Abstract
Background
Programs targeting the standard modifiable cardiovascular risk factors (SMuRFs: hypertension, diabetes mellitus, hypercholesterolemia, smoking) are critical to tackling coronary heart disease at a community level. However, myocardial infarction in SMuRF‐less individuals is not uncommon. This study uses 2 sequential large, multicenter registries to examine the proportion and outcomes of SMuRF‐less ST‐segment–elevation myocardial infarction (STEMI) patients.
Methods and Results
We identified 3081 STEMI patients without a prior history of cardiovascular disease in the Australian GRACE (Global Registry of Acute Coronary Events) and CONCORDANCE (Cooperative National Registry of Acute Coronary Syndrome Care) registries, encompassing 42 hospitals, between 1999 and 2017. We examined the proportion that were SMuRF‐less as well as outcomes. The primary outcome was in‐hospital mortality, and the secondary outcome was major adverse cardiovascular events (death, myocardial infarction, or heart failure, during the index admission). Multivariate regression models were used to identify predictors of major adverse cardiovascular events. Of STEMI patients without a prior history of cardiovascular disease 19% also had no history of SMuRFs. This proportion increased from 14% to 23% during the study period (P=0.0067). SMuRF‐less individuals had a higher in‐hospital mortality rate than individuals with 1 or more SMuRFs. There were no clinically significant differences in major adverse cardiovascular events at 6 months between the 2 groups.
Conclusions
A substantial and increasing proportion of STEMI presentations occur independently of SMuRFs. Discovery of new markers and mechanisms of disease beyond standard risk factors may facilitate novel preventative strategies. Studies to assess longer‐term outcomes of SMuRF‐less STEMI patients are warranted.
Keywords: atherosclerosis, mortality, risk factor, ST‐segment–elevation myocardial infarction
Subject Categories: Mortality/Survival, Quality and Outcomes, Acute Coronary Syndromes, Atherosclerosis, Percutaneous Coronary Intervention
Clinical Perspective
What Is New?
The proportion of ST‐segment–elevation myocardial infarction patients without standard modifiable cardiovascular risk factors is not insubstantial and has been increasing in recent years.
ST‐segment–elevation myocardial infarction patients without standard modifiable cardiovascular risk factors have a higher in‐hospital mortality rate than ST‐segment–elevation myocardial infarction patients with 1 or more standard modifiable cardiovascular risk factors.
What Are the Clinical Implications?
Discovery of new markers and mechanisms of coronary artery disease beyond standard risk factors may facilitate new preventative strategies.
Introduction
The standard modifiable cardiovascular risk factors (SMuRFs) of diabetes mellitus, hyperlipidemia, hypertension, and cigarette smoking are central elements of the well‐established Framingham cardiovascular risk score and many subsequent validated risk scores.1, 2, 3, 4 Primary and secondary prevention programs that utilize and target susceptible individuals’ modifiable risk factors for coronary heart disease (CHD) have resulted in a substantial reduction in morbidity and mortality due to CHD, particularly in high‐income and some middle‐income countries.5 However, CHD remains a leading cause of death in all regions of the world.6, 7, 8 ST‐segment–elevation myocardial infarction (STEMI) has a higher in‐hospital mortality rate than non–ST‐segment–elevation acute coronary syndromes.9, 10 In a recent large, single‐center cohort study, we reported that 25% of first‐presentation STEMI patients, confirmed to be a result of atherosclerosis, had no known SMuRFs at the time of their event. Furthermore, the proportion of the STEMI cohort that was SMuRF‐less increased significantly during the study period (2006‐2014).11
To date, in the fields of clinical and biomedical research, there has been a lack of attention paid to the SMuRF‐less subgroup of STEMI patients. An example of this lack of attention is the traditional means of reporting risk factor profiles in STEMI clinical trials. Frequently the proportion of patients with diabetes mellitus, hypertension, smoking, and hypercholesterolemia are reported, but it is often difficult to extract information regarding the proportion without any traditional risk factors. For those that do report this, the proportion of SMuRF‐lessness is similar to what we observed.12 Given the absolute number of individuals that this proportion represents, and the mortality and morbidity of myocardial infarction, it is critical to determine any changing patterns on a broader scale than our single center and to formally study patterns of management and specific outcomes of SMuRF‐less STEMI patients.
The Australian GRACE (Global Registry of Acute Coronary Events) and CONCORDANCE (Cooperative National Registry of Acute Coronary Syndrome Care) registries capture a large, unbiased population of patients presenting with acute coronary syndrome (ACS) to 42 hospitals across Australia, from 1999 to 2017 and provide us with an opportunity to examine our 3 key questions: (1) What is the proportion of STEMI patients who are SMuRF‐less? (2) Is this changing over time? (3) What are the short‐ and longer‐term cardiovascular outcomes of SMuRF‐less STEMI patients versus those with traditional risk factors, in a broad, representative, contemporary Australian context?
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Populations
Full details of the GRACE and CONCORDANCE registry methods have been published previously.13, 14 GRACE and CONCORDANCE employed closely aligned inclusion and exclusion criteria designed to reflect an unbiased population of patients with ACS.13, 14 In addition to symptoms consistent with myocardial ischemia, patients were required to have either ECG changes consistent with ACS, elevation of serum cardiac biomarkers of myocardial necrosis or documented coronary artery disease including STEMI, non–ST‐segment–elevation myocardial infarction, and unstable angina. To enroll an unselected population, the first 10‐20 consecutive eligible patients were recruited from each site per month. Hospitals in both registries were selected to represent regional and metropolitan acute‐care facilities with and without catheterization laboratory facilities.
Inclusion Criteria
Patients included in this analysis were at least 18 years of age, presented with symptoms suggestive of coronary ischemia, and had a final diagnosis of STEMI. For this analysis, we limited our study to the Australian population enrolled in the GRACE registry between 1999 and 2007 and the CONCORDANCE registry between 2009 and 2017. No equivalent ACS registry data were available from the participating hospitals in 2008. An opt‐out informed consent process was applied with a waiver of consent for patients who were too ill to provide informed consent. All participating hospitals received human research ethics approval to participate in the registries. Approval for this analysis was granted by the lead ethics committee, Concord Hospital, Sydney Local Health District.
Exclusion Criteria
ACSs precipitated by a significant noncardiovascular comorbidity such as anemia or trauma were excluded.13 For the purposes of this study, only patients presenting with their first cardiovascular event were included in the analysis. Therefore, patients with a known history of CHD (percutaneous cardiovascular intervention, coronary artery bypass graft, ACS, or history of angina pectoris), cerebrovascular disease, or peripheral vascular disease or a final diagnosis other than STEMI, were excluded.
Data Sources
Data were collected by trained coordinators using standardized case report forms. Demographic characteristics, medical history, presenting symptoms, biochemical and electrocardiographic findings, treatment practices, and a variety of hospital outcome data were collected from the medical record. If a patient was transferred to an additional facility, data were collected from each facility. Patients were recontacted at 6, 12, and 24 months postdischarge for collection of data on unscheduled readmissions, unscheduled procedures (coronary angiography, percutaneous coronary intervention, coronary artery bypass graft surgery), and complications. To ensure data quality, a random sample of submitted case report forms were audited. In this analysis we report outcomes at 6 months only because of lower follow‐up rates at later time points. Standardized definitions for all patient‐related variables and clinical diagnoses were used across the 2 registries. We refer to a known history of hypertension, hyperlipidemia, diabetes mellitus, and current smoking as the 4 SMuRFs for cardiovascular disease (CVD). A patient was considered to be a current smoker if he or she had regularly smoked within the past 12 months. A patient was considered to have a known history of hyperlipidemia, diabetes mellitus, or hypertension if he or she identified as having a history of the same or was on pharmacological treatment for these conditions at the time of presentation. For the purposes of the study, primary analysis was focused on patients with a final diagnosis of STEMI presenting for the first time with CVD. A secondary analysis examined the proportion of SMuRF‐less patients in the registry over the same study period as the primary analysis with a final diagnosis non–ST‐segment–elevation acute coronary syndrome in the absence of a prior history of CVD as defined above.
Outcomes
The primary outcome was in‐hospital mortality, and the secondary outcome was major adverse cardiovascular events (MACE) defined as death, myocardial infarction, or heart failure during the index admission and at 6 months. The study index date for both in‐hospital and 6‐month outcomes was the admission date. The 6‐month MACE was based on postdischarge events only because not all subjects were able to be followed to 6 months.
Statistical Analyses
Categorical variables are summarized using frequencies and percentages, whereas numerical variables are summarized using mean, SD, median, and quartiles. Multivariable logistic regression analyses were performed to estimate the adjusted odds ratio and 95% CI for the binary outcomes in‐hospital mortality, in‐hospital MACE, and 6‐month MACE. The predictor variables were the components of the GRACE risk score (age, heart rate, systolic blood pressure, creatinine, Killip class, cardiac arrest, ST deviation, and initial positive biomarkers). These analyses were done within the framework of a generalized estimating equation. The Cochrane‐Armitage trend test was used to determine if there was a trend across the study period for the proportion of ACS subjects who were SMuRF‐less. All statistical tests were 2‐tailed with the significance level set at 0.05. All statistical analyses are conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
Of the 13 911 patients enrolled in the GRACE Australia and CONCORDANCE ACS registry between 1999 and 2017, 3081 patients presented with their first cardiovascular event, had a final diagnosis of STEMI, and were included in the current analysis. The median age was 61 years (interquartile range 52 to 69 years), and 2310 (75%) were male. Of the total 3081 patients, 591 (19%) had 0 SMuRFs. The most common SMuRF was hypertension (44%), followed by current smoking (40%), dyslipidemia (37%), and diabetes mellitus (18%) (Table 1). The proportion of SMuRF‐less STEMI patients was substantially higher in men than women (20% versus 15%; Table 1). In STEMI patients with and without SMuRFs there were small differences in the mean age (60.4 years [SD 12.9] versus 61.7 years [SD 12.4]), the proportion female (26% versus 19%), mean body mass index (28.5 [SD 5.43] versus 27.0 [SD 4.37]), history of atrial fibrillation (3% versus 1%), and the proportion of patients with a family history of CHD (36% versus 33%).
Table 1.
Baseline (Preadmission) Characteristics
| Variable | Statistic (Level) | 0 SMuRFs (N=591) | >0 SMuRFs (N=2490) | Overall (N=3081) |
|---|---|---|---|---|
| Age | Mean (SD) | 61.7 (12.39) | 60.4 (12.88) | 60.6 (12.80) |
| Median (IQR) | 62 (53‐70) | 60 (51‐69) | 60 (52‐69) | |
| Sex | Female (%) | 115 (19) | 656 (26) | 771 (25) |
| Male (%) | 476 (81) | 1834 (74) | 2310 (75) | |
| SMuRFs, n (%) | ||||
| Diabetes mellitus | 0 (0) | 554 (22) | 554 (18) | |
| Hypertension | 0 (0) | 1363 (55) | 1363 (44) | |
| Dyslipidemia | 0 (0) | 1126 (45) | 1126 (37) | |
| Current smoking | 0 (0) | 1243 (50) | 1243 (40) | |
| BMI | Mean (SD) | 27.0 (4.37) | 28.5 (5.43) | 28.2 (5.28) |
| Median (IQR) | 26.8 (24.2‐30.0) | 27.8 (24.8‐31.4) | 27.8 (24.8‐31.6) | |
| Family history CHD, n (%) | 180 (33) | 790 (36) | 970 (35) | |
| Previous atrial fibrillation, n (%) | 6 (1) | 86 (3) | 92 (3) | |
| Prehospitalization medications, n (%) | ||||
| Statin | 0 (0) | 489 (20) | 489 (16) | |
| Aspirin | 33 (6) | 339 (14) | 372 (12) | |
| P2Y12 inhibitor | 7 (1) | 45 (2) | 52 (2) | |
| β‐blocker | 0 (0) | 219 (9) | 219 (7) | |
| ACE inhibitor | 0 (0) | 344 (14) | 344 (11) | |
| ARB| | 0 (0) | 429 (17) | 429 (14) | |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin II receptor antagonist blocker; BMI, body mass index; CHD, coronary heart disease; IQR, interquartile range; SMuRFs, standard modifiable cardiovascular risk factors.
Presenting Characteristics and In‐Hospital Management
In patients with and without SMuRFs, there were no significant differences in hemodynamic observations, Killip Class, cardiac arrest on admission, and GRACE risk score. There was also no significant difference in the incidence of significant multivessel disease (Table 2). A higher proportion of SMuRF‐less patients received primary percutaneous coronary intervention than patients with 1 or more SMuRF (49% versus 45%), and a smaller proportion received thrombolysis (32% versus 37%). The proportion of SMuRF‐less patients who were transferred to another hospital was lower than those with 1 or more SMuRFs (37% versus 44%). There was no clinically significant difference in evidence‐based secondary prevention medications prescribed at time of discharge between those with and without SMuRFs (Table 3). We observed higher in‐hospital and discharge use of angiotensin receptor blocker therapy use in patients with 1 or more SMuRFs. In secondary prevention angiotensin receptor blockers are second‐line therapy generally reserved for patients unable to tolerate angiotensin‐converting enzyme inhibitors. The higher rate of use of angiotensin receptor blockers in those with 1 or more SMuRFs may reflect a known intolerance to angiotensin‐converting enzyme inhibitor administration.
Table 2.
Patient Characteristics on Presentation
| Variable | Statistic (Level) | 0 SMuRFs (N=591) | >0 SMuRFs (N=2490) | Overall (N=3081) |
|---|---|---|---|---|
| Systolic blood pressure at time of presentation, mm Hg | Mean (SD) | 137.6 (27.39) | 137.6 (27.76) | 137.6 (27.68) |
| Median (IQR) | 136 (119‐154) | 135 (119‐155) | 136 (119‐155) | |
| Diastolic blood pressure at time of presentation, mm Hg | Mean (SD) | 82.8 (17.33) | 82.1 (17.44) | 82.2 (17.42) |
| Median (IQR) | 81 (70‐93) | 80 (70‐93) | 80 (70‐93) | |
| Heart rate, beats/min | Mean (SD) | 77.4 (21.26) | 78.9 (19.54) | 78.6 (19.89) |
| Median (IQR) | 73 (63‐88) | 77 (65‐90) | 76 (65‐90) | |
| Killip class, n (%) | ||||
| 1 | 542 (92) | 2234 (90) | 2776 (91) | |
| 2 | 31 (5) | 174 (7) | 205 (7) | |
| 3 | 9 (2) | 45 (2) | 54 (2) | |
| 4 | 6 (1) | 21 (1) | 27 (1) | |
| Cardiac arrest on admission, n (%) | 44 (7) | 184 (7) | 228 (7) | |
| Serum creatinine, μmol/L | Mean (SD) | 89.7 (24.77) | 91.0 (40.80) | 90.7 (38.24) |
| Median (IQR) | 87 (74‐100) | 84 (72‐100) | 85 (72‐100) | |
| Grace risk score | Mean (SD) | 110.5 (29.67) | 108.8 (30.02) | 109.15 (29.59) |
| Median (IQR) | 108.2 (90.04‐127.4) | 106.0 (87.90‐126.53) | 106.5 (88.58‐126.81) | |
| Culprit lesion territory, n (%) | ||||
| Left main | 0 (0) | 5 (1) | 5 (1) | |
| Left anterior descending | 34 (46) | 144 (37) | 178 (38) | |
| Circumflex artery | 8 (11) | 47 (12) | 55 (12) | |
| Right coronary | 22 (30) | 151 (39) | 173 (37) | |
| Unknown | 10 (14) | 42 (11) | 52 (11) | |
| Multivessel disease, >50% stenosis | 216 (38) | 1025 (42) | 1241 (41) | |
| Left ventricular function | Normal, n (%) | 117 (36) | 509 (36) | 626 (36) |
| Mild impairment, n (%) | 116 (36) | 493 (34) | 609 (35) | |
| Moderate impairment, n (%) | 65 (20) | 353 (25) | 418 (24) | |
| Severe impairment, n (%) | 25 (8) | 76 (5) | 101 (6) | |
IQR indicates interquartile range; SMuRFs, standard modifiable cardiovascular risk factors.
Table 3.
Medical Management and Cardiac Procedures
| Variable | 0 SMuRFs (N=91) | >0 SMuRFs (N=2490) | Overall (N=3081) |
|---|---|---|---|
| In‐hospital treatment, n (%) | |||
| Statin | 535 (91) | 2267 (91) | 2802 (91) |
| Aspirin | 571 (97) | 2437 (98) | 3008 (98) |
| P2Y12 receptor inhibitor | 502 (85) | 2077 (83) | 2579 (84) |
| ACE inhbitor | 446 (76) | 1728 (70) | 2174 (71) |
| ARB | 5 (1) | 317 (13) | 322 (10) |
| Heparin | 461 (78) | 1939 (78) | 2400 (78) |
| Low‐molecular‐weight heparin | 203 (34) | 793 (32) | 996 (32) |
| Thrombolysis | 188 (32) | 919 (37) | 1107 (36) |
| Primary PCI in STEMI patients | 291 (49) | 1099 (44) | 1390 (45) |
| Hospital transfer | 219 (37) | 1086 (44) | 1305 (42) |
| PCI | 415 (70) | 1708 (69) | 2123 (69) |
| CABG | 25 (4) | 150 (6) | 175 (6) |
| Discharge medications, n (%) | |||
| Statin | 495 (91) | 2152 (92) | 2647 (92) |
| Aspirin | 486 (89) | 2122 (91) | 2608 (90) |
| P2Y12 inhibitor | 437 (80) | 1813 (78) | 2250 (78) |
| β‐blocker | 453 (83) | 1953 (84) | 2406 (83) |
| ACE inhibitor | 390 (71) | 1588 (68) | 1978 (69) |
| ARB | 9 (2) | 256 (11) | 265 (9) |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin II receptor antagonist blocker; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; SMuRFs, standard modifiable cardiovascular risk factors; STEMI, ST‐segment–elevation myocardial infarction.
Changing Proportion of SMuRF‐Less STEMI
During the study period from 1999 to 2017, the proportion of STEMI patients with 0 SMuRFs increased from 14% to 23% (Cochrane‐Armitage trend test, P=0.0067; Figure 1). Although this current study is focused on the STEMI cohort, we also performed a simple analysis of the SMuRF‐less proportion in the 3773‐subject subgroup of non–ST‐segment–elevation acute coronary syndrome GRACE/CONCORDANCE subjects without prior CVD. It is important to note that this group is not broadly representative of all non–ST‐segment–elevation acute coronary syndromes, as it was intentionally enriched for patients with high‐risk features based on their clinical presentation. Although the overall proportion (18%) was similar to that in the STEMI cohort, there was no statistically significant change in the SMuRF‐less proportion during the study period.
Figure 1.
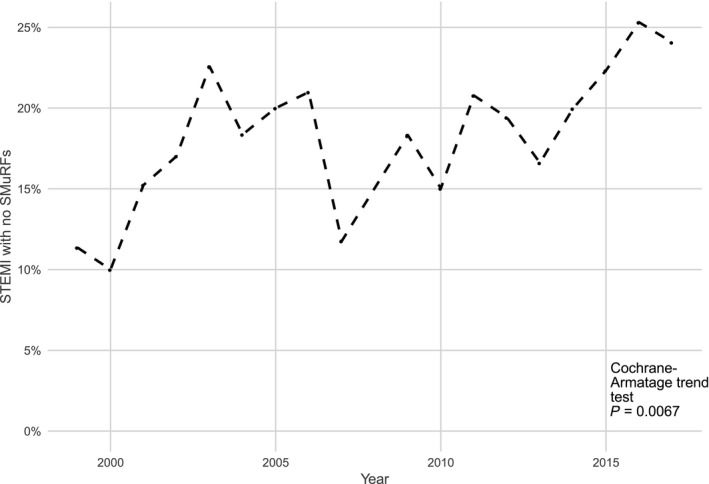
Increasing proportion of ST‐segment–elevation myocardial infarction (STEMI) patients with 0 standard modifiable cardiovascular risk factors (SMuRFs) during the study period. Cochrane‐Armatage trend test P=0.0067.
In‐Hospital Outcomes
The unadjusted in‐hospital mortality rate for SMuRF‐less STEMI patients was higher than that in patients with 1 or more SMuRFs (6% versus 4%). However, the rates of recurrent ischemic symptoms (12% versus 16%) and clinical heart failure (7% verse 11%) were lower in SMuRF‐less patients compared with patients with 1 or more SMuRFs. There were no differences in the rates of recurrent in‐hospital myocardial infarction, cardiogenic shock, or major bleeding between the groups (Table 4). In a multivariable analysis SMuRF‐less status, cardiac arrest on admission, age greater than 70 years, heart rate greater than 90 beats/min, systolic blood pressure less than 155 mm Hg, and creatinine greater than 100 mmol/L predicted in‐hospital mortality (Figure 2A). In a separate multivariable analysis, age greater than 70 years, heart rate greater than 90 beats/min, systolic blood pressure less than 155 mm Hg, cardiac arrest on admission, Killip class greater than 1, and negative cardiac biomarkers, but not SMuRF‐less status, were all predictors of MACE, defined as death, myocardial infarction, or heart failure during the index admission (Figure 2B).
Table 4.
Unadjusted In‐Hospital Outcomes
| Variable | Statistic (Level) | 0 SMuRFs (N=91) | >0 SMuRFs (N=2490) | Overall (N=3081) |
|---|---|---|---|---|
| In‐hospital MACE, % | 88 (16%) | 406 (18%) | 494 (18%) | |
| In‐hospital death, % | 36 (6) | 107 (4) | 143 (5) | |
| Myocardial infarction, % | 11 (2) | 54 (2) | 65 (2) | |
| Cardiogenic shock, % | 38 (6) | 117 (5) | 155 (5) | |
| In‐hospital death or myocardial infarction, % | 44 (8) | 154 (7) | 198 (7) | |
| Recurrent ischemic symptoms, % | 68 (12) | 386 (16) | 454 (15) | |
| Heart failure, % | 43 (7) | 277 (11) | 320 (10) | |
| Major bleeding, % | 40 (7) | 149 (6) | 189 (6) | |
| Stroke, % | 2 (0) | 16 (1) | 18 (1) | |
| Length of stay, d | Mean, SD | 6.7 (17.13) | 6.4 (9.90) | 6.4 (11.62) |
| Median, IQR | 4 (3‐6) | 4 (3‐7) | 4 (3‐6) |
IQR indicates interquartile range; MACE, major adverse cardiovascular events (death/myocardial infarction/heart failure/shock); SMuRFs, standard modifiable cardiovascular risk factors.
Figure 2.
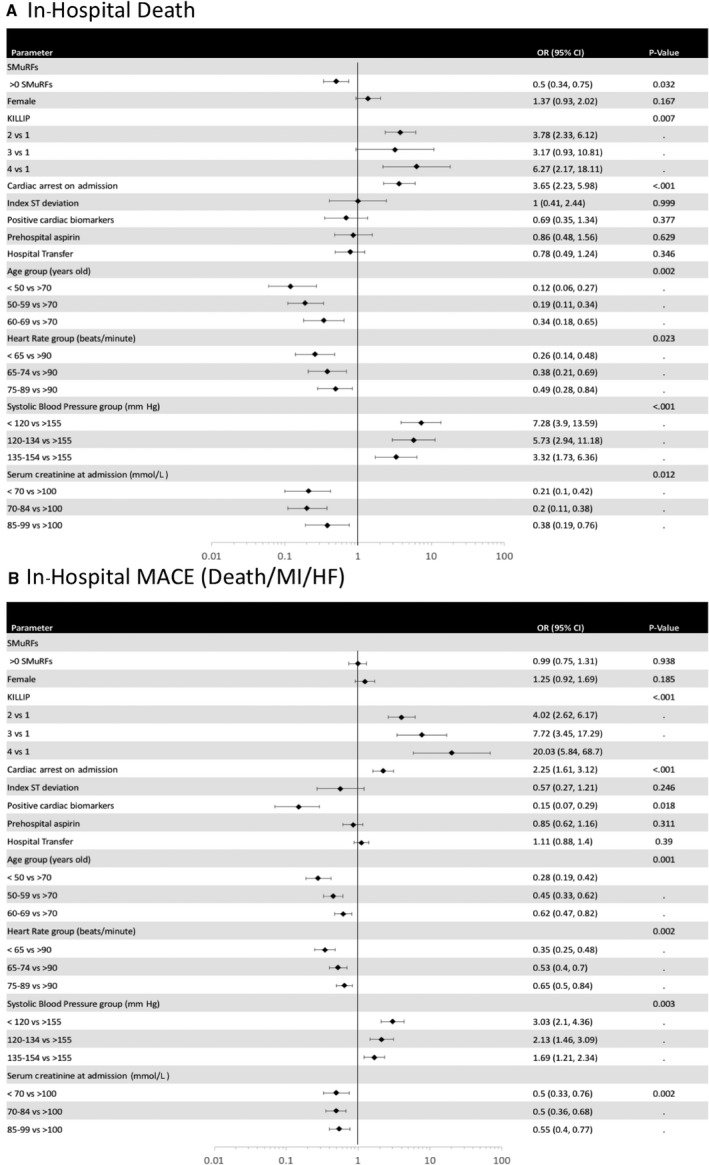
Multivariate odds ratios for in‐hospital (A) death and (B) major adverse cardiac events (MACE: death/recurrent myocardial infarction/heart failure/cardiogenic shock). Bars indicate 95% CIs. HF indicates heart failure; MI, myocardial infarction; OR, odds ratio; SMuRFs, standard modifiable cardiovascular risk factors (hypertension, diabetes mellitus, hypercholesterolemia, and smoking).
Posthospital Outcomes
Six‐month follow‐up data were available for 2487 of the original 3081 cohort (Table 5). There were no clinically significant differences in the unadjusted rates of recurrent myocardial infarction, heart failure, or MACE in the first 6 months postdischarge. In a multivariable analysis, older age, higher heart rate, and higher Killip class but not SMuRF status were predictors of MACE at 6 months (Table 6).
Table 5.
Unadjusted Outcomes 6 Months Postdischarge
| Variable | 0 SMuRFs n (%) N=471 | >0 SMuRFs n (%) N 016 | Overall n (%) N=2487 |
|---|---|---|---|
| MACE, % | 20 (6) | 101 (7) | 121 (7) |
| Death/MI, % | 16 (4) | 76 (4) | 92 (4) |
| Death, % | 9 (2) | 48 (2) | 57 (2) |
| MI, % | 7 (2) | 34 (2) | 41 (2) |
| Recurrent revascularization, % | 14 (3) | 62 (3) | 76 (3) |
| CHF, % | 6 (2) | 29 (2) | 35 (2) |
| Recurrent angina, % | 24 (7) | 98 (7) | 122 (7) |
| Major bleeding, % | 2 (1) | 6 (0) | 8 (0) |
| Stroke, % | 0 (0) | 15 (1) | 15 (1) |
CHF indicates congestive heart failure; MACE, major adverse cardiovascular events (death/myocardial infarction/heart failure/shock); MI, myocardial infarction; SMuRFs, standard modifiable cardiovascular risk factors.
Table 6.
Multivariable Logistic Regression Model for 6‐Month MACE
| Parameter | Effect | Odds Ratio (95% CI) | P Value |
|---|---|---|---|
| SMuRFs | >0 SMuRFs | 1.27 (0.72, 2.26) | 0.407 |
| 0 SMuRFS | Ref | ||
| Killip class | 2 | 2.8 (1.58, 4.97) | 0.013 |
| 3 | 8.42 (2.71, 26.18) | ||
| 4 | 2.59 (0.57, 11.8) | ||
| 1 | Ref | ||
| Cardiac arrest on admission | Yes | 0.35 (0.13, 0.94) | 0.019 |
| No | Ref | ||
| Index ST deviation | Yes | 0.79 (0.27, 2.31) | 0.696 |
| No | Ref | ||
| Positive cardiac biomarkers | Yes | 0.21 (0.07, 0.61) | 0.095 |
| No | Ref | ||
| Prehospital aspirin | Yes | 1.25 (0.77, 2.05) | 0.388 |
| No | Ref | ||
| Hospital transfer | Yes | 0.82 (0.53, 1.28) | 0.371 |
| No | Ref | ||
| Age group, y | <50 | 0.29 (0.17, 0.51) | 0.010 |
| 50 to 59 | 0.22 (0.12, 0.42) | ||
| 60 to 69 | 0.41 (0.23, 0.74) | ||
| 70+ | Ref | ||
| Heart rate group, bpm | <65 | 0.54 (0.31, 0.94) | 0.094 |
| 65 to 74 | 0.37 (0.17, 0.83) | ||
| 75 to 89 | 0.68 (0.38, 1.22) | ||
| 90+ | Ref | ||
| Systolic blood pressure group, mm Hg | <120 | 1.18 (0.68, 2.05) | 0.798 |
| 120 to 134 | 1.12 (0.56, 2.23) | ||
| 135 to 154 | 0.89 (0.5, 1.59) | ||
| 155+ | Ref | ||
| Serum creatinine at admission, mmol/L | <70 | 0.59 (0.35, 1) | 0.033 |
| 70 to 84 | 0.54 (0.37, 0.78) | ||
| 85 to 99 | 0.42 (0.25, 0.69) | ||
| 100+ | Ref |
MACE indicates major adverse cardiovascular events (death/myocardial infarction/heart failure/shock); SMuRFs, standard modifiable cardiovascular risk factors.
Discussion
This large, multicenter study highlights the importance of the often‐overlooked subgroup of STEMI patients with atherosclerosis not predicted by SMuRFs. We validate our previous published findings, from a single‐center study, that the proportion of SMuRF‐less STEMI patients is not insubstantial and has been significantly increasing in recent years. The relevance of this underappreciated group of STEMI patients is further highlighted by the observed higher in‐hospital mortality in this group.
The substantial proportion with no SMuRFs at the time of their index event is consistent with previously published studies at 18% overall12, 15, 16, 17 and represents a significant burden of CVD at a national and global scale, with an estimated 7.3 million acute myocardial infarctions per year worldwide.5. The STEMIs in the SMuRF‐less group were not explained by obesity, family history of premature coronary artery disease, or age (with body mass index being less in the SMuRF‐less group than the explained STEMI group, similar rates of relevant family history, and similar ages). It is interesting to observe that the proportion of SMuRF‐less STEMI patients was a third higher in men than women.
The YOUNG‐MI registry similarly found that 17% of ACS patients aged less than 50 years old presenting with a type 1 acute myocardial infarction had no SMuRFs and in addition found that fewer than 50% of patients in their cohort would have met the criteria for primary prevention statin therapy according to current American guidelines.17 Although it is essential that we continue at a community and primary healthcare level to identify and address the burden of known risk factors for atherosclerosis, parallel efforts should continue toward unraveling the biological mechanisms underlying disease in SMuRF‐less individuals. New technologies and data science advances in omics and multiomics approaches will allow novel discovery approaches to be adopted in accurately phenotyped cohorts with the potential to identify as yet unknown biological networks and processes.18 Polygenic risk scores have been developed that can stratify an individual's risk largely independent of the individual's SMuRFs and can improve risk prediction over traditional risk factor–derived scores.19 Future cohort studies such as GRACE and CONCORDANCE registries would benefit from building biobanks into their study design to enable validation of these scores as well as identification of new biomarkers.
The ultimate marker of risk for myocardial infarction is a noninvasive measure of early atherosclerotic disease itself, integrating not just the “attacking” risk factors but also the host “response.”18 Currently, in clinical practice, cardiac computed tomography—both coronary calcium score and coronary angiography—are all that we have available. Research tools measuring early vascular dysfunction or disease include carotid intimal medial thickness and brachial artery reactivity.20, 21 But there is an absence of circulating blood markers of atherosclerosis “activity,” with the nonspecific inflammatory marker high‐sensitive C‐reactive protein being the closest clinically available measure that we have. Application of noninvasive imaging of subclinical vascular disease supports the importance of the problem. One recent study demonstrated evidence of atherosclerosis in 50% of adults without SMuRFs.21 Another study showed that in asymptomatic adults with no SMuRFs, 32% had evidence of coronary artery calcification, and 12% had moderate or severe coronary artery calcification, defined as a coronary artery calcium score greater than 100 Agatston units.22 Currently, international guidelines do not recommend screening with coronary artery calcium or computed tomographic coronary angiography in patients deemed at low risk based on traditional risk factor scores.23, 24, 25 Data highlighting the burden of disease in the SMuRF‐less population and the dramatic difference earlier detection and targeted prevention would make suggest the need for more widely accessible markers of subclinical disease particularly relevant for these individuals.
This is the first report, in a large, multicenter study, of an increase in the proportion of SMuRF‐less STEMI patients. This increase may be explained by a simple competing risks principle, with improved identification and effective management of SMuRFs in primary care effectively increasing the relative proportion of patients with minimal risk factors that have received little recognition to date. Alternatively, it is plausible that environmental factors such as air particulate matter and heavy metal exposure levels or other unknown factors may have changed and may be contributing to atherosclerotic events in individuals without SMuRFs.26, 27, 28
A major goal of these analyses was to investigate the outcomes of SMuRF‐less STEMI patients compared with their counterparts whose myocardial infarctions were more easily explained by traditional risk factors. We confirmed previous observations that SMuRF‐less myocardial infarction patients were more likely to die in hospital than those with 1 or more SMuRFs.15, 16, 29 This does not appear to reflect treatment differences (Table 3). These data need to be more widely communicated to front‐line physicians to ensure that timely evidence‐based care is provided to this vulnerable group of patients. Despite the higher in‐hospital mortality in SMuRF‐less individuals, the rates of MACE, including in‐hospital recurrent myocardial infarctions and cardiogenic shock were similar. The higher in‐hospital mortality rate may reflect differing underlying biological processes or a reduced capacity to tolerate myocardial ischemia, and mechanisms of in‐hospital mortality in SMuRF‐less individuals warrant further investigation.30
There are a number of limitations in our study deserving of mention. SMuRFs were documented as categorical variables, although each SMuRF is actually a continuous variable.31 Identification of SMuRF status is based on interrogation of medical records and was not independently validated with biochemical testing; thus, it may be susceptible to underdiagnoses. Furthermore, less commonly used risk factors such as lipoprotein(a), high‐sensitivity C‐reactive protein, coronary artery calcium score, and genetic risk scores were not available and therefore not assessed in this study.32 However, this real‐world study reflects the information that is readily available to clinicians. A total of 19% of subjects were lost to follow‐up at 6 months; this may have resulted in a potential bias. However, as shown in Table S1, there were only subtle differences in the demographic and clinical features between the 2 groups, with a slightly higher Grace Risk Score, creatinine, and Killip class in those lost to follow‐up compared with those who were able to be contacted for follow‐up. It was due to the loss to follow‐up that the 6‐month data were considered secondary and treated as descriptive. The specific timing of events within the 6‐month follow‐up period was not captured, and we were unable to assess the proximity of events to the index event or to learn whether there was an association between length of stay and early postdischarge events. Continued efforts are required to examine progression of atherosclerosis and related outcomes in SMuRF‐less subjects.
Conclusions
In these multicenter registry studies spanning nearly 20 years, 19% of STEMI patients without a prior history of CVD also had no prior history of SMuRFs. The proportion of STEMI patients with no SMuRFs increased from 14% to 23% during the study period. SMuRF‐less STEMI patients had higher in‐hospital mortality rates when compared with patients with 1 or more SMuRFs. Our study highlights the need for ongoing investigation and for discovery of novel risk factors, biomarkers, biological processes, and treatment targets in order to better address the changing nature of coronary artery disease in the 21st century.
Sources of Funding
The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article. The first author is supported by a University of Sydney Postgraduate Research Scholarship funded by Heart Research Australia. The senior author is supported by a National Health and Medical Research Council practitioner fellowship (grant number APP11359290) and Heart Research Australia. GRACE was supported by an unrestricted grant from Sanofi Aventis. The CONCORDANCE Registry is funded by unrestricted grants from the Heart Foundation of Australia, Sanofi, Astra Zeneca, Eli Lilly, Boehringer Ingelheim, and the Merck Sharp and Dohme/Schering Plough Joint Venture.
Disclosures
None.
Supporting information
Table S1. Baseline, Presentation, and In‐Hospital Management Characteristics of Subjects With 6‐Month Follow‐Up Data Versus Those Without 6‐Month Follow‐Up Data
(J Am Heart Assoc. 2019;8:e013296 DOI: 10.1161/JAHA.119.013296.)
References
- 1. Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J III. Factors of risk in the development of coronary heart disease—six year follow‐up experience. The Framingham Study. Ann Intern Med. 1961;55:33–50. [DOI] [PubMed] [Google Scholar]
- 2. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 3. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. [DOI] [PubMed] [Google Scholar]
- 4. Dawber TR, Moore FE, Mann GV. Coronary heart disease in the Framingham study. Am J Public Health Nations Health. 1957;47:4–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roth GA, Johnson C, Abajobir A, Abd‐Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. AIHW . Cardiovascular disease, diabetes and chronic kidney disease—Australian facts: prevalence and incidence. Cardiovascular, diabetes and chronic kidney disease series no. 2. (Cat. no. CDK 2). Canberra: Australian Institute of Health and Welfare; 2014.
- 7. Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, Casey DC, Charlson FJ, Chen AZ, Coates MM, et al. Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980‐2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, Blackman DJ, Dalby M, Fairbrother KL, Banya W, Wang D, Flather M, Hetherington SL, Kelion AD, Talwar S, Gunning M, Hall R, Swanton H, McCann GP. Randomized trial of complete versus lesion‐only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steg PG, Goldberg RJ, Gore JM, Fox KA, Eagle KA, Flather MD, Sadiq I, Kasper R, Rushton‐Mellor SK, Anderson FA. Baseline characteristics, management practices, and in‐hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE). Am J Cardiol. 2002;90:358–363. [DOI] [PubMed] [Google Scholar]
- 10. Hasdai D, Behar S, Wallentin L, Danchin N, Gitt AK, Boersma E, Fioretti PM, Simoons ML, Battler A. A prospective survey of the characteristics, treatments and outcomes of patients with acute coronary syndromes in Europe and the Mediterranean basin; the Euro Heart Survey of Acute Coronary Syndromes (Euro Heart Survey ACS). Eur Heart J. 2002;23:1190–1201. [DOI] [PubMed] [Google Scholar]
- 11. Vernon ST, Coffey S, Bhindi R, Soo Hoo SY, Nelson GI, Ward MR, Hansen PS, Asrress KN, Chow CK, Celermajer DS, O'Sullivan JF, Figtree GA. Increasing proportion of ST elevation myocardial infarction patients with coronary atherosclerosis poorly explained by standard modifiable risk factors. Eur J Prev Cardiol. 2017:24:1824–1830. [DOI] [PubMed] [Google Scholar]
- 12. Khot UN, Khot MB, Bajzer CT, Sapp SK, Ohman EM, Brener SJ, Ellis SG, Lincoff AM, Topol EJ. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290:898–904. [DOI] [PubMed] [Google Scholar]
- 13. Fox KA, Eagle KA, Gore JM, Steg PG, Anderson FA. The Global Registry of Acute Coronary Events, 1999 to 2009–GRACE. Heart. 2010;96:1095–1101. [DOI] [PubMed] [Google Scholar]
- 14. Hyun K, Redfern J, Woodward M, D'Souza M, Shetty P, Chew D, Kangaharan N, Farshid A, Alford K, Briffa T, Brieger D. Socioeconomic equity in the receipt of in‐hospital care and outcomes in Australian acute coronary syndrome patients: the CONCORDANCE Registry. Heart Lung Circ. 2018;27:1398–1405. [DOI] [PubMed] [Google Scholar]
- 15. Wang JY, Goodman SG, Saltzman I, Wong GC, Huynh T, Dery JP, Leiter LA, Bhatt DL, Welsh RC, Spencer FA, Fox KA, Yan AT; Global Registry of Acute Coronary Events (GRACE/GRACE‐2), Canadian Registry of Acute Coronary Events (CANRACE) Investigators . Cardiovascular risk factors and in‐hospital mortality in acute coronary syndromes: insights from the Canadian Global Registry of Acute Coronary Events. Can J Cardiol. 2015;31:1455–1461. [DOI] [PubMed] [Google Scholar]
- 16. Canto JG, Kiefe CI, Rogers WJ, Peterson ED, Frederick PD, French WJ, Gibson CM, Pollack CV Jr, Ornato JP, Zalenski RJ, Penney J, Tiefenbrunn AJ, Greenland P. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA. 2011;306:2120–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh A, Collins BL, Gupta A, Fatima A, Qamar A, Biery D, Baez J, Cawley M, Klein J, Hainer J, Plutzky J, Cannon CP, Nasir K, Di Carli MF, Bhatt DL, Blankstein R. Cardiovascular risk and statin eligibility of young adults after an MI: partners YOUNG‐MI registry. J Am Coll Cardiol. 2018;71:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vernon ST, Hansen T, Kott KA, Yang JY, O'Sullivan JF, Figtree GA. Utilizing state‐of‐the‐art “omics” technology and bioinformatics to identify new biological mechanisms and biomarkers for coronary artery disease. Microcirculation. 2018;26:e12488. [DOI] [PubMed] [Google Scholar]
- 19. Inouye M, Abraham G, Nelson CP, Wood AM, Sweeting MJ, Dudbridge F, Lai FY, Kaptoge S, Brozynska M, Wang T, Ye S, Webb TR, Rutter MK, Tzoulaki I, Patel RS, Loos RJF, Keavney B, Hemingway H, Thompson J, Watkins H, Deloukas P, Di Angelantonio E, Butterworth AS, Danesh J, Samani NJ. Genomic risk prediction of coronary artery disease in 480,000 adults: implications for primary prevention. J Am Coll Cardiol. 2018;72:1883–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard‐Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial‐dependent flow‐mediated vasodilation of the brachial artery. J Am Coll Cardiol. 2002;39:257. [DOI] [PubMed] [Google Scholar]
- 21. Fernández‐Friera L, Fuster V, López‐Melgar B, Oliva B, García‐Ruiz JM, Mendiguren J, Bueno H, Pocock S, Ibáñez B, Fernández‐Ortiz A, Sanz J. Normal LDL‐Cholesterol Levels Are Associated With Subclinical Atherosclerosis in the Absence of Risk Factors. J Am Coll Cardiol. 2017;70:2979–2991. [DOI] [PubMed] [Google Scholar]
- 22. Silverman MG, Blaha MJ, Krumholz HM, Budoff MJ, Blankstein R, Sibley CT, Agatston A, Blumenthal RS, Nasir K. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the Multi‐Ethnic Study of Atherosclerosis. Eur Heart J. 2014;35:2232–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alliance NVDP . Guidelines for the management of absolute cardiovascular disease risk. 2012. Available at: https://www.heartfoundation.org.au/images/uploads/publications/Absolute-CVD-Risk-Full-Guidelines.pdf. Accessed April 23, 2017.
- 24. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M‐T, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen M‐L, Löllgen H, Marques‐Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S, De Backer G, Roffi M, Aboyans V, Bachl N, Bueno H, Carerj S, Cho L, Cox J, De Sutter J, Egidi G, Fisher M, Fitzsimons D, Franco OH, Guenoun M, Jennings C, Jug B, Kirchhof P, Kotseva K, Lip GYH, Mach F, Mancia G, Bermudo FM, Mezzani A, Niessner A, Ponikowski P, Rauch B, Rydén L, Stauder A, Turc G, Wiklund O, Windecker S, Zamorano JL, Zamorano JL, Aboyans V, Achenbach S, Agewall S, Badimon L, Barón‐Esquivias G, Baumgartner H, Bax JJ, Bueno H, Carerj S, Dean V, Erol Ç, Fitzsimons D, Gaemperli O, Kirchhof P, Kolh P, Lancellotti P, Lip GYH, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Roffi M, Torbicki A, Carneiro AV, Windecker S, Metzler B, Najafov R, Stelmashok V, De Maeyer C, Dilić M, Gruev I, Miličić D, Vaverkova H, Gustafsson I, Attia I, Duishvili D, Ferrières J, Kostova N, Klimiashvili Z, Hambrecht R, Tsioufis K, Szabados E, Andersen K, Vaughan C, Zafrir B, Novo S, Davletov K, Jashari F, Kerimkulova A, Mintale I, Saade G, Petrulioniene Z, Delagardelle C, Magri CJ, Rudi V, Oukerraj L, Çölkesen BE, Schirmer H, dos Reis RP, Gherasim D, Nedogoda S, Zavatta M, Giga V, Filipova S, Padial LR, Kiessling A, Mach F, Mahdhaoui A, Ural D, Nesukay E, Gale C. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 26. McGuinn LA, Ward‐Caviness C, Neas LM, Schneider A, Di Q, Chudnovsky A, Schwartz J, Koutrakis P, Russell AG, Garcia V, Kraus WE, Hauser ER, Cascio W, Diaz‐Sanchez D, Devlin RB. Fine particulate matter and cardiovascular disease: comparison of assessment methods for long‐term exposure. Environ Res. 2017;159:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martinelli N, Olivieri O, Girelli D. Air particulate matter and cardiovascular disease: a narrative review. Eur J Intern Med. 2013;24:295–302. [DOI] [PubMed] [Google Scholar]
- 28. Lamas GA, Navas‐Acien A, Mark DB, Lee KL. Heavy metals, cardiovascular disease, and the unexpected benefits of chelation therapy. J Am Coll Cardiol. 2016;67:2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roe MT, Halabi AR, Mehta RH, Chen AY, Newby LK, Harrington RA, Smith SC Jr, Ohman EM, Gibler WB, Peterson ED. Documented traditional cardiovascular risk factors and mortality in non‐ST‐segment elevation myocardial infarction. Am Heart J. 2007;153:507–514. [DOI] [PubMed] [Google Scholar]
- 30. Iliodromitis EK, Lazou A, Kremastinos DT. Ischemic preconditioning: protection against myocardial necrosis and apoptosis. Vasc Health Risk Manag. 2007;3:629–637. [PMC free article] [PubMed] [Google Scholar]
- 31. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 32. Folsom AR, Chambless LE, Ballantyne CM, Coresh J, Heiss G, Wu KK, Boerwinkle E, Mosley TH Jr, Sorlie P, Diao G, Sharrett AR. An assessment of incremental coronary risk prediction using C‐reactive protein and other novel risk markers: the Atherosclerosis Risk in Communities Study. Arch Intern Med. 2006;166:1368–1373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline, Presentation, and In‐Hospital Management Characteristics of Subjects With 6‐Month Follow‐Up Data Versus Those Without 6‐Month Follow‐Up Data


