PR interval, ms | 192.5±54.7 | 181.6±39.8 | 207.5±67.5 | <0.001 |
| QRSd, ms | 153.5±22.7 | 163.9±20.4 | 142.2±19.5 | <0.001 |
| QRS <150 ms, n (%) | 169 (44.47) | 45 (22.84) | 124 (67.76) | <0.001 |
| LBBB, n (%) | 239 (62.89) | 151 (76.65) | 88 (48.09) | <0.001 |
| RBBB, n (%) | 33 (8.68) | 1 (0.51) | 32 (17.49) | <0.001 |
| NICD, n (%) | 59 (15.53) | 5 (2.54) | 54 (29.51) | <0.001 |
| RV‐paced, n (%) | 49 (12.89) | 40 (20.30) | 9 (4.92) | <0.001 |
| Vectorcardiography variable | ||||
| QRSarea, μVs | 113.4±56.5 | 156.9±41.7 | 66.6±22.7 | <0.001 |
ACEI indicates angiotensin receptor converting enzyme inhibitor; AF, atrial fibrillation; ARA, angiotensin receptor antagonist; CABG, coronary artery bypass graft; CRT‐D, cardiac resynchronization therapy with defibrillation; CRT‐P, cardiac resynchronization therapy‐pacing; LBBB, left bundle branch block; MRA, mineralocorticoid receptor antagonist; LVEF, left ventricular ejection fraction; NICD, nonspecific conduction delay; NYHA, New York Heart Association; QRSarea, QRS area; QRSd, QRS duration; RBBB, right bundle branch block; RV, right ventricular.
Expressed as a percentage of CRT‐D devices.
Pre‐CRT QRSarea
Over a median follow‐up period of 3.8 years (interquartile range 2.3–5.3), 135/380 (36%) patients died, 70/380 (18%) from cardiac causes and 31/380 (8%) from noncardiac causes (Table 2). The cause of death was unknown in 34/380 (9%).
Table 2.
Clinical Outcomes
| All | Pre‐CRT | Post‐CRT | |||
|---|---|---|---|---|---|
| QRSarea ≥102 μVs | QRSarea <102 μVs | QRSarea Reduction ≥45 μVs | QRSarea Reduction <45 μVs | ||
| N | 380 | 197 | 183 | 177 | 203 |
| Mortality end points | |||||
| Cardiac mortality | 70 (18.4) | 21 (10.7) | 49 (26.8) | 11 (6.21) | 59 (29.1) |
| Sudden cardiac deatha | 5 (7.14) | 1 (4.76) | 4 (8.16) | 0 | 5 (8.47) |
| Death from pump failurea | 63 (90.0) | 19 (90.5) | 44 (89.8) | 10 (90.9) | 53 (89.8) |
| Total mortality | 135 (35.5) | 55 (27.9) | 80 (43.7) | 42 (23.7) | 93 (45.8) |
| Total mortality or HF hospitalization | 165 (43.4) | 66 (33.5) | 99 (54.1) | 53 (29.9) | 112 (55.2) |
| Total mortality or hospitalization for MACE | 185 (48.7) | 74 (37.6) | 111 (60.7) | 59 (33.3) | 126 (62.1) |
| Ventricular arrhythmic events | |||||
| All VT/VF | 32 (8.42) | 12 (6.09) | 20 (10.9) | 7 (3.95) | 25 (12.3) |
| VT/VF treated with ATP only | 6 (15.8) | 4 (2.03) | 2 (1.09) | 3 (1.69) | 3 (1.48) |
| Appropriate shocks (with or without ATP) | 19 (5.0) | 7 (3.55) | 14 (7.65) | 2 (1.13) | 17 (8.37) |
| Inappropriate shocks | 1 (0.26) | 0 | 1 (0.5) | 0 | 1 (0.5) |
Clinical outcomes, expressed as n (%), according to pre‐implantation QRS area (QRSarea) and postimplantation change in QRSarea. ATP indicates antitachycardia pacing; CRT, cardiac resynchronization therapy; HF, heart failure; MACE, major adverse cardiac events; VF, ventricular fibrillation; VT, ventricular tachycardia.
Expressed as a percentage of cardiac deaths.
The AUC for predicting cardiac mortality was higher for QRSarea than for QRSd or QRS morphology (0.71, 0.60, and 0.53, respectively; P<0.001 for comparison) (Figure 2) and for QRSd and QRS morphology combined (AUC: 0.66; P=0.002). In Kaplan–Meier survival analyses, QRSarea ≥102 μVs was associated with a lower cardiac mortality (P<0.001), total mortality (P=0.001), total mortality or HF hospitalization, and total mortality or MACE (both P<0.001) (Table 2 and Figure 3). In univariable Cox proportional hazards analyses, QRSarea predicted cardiac mortality, total mortality, total mortality or HF hospitalization, and total mortality or MACE (all P<0.001) (Table 3). In multivariate analyses, QRSarea (per μVs) predicted cardiac mortality (adjusted hazard ratio [HR]: 0.99 [95% CI]: 0.98–0.99), independent of all baseline variables, including QRSd and QRS morphology (Table 4).
Figure 2.
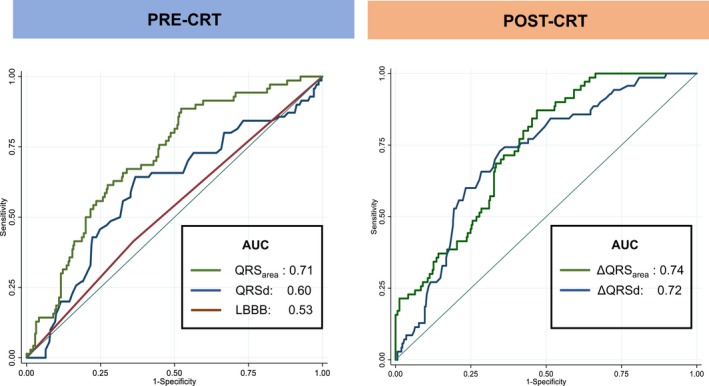
Receiver‐operator characteristic curves. Graphs show areas under the receiver‐operator characteristic curves (AUC) for QRSd, QRS area, and QRS morphology (LBBB) in the whole cohort. AUC indicates area under the curve; CRT, cardiac resynchronization therapy; LBBB, left bundle branch block.
Figure 3.
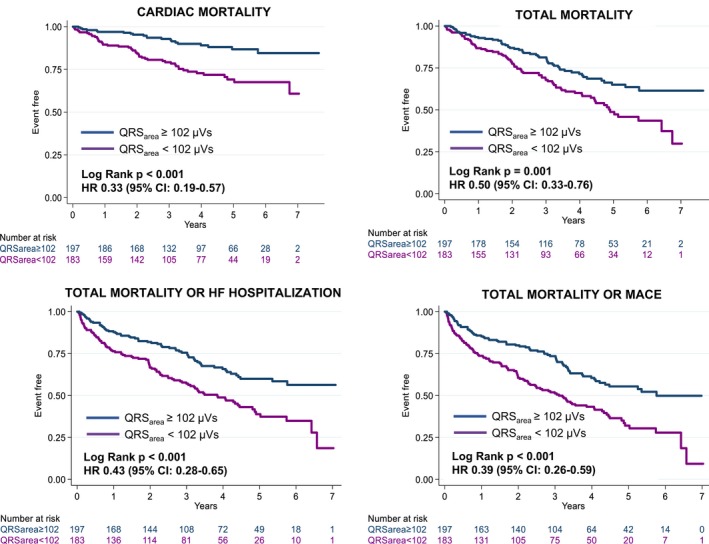
Clinical outcomes according to pre‐implantation QRS area. Kaplan–Meier survival curves for the various end points according to precardiac resynchronization therapy QRS area. Results of univariate Cox proportional hazard models are expressed in terms of hazard ratio (HR) (95% CI). HF indicates heart failure; MACE, major adverse cardiac events.
Table 3.
Univariable Analyses of Predictors of Clinical Outcomes After CRT
| Cardiac Mortality | Total Mortality | Total Mortality or HF Hospitalization | Total Mortality or MACE | SCD, VT/VF or Shock | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | ||||||
| Pre‐CRT | ||||||||||||||||||||
| QRSarea (≥102 μVs) | 0.33 | 0.19 | 0.57 | <0.001 | 0.50 | 0.33 | 0.76 | <0.001 | 0.43 | 0.28 | 0.65 | <0.001 | 0.39 | 0.26 | 0.59 | <0.001 | 0.50 | 0.25 | 0.98 | 0.042 |
| QRSd (≥150 ms) | 0.37 | 0.22 | 0.64 | <0.001 | 0.60 | 0.39 | 0.92 | 0.018 | 0.60 | 0.40 | 0.91 | 0.016 | 0.53 | 0.35 | 0.80 | 0.002 | 0.38 | 0.19 | 0.75 | 0.006 |
| LBBB | 0.80 | 0.47 | 1.36 | 0.408 | 0.75 | 0.49 | 1.15 | 0.192 | 0.67 | 0.44 | 1.02 | 0.061 | 0.82 | 0.54 | 1.25 | 0.355 | 0.69 | 0.36 | 1.31 | 0.251 |
| Post‐CRT | ||||||||||||||||||||
| ΔQRSarea, μVs | 1.02 | 1.01 | 1.02 | <0.001 | 1.01 | 1.01 | 1.01 | <0.001 | 1.01 | 1.01 | 1.01 | <0.001 | 1.01 | 1.01 | 1.01 | <0.001 | 1.01 | 1.01 | 1.02 | 0.003 |
| QRSarea reduction ≥45 μVs | 0.19 | 0.10 | 0.36 | <0.001 | 0.45 | 0.31 | 0.65 | <0.001 | 0.44 | 0.32 | 0.61 | <0.001 | 0.43 | 0.31 | 0.58 | <0.001 | 0.26 | 0.11 | 0.58 | 0.001 |
| ΔQRSd, ms | 1.02 | 1.01 | 1.03 | <0.001 | 1.01 | 1.01 | 1.02 | <0.001 | 1.01 | 1.01 | 1.02 | <0.001 | 1.01 | 1.02 | 1.02 | <0.001 | 1.01 | 1.01 | 1.03 | 0.003 |
| QRSd reductiona | 0.23 | 0.14 | 0.39 | <0.001 | 0.48 | 0.34 | 0.68 | <0.001 | 0.48 | 0.35 | 0.65 | <0.001 | 0.51 | 0.38 | 0.68 | <0.001 | 0.33 | 0.17 | 0.67 | 0.018 |
Results of univariate Cox proportional hazards models, expressed as hazard ratio (HR) and 95% CI, for QRS area (QRSarea) and QRS duration (QRSd) using specified cutoffs (in parentheses), and for QRS morphology (LBBB). Results of analyses using continuous and dichotomous variables are shown. CRT indicates cardiac resynchronization therapy; HF, heart failure; LBBB, left bundle branch block; MACE, major adverse cardiac events; SCD, sudden cardiac death; VF, ventricular fibrillation; VT, ventricular tachycardia.
Refers to any QRSd reduction below baseline.
Table 4.
Univariate and Multivariate Analysis of Pre‐Implantation Variables in Relation to Cardiac Mortality
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |||
| Age, y | 1.02 | 1.00 | 1.04 | 0.044 | 1.02 | 1.00 | 1.05 | 0.097 |
| Sex (male) | 2.25 | 1.21 | 4.19 | 0.011 | 1.57 | 0.80 | 3.07 | 0.186 |
| NYHA class (I, II) | 0.61 | 0.32 | 1.14 | 0.122 | ||||
| Ischemic cause | 1.73 | 1.07 | 2.80 | 0.026 | 1.07 | 0.63 | 1.80 | 0.813 |
| Comorbidities | ||||||||
| Diabetes mellitus | 2.08 | 1.28 | 3.38 | 0.003 | 1.56 | 0.95 | 2.58 | 0.081 |
| Hypertension | 1.25 | 0.76 | 2.08 | 0.380 | ||||
| CABG | 1.46 | 0.83 | 2.54 | 0.187 | ||||
| CRT‐D | 0.79 | 0.49 | 1.26 | 0.327 | ||||
| Upgrades | 1.18 | 0.67 | 2.09 | 0.564 | ||||
| LVEF (%) | 0.98 | 0.96 | 1.01 | 0.141 | ||||
| Medication | ||||||||
| ACEI/ARA | 0.56 | 0.29 | 1.10 | 0.092 | 0.63 | 0.31 | 1.27 | 0.196 |
| β‐Blocker | 0.80 | 0.48 | 1.33 | 0.389 | ||||
| MRA | 1.24 | 0.78 | 1.98 | 0.371 | ||||
| ECG variables | ||||||||
| AF/flutter | 1.62 | 1.01 | 2.62 | 0.047 | 1.03 | 0.60 | 1.76 | 0.925 |
| PR interval, ms | 1.00 | 1.00 | 1.01 | 0.153 | ||||
| LBBB | 0.79 | 0.49 | 1.27 | 0.331 | ||||
| RBBB | 2.50 | 1.34 | 4.65 | 0.004 | 0.92 | 0.42 | 2.02 | 0.831 |
| RV‐paced | 0.56 | 0.22 | 1.38 | 0.207 | ||||
| NICD | 1.08 | 0.58 | 2.01 | 0.806 | ||||
| QRSd, ms | 0.99 | 0.98 | 1.00 | 0.075 | 1.01 | 0.99 | 1.02 | 0.219 |
| QRSarea, μVs | 0.99 | 0.98 | 0.99 | <0.001 | 0.98 | 0.98 | 0.99 | <0.001 |
ACEI indicates angiotensin receptor converting enzyme inhibitor; AF, atrial fibrillation; ARA, angiotensin receptor antagonist; CABG, coronary artery bypass graft; CRT‐D, cardiac resynchronization therapy with defibrillation; HR, hazard ratio; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NICD, nonspecific intraventricular conduction delay; NYHA, New York Heart Association; QRSarea, QRS area; QRSd, QRS duration; RBBB, right bundle branch block; RV, right ventricular.
Post‐CRT QRSarea
The QRSarea decreased by 41.0 μVs (interquartile range: −79 to −4) after CRT (Figure 4). The cutoff of ΔQRSarea derived from Liu method was −45 μVs ([−60] to [−31] μVs). As shown in Table 5, the ΔQRSarea groups were well matched for age, New York Heart Association class, hypertension and diabetes mellitus status, device type, left ventricular ejection fraction, and medical therapy (Table 5). A QRSarea reduction ≥45 μVs group had a lower proportion of men (P<0.001), and most had nonischemic cardiomyopathy (P<0.001). As expected from the ΔQRSarea grouping, there were significant differences in ECG and vectorcardiography variables.
Figure 4.
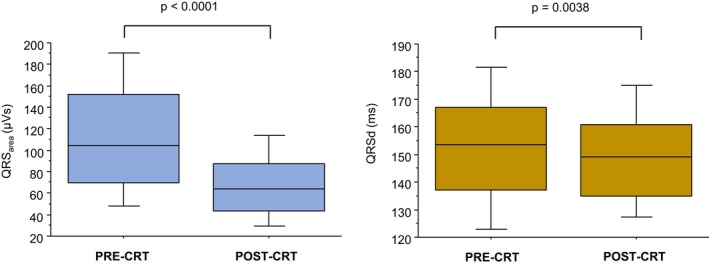
Postimplantation changes in QRS area and QRS duration. Box‐and‐whisker plots of QRS area (left) and QRS duration (QRSd) (right) before and after CRT implantation. The horizontal line denotes the median, whereas the lower and upper limits of the box denote the first and third quartiles. The limits of the vertical bar denote maximum and minimum. CRT indicates cardiac resynchronization therapy.
Table 5.
Characteristics of the Study Group According to Post‐Implantation Change in QRS Area
| QRSarea Reduction ≥45 μVs | QRSarea Reduction <45 μVs | P Value | |
|---|---|---|---|
| N | 177 | 203 | |
| Age, y | 72.1±13.1 | 72±11.7 | 0.979 |
| Sex (male), n (%) | 103 (58.19) | 158 (77.83) | <0.001 |
| NYHA class, n (%) | |||
| I | 14 (8.48) | 12 (6.35) | 0.322 |
| II | 36 (21.82) | 51 (26.98) | |
| III | 110 (66.67) | 115 (60.85) | |
| IV | 5 (3.03) | 11 (5.82) | |
| Cause, n (%) | |||
| Ischemic | 67 (37.85) | 115 (56.65) | <0.001 |
| Nonischemic | 110 (62.15) | 88 (43.35) | |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 44 (24.86) | 45 (22.17) | 0.537 |
| Hypertension | 50 (28.25) | 56 (27.59) | 0.886 |
| CABG | 17 (9.6) | 47 (23.15) | <0.001 |
| Device type, n (%) | |||
| CRT‐D | 90 (51.14) | 119 (58.62) | 0.144 |
| CRT‐P | 86 (48.86) | 84 (41.38) | |
| Upgrades, n (%) | |||
| Pacemaker to CRT‐D | 14 (35) | 25 (59.52) | 0.026 |
| Pacemaker to CRT‐P | 26 (65) | 17 (40.48) | |
| LVEF | 25.3±9.1 | 26.2±10.7 | 0.429 |
| Medication, n (%) | |||
| ACEI/ARA | 154 (88) | 183 (91.04) | 0.334 |
| β‐Blocker | 125 (71.43) | 152 (75.62) | 0.357 |
| MRA | 82 (46.86) | 85 (42.29) | 0.374 |
| ECG variables | |||
| Sinus rhythm, n (%) | 139 (78.53) | 130 (64.04) | 0.002 |
| AF/flutter, n (%) | 38 (21.47) | 73 (35.96) | |
| PR interval, ms | 178.3±34.5 | 208.8±67.6 | <0.001 |
| QRSd, ms | 162.1±20.6 | 145.9±21.8 | <0.001 |
| LBBB, n (%) | 134 (75.71) | 105 (51.72) | <0.001 |
| RBBB, n (%) | 2 (1.13) | 31 (15.27) | <0.001 |
| NICD, n (%) | 10 (5.65) | 49 (24.14) | <0.001 |
| RV‐paced, n (%) | 31 (17.51) | 18 (8.87) | 0.012 |
| Vectorcardiography variable | |||
| QRSarea, μVs | 153.9±47.5 | 78.1±36.3 | <0.001 |
| Circumferential lead positions | |||
| Anterior | 6 (3.39) | 8 (3.94) | 0.498 |
| Anterolateral | 28 (15.8) | 37 (18.2) | |
| Lateral | 73 (41.2) | 86 (42.3) | |
| Posterolateral | 24 (13.6) | 16 (9.03) | |
| Posterior | 46 (26.0) | 56 (27.6) | |
| Longitudinal lead positions | |||
| Basal | 19 (10.7) | 12 (5.91) | 0.249 |
| Mid | 99 (55.9) | 121 (59.6) | |
| Apical | 59 (33.3) | 70 (34.5) | |
ACEI indicates angiotensin receptor converting enzyme inhibitor; AF, atrial fibrillation; ARA, angiotensin receptor antagonist; CABG, coronary artery bypass graft; CRT‐D, cardiac resynchronization therapy with defibrillation; CRT‐P, cardiac resynchronization therapy‐pacing; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NICD, nonspecific intraventricular conduction delay; NYHA, New York Heart Association; QRSarea, QRS area; QRSd, QRS duration; RBBB, right bundle branch block; RV, right ventricular.
Cardiac mortality was 11/177 (6.21%) in patients with QRSarea reduction ≥45 μVs and 59/203 (29.1%) in patients with QRSarea reduction <45 μVs. (Table 2). The AUC for predicting cardiac mortality for ΔQRSarea and ΔQRSd were similar (0.74 versus 0.72; P=0.425 for comparison) (Figure 2). In Kaplan–Meier survival analyses, a QRSarea reduction ≥45 μVs was associated with a lower cardiac mortality, total mortality, total mortality or HF hospitalization, and total mortality or MACE, compared with a QRSarea reduction <45 μVs (all P<0.001) (Figures 5 and 6). In univariate analyses, a QRSarea reduction ≥45 μVs was a strong predictor of cardiac mortality (HR: 0.19, 95% CI: 0.10–0.36), as well as other end points (all P<0.001) (Table 3).
Figure 5.
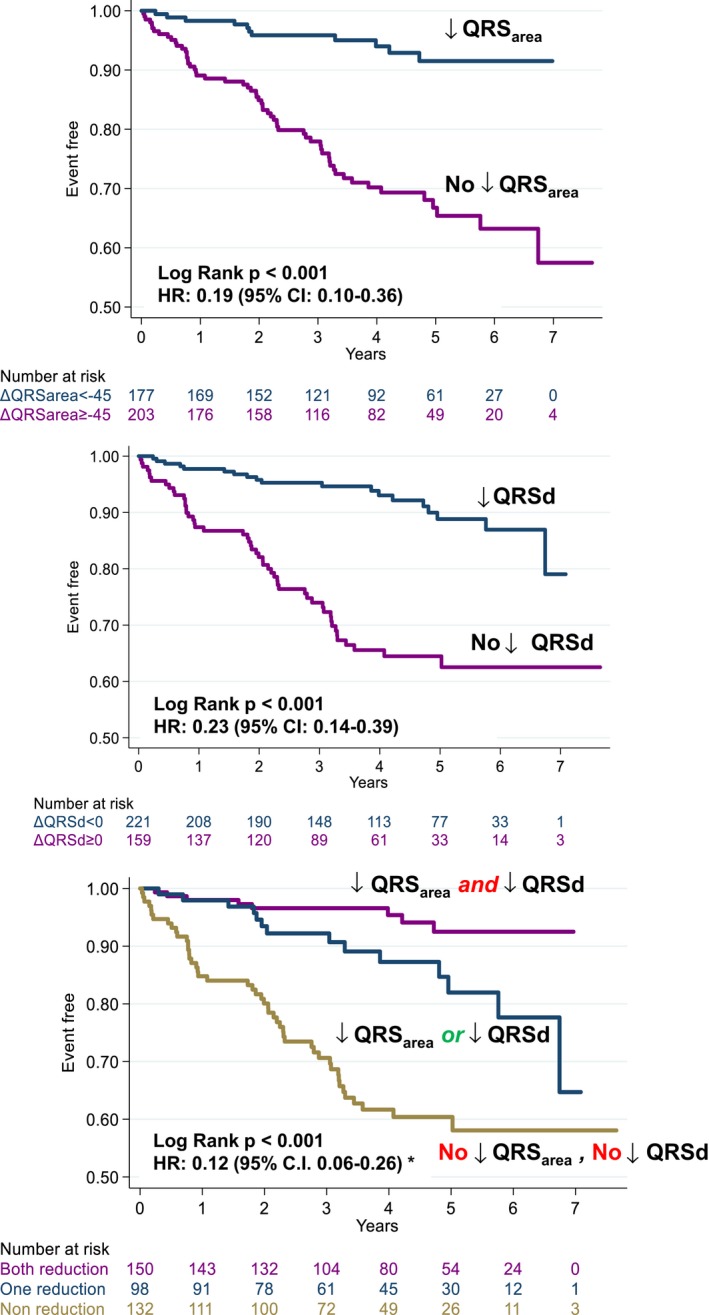
QRS area and QRS duration in relation to cardiac mortality. Kaplan–Meier survival curves and univariate HR and (95% CI for QRS area (QRS area) and QRS duration (QRSd) in relation to cardiac mortality. *Refers to the interaction between changes in QRSarea and QRSd after CRT. HR indicates hazard ratios.
Figure 6.
Secondary clinical end points according to changes in QRS area and QRS duration. Kaplan–Meier survival curves for the various end points according to postcardiac resynchronization therapy reductions in QRS area (≥45 μVs) QRS duration (QRSd; to any value below baseline). HF indicates heart failure; MACE, major adverse cardiac events.
Interaction of ΔQRSarea and ΔQRSd
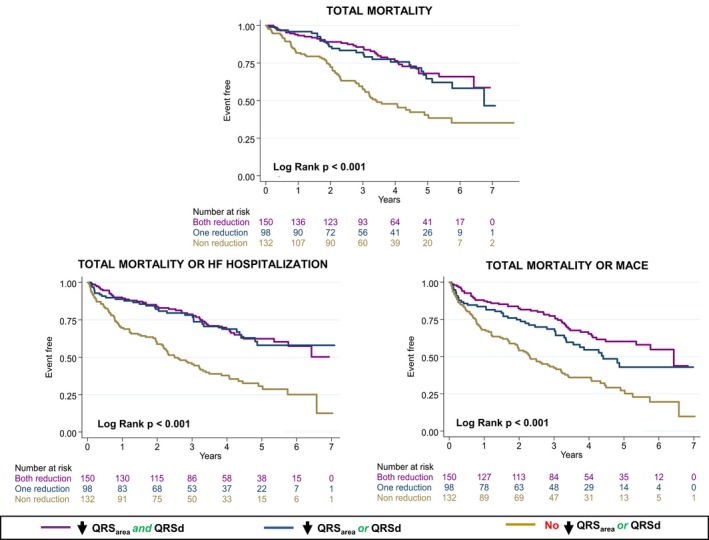
As shown in Figures 2 and 5, ΔQRSarea and ΔQRSd were comparable predictors of cardiac mortality. In Cox proportional hazard analyses, a significant interaction between ΔQRSarea and ΔQRSd emerged with respect to cardiac mortality (HR: 0.12, 96% CI 0.06–0.26). A similar trend was observed for total mortality, total mortality or HF hospitalization, and total mortality or MACE (Figure 6).
Lead Positions
Most LV leads were deployed in a lateral or posterolateral position (Table 5). As shown in Figure 7, there was considerable interindividual variability in ΔQRSarea and ΔQRSd within each LV lead position, but no significant differences emerged in ΔQRSarea or ΔQRSd between the different LV lead positions.
Figure 7.
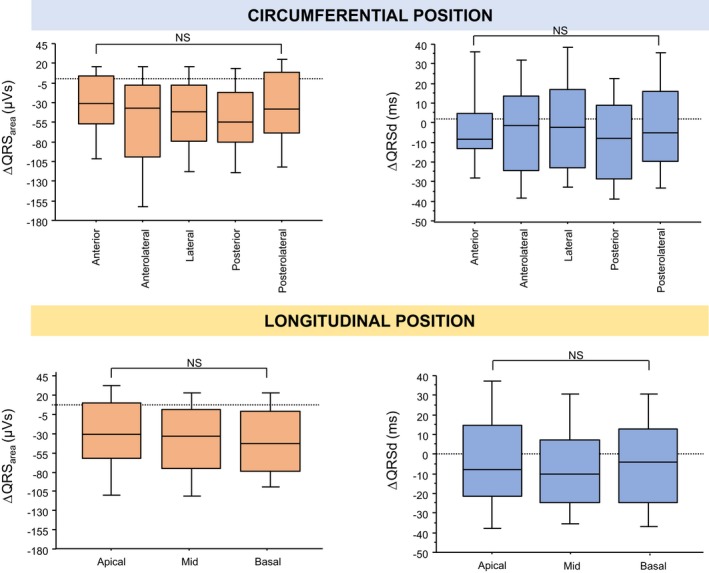
Postimplantation changes QRS area and QRS duration according to left ventricular lead position. The figure shows post– cardiac resynchronization therapy changes in QRS area (ΔQRS area) and QRS duration (ΔQRSd) according to circumferential (upper panel) and longitudinal (lower panel) left ventricular lead positions. In the box‐and‐whisker plots, the horizontal line denotes the median, whereas the lower and upper limits of the box denote the first and third quartiles. The limits of the vertical bar denote maximum and minimum. NS indicates not significant.
Arrhythmic events
As shown in Table 3 and Figure 8, both QRSd and QRSarea predicted the combined end point of sudden cardiac death, ventricular tachycardia/ventricular fibrillation or shock, but no such relationship was observed for QRS morphology. A QRSarea reduction ≥45 μVs (HR: 0.26, 95% CI 0.11–0.58) and QRSd reduction (HR: 0.33, 95% CI 0.17–0.67) predicted this combined end point. Concomitant reductions in QRSarea and QRSd were associated with the lowest risk of the arrhythmic end point (HR: 0.12, 95% CI 0.04–0.41).
Figure 8.
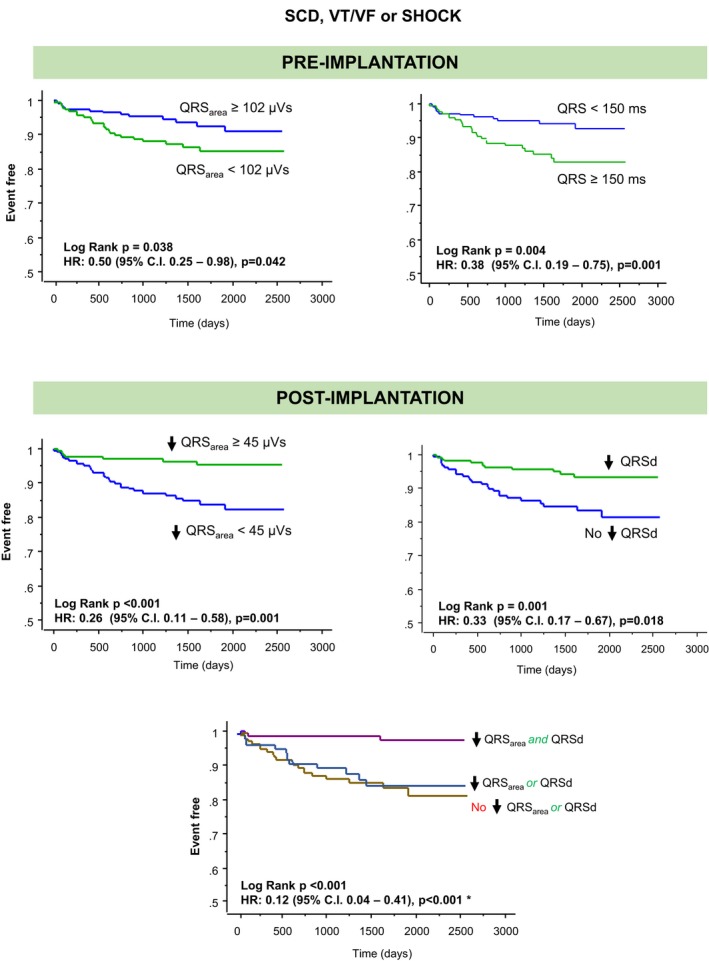
Sudden cardiac death and ventricular arrhythmias according to postimplantation changes in QRS area and QRS duration. Kaplan–Meier survival curves for the combined end point of sudden cardiac death (SCD), ventricular tachycardia (VT)/ventricular fibrillation (VF), or shock according to postimplantation reductions in QRS area (QRS area, ≥45 μVs) QRS duration (QRSd to any value below baseline). *Refers to the comparison of the group with concomitant reductions in QRS area (≥45 μVs) and QRSd against the group with no reductions in either variable. HR indicates hazard ratio.
Discussion
This is the first study to explore both pre‐ and postimplantation QRSarea in relation to long‐term, cause‐specific mortality, as well as long‐term HF hospitalization, MACE, and ventricular arrhythmias after CRT. Several findings have emerged. First, pre‐implantation QRSarea was superior to QRSd and QRS morphology in predicting cardiac mortality after CRT. Second, a QRSarea reduction after CRT was associated with favorable outcomes, independent of baseline QRSd or QRS morphology. Third, the best outcomes after CRT were observed in patients exhibiting concomitant reductions in QRSarea and QRSd.
Pre‐CRT QRSarea
This study provides an external validation of the findings of 2 observational studies showing that QRSarea is superior to QRSd and QRS morphology in predicting total mortality after CRT.11, 12 We found that QRSarea (<102 μVs) predicted total mortality, with an AUC of 0.71, which is higher than the AUC of 0.61 identified by van Stipdonk et al using a cutoff of 109 μVs.11 Emerek et al found that a QRSarea ≤95 μVs was associated with a higher total mortality than a QRSarea >95 μVs, with an unadjusted HR of 2.11 (P<0.001).12 Using a cutoff of 102 μVs, we have found an unadjusted HR of 1.73 (P=0.002) for total mortality and 2.77 for cardiac mortality (P<0.001).
Previous studies on QRSarea 11, 12 did not address cause‐specific mortality and only 1 year follow‐up data were provided with respect to HF hospitalization.11 We found that, in addition to predicting total mortality, QRSarea predicted cardiac mortality, total mortality or HF hospitalization, and total mortality or MACE. The relation between a high QRSarea and better clinical outcomes after CRT is not unexpected, because QRS area correlates with electrical dyssynchrony,8 the natural substrate of CRT.
Post‐CRT ΔQRSarea
In an acute hemodynamic study of 25 patients with LBBB, De Pooter et al showed that ΔQRSarea correlated with ΔLV dP/dtmax.8 This is consistent with our finding that a reduction in QRSarea was associated with a lower cardiac mortality, as well as other end points. While De Pooter et al8 found that ΔQRSarea after CRT was a stronger correlate of ΔLV dP/dtmax than ΔQRSd, we found that both ΔQRSd and ΔQRSarea were comparable in predicting long‐term clinical outcomes. Importantly, the combination of ΔQRSd and ΔQRSarea had additive effects in predicting cardiac mortality: patients who exhibited reductions in both variables experienced the best outcomes after CRT, whereas patients who did not exhibit reductions in either experienced the worst outcomes.
QRSd
Randomized, controlled trials of CRT19, 20 adopted a QRSd ≥120 ms as an indication for CRT. In COMPANION (Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure), patients without LBBB and those with QRSd ≤147 ms did not derive a benefit.19 Similarly, in the MADIT‐CRT (Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy) trial, patients with a QRSd <150 ms derived no survival benefit from CRT.21, 22 In the present study, we found that a QRSd ≥150 ms was associated with a lower cardiac mortality, compared with a QRSd <150 ms. The ability of QRSd to predict cardiac mortality, however, was relatively weak (AUC: 0.60).
Meta‐analyses of observational studies have shown an inconsistent relationship between post‐CRT ΔQRSd23, 24 and “clinical response.” In these meta‐analyses, however, “clinical response” was defined in terms of symptoms, echocardiographic variables, and/or hard end points, assuming that these are identical, interchangeable measures. On the other hand, studies focusing on hard end points do indeed support a relationship between a QRSd reduction and better outcomes after CRT. The REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) study, the only randomized controlled trial to address ΔQRSd after CRT, explored acute ΔQRSd in the CRT‐treated group in relation to the primary end point of the clinical composite score, as well as LV reverse remodeling.25 Although not designed to address hard end points, REVERSE reported an association between ΔQRSd and total mortality or HF hospitalization over a relatively short follow‐up (12 months in North America and for 24 months in Europe) on univariate analyses, but not in a multivariate model that corrected for baseline QRSd. Importantly, however, the CRT‐treated group in REVERSE only had 4 deaths over 24 months, raising the possible play of statistical underpowering. In contrast, in an observational study, Appert et al showed that a lack of postoperative QRSd reduction was independently associated with an increased risk of total mortality over a median follow‐up period of 48 months.6 In a similar study, Jastrzebski et al showed that a QRSd reduction predicted death from any cause or urgent heart transplantation and death from any cause/urgent heart transplantation or hospital admission for HF over an average follow‐up period of 46 months.5 In the present study, in which 135 deaths occurred over a median follow‐up of 3.8 years, a QRSd reduction below baseline predicted cardiac mortality, total mortality, total mortality or HF hospitalization, and total mortality or MACE.
QRS Morphology
Observational studies26, 27 as well as large registries28 and subanalyses of randomized, controlled trials19, 22, 25, 29 have shown that patients with a LBBB morphology derive the most benefit from CRT. While some studies have suggested that a LBBB defined using “strict” criteria, with notching and/or slurring of the QRS complex, is associated with a better left ventricular ejection fraction response to CRT,10, 27 this is not a consistent finding.30, 31 Moreover, Emerek et al found that “strict” (Strauss) criteria of LBBB was not predictive of clinical outcomes after CRT.12 In the present study, a conventionally defined LBBB did not predict cardiac mortality after CRT (AUC: 0.53).
Lead Position
We have observed a considerable interindividual variability in QRSarea at a given LV lead position. In this regard, De Pooter et al also found a similar interindividual variability in QRSarea in CRT recipients with a LBBB.8 Crucially, they also found that QRSarea and the acute hemodynamic response to CRT in a given patient could be improved by changing the LV lead position. Together, these findings make the case for optimization of QRSarea in CRT recipients. To date, however, no studies have prospectively explored this issue.
Arrhythmic events
Several studies have suggested that QRSd predicts sudden cardiac death.32, 33 In contrast, no studies have explored QRSarea or ΔQRSarea in relation to sudden cardiac death or ventricular tachycardia/ventricular fibrillation. Although pre‐implantation QRSarea did not predict this end point, its reduction was associated with a 74% reduction in the end point. Moreover, concomitant with QRSarea reduction, a QRd reduction was associated with an 88% lower risk of the combined end point. This novel finding, which was not anticipated, could speculatively relate to a greater dispersion of depolarization in relation to arrhythmic events. The physiological basis for this empirical finding requires further study.
Clinical Perspectives
Attention has recently focused on ECG imaging using body surface mapping as a tool for identifying electrical dyssynchrony and to predict response to CRT.34, 35 Although there is a proof‐of‐principle and encouraging clinical data to support the use of this technique in CRT, it requires specialized acquisition. Importantly, data on body surface mapping in relation to long‐term outcomes after CRT are lacking. In contrast, QRSarea can be readily derived from the standard 12‐lead ECG and crucially, is now known to predict long‐term clinical outcomes. The role of QRSarea in patient selection and CRT optimization requires further investigation.
Limitations
This study has all the limitations of an observational study. Although we have corrected for potential confounders using statistical means, unobserved variables may have contributed to outcomes. Importantly, vectorcardiographies were derived retrospectively from 12‐lead ECGs undertaken before implantation. Inconsistencies in electrode position could conceivably influence vectorcardiography analysis.36 Notwithstanding, all ECGs were acquired by trained cardiac technicians using a standardized operating procedure in routine clinical practice. Consequently, our results should be generalizable to a “real‐world” environment. Unfortunately, we did not systematically collect data on device programming. In this respect, variable programming at implantation and follow‐up could account for variations in ECG and vectorcardiography variables, as well as outcomes. Although the AUCs for pre‐implant QRSarea and ΔQRSarea did not exceed 0.74, these values are comparable to those found in other studies11 and exceed the AUCs for QRSd and LBBB.
Conclusions
Pre‐implantation QRSarea was superior to QRSd and QRS morphology in predicting clinical outcomes after CRT. A concomitant reduction in QRSd and QRSarea after CRT was associated with the lowest risk of cardiac and total mortality, as well as ventricular arrhythmias. These findings add support for the use of QRSarea and QRSd in the risk stratification and optimization of CRT recipients.
Sources of Funding
Okafor was supported by an unrestricted educational grant from Medtronic Plc.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e013539 DOI: 10.1161/JAHA.119.013539.)
References
- 1. Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol. 2014;64:1047–1058. [DOI] [PubMed] [Google Scholar]
- 2. Fornwalt BK, Sprague WW, BeDell P, Suever JD, Gerritse B, Merlino JD, Fyfe DA, Leon AR, Oshinski JN. Agreement is poor among current criteria used to define response to cardiac resynchronization therapy. Circulation. 2010;121:1985–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Auricchio A, Prinzen FW. Enhancing response in the cardiac resynchronization therapy patient: the 3B perspective‐bench, bits, and bedside. JACC Clin Electrophysiol. 2017;3:1203–1219. [DOI] [PubMed] [Google Scholar]
- 4. Chung E, Leon A, Tavazzi L, Sun J, Nihoyannopoulos P, Merlino J, Abraham W, Guio S, Leclerq C, Bax J, Yu C‐M, Gorcsan J III, Sutton M, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. [DOI] [PubMed] [Google Scholar]
- 5. Jastrzebski M, Baranchuk A, Fijorek K, Kisiel R, Kukla P, Sondej T, Czarnecka D. Cardiac resynchronization therapy‐induced acute shortening of QRS duration predicts long‐term mortality only in patients with left bundle branch block. Europace. 2019;21:281–289. [DOI] [PubMed] [Google Scholar]
- 6. Appert L, Menet A, Altes A, Ennezat PV, Bardet‐Bouchery H, Binda C, Guyomar Y, Delelis F, Castel AL, Le Goffic C, Guerbaai RA, Graux P, Tribouilloy C, Marechaux S. Clinical significance of electromechanical dyssynchrony and QRS narrowing in patients with heart failure receiving cardiac resynchronization therapy. Can J Cardiol. 2019;35:27–34. [DOI] [PubMed] [Google Scholar]
- 7. Mafi Rad M, Wijntjens GW, Engels EB, Blaauw Y, Luermans JG, Pison L, Crijns HJ, Prinzen FW, Vernooy K. Vectorcardiographic QRS area identifies delayed left ventricular lateral wall activation determined by electroanatomic mapping in candidates for cardiac resynchronization therapy. Heart Rhythm. 2016;13:217–225. [DOI] [PubMed] [Google Scholar]
- 8. De Pooter JAN, El Haddad M, De Buyzere M, Aranda HA, Cornelussen R, Stegemann B, Rinaldi CA, Sterlinski M, Sokal A, Francis DP, Jordaens LUC, Stroobandt RX, Van Heuverswyn F, Timmermans F. Biventricular paced QRS area predicts acute hemodynamic CRT response better than QRS duration or QRS amplitudes. J Cardiovasc Electrophysiol. 2017;28:192–200. [DOI] [PubMed] [Google Scholar]
- 9. Engels EB, Strik M, van Middendorp LB, Kuiper M, Vernooy K, Prinzen FW. Prediction of optimal cardiac resynchronization by vectors extracted from electrograms in dyssynchronous canine hearts. J Cardiovasc Electrophysiol. 2017;28:944–951. [DOI] [PubMed] [Google Scholar]
- 10. van Deursen CJM, Vernooy K, Dudink E, Bergfeldt L, Crijns HJGM, Prinzen FW, Wecke L. Vectorcardiographic QRS area as a novel predictor of response to cardiac resynchronization therapy. J Electrocardiol. 2015;48:45–52. [DOI] [PubMed] [Google Scholar]
- 11. van Stipdonk AMW, Ter Horst I, Kloosterman M, Engels EB, Rienstra M, Crijns H, Vos MA, van Gelder IC, Prinzen FW, Meine M, Maass AH, Vernooy K. QRS area is a strong determinant of outcome in cardiac resynchronization therapy. Circ Arrhythm Electrophysiol. 2018;11:e006497. [DOI] [PubMed] [Google Scholar]
- 12. Emerek K, Friedman DJ, Sørensen PL, Hansen SM, Larsen JM, Risum N, Thøgersen AM, Graff C, Kisslo J, Søgaard P, Atwater BD. Vectorcardiographic QRS area is associated with long‐term outcome after cardiac resynchronization therapy. Heart Rhythm. 2018;16:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lux R, Urie P, Burgess M, Abildskov L. Variability of the body surface distributions of QRS, ST‐T and QRST deflection areas with varied activation sequence in dogs. Cardiovasc Res. 1980;14:607–612. [DOI] [PubMed] [Google Scholar]
- 14. National Institute of Health and Care Excellence . NICE technology appraisal [TA 314]: implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure (review of TA95 and TA120). 2014. London, United Kingdom. Available at: https://www.nice.org.uk/guidance/ta314. Accessed February 1, 2019.
- 15. Leyva F, Zegard A, Taylor RJ, Foley PWX, Umar F, Patel K, Panting J, van Dam P, Prinzen FW, Marshall H, Qiu T. Long‐term outcomes of cardiac resynchronization therapy using apical versus nonapical left ventricular pacing. J Am Heart Assoc. 2018;7:e008508 DOI: 10.1161/JAHA.117.008508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Linde C, Abraham WT, Gold MR, Daubert C. Cardiac resynchronization therapy in asymptomatic or mildly symptomatic heart failure patients in relation to etiology: results from the REVERSE (REsynchronization reVErses Remodeling in Systolic Left vEntricular Dysfunction) study. J Am Coll Cardiol. 2010;56:1826–1831. [DOI] [PubMed] [Google Scholar]
- 17. Rockman HA, Juneau C, Chatterjee K, Rouleau JL. Long‐term predictors of sudden and low output death in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1989;64:1344–1348. [DOI] [PubMed] [Google Scholar]
- 18. Liu X. Classification accuracy and cut point selection. Stat Med. 2012;31:2676–2686. [DOI] [PubMed] [Google Scholar]
- 19. Bristow M, Saxon L, Boehmer J, Krueger S, Kass D, De Marco T, Carson P, DiCarlo L, DeMets D, White B. Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 20. Cleland J, Daubert J, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 21. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NAM, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W. Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med. 2009;361:1329–1338. [DOI] [PubMed] [Google Scholar]
- 22. Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, Brown M, Cannom D, Daubert JP, Eldar M, Gold MR, Goldberger JJ, Goldenberg I, Lichstein E, Pitschner H, Rashtian M, Solomon S, Viskin S, Wang P, Moss AJ; Madit‐CRT Investigators . Effectiveness of cardiac resynchronization therapy by QRS morphology in the multicenter automatic defibrillator implantation trial‐cardiac resynchronization therapy (MADIT‐CRT). Circulation. 2011;123: 1061–1072. [DOI] [PubMed] [Google Scholar]
- 23. Kashani A, Barold SS. Significance of QRS complex duration in patients with heart failure. J Am Coll Cardiol. 2005;46:2183–2192. [DOI] [PubMed] [Google Scholar]
- 24. Bryant AR, Wilton SB, Lai MP, Exner DV. Association between QRS duration and outcome with cardiac resynchronization therapy: a systematic review and meta‐analysis. J Electrocardiol. 2013;46:147–155. [DOI] [PubMed] [Google Scholar]
- 25. Gold MR, Thebault C, Linde C, Abraham WT, Gerritse B, Ghio S, St John Sutton M, Daubert JC. Effect of QRS duration and morphology on cardiac resynchronization therapy outcomes in mild heart failure: results from the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study. Circulation. 2012;126:822–829. [DOI] [PubMed] [Google Scholar]
- 26. Sweeney MO, van Bommel RJ, Schalij MJ, Borleffs CJ, Hellkamp AS, Bax JJ. Analysis of ventricular activation using surface electrocardiography to predict left ventricular reverse volumetric remodeling during cardiac resynchronization therapy. Circulation. 2010;121:626–634. [DOI] [PubMed] [Google Scholar]
- 27. Tian Y, Zhang P, Li X, Gao Y, Zhu T, Wang L, Li D, Wang J, Yuan C, Guo J. True complete left bundle branch block morphology strongly predicts good response to cardiac resynchronization therapy. Europace. 2013;15:1499–1506. [DOI] [PubMed] [Google Scholar]
- 28. Bilchick Kenneth C, Kamath S, DiMarco John P, Stukenborg George J. Bundle‐branch block morphology and other predictors of outcome after cardiac resynchronization therapy in Medicare patients. Circulation. 2010;122:2022–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, Yee R, Healey JS, Rouleau JL; Resynchronization‐Defibrillation for Ambulatory Heart Failure Trial I . Cardiac‐resynchronization therapy for mild‐to‐moderate heart failure. N Engl J Med. 2010;363:2385–2395. [DOI] [PubMed] [Google Scholar]
- 30. Bertaglia E, Migliore F, Baritussio A, De Simone A, Reggiani A, Pecora D, D'Onofrio A, Rapacciuolo A, Savarese G, Pierantozzi A, Marenna B, Ruffa F, Campari M, Malacrida M, Stabile G. Stricter criteria for left bundle branch block diagnosis do not improve response to CRT. Pacing Clin Electrophysiol. 2017;40:850–856. [DOI] [PubMed] [Google Scholar]
- 31. Caputo ML, van Stipdonk A, Illner A, D'Ambrosio G, Regoli F, Conte G, Moccetti T, Klersy C, Prinzen FW, Vernooy K, Auricchio A. The definition of left bundle branch block influences the response to cardiac resynchronization therapy. Int J Cardiol. 2018;269:165–169. [DOI] [PubMed] [Google Scholar]
- 32. Kurl S, Makikallio TH, Rautaharju P, Kiviniemi V, Laukkanen JA. Duration of QRS complex in resting electrocardiogram is a predictor of sudden cardiac death in men. Circulation. 2012;125:2588–2594. [DOI] [PubMed] [Google Scholar]
- 33. Morin DP, Oikarinen L, Viitasalo M, Toivonen L, Nieminen MS, Kjeldsen SE, Dahlof B, John M, Devereux RB, Okin PM. QRS duration predicts sudden cardiac death in hypertensive patients undergoing intensive medical therapy: the LIFE study. Eur Heart J. 2009;30:2908–2914. [DOI] [PubMed] [Google Scholar]
- 34. Ploux S, Eschalier R, Whinnett ZI, Lumens J, Derval N, Sacher F, Hocini M, Jais P, Dubois R, Ritter P, Haissaguerre M, Wilkoff BL, Francis DP, Bordachar P. Electrical dyssynchrony induced by biventricular pacing: implications for patient selection and therapy improvement. Heart Rhythm. 2015;12:782–791. [DOI] [PubMed] [Google Scholar]
- 35. Gage RM, Curtin AE, Burns KV, Ghosh S, Gillberg JM, Bank AJ. Changes in electrical dyssynchrony by body surface mapping predict left ventricular remodeling in patients with cardiac resynchronization therapy. Heart Rhythm. 2017;14:392–399. [DOI] [PubMed] [Google Scholar]
- 36. Tomlinson DR, Bashir Y, Betts TR, Rajappan K. Accuracy of manual QRS duration assessment: its importance in patient selection for cardiac resynchronization and implantable cardioverter defibrillator therapy. Europace. 2009;11:638–642. [DOI] [PubMed] [Google Scholar]


