Abstract
Background
The relation of sex to clinical presentation and course in hypertrophic cardiomyopathy (HCM) remains incompletely resolved. We assessed differences in clinical outcomes between men and women within our large HCM cohort.
Methods and Results
Of 2123 consecutive patients, a minority (38%) were women who were diagnosed with HCM at older ages or referred for subspecialty evaluation later than men (50±19 versus 44±16 and 55±18 versus 49±16; P<0.001). Women more commonly developed advanced New York Heart Association class III/IV symptoms (53% versus 35% in men; P<0.001), predominantly secondary to outflow obstruction. While end‐stage heart failure with systolic dysfunction (ejection fraction <50%) was similar in men (5% versus 4% in women; P=0.33), women were 3‐fold more likely to develop heart failure with preserved systolic function (7.5% versus 2.6%; P=0.002). Sudden death events terminated by defibrillator therapy were similar in women (0.9%/year) versus men (1.0%/year; hazard ratio, 0.92; 95% CI, 0.6–1.5; P=0.73). HCM mortality was uncommon, with identical rates in both sexes (0.3%/year; hazard ratio, 1.5; 95% CI, 0.7–3.4;, P=0.25). Age‐adjusted all‐cause mortality also did not differ between women and men (1.7% versus 1.3%/year; hazard ratio, 1.32; 95% CI, 0.92–1.91; P=0.13).
Conclusions
Survival was not less favorable in women with HCM. Contemporary treatments including surgical myectomy to reverse heart failure and defibrillators to prevent sudden death, were effective in both sexes contributing to low mortality. However, despite more frequent outflow obstruction, women with HCM are underrecognized and referred to centers later than men, often with more advanced heart failure. Greater awareness of HCM in women should lead to earlier diagnosis and treatment, with implications for improved quality of life.
Keywords: heart failure, hypertrophic cardiomyopathy, implantable defibrillator, sex, surgical myectomy
Subject Categories: Cardiomyopathy, Women
Clinical Perspective
What Is New?
There is significant delay in hypertrophic cardiomyopathy diagnosis in women, on average 6 years later than in men. Left ventricular outflow obstruction is more common and responsible for more advanced heart failure symptoms.
Therapeutic options are equally effective in women and in men with hypertrophic cardiomyopathy, including implantable defibrillators terminating potentially lethal ventricular tachyarrhythmias, or septal myectomy (and alcohol septal ablation) for relieving outflow obstruction and averting heart failure morbidity and death.
Contemporary therapeutic strategies contribute importantly to low mortality. No significant differences were evident between men and women regarding either hypertrophic cardiomyopathy death or age‐adjusted all‐cause mortality.
What Are the Clinical Implications?
Greater awareness of hypertrophic cardiomyopathy in women is needed to allow for earlier diagnosis and treatment, particularly for progressive heart failure.
Management strategies are equally effective in women and men and should be implemented for sudden death prevention and heart failure reversal to permit extended survival and quality of life.
Introduction
The past 25 years have witnessed a transformation in our understanding and management of hypertrophic cardiomyopathy (HCM). Once thought to be a rare disorder with high rates of sudden and heart failure–related death, we now recognize HCM to be a relatively common, global disease compatible with normal or extended life expectancy attributable to contemporary treatment strategies.1, 2, 3, 4, 5, 6, 7
HCM is inherited as an autosomal dominant disease and characterized by vast clinical and phenotypic heterogeneity.8, 9, 10, 11 However, historically, women have been underrepresented in HCM clinical cohort reports, and there is some uncertainty as to whether women with this disease are at greater mortality risk from heart failure or other disease complications.12, 13, 14, 15, 16, 17, 18 Therefore, we have taken this opportunity to access our large database to evaluate whether there are sex‐related differences in clinical presentation or outcome for patients with HCM.
Methods
The 2123 study patients were evaluated consecutively at the Tufts HCM Institute from 2001 to 2016. For the overall cohort, median follow‐up duration from study entry (initial clinical evaluation at Tufts) to most recent contact (or death) was 3.9 (interquartile range, 2.0–6.9; mean, 4.7±3.5 years) years, encompassing 10 188 patient‐years. The most recent clinical assessment was obtained by hospital visit or telephone contact. The authors declare that all supporting data are available within the article.
Definitions
Clinical diagnosis of HCM was based on the 2‐dimensional echocardiographic and/or cardiovascular magnetic resonance identification of a hypertrophied nondilated left ventricle (wall thickness ≥13 mm) in the absence of another cardiac or systemic disease capable of producing the magnitude of hypertrophy evident.1 Patients with known phenocopies of HCM (eg, Fabry, LAMP2, PRKAG2, or amyloidosis) were excluded. Atherosclerotic coronary artery disease was defined as ≥50% narrowing in ≥1 epicardial artery by coronary arteriogram or CT angiography.
Advanced heart failure: New York Heart Association (NYHA) functional class III or IV heart failure symptoms, refractory to maximum medical management, due to either outflow obstruction or, alternatively, evolution to the end stage in the absence of outflow obstruction with reduced (or preserved) ejection fraction.
Sudden cardiac death: unexpected sudden collapse occurring <1 hour from the onset of symptoms in patients who had previously experienced a relatively stable or uneventful clinical course.
Heart failure‐related death: event occurring in the context of cardiac decompensation and progressive disease course before death, particularly if complicated by pulmonary edema, evolution to the end stage, and/or requiring hospitalization for heart failure.
Stroke‐related death: judged as the consequence of an embolic event, usually in the setting of atrial fibrillation.
Imaging
Transthoracic echocardiographic studies were performed in a standard fashion. Left ventricular (LV) wall thickness was taken as the maximal end‐diastolic dimension (usually ventricular septum). Continuous‐wave Doppler estimated peak instantaneous LV outflow gradient, with obstruction defined as a subaortic gradient ≥30 mm Hg. Patients with gradients <50 mm Hg at rest underwent symptom‐limited exercise (stress) testing with echocardiography on a standard Bruce protocol.9
Cardiovascular magnetic resonance studies were performed in 1232 study patients with a 1.5‐T clinical scanner. Late gadolinium enhancement quantification was performed by manually adjusting the gray‐scale threshold to visually define areas of late gadolinium enhancement, which were summed and expressed as the proportion of total LV myocardium.
Genetic testing was performed in 344 patients by commercially available panels that included the most common HCM‐associated myofilament‐encoding genes. Sarcomere protein variants were classified as pathogenic according to 2015 American College of Medical Genetics Guidelines.
This study was reviewed and approved by the Institutional Review Board at Tufts Medical Center, allowing retrospective review of medical records and granting a waiver of informed consent in accordance with 45 CFR 46.116 (d). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Statistical Analyses
Data are expressed as mean±SD for continuous variables and proportions for categorical variables. Student's t‐test or Wilcoxon rank‐sum tests addressed statistical significance of continuous variables; chi‐square or Fisher exact tests analyzed categorical variables. Tests were 2‐sided; P<0.05 was considered statistically significant.
For survival and event analyses, the fraction of patients at follow‐up intervals was estimated using the Kaplan‐Meier method. To adjust all‐cause mortality for age, Cox proportional hazards regression models were used. Expected fractions surviving at each time after the initial visit were computed by assigning probability of survival (appropriate to age and sex) based on the US general population.19 Actual and expected survival fractions were compared using 1‐sample log‐rank tests, which also provide an estimate for the standardized mortality ratio and 95% CI.
End points were total (all‐cause) and HCM‐related mortality, including sudden, heart failure, and embolic stroke–related death. Appropriate implantable cardioverter defibrillator (ICD) discharges to terminate ventricular fibrillation or sustained rapid ventricular tachycardia were regarded as aborted sudden death events. Statistical calculations were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Baseline Characteristics
Demographics of the study population are summarized in Table. Among the 2123 study patients, there was a predominance of men with HCM (n=1329; 62%) compared with women (n=794; 38%), which was similar throughout the 15‐year study period (P=0.13; Figures 1 and 2). At the time of diagnosis, women with HCM were 50±19 years of age, an average of 6 years older than men (44±16 years; <0.001), and were referred for subspecialty evaluation at older ages than men (55±18 versus 49±16 years; <0.001). Women represented the majority of patients diagnosed ≥60 years of age (286 patients [56%] versus 226 patients [44% for men]; P<0.001) but were less common than men in younger age groups (comprising 26% to 34% of the cohort; Figure 3).
Table 1.
Demographics and Clinical Features in 2123 HCM Patients by Sex
| Parameter | Female Patients (n=794; 38%) | Male Patients (n=1329; 62%) | P Value |
|---|---|---|---|
| Age at diagnosis, y | 50±19 | 44±16 | <0.001 |
| Age at first visit, y | 55±18 | 49±16 | <0.001 |
| Age at last evaluation, y | 60±17 | 54±16 | <0.001 |
| Family history of HCM, n (%) | 218 (27) | 292 (22) | 0.005 |
| Family history—sudden death, n (%) | 97 (12) | 129 (10) | 0.08 |
| Syncope, n (%) | 85 (11) | 174 (13) | 0.11 |
| NSVT on ambulatory monitoring, n (%) | 100 (13) | 220 (17) | 0.01 |
| Maximum LV wall thickness, mm | 18.7±4.2 | 19.1±4.6 | 0.03 |
| No. (%) with left ventricle ≥30 mm | 39 (5) | 88 (7) | 0.13 |
| No. (%) LV apical aneurysm | 25 (3) | 48 (4) | 0.62 |
| Ejection fraction, % | 64±7 | 63±6 | <0.001 |
| Left atrial dimension, mm | 40±7 | 42±7 | <0.001 |
| Peak LV outflow gradient, ≥30 mm Hg rest, n (%) | 357 (45) | 408 (31) | <0.001 |
| Peak LV outflow gradient <30 mm Hg rest, ≥50 mm Hg exercise, n (%) | 156 (20) | 349 (26) | <0.001 |
| Nonobstructive, n (%) | 281 (35) | 572 (43) | 0.01 |
| LVED, mm | 39±7 | 43±6 | <0.001 |
| Contrast CMR | |||
| No. CMR studies | 455 | 777 | |
| No. (%) with LGE | 244 (54) | 518 (67) | <0.001 |
| % LGE (in patients with LGE) | 5.4±5.2 | 6.0±6.0 | 0.20 |
| No. (%) LGE ≥15% of LV | 16 (4) | 52 (7) | 0.02 |
| NYHA functional class, initial evaluation, n (%) | |||
| I | 214 (27) | 618 (47) | <0.001 |
| II | 268 (34) | 406 (31) | 0.28 |
| III/IV | 312 (39) | 306 (23) | <0.001 |
| No. (%) with atrial fibrillation | 197 (25) | 348 (26) | 0.33 |
| No. (%) with CAD | 47 (7) | 96 (6) | 0.71 |
| No. (%) with HTN | 268 (34) | 431 (32) | 0.56 |
| No. (%) with resistant HTN | 5 (<1) | 7 (<1) | 0.77 |
| Reason leading to diagnosis, n (%) | |||
| Symptoms/event | 404 (51) | 606 (46) | 0.02 |
| Screening due to family history | 74 (9) | 121 (9) | 0.88 |
| Asymptomatic with abnormal ECG or murmur leading to diagnosis | 182 (23) | 420 (32) | <0.001 |
| Symptoms at first evaluation, n (%) | |||
| Dyspnea | 580 (73) | 725 (55) | <0.001 |
| Chest pain | 210 (26) | 289 (22) | 0.04 |
| Fatigue | 339 (29) | 235 (18) | <0.001 |
| Palpitations | 129 (16) | 159 (12) | 0.006 |
| Refractory HF during clinical course, n (%) | 420 (53) | 461 (35) | <0.001 |
| Refractory HF attributable to LVOT obstruction | 388 (49) | 425 (32) | <0.001 |
| Refractory HF in absence of LVOT obstruction | 32 (4) | 36 (3) | 0.10 |
| No. (%) septal myectomy* | 283 (36) | 347 (26) | <0.001 |
| No. (%) alcohol septal ablations* | 89 (11) | 58 (4) | <0.001 |
| No. (%) heart transplants | 13 (1.6) | 18 (1.4) | 0.58 |
| Age at heart transplant, y | 44±14 | 48±14 | 0.43 |
| Primary prevention ICD, n (%) | 191 (24) | 336 (25) | 0.54 |
| Age at ICD implantation, y | 42±17 | 43±16 | 0.92 |
| Appropriate primary prevention ICD interventions, n (%) | 25 (13) | 57 (17) | 0.20 |
| Resuscitated cardiac arrest,† n (%) | 14 (1.7) | 22 (1.6) | 0.86 |
| Sudden death rate,‡ %/y | 0.9%/year | 0.8%/year | 0.73 |
| ICD‐related complications, n (%) | 34 (18) | 50 (15) | 0.45 |
| Inappropriate shock | 21 (11) | 40 (12) | 0.89 |
| Device lead fracture | 8 (4) | 7 (2) | 0.42 |
| Device/lead infection | 11 (6) | 10 (3) | 0.08 |
| Drug therapy, n (%) | |||
| Beta‐blockers | 657 (82) | 987 (74) | <0.001 |
| Calcium antagonists | 375 (47) | 500 (37) | <0.001 |
| Disopyramide | 100 (13) | 93 (7) | <0.001 |
| ACE/ARB | 229 (28) | 391 (29) | 0.81 |
| Amiodarone | 127 (16) | 155 (12) | 0.07 |
| Genetic testing performed, n (%) | 123 (15) | 228 (17) | 0.33 |
| Pathogenic mutation | 74 (60) | 101 (44) | <0.001 |
| MYBPC3 | 37 | 51 | |
| MYH7 | 24 | 28 | |
| TNNT2 | 6 | 6 | |
| MYL2, MYL3 | 1 | 3 | |
| TPM1 | 1 | 3 | |
| TNNI | 3 | 7 | |
| MYBPC3+TNNI | 1 | 1 | |
| MYH7+MYBPC3 | 1 | 2 | |
| NYHA‐functional class, last evaluation,§ n (%) | |||
| I | 305 (42) | 732 (58) | <0.001 |
| II | 365 (50) | 480 (38) | <0.001 |
| III/IV | 54 (8) | 52 (5) | 0.002 |
| Nonfatal adverse events, n (%) | 56 (7) | 93 (7) | 1 |
| Deaths, n (%) | 70 (9) | 65 (5) | <0.001 |
| Age at death, y | 67±15 | 59±5 | 0.05 |
| Noncardiac death,║ n (%) | 55 (7) | 46 (4) | <0.001 |
| Cardiac, non‐HCM,¶ n (%) | 4 (0.4) | 3 (0.2) | 0.68 |
| Unknown, n (%) | 2 (0.3) | 4 (0.3) | 1 |
| HCM‐related death, n (%) | 13 (1.6) | 15 (1.1) | 0.33 |
| Sudden | 1 | 4 | |
| Heart failure | 6 | 5 | |
| Posttransplant | 2 | 1 | |
| Postoperative | 3 | 3 | |
| Embolic stroke death | 1 | 2 | |
| Age at HCM death, y | 56±11 | 53±16 | 0.54 |
| HCM mortality rate, %/y | 0.3 | 0.3 | 0.88 |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CAD, coronary artery disease; CMR, cardiovascular magnetic resonance; HCM, hypertrophic cardiomyopathy; HF, heart failure; HTN, hypertension; ICD, implantable cardioverter defibrillator; LGE, late gadolinium enhancement; LV, left ventricular; LVED, left ventricular end‐diastolic dimension; LVOT, left ventricular outflow tract; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association.
Includes 15 female and 6 male patients with unsuccessful alcohol septal ablation prior to myectomy.
Includes 9 female and 4 male patients with subsequent secondary prevention appropriate ICD interventions.
Includes sudden death, appropriate ICD intervention, and resuscitated out‐of‐hospital arrest.
In 1988 surviving patients.
Most commonly, pulmonary disease (n=3 men, 8 women), cancer (n=12 men, 6 women), and multiorgan noncardiac comorbidities often associated with advanced age (n=12 women, n=9 men).
CAD related in 4 men, postoperative aortic valve replacement in 2 women, and postoperative mitral valve replacement/coronary artery bypass graft in 1 woman.
Figure 1.
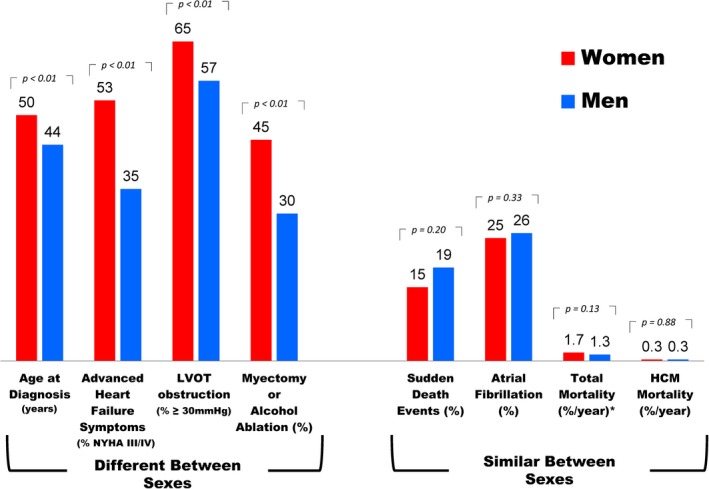
Clinical and demographic comparison between women and men with hypertrophic cardiomyopathy. LVOT indicates left ventricular outflow tract; NYHA, New York Heart Association functional class.
Figure 2.
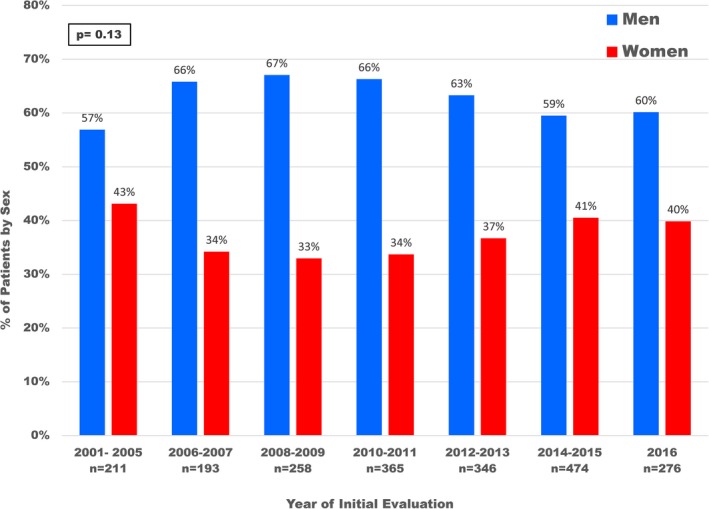
Distribution by sex according to year of initial evaluation in 2123 hypertrophic cardiomyopathy patients.
Figure 3.
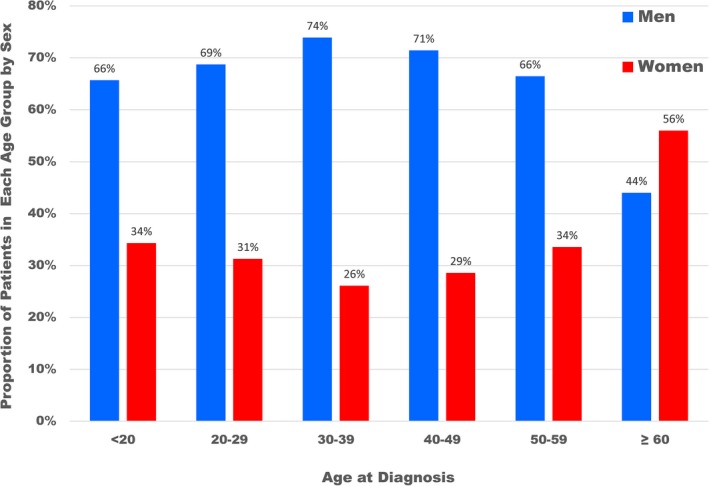
Distribution by sex within each age group at initial hypertrophic cardiomyopathy diagnosis in 2123 patients.
Determinants of the initial HCM diagnosis differed among men and women. In women, heart failure symptoms led to diagnosis most commonly (51% versus 46% in men; P=0.02) while in men diagnosis was raised more commonly by an abnormal ECG or for evaluation of a heart murmur (32% versus 23%; P<0.01). Family HCM screening led to a diagnosis in a similar proportion of men and women (9% versus 9%; P=0.88).
Morphology
Compared with men, women with HCM had smaller LV end‐diastolic and left atrial transverse dimensions (39±7 versus 43±6 and 40±7 versus 42±7; P<0.001 for both), with slightly less maximum LV wall thickness (usually in ventricular septum; 18.7±4.2 versus 19.1±4.6; P=0.03). There was no significant difference in the prevalence of extreme hypertrophy, that is, LV wall thickness ≥30mm (5% in women versus 7% in men; P=0.13) nor LV apical aneurysm with regional scarring (3% in women versus 4% in men; P=0.62).20
Women more commonly demonstrated dynamic LV outflow tract obstruction caused by mitral valve systolic anterior motion than did men (65% versus 57%; P<0.001), present most commonly at rest (45% versus 31% in men; P<0.001), and less frequently provoked with stress exercise echocardiography (20% versus 26% in men; P<0.001).
A total of 351 patients underwent clinical genetic testing, similar in women and men (15% versus 17%; P=0.33). However, women were more likely to have a HCM pathologic (disease‐causing) sarcomere variant identified (60% versus 44%), most commonly in the MYBC3 and MYH7 genes (Table).
Heart Failure
Overall by sex
At the time of initial evaluation at our center, women more commonly had heart failure symptoms than men, NYHA functional classes II to IV, characteristically with exertional dyspnea or fatigue, with or without chest pain (73% women versus 53% men; P<0.001). Similarly, during follow‐up, women were more likely to develop advanced drug refractory heart failure (NYHA class III/IV, 53% women versus 35% men; P<0.001; Figure 4), an average of 6 years later than in men (58±16 versus 52±14 years in men; P<0.001). Nevertheless, a substantial proportion of women developed advanced heart failure at a relatively young age, including 48% who were <50 years of age (range, 14–49).
Figure 4.
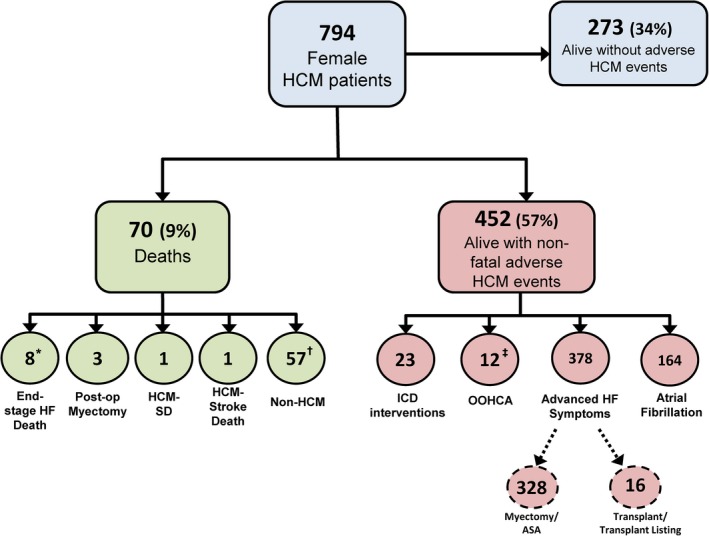
Flow diagram summarizing clinical outcome of women with hypertrophic cardiomyopathy (HCM). *Includes 2‐post‐transplant deaths. †Includes 2 patients in whom the cause of death was unknown and one with resuscitated cardiac arrest 2 years prior to death. ‡Includes 9 with subsequent appropriate defibrillator interventions. HF indicates heart failure; ICD, implantable cardioverter defibrillator; OOHCA, out‐of‐hospital cardiac arrest; SD, sudden death.
The rate of heart failure progression was 4.8%/year in women versus 3.4%/year in men (hazard ratio [HR], 1.6; 95% CI, 1.2–2.1; P<0.001; Figure 5) despite greater use of beta‐blockers, verapamil, and disopyramide in women (Table). Advanced heart failure symptoms were predominantly secondary to outflow obstruction in 92% of women and 92% of men (P=0.71).
Figure 5.
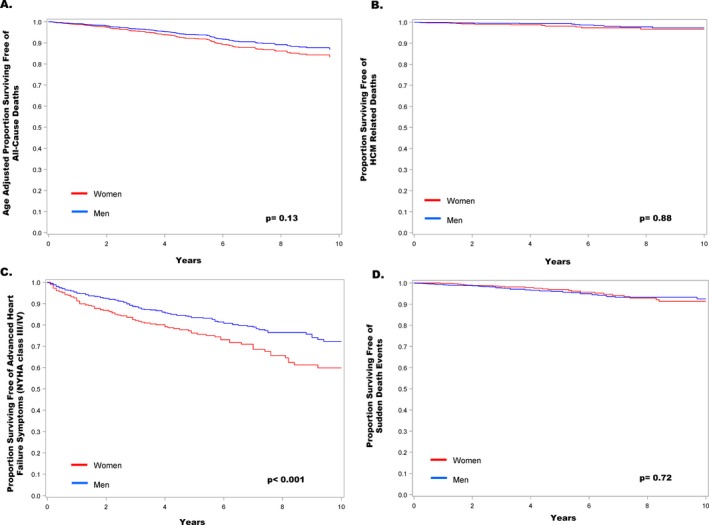
Survival free of events in men and women with hypertrophic cardiomyopathy. A, Comparison of total (all‐cause) mortality adjusted for age. B, Comparison of hypertrophic cardiomyopathy‐related deaths by sex. C, Freedom from development of advanced heart failure symptoms (NYHA class I/II to III/IV). D, Freedom from sudden death events (including appropriate defibrillator intervention for ventricular tachyarrhythmias and resuscitated cardiac arrest).
Obstructive HCM
At initial evaluation, women with obstructive HCM were older (58±16 versus 51±15 years in men; P<0.001), more symptomatic (NYHA class 2.4±0.7 versus 2.1±0.8 years; P<0.001), and had higher outflow gradients (78±31 versus 73±30 mm Hg; P<0.001). The proportion of women with obstructive HCM who ultimately developed advanced heart failure symptoms (75% versus 56%; P<0.001), as well as the rate of heart failure progression from NYHA class I/ II to class III/IV (7%/year versus 5%/yr; P=0.002) was substantially higher in women than in men.
Consistent with the higher prevalence of heart failure in women, both surgical myectomy (36% versus 26%; P<0.001) and alcohol septal ablation (11% versus 4%, P<0.001) were performed more commonly in women. Of 283 women who underwent septal myectomy with advanced NYHA class III/IV heart failure symptoms, 266 (94%) improved to NYHA class I/II, at the end of follow‐up, while 17 patients (6%) were nonresponders with persistent severe heart failure symptoms despite successful relief of subaortic obstruction. Similarly, of the 347 men with obstruction and NYHA III/IV symptoms who underwent myectomy, 335 (97%) improved to NYHA class I/II, and 12 (3%) were nonresponders (P=0.18).
Both men and women demonstrated substantial reduction or abolition of outflow gradients on the most recent echocardiogram 2.5±2.0 years after surgery (from 67±40 mm Hg to 2±6 mm Hg in women versus 48±41 mm Hg to 2±7 mm Hg in men; P=0.86). Operative mortality was low and did not differ between women and men (1.1% versus 0.9%; P=0.56).
Alcohol septal ablation was performed at older ages in 89 women (67±13 years) compared with 58 men (61±12; P=0.002) usually due to perceived increase in surgical risk or particularly advanced age. Alcohol septal ablation failed to adequately relieve outflow obstruction and symptoms in similar proportions of men and women treated (17% versus 20%; P=0.82), leading to either repeat ablation (n=7) or ultimately myectomy (n=21).
Nonobstructive HCM
At initial evaluation, women with nonobstructive HCM were older (51±19 versus 47±17 years; P=0.003) and more symptomatic then men (NYHA class 1.6±0.7 versus 1.3±0.5 years; P<0.001). The majority of women have remained asymptomatic or minimally symptomatic (NYHA class II) over follow‐up (88% of women versus 94% of men; P=0.002). However, the proportion of women with nonobstructive HCM who ultimately developed advanced refractory heart failure symptoms (12%) exceeded that of men 2‐fold (6%; P=0.002).
Development of end‐stage HCM with global systolic dysfunction and ejection fraction <50% occurred with similar frequency in both sexes (5% women, 4% men; P=0.33), although women with no obstruction were 2.8 times more likely to experience advanced drug‐refractory heart failure associated with preserved systolic function and ejection fraction ≥50% (7.5% versus 2.6%; P=0.002).
A similar proportion of women and men with nonobstructive HCM and unrelenting drug‐refractory heart failure symptoms were listed for heart transplant (57% women versus 63% men; P=0.80) and ultimately underwent transplant (65% versus 82%; P=0.19). Survival following transplant was also similar in both sexes: 85% of women at 6.4±4.1 years, and 94% of men (P=0.56); each is currently physically active without cardiovascular symptoms.
Eight women died of end‐stage heart failure, including 2 with transplant‐related complications. Of 6 other patients, 2 died awaiting transplant; 2 were not considered for transplant listing because of medication nonadherence (n=1) or irreversible pulmonary hypertension (n=1); and 2 patients declined transplant.
Sudden death
There was no difference in primary prevention ICD placements between women (24%) and men (25%; P=0.54), recommended on the basis of ≥1 HCM risk marker considered major within the individual patient clinical profile. Of 191 women implanted with a prophylactic ICD, 25 (13%) experienced ≥1 appropriate ICD intervention to terminate ventricular tachycardia/ventricular fibrillation and restore sinus rhythm, compared with 57 men (17%; P=0.20). Despite women presenting at older ages, the age at first appropriate ICD intervention was similar in women and men (44±15 years in women versus 46±17 in men; P=0.53).
Sudden cardiac death, resuscitated out‐of‐hospital cardiac arrest, and appropriate ICD interventions for ventricular tachycardia/ventricular fibrillation occurred in 5% of women (0.9%/year), versus 6% of men (0.8%/year; HR, 0.92; 95% CI, 0.6–1.5; P=0.73). One woman (and 4 men) ultimately died suddenly attributable to HCM (0.1% women versus 0.3% men; P=1.0). The only sudden death in a woman was in setting of a LV apical aneurysm (in 2004), well before this abnormality became an established risk marker and ICD indication.20
Atrial fibrillation
Symptomatic atrial fibrillation occurred at similar frequency in women and men (25% versus 26%; P=0.33), more frequently paroxysmal in women (86% versus 76%; P=0.004). Prophylactic anticoagulation to reduce stroke risk (91% in both groups; P=1.0), radiofrequency catheter ablation (17% versus 15%; P=0.60), and Maze procedure during surgical myectomy (17% versus 21%; P=0.28) were similar in women and men. Over follow‐up of 3.2±2.0 years, women trended toward less recurrent atrial fibrillation than men after the Maze procedure (19% versus 42%; P=0.06), but there was no difference between the sexes after catheter‐ablation (76% versus 64%; P=0.53). Thirteen women (7%) experienced thromboembolic strokes (one fatal), no different than men (4%; P=0.15); only 4 of the 13 occurred on prophylactic anticoagulation.
Mortality
Of the 794 women with HCM, 724 have survived over the follow‐up period; 70 (9%) died (Figure 4). Death attributable to HCM did not differ between the sexes: 13 women (1.6%) at 53±16 years of age, compared with 15 men (1.1%) at 56± 12 years (P=0.54), and 0.3%/year in both (HR, 1.5; 95% CI, 0.7–3.4; P=0.25). In women, 8 of the 13 deaths were related directly to end‐stage heart failure in the absence of LV outflow obstruction, including 6 who had either declined heart transplant, were ineligible, or waitlisted; and 2 others died of transplant‐related complications. The other 5 deaths were 1 sudden and arrhythmic (with LV apical aneurysm but without an ICD), 1 attributable to embolic stroke, and 3 postoperatively after myectomy (Table).
When adjusted for age at initial evaluation, there was no difference between women and men in all‐cause (total) mortality (1.7%/year versus 1.3%/year; HR, 1.32; 95% CI, 0.92–1.91; P=0.13; Figure 4), nor with respect to the mortality expected in an age‐ and sex‐matched general US population (P=0.38 and 0.37, respectively). While unadjusted total mortality in women (9%) exceeded that in men (5%; P=0.001), that difference was attributable to a greater number of non‐HCM related deaths in women, most commonly chronic pulmonary disease (n=8), cancer (n=6), or multiple noncardiac abnormalities (n=12).
Discussion
In HCM, there remains some uncertainty as to whether sex differences relate to mortality risk from heart failure or other disease complications.12, 13, 14 Therefore, in the present analysis of a large consecutive tertiary referral HCM cohort, we focused on differences in clinical course of heart failure, mortality, and other disease complications in women versus men as well as the effectiveness of contemporary treatments.1
Our data showed no difference between men and women with regard to deaths directly attributable to HCM. In addition, we found no significant difference in men and women for total (all‐cause) mortality after adjustment for patient age. Notably, the excess absolute number of all‐cause deaths observed in women was due largely to comorbid conditions such as obstructive pulmonary disease and cancer. Furthermore, we found no evidence that life expectancy is significantly altered in HCM solely on the basis of sex, given the low HCM‐related mortality rates of 0.3%/year in both women and men and lack of difference in mortality for women with HCM compared with the general US population.19
Our low mortality rates in women diverge from the portrayal by other HCM investigators in which women are reported to have a generally unfavorable outcome compared with men.12, 13, 14 This discrepancy appears to be largely attributable to differences in study designs and treatment strategies.
For example, in contrast to a report from the Mayo Clinic12 in which the primary end point was all‐cause mortality, in the present study we focused on the clinical course of individual patients with detailed follow‐up related specifically to HCM outcome and mortality. It is noteworthy, however, that our present cohort is smaller in size with shorter follow‐up,12 and therefore a trend toward increased all‐cause mortality in women cannot be excluded. While both studies have merit and are difficult to compare head to head, we believe our present methodology contributes a unique understanding of contemporary treatment benefit in HCM most importantly resulting in low disease‐related mortality. Furthermore, our data, showing low mortality in women attributable to effective management strategies, is supported by another recent Mayo Clinic report showing men and women with similar longevity and symptom relief specifically following myectomy surgery for obstructive HCM.18
Therapeutic options that aborted potentially lethal tachyarrhythmic events with implantable defibrillators, or averted heart failure death and morbidity with myectomy (and alcohol septal ablation) or heart transplant, contributed importantly to low HCM‐related mortality in women. Indeed, heart failure–related death has become exceedingly rare in HCM (involving only 1% of the women in this cohort) and was virtually confined to clinical circumstances surrounding heart transplant including listing for transplant. Furthermore, only 1 woman in the study population incurred sudden cardiac death (0.1%), in a patient with an LV apical aneurysm who presented well before this abnormality became an established risk marker and ICD indication in HCM.20
We found no evidence of sex‐related bias in recommendations for invasive therapeutic options such as myectomy, alcohol septal ablation, catheter ablation for atrial fibrillation, or heart transplant. This experience in HCM contrasts sharply with the management of other cardiovascular diseases, for which women are less likely to be referred for invasive treatment interventions including cardiac catheterization and revascularization for coronary artery disease21 or ICDs for primary sudden death prevention,22 anticoagulation and catheter ablation for atrial fibrillation,23 and heart transplant for end‐stage heart failure.24
Progression to severe drug‐refractory heart failure (classes III‐IV) predominated in women because of more frequent underlying LV outflow obstruction (at rest or, less commonly, with exercise provocation).11 Nevertheless, with surgical septal myectomy (or alcohol septal ablation), both women and men reliably experienced relief of outflow gradients and reversibility of heart failure symptoms; that is, >90% of patients improved by ≥1 NYHA functional class (with 70% returned to normal activity in class I). Indeed, delayed recognition of HCM can unnecessarily expose many symptomatic women to years of diminished quality of life without the benefit of myectomy (or alcohol septal ablation) and the opportunity to experience extended periods of life without significant functional impairment.2, 3, 11 This is particularly relevant considering that about one half of symptomatic women first experienced limiting heart failure symptoms attributable to outflow obstruction at relatively young ages.
While 90% of women with nonobstructive HCM have survived with no or only mild symptoms, they nevertheless more frequently experienced advanced heart failure than did men, including an almost 3‐fold greater likelihood associated with preserved systolic function (ejection fraction ≥50%). This clinical profile in HCM is similar to non‐HCM populations in which heart failure with preserved ejection fraction is twice as common in women, although systolic dysfunction occurs in similar frequency by sex.25
Less frequent HCM diagnosis in women was associated with a delay in clinical recognition, potentially related to societal bias affecting diagnosis and evaluation of HCM patients by sex.21, 26, 27 Given that HCM is a genetic heart disease with an autosomal dominant inheritance pattern, it would be our expectation that women with clinically established HCM should comprise about 50% of the cohort. The fact that only a little more than one third of our patients were women represents significant underrepresentation within the HCM study group. As reported in atherosclerotic coronary artery disease populations and non‐HCM congestive heart failure,21, 26, 27 early symptoms of angina or atypical chest pain, exertional dyspnea, and fatigue in women are not uncommonly misinterpreted, ignored, or inappropriately attributed to other causes. Women with such complaints may be less likely to seek medical diagnosis and care.21, 27 Also, HCM frequently has been considered a disease more commonly affecting men such as in the competitive athlete population.1, 28 Sex as a determinant of less frequent HCM diagnosis is reminiscent of recent observations that HCM is underrecognized in black patients.29
To explain the significant delay in HCM diagnosis and recognition of heart failure symptoms in women (by 6 years on average compared with men), we cannot exclude the possibility that biologic variability or genetic and/or hormonal factors are in part responsible for the timing and onset of heart failure symptoms. Of note, both atherosclerotic coronary artery disease and heart failure with preserved ejection fraction occur in women at more advanced ages than men, usually after menopause and associated with a burden of cardiovascular risk factors.21, 24, 25, 27 However, our observation that about 50% of women <50 years of age with HCM developed heart failure appears to exclude menopause as a major mechanism for this clinical course.
In conclusion, in this large consecutive HCM patient cohort analysis utilizing effective treatment interventions, we found no compelling evidence that this disease in women was associated with greater heart failure mortality, arrhythmic sudden death, nor other disease‐related complications than in men. We have, however, documented a significant delay in HCM diagnosis occurring in women with more frequent heart failure and progression to unrelenting symptoms consistent with NYHA functional class III to IV, predominantly driven by LV outflow obstruction.
Importantly, there were no treatment discrepancies identified between the sexes, and heart failure caused by LV outflow obstruction in women and men was equally amenable to reversal with low‐risk surgical septal myectomy. Therefore, these data underscore the importance of increased awareness of HCM in women with implications for earlier diagnosis and effective treatment, and the potential for survival with improved quality of life.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e012041 DOI: 10.1161/JAHA.119.012041.)
References
- 1. Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379:655–668. [DOI] [PubMed] [Google Scholar]
- 2. Maron BJ, Rowin EJ, Casey SA, Maron MS. How hypertrophic cardiomyopathy became a contemporary treatable genetic disease with low mortality. JAMA Cardiol. 2016;1:98–105. [DOI] [PubMed] [Google Scholar]
- 3. Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, Gersh BJ, Ackerman MJ, McCully RB, Dearani JA, Schaff HV, Danielson GK, Tajik AJ, Nishimura RA. Long‐term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470–476. [DOI] [PubMed] [Google Scholar]
- 4. Desai MY, Bhonsale A, Smedira NG, Naji P, Thamilarasan M, Lytle BW, Lever HM. Predictors of long‐term outcomes in symptomatic hypertrophic obstructive cardiomyopathy patients undergoing surgical relief of left ventricular outflow tract obstruction. Circulation. 2013;128:209–216. [DOI] [PubMed] [Google Scholar]
- 5. Woo A, Williams WG, Choi R, Wigle ED, Rozenblyum E, Fedwick K, Siu S, Ralph‐Edwards A, Rakowski H. Clinical and echocardiographic determinants of long‐term survival after surgical myectomy in obstructive hypertrophic cardiomyopathy. Circulation. 2005;111:2033–2041. [DOI] [PubMed] [Google Scholar]
- 6. Maron BJ, Dearani JA, Ommen SR, Maron MS, Schaff HV, Nishimura RA, Ralph‐Edwards A, Rakowski H, Sherrid MV, Swistel DG, Balaram S, Rastegar H, Rowin EJ, Smedira NG, Lytle BW, Desai MY, Lever HM. Low operative mortality achieved with surgical septal myectomy: role of dedicated hypertrophic cardiomyopathy centers in the management of dynamic subaortic obstruction. J Am Coll Cardiol. 2015;66:1307–1308. [DOI] [PubMed] [Google Scholar]
- 7. Rowin EJ, Maron BJ, Abt P, Kiernan MS, Vest A, Costantino F, Maron MS, DeNofrio D. Impact of advanced therapies for improving survival to heart transplant in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2018;121:986–996. [DOI] [PubMed] [Google Scholar]
- 8. Burke MA, Cook SA, Seidman JG, Seidman CE. Clinical and mechanistic insights into the genetics of cardiomyopathy. J Am Coll Cardiol. 2016;68:2871–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maron MS, Rowin EJ, Olivotto I, Casey SA, Arretini A, Tomberli B, Garberich RF, Link MS, Chan RHM, Lesser JR, Maron BJ. Contemporary natural history and management of nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2016;67:1399–1409. [DOI] [PubMed] [Google Scholar]
- 10. Sherrid MV, Balaram S, Kim B, Axel L, Swistel DG. The mitral valve in obstructive hypertrophic cardiomyopathy: a test in context. J Am Coll Cardiol. 2016;67:1846–1858. [DOI] [PubMed] [Google Scholar]
- 11. Maron BJ, Rowin EJ, Udelson JE, Maron MS. Clinical spectrum and management of heart failure in hypertrophic cardiomyopathy. JACC Heart Fail. 2018;6:353–363. [DOI] [PubMed] [Google Scholar]
- 12. Geske JB, Ong KC, Siontis KC, Hebl VB, Ackerman MJ, Hodge DO, Miller VM, Nishimura RA, Oh JK, Schaff HV, Gersh BJ, Ommen SR. Women with hypertrophic cardiomyopathy have worse survival. Eur Heart J. 2017;38:3434–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olivotto I, Maron MS, Adabag AS, Casey SA, Vargiu D, Link MS, Udelson JE, Cecchi F, Maron BJ. Gender‐related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:480–487. [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Wang J, Zou Y, Bao J, Sun K, Zhu L, Tian T, Shen H, Zhou X, Ahmad F, Hui R, Song L. Female sex is associated with worse prognosis in patients with hypertrophic cardiomyopathy in China. PLoS One. 2014;9:e102969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bos JM, Theis JL, Tajik AJ, Gersh BJ, Ommen SR, Ackerman MJ. Relationship between sex, shape, and substrate in hypertrophic cardiomyopathy. Am Heart J. 2008;155:1128–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Velzen HG, Schinkel AFL, Baart SJ, Huurman R, van Slegtenhorst MA, Kardys I, Michels M. Effect of gender and genetic mutations on outcomes in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2018;122:1947–1954. [DOI] [PubMed] [Google Scholar]
- 17. Nijenkamp L, Bollen IAE, van Velzen HG, Regan JA, van Slegtenhorst M, Niessen HWM, Schinkel AFL, Krüger M, Poggesi C, Ho CY, Kuster DWD, Michels M, van der Velden J. Sex differences at the time of myectomy in hypertrophic cardiomyopathy. Circ Heart Fail. 2018;11:e004133. [DOI] [PubMed] [Google Scholar]
- 18. Meghji Z, Nguyen A, Fatima B, Geske JB, Nishimura RA, Ommen SR, Lahr BD, Dearani JA, Schaff HV. Survival differences in women and men after septal myectomy for obstructive hypertrophic cardiomyopathy. JAMA Cardiol. 2019;4:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arias E, Heron M, Xu J. United States life tables, 2014. Natl Vital Stat Rep. 2017;66:1–64. [PubMed] [Google Scholar]
- 20. Rowin EJ, Maron BJ, Haas TS, Garberich RF, Wang W, Link MS, Maron MS. Hypertrophic cardiomyopathy with left ventricular apical aneurysm: implications for risk stratification and management. J Am Coll Cardiol. 2017;69:761–773. [DOI] [PubMed] [Google Scholar]
- 21. Jneid H, Fonarow GC, Cannon CP, Hernandez AF, Palacios IF, Maree AO, Wells Q, Bozkurt B, Labresh KA, Liang L, Hong Y, Newby LK, Fletcher G, Peterson E, Wexler L. Sex differences in medical care and early death after acute myocardial infarction. Circulation. 2008;118:2803–2810. [DOI] [PubMed] [Google Scholar]
- 22. Al‐Khatib SM, Hellkamp AS, Hernandez AF, Hernandez AF, Palacios IF, Maree AO, Wells Q, Bozkurt B, Labresh KA, Liang L, Hong Y, Newby LK, Fletcher G, Peterson E, Wexler L. Trends in use of implantable cardioverter‐defibrillator therapy among patients hospitalized for heart failure: have the previously observed sex and racial disparities changed over time? Circulation. 2012;125:1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng EY, Kong MH. Gender differences of thromboembolic events in atrial fibrillation. Am J Cardiol. 2016;117:1021–1027. [DOI] [PubMed] [Google Scholar]
- 24. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics‐2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pedrotty DM, Jessup M. “Frailty, thy name is woman”: syndrome of women with heart failure with preserved ejection fraction. Circ Cardiovasc Qual Outcomes. 2015;8:S48–S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. George J, Rapsomaniki E, Pujades‐Rodriguez M, Shah AD, Denaxas S, Herrett E, Smeeth L, Timmis A, Hemingway H. How does cardiovascular disease first present in women and men? Incidence of 12 cardiovascular diseases in a contemporary cohort of 1,937,360 people. Circulation. 2015;132:1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McSweeney JC, Rosenfeld AG, Abel WM, Braun LT, Burke LE, Daugherty SL, Fletcher GF, Gulati M, Mehta LS, Pettey C, Reckelhoff JF. Preventing and experiencing ischemic heart disease as a woman: state of the science: a scientific statement from the American Heart Association. Circulation. 2016;133:1302–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maron BJ, Haas TS, Ahluwalia A, Murphy CJ, Garberich RF. Demographics and epidemiology of sudden deaths in young competitive athletes: from the United States National Registry. Am J Med. 2016;129:1170–1177. [DOI] [PubMed] [Google Scholar]
- 29. Wells S, Rowin EJ, Bhatt V, Maron MS, Maron BJ. Association between race and clinical profile of patients referred for hypertrophic cardiomyopathy. Circulation. 2018;137:1973–1975. [DOI] [PubMed] [Google Scholar]


