Abstract
Background
Depression is associated with cardiovascular diseases, but the evidence is scarce regarding depression and risk of abdominal aortic aneurysms (AAA). The aim was to determine whether individuals with depressive symptoms have increased risk of AAA.
Methods and Results
This population‐based prospective study included 59 136 participants (52.4% women) aged 50 to 106 years from the HUNT (Norwegian Nord‐Trøndelag Health Study). Symptoms of depression were assessed using the depression subscale of the Hospital Anxiety and Depression Scale (HADS). During a median follow‐up of 13 years, there were 742 incident cases of AAA (201 women). A total of 6401 individuals (12.3%) reported depressive symptoms (defined as HADS depression scale [HADS‐D]) ≥8) (52.5% women). The annual incidence rate of AAA was 1.0 per 1000 individuals. At all ages, the estimated proportion of individuals diagnosed with AAA was higher among those with depressive symptoms (log‐rank test, P<0.001). People with HADS‐D ≥8 were older than those with HADS‐D<8 (median 57.8 versus 52.3 years, P<0.001) and a statistically significantly higher proportion of them (P<0.001) were smokers, overweight or obese, and reported a history of coronary heart disease, diabetes mellitus, and hypertension. In a Cox proportional hazard regression model adjusted for these factors, individuals with depressive symptoms had a ≈30% higher risk of AAA than those without (hazard ratio, 1.32, 95% CI 1.08–1.61, P=0.007).
Conclusions
This study shows that individuals with depressive symptoms have significantly higher risk of incident AAA, after adjustments for established risk factors.
Keywords: abdominal aortic aneurysm, depression, depressive symptoms, risk factors, HADS score, HUNT study
Subject Categories: Aneurysm, Risk Factors, Vascular Disease
Clinical Perspective
What Is New?
This large prospective population‐based study including more than 50 000 individuals used the Hospital Anxiety and Depression Scale depression score to examine a potential association between depressive symptoms and risk of abdominal aortic aneurysm.
Our study shows that individuals with depressive symptoms have significantly higher risk of incident abdominal aortic aneurysms, after adjusting for established risk factors for the disease.
What Are the Clinical Implications?
Our study provides new support to consider assessment of symptoms of depression in the risk evaluation of development of abdominal aortic aneurysms.
Assessing symptoms of depression in the risk evaluation of development of abdominal aortic aneurysms could be important when screening subgroups at increased risk of the disease.
Introduction
Abdominal aortic aneurysm (AAA), a localized widening of the abdominal aorta, is a potentially life‐threatening disease occurring in 1% to 3% of the adult population.1, 2, 3 Untreated ruptured AAA is associated with almost 100% mortality. Smoking, age, heredity, and male sex have consistently been identified as risk factors for the disease, and strong associations have also been found with coronary artery disease, hypertension, and hyperlipidemia.4, 5, 6 Diabetes mellitus has been associated with a lower risk of AAA in some studies.7 The pathogenesis of AAA is not fully understood, and it is highly likely that unknown factors that have yet to be discovered may influence development of this disease.8
Several studies have found that depression is associated with risk of cardiovascular disease (CVD).9, 10, 11, 12, 13, 14 A number of these studies have applied the Hospital Anxiety and Depression Scale (HADS)15 to measure symptoms of depression.13, 14 To our knowledge, only 1 previous study has reported a possible association between depression and development of AAA. This study showed a weak but increased risk of AAA in a subgroup with a history of depression.12
The role of depression in development of CVD has often been attributed to a high prevalence of established cardiovascular risk factors, such as smoking, low physical activity, coronary heart disease, and diabetes mellitus, among individuals with depression.9 The amount of adjustments for cardiovascular risk factors has varied in previous studies.9, 10 However, functional biological changes that occur during depression may also affect the cardiovascular system.16, 17 Previous studies have shown that depression is associated with low‐grade chronic inflammation17 and elevated inflammatory markers.16, 18, 19, 20 Inflammation seems to be a crucial part of the pathophysiology behind development of AAA.21, 22, 23 Thus, there could also be a biological link between depression and AAA, mediated through inflammation.
The aim of the present study was to examine whether individuals with symptoms of depression have an increased risk of AAA. The results are based on a large population‐based prospective study from Norway.
Methods
Because of the sensitive nature of the data collected for this study from the HUNT study database, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be directed to the HUNT (Nord‐Trøndelag Health) study.24 The HUNT databank has precise information on all data exported to different projects and is able to reproduce these on request.
The HUNT Study
The HUNT Study is a large multiphase population‐based health study conducted in the county of Nord‐Trøndelag in central Norway. The overall response rate was 89.4% in HUNT1 (1984–1986) (77 212 participants), 69.5% in HUNT2 (August 1995–1997) (65 237 participants), and 54.1% in HUNT3 (2006–2008) (50 807 participants).25 The population of Nord‐Trøndelag County (n=127 000 during HUNT2) is representative of the Norwegian population as a whole. The population has been found to be relatively stable (net out migration 0.3% per year) and homogeneous (<3% nonwhite), which makes it suitable for epidemiological studies.25, 26
Study Population
The present study population includes 59 136 individuals who participated in HUNT2 and/or HUNT3 (53.8% participated in both), with no prior diagnosis of AAA, and who reached the age of 50 years before the study ended on December 31, 2014. Among the 59 136 individuals (52.4% women), 742 incident (27.1% women) AAAs were detected (1.2%). Median age at initial HUNT participation was 53.7 years (range 31.6–101.4 years). For each HUNT survey, participants completed questionnaires regarding clinical and demographic parameters and underwent clinical examination. The date of AAA diagnosis was obtained from hospital medical records. The unique personal identification number was used to link data on AAA diagnoses and death with exposure data from the HUNT study. In total, 622 cases of AAA (166 women) were identified among the 50 657 (51.2% women) individuals with complete data on all risk factors.
Follow‐Up Period
The primary end point of this study was a diagnosis of AAA. Since only 3 cases of AAA were diagnosed before age 50 years, each individual was considered to be at risk of incident AAA from the age of 50 years, or from the date of initial HUNT participation at age >50 years, until the date of diagnosis of AAA, time of death, or closing date of the study. Median age at start of follow‐up was 53.7 years (50–101.4 years), and median follow‐up time was 13 years (range 1 month–20 years). The Norwegian Cause of Death Registry was used to acquire information on dates of death. A total of 11 919 deaths were attributable to causes other than AAA (Figure 1). At the end of the study period the oldest person was 106 years old.
Figure 1.
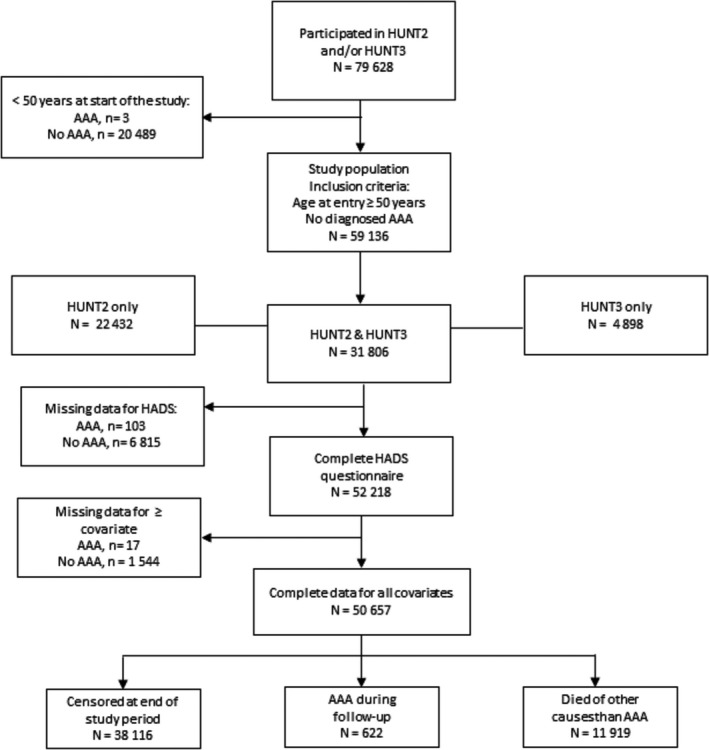
Flow chart of the study population. AAA indicates abdominal aortic aneurysm; HUNT, Norwegian Nord‐Trøndelag Health Study.
Diagnosis of AAA
National screening for AAA is not implemented in Norway, and no ultrasound of the abdominal aorta was performed in HUNT. All patients diagnosed with AAA according to the International Classification of Diseases, Ninth and Tenth Revisions (ICD‐9, ICD‐10 codes, 441.3–4 and I71.3–4) in the period 1995 through 2014 in Nord‐Trøndelag were identified. This information was obtained from hospital and outpatient registries from the 3 hospitals responsible for all outpatient care, follow‐up, and treatment of AAA in the region (St. Olavs Hospital, Levanger Hospital, and Namsos Hospital). All diagnoses were manually verified using patient charts. No distinction was made between symptomatic and asymptomatic AAA. From the total number of 742 AAAs, 134 ruptures (18%) were identified.
Assessment of Depressive Symptoms
A Norwegian version of the HADS questionnaire,15 a self‐reporting instrument used to measure severity of depressive symptoms, was included in HUNT2 and HUNT3, but not in HUNT1. This questionnaire can be used to assess symptoms of both anxiety (HADS‐A) and depression (HADS‐D) during the preceding week. It includes 7 questions on a Likert‐based scale of 0 to 3, with 2 separate ordinal subscales for anxiety and depression ranging from 0 to a maximum score of 21. A cut‐off point of ≥8 on the HADS‐D score has been shown to have a specificity of 0.79 and a sensitivity of 0.83 for clinical depression.27, 28 Accordingly, a HADS‐D score ≥8 was considered to reflect depressive symptoms in the main analyses. As an alternative to this dichotomization and to distinguish between moderate and severe depressive symptoms, we also performed additional analyses where HADS‐D was included as a continuous variable or categorized into 3 groups (<8, 8–10, ≥11, respectively). The low number of AAAs with high HADS‐D scores made it difficult to define more than 3 categories in our additional analyses, because only 5 of 622 AAA individuals had a HADS‐D score ≥15. The HADS anxiety score (HADS‐A) was not taken into consideration.
Assessment of Other Risk Factors
Results from clinical examinations and questionnaires provided data on other potential risk factors. The average of the second and third measurements of systolic and diastolic blood pressure was calculated. Hypertension (yes, no) was defined as systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg or use of antihypertensive medication (self‐reported). Height and weight were measured by the HUNT study. Body mass index (BMI) was computed as weight/height2 (kg/m2), and divided into 4 categories (<25, 25–30, 30–35, and ≥35 kg/m2). Coronary heart disease (CHD, yes, no) was defined as a composite variable of self‐reported history of myocardial infarction or angina pectoris. Diabetes mellitus (yes, no) and smoking status (never, past, current) were defined according to questionnaire responses (self‐reported). Blood samples were nonfasting, and serum was separated at the screening site and immediately placed in a refrigerator (−48°C). Serum total cholesterol was measured at a single laboratory (Levanger Hospital) as previously described.25, 26
Statistical Analyses
Descriptive statistics at start of follow‐up are presented as mean (SD), median (interquartile range), or as frequencies. The groups defined by HADS‐D (<8, ≥8) were compared by differences in mean values (95% CI) for continuous variables, and differences in proportions (95% CI) for categorical variables. The univariate Kaplan–Meier method was used to estimate proportions of disease‐free individuals at any point in time (ages) during the follow‐up period, and the log‐rank test was used to compare the 2 groups defined by HADS‐D (<8 versus ≥8). The number of individuals at risk of AAA at different ages is presented in the Kaplan–Meier plot.
Hazard ratios (HR) with 95% CI for incident AAA were calculated as estimates of relative risk in a multiple Cox proportional hazard regression model. Attained age was used as a time scale. Adjustments were made for sex, total cholesterol, BMI, and smoking, as well as indicator variables (yes, no) for CHD, diabetes mellitus, and hypertension. A variable representing age at measured HADS‐D (<50, 50–59, 60–69, 70–79, and ≥80 years) was also adjusted for in the regression model. Cholesterol was included in the model as a continuous variable (single term for linear trend), whereas the other continuous variables (age at HADS‐D measurement, BMI) were categorized to allow for a nonlinear association. All risk factors, except sex, were treated as time‐dependent covariates to allow for a shift in the exposure category for individuals who participated in HUNT more than once (the most recent measure that was used during follow‐up defined the risk factor level). The indicator variable for depressive symptoms was defined in terms of exposure at any time (HADS‐D ≥8), but was not updated if the second measurement was <8 (defined as never, ever).
Additional analyses were performed to explore a potential linear trend (HADS‐D as a continuous variable) or heterogeneity in risk of AAA according to increasing severity of depressive symptoms (HADS‐D <8, 8–10, ≥11). To distinguish between intermittent and persistent depressive symptoms, a variable based on the HADS‐D scores in both surveys was constructed and divided into never (<8 at both), intermittent (≥8 at first or second), or persistent (≥8 at both) depressive symptoms. Because of a low number of AAA cases among those with 2 HUNT participations and a HADS‐D score ≥8 (at 1 or both occasions), only age was adjusted for in the analysis of this variable (20 366 individuals were included).
The Cox regression analyses included individuals with complete information on all covariates. To test for heterogeneity regarding association with HADS‐D across subgroups, interaction terms between HADS‐D and each adjustment factor were included in the model, 1 at a time. The proportional hazard assumption was evaluated using log‐minus‐log plots. For adjustment factors that showed deviation from the proportional hazard assumption (smoking status, diabetes mellitus), additional analyses stratified by each of these factors were performed to evaluate whether and to what extent this affected the HR of HADS‐D. All statistical analyses were performed using Stata (Version IC.15.1 Stata Corp, College Station, TX). A P<0.05 was considered statistically significant (2‐sided tests).
Missing Values and Sensitivity Analyses
The proportion of missing values was generally low (range 0.1–11.7%, Table 1, Table S1), but was highest for HADS‐D (11.7%) and smoking (2.5%). Additional analyses identical to the final adjusted Cox regression model was performed based on multiple imputation for missing values (Table S2). Imputation was carried out using chained equations (n=50 data sets), under the assumption of “missing at random” (Table S1). Sensitivity analyses were performed where continuous variables for systolic and diastolic blood pressure, BMI, and age at HADS measurements replaced the categorical variables used in the main model.
Table 1.
Demographic and Clinical Characteristics at Start of Follow‐Upa, Total and by HADS‐D
| Total (N=59 136) | HADS‐D <8 (N=45 817) | HADS‐D ≥8 (N=6401) | Difference (95% CI) HADS‐D ≥8 vs <8b | |
|---|---|---|---|---|
| Age (y) at measured HADS‐D, mean (SD) | 52 218 (56.8 (13.3) | 4.00 (3.70, 4.40) | ||
| <50 | 21 474 (41.1) | 55.5 (12.7) | 59.5 (12.5) | |
| 50–60 | 12 343 (23.6) | 19 536 (42.6) | 1938 (30.3) | |
| 60–70 | 9113 (17.5) | 10 786 (23.5) | 1557 (24.3) | |
| 70–80 | 6931 (13.3) | 7860 (17.2) | 1253 (19.6) | |
| ≥80 | 2357 (4.5) | 5775 (12.6) | 1156 (18.0) | |
| Missing | 6918 (11.7) | 1860 (4.1) | 497 (7.8) | |
| Sex | ||||
| Women | 30 982 (52.4) | 24 044 (52.5) | 3291 (51.4) | −0.01 (−0.02, 0.002) |
| Men | 28 154 (47.6) | 21 773 (47.5) | 3110 (48.6) | |
| Smoking | ||||
| Never | 22 639 (32.28) | 17 703 (39.3) | 2144 (34.1) | −0.05 (−0.06, −0.04) |
| Past | 18 224 (30.82) | 14 352 (31.8) | 2004 (31.9) | 0.0007 (−0.01, 0.01) |
| Current | 16 913 (28.60) | 13 024 (28.9) | 2132 (34.0) | 0.05 (0.04, 0.06) |
| Missing | 1360 (2.3) | |||
| BMI (kg/m2), mean (SD) | 26.9 (4.1) | 0.40 (0.24, 0.45) | ||
| <25 | 19 923 (33.69) | 26.8 (4.0) | 27.1 (4.4) | |
| 25–29 | 26 926 (45.53) | 15 775 (34.7) | 2063 (32.9) | |
| 30–35 | 9171 (15.51) | 21 118 (46.4) | 2794 (44.6) | |
| ≥35 | 2429 (4.11) | 6872 (15.1) | 1070 (17.0) | |
| Missing | 687 (1.16) | 1731 (3.8) | 343 (5.5) | |
| CHD | ||||
| Yes | 4841 (8.19) | 3135 (6.9) | 787 (12.3) | 0.05 (0.05, 0.06) |
| No | 54 (91.71) | 42 660 (93.1) | 5607 (87.7) | |
| Missing | 61 (0.10) | |||
| Diabetes mellitus | ||||
| Yes | 2396 (4.1) | 157 (3.5) | 340 (5.3) | 0.020 (0.013, 0.024) |
| No | 56 593 (95.9) | 1589 (96.5) | 6032 (94.7) | |
| Missing | 147 (0.25) | |||
| Blood pressure (mm Hg) | ||||
| Diastolic, mean (SD) | 81.2 (12.3) | 81.2 (12.1) | 81.6 (12.5) | 0.44 (0.13, 0.77) |
| Systolic, mean (SD) | 139.5 (12.3) | 138.6 (21.6) | 140.3 (22.7) | 1.74 (1.17, 2.31) |
| Combined variable (c) | ||||
| Hypertension | 29 847 (50.47) | 0.05 (0.04, 0.07) | ||
| Not hypertension | 29 249 (49.46) | 22 425 (49.0) | 3481 (54.4) | |
| Missing | 40 (0.07) | 23 388 (51.0) | 2913 (45.6) | |
| Cholesterol (mmol/L) | ||||
| Mean (SD) | 6.1 (1.2) | 6.04(1.2) | 6.15 (1.2) | 0.10 (0.07, 0.14) |
| Missing | 1047 (1.8) | |||
BMI indicates body mass index (kg/m2); CHD, coronary heart disease;HADS‐D, Hospital Anxiety and Depression Scale–Depression.
Median age at start of follow‐up was 53.7 years (range 50–101.4).
Differences are given in proportion or mean values with 95% CIs (missing values excluded).
Combined variable of systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg or reported use of antihypertensive medication.
Ethics
The HUNT Study board of directors and the Regional Ethics Committee (2014/175/REK Midt‐Norge) approved this study. The present study was conducted in accordance with the Helsinki Declaration. Every participant included in the HUNT study provided written informed consent. The HUNT databank has precise information on all data exported to different projects and is able to reproduce these on request. Requests to access the data set from qualified researchers may be directed to the HUNT study.24
Results
Characteristics of the Study Population
Table 1 shows characteristics of the study population at start of follow‐up, total, and by HADS‐D. In total, 22 432 of the individuals in the present study participated in HUNT2 only (38%), 4898 in HUNT3 only (8%), whereas 31 806 participated in both surveys (54%) (Figure 1). The median HADS‐D score among the 52 218 individuals who responded to the questionnaire was 3.0 (interquartile range 1.0–6.9). In all, 12.3% of the study population reported depressive symptoms (HADS‐D ≥8) (men, 12.5% versus women, 12.0%, P=0.11). A HADS‐D score ≥8 was associated with several known risk factors for AAA. People with HADS‐D ≥8 were older than those with HADS‐D <8 (median 57.8 years [interquartile range 50.0–65.0] versus 52.3 years [interquartile range 50.0–70.4] years, P<0.001), and a statistically significantly higher (P<0.001) proportion of them were smokers (34.0% versus 28.9%), overweight or obese (32.5% versus 18.9%), had higher mean cholesterol levels (6.15 [95% CI, 6.12–6.18]) versus 6.04 [95% CI, 6.03–6.05]), and reported a history of CHD (12.3% versus 6.9%), diabetes mellitus (5.3% versus 3.5%), and hypertension (54.4% versus 49.0%).
Depressive Symptoms and Risk of AAA
The crude annual incidence rate of AAA was 1.0 per 1000 person‐years, and higher for men than women (1.59 versus 0.49 per 1000). Figure 2 shows the results from the univariate Kaplan–Meier analysis of the association between HADS‐D (missing values excluded) and AAA. At all ages and in both groups defined by HADS‐D, the estimated proportion of individuals diagnosed with AAA was low (<10%), albeit significantly higher in the group of individuals with HADS‐D ≥8 (P<0.001, log‐rank test). At the age of 60, 70, 80, and 90 years, the difference in estimated proportions with AAA was 0.2% (HADS‐D ≥8) versus 0.1% (HADS‐D<8), 1.3% versus 0.7%, 4.2% versus 2.7%, and 7.8% versus 5.7%, respectively. Among the individuals diagnosed with AAA, the median time from completion of the HADS‐D questionnaire (HUNT participation) to the date of AAA diagnosis was 10.5 years (range 0.3–18.6 years), and similar between those with and without depressive symptoms. Mean age at diagnosis of AAA was 76.1 years (50.5–100.3 years), and women were 2 years older than men when diagnosed (77.6 versus 75.6 years, P=0.005).
Figure 2.

The Kaplan–Meier curve of incident AAAs and numbers at risk during follow‐up according to HADS‐D <8 vs ≥8. Numbers at risk of AAA are shown among individuals with no missing values for HADS‐D during follow‐up. AAA indicates abdominal aortic aneurysm; HADS‐D, Hospital Anxiety and Depression Scale–Depression.
In a univariable Cox regression analysis (Table 2), individuals with HADS‐D ≥8 had a 46% higher hazard of incident AAA than those with HADS‐D <8 (HR, 1.46; 95% CI 1.2–1.8). Adjusted for sex, age at measurement, and known factors associated with AAA (smoking, hypertension, cholesterol levels, BMI, CHD, and diabetes mellitus), individuals with HADS‐D ≥8 had a 31% higher hazard of AAA than those with HADS<8 (HR, 1.32, 95% CI 1.08–1.61). Current smoking was associated with a considerable increase in risk of AAA (HR 8.72, 95% CI 6.55–11.61). The proportion of smokers was higher among those with depressive symptoms, but introduction of smoking as the last factor in the adjusted model only moderately reduced the HR for HADS‐D (from HR 1.41 to HR 1.32). Incident AAA was >2.5 times more likely to occur in men than women (HR, 2.64, 95% CI 2.18–3.20). Significant associations were also observed for hypertension, CHD, and increasing cholesterol levels, while a borderline protective effect was found for diabetes mellitus in the fully adjusted model. No significant association was found with BMI. Additional analyses with smoking status and diabetes mellitus as stratification variables because of deviation from proportional hazard assumption did not change the risk estimates for HADS‐D notably.
Table 2.
HR with 95% CI for the Association Between HADS‐D and AAA Developmenta
| No. of AAA n | Univariable HR (95% CI) | Multivariable HR (95% CI)b | |
|---|---|---|---|
| HADS‐D | |||
| <8 | 513 | 1.00 | 1.0 |
| ≥8 | 109 | 1.46 (1.19–1.78) | 1.32 (1.08–1.61) |
| P value | <0.001 | 0.007 | |
| Sex | |||
| Women | 161 | 1.00 | 1.00 |
| Men | 461 | 3.51 (2.93–4.20) | 2.64 (2.18–3.20) |
| P value | <0.001 | <0.001 | |
| Smoking | |||
| Never | 61 | 1.00 | 1.00 |
| Past | 204 | 4.78 (3.60–6.35) | 3.17 (2.36–4.25) |
| Current | 357 | 11.20 (8.47–14.80) | 8.72 (6.56–11.61) |
| P value | <0.001 | <0.001 | |
| CHD | |||
| No | 460 | 1.00 | 1.00 |
| Yes | 162 | 2.83 (2.383.37) | 2.39 (2.00–2.85) |
| P value | <0.001 | <0.001 | |
| Diabetes mellitus | |||
| No | 601 | 1.00 | 1.00 |
| Yes | 21 | 0.81 (0.57–1.15) | 0.75 (0.52–1.08) |
| P value | 0.25 | <0.12 | |
| Hypertension | |||
| No | 174 | 1.00 | 1.00 |
| Yes | 448 | 1.25 (1.04–1.51) | 1.35 (1.12–1.62) |
| P value | 0.016 | 0.002 | |
| BMI, m/kg2 | |||
| <25 | 196 | 1.00 | 1.00 |
| 25–29 | 305 | 0.88 (0.74–1.06 | 0.96 (0.80–1.16) |
| 30–34 | 103 | 0.84 (0.67–1.06 | 1.10 (0.87–1.41) |
| ≥35 | 18 | 0.71 (0.46–1.08) | 1.19 (0.77–1.84) |
| P value | 0.25 | 0.56 | |
| Cholesterol (mmol/L) | 622 | 0.97 (0.91–1.03) | 1.10 (1.03–1.17) |
| P value | 0.39 | 0.005 | |
| Age (y) at measurement | |||
| <50 | 38 | 1.89 (0.98–3.65) | 1.43 (0.75–2.75) |
| 50–59 | 128 | 1.16 (0.77–1.77) | 0.98 (0.65–1.49) |
| 60–69 | 248 | 1.00 | 1.00 |
| 70–79 | 185 | 1.26 (0.99–1.60) | 1.17 (0.92–1.48) |
| ≥80 | 23 | 0.69 (0.48–0.98) | 0.74 (0.52–1.06) |
| P value | 0.02 | 0.13 | |
Number of individuals, n=50 657 (622 AAA). AAA, indicates abdominal aortic aneurysm; BMI, body mass index (kg/m2); CHD, coronary heart disease; HADS‐D, Hospital Anxiety and Depression Scale–Depression; HR, hazard ratios.
HR estimates with 95% CIs calculated in a Cox proportional hazard regression model with attained age as time scale.
Adjusted for age at HADS‐D measure (HUNT participation), sex, diabetes mellitus, BMI category, coronary heart disease, hypertension, total cholesterol, and smoking.
Table 3 presents HRs for AAA in relation to HADS‐D measured as a continuous variable, as an ordinal categorical variable (<8, 8–11, ≥11), and with combined data from HUNT2 and HUNT3 (never, intermittent, persistent). A significant linear trend was observed between increasing HADS‐D and risk of AAA in the unadjusted analysis, but not in the adjusted analyses. Moreover, individuals with severe depressive symptoms (HADS‐D ≥11) did not have higher risk of AAA than individuals with less severe (HADS‐D 8–10) symptoms (HR of 1.02 versus 1.42, compared with HADS‐D <8) (Figure 3). The number of individuals with AAA and a HADS‐D ≥11 was low (n=29). Among individuals with valid HADS‐D in both HUNT2 and HUNT3 (n=20 366), 82% reported no depressive symptoms (HADS‐D <8 at both surveys), 13% intermittent symptoms (HADS‐D ≥8 at only 1 survey), and 4.2% persistent symptoms (HADS‐D≥8 at both surveys). Those with persistent symptoms did not have significantly higher risk of AAA than those with intermittent symptoms, but both had higher risk compared with individuals with no depressive symptoms (HR, 1.50 and 1.41, respectively).
Table 3.
HR With 95% CI for the Association Between HADS‐D (Continuous, 3 Categories, Combined Values from HUNT2 and HUNT3), and Risk of AAAa
| Factors | No. of AAA n | Univariable HR (95% CI) | Multivariable HR (95% and CI)b |
|---|---|---|---|
| HADS‐D, continuous (0–21) | 622 | 1.03 (1.01–1.06) | 1.02 (0.99–1.04) |
| P for trend | 0.007 | 0.16 | |
| HADS‐D (3 categories) | |||
| <8 | 513 | 1.00 | 1.00 |
| 8–10 | 80 | 1.55 (1.24–1.94) | 1.43 (1.15–1.79) |
| ≥11 | 29 | 1.21 (0.81–1.79) | 1.03 (0.70–1.53) |
| P for trend | 0.0005 | 0.007 | |
| HADS‐D (combined)c | |||
| Never | 120 | 1.00 | … |
| Intermittent | 31 | 1.41 (0.95–2.1) | |
| Persistent | 11 | 1.50 (0.81–2.8) | |
| P for trend | 0.13 | ||
Number of individuals (continuous vs 3 categories), n=50 657. Number of individuals (combined categories), n=20 366. AAA, abdominal aortic aneurysm; HADS‐D, Hospital Anxiety and Depression Scale–Depression; HR, hazard ratios; HUNT, Norwegian Nord‐Trøndelag Health Study.
HR estimates with 95% CIs calculated in a Cox proportional hazard regression model with attained age as time scale.
HR estimates with 95% CI for all 3 HADS‐D variables (continuous vs 3 categories, combined categories) in separate models, but equally adjusted for age at HADS‐D measure (HUNT participation), sex, diabetes mellitus, body mass index, coronary heart disease, hypertension, total cholesterol, and smoking.
Combined categories of HADS‐D were defined as, never (<8, <8), intermittent (<8,≥8 or ≥8,<8), persistent (≥8, ≥8).
Figure 3.

HR with 95% CI for association between HADS‐D (3 categories, with <8 as reference group) and risk of AAA. HR for dichotomized HADS‐D (≥8 vs <8) and HR for linear trend (per unit increase) are also shown. HR values were calculated in a Cox proportional hazard regression model with attained age as time scale, adjusted for age at HADS‐D measure (HUNT participation), sex, diabetes mellitus, body mass index category, coronary heart disease, hypertension, total cholesterol, and smoking category. AAA indicates abdominal aortic aneurysm; HR, hazard ratio; HUNT, Norwegian Nord‐Trøndelag Health Study; HUNT, Norwegian Nord‐Trøndelag Health Study.
Subgroup Analyses
The results from the analyses of the association between HADS‐D and AAA analyzed in subgroups of each of the included covariates are presented in Table 4. The proportion with AAA was <10% in all age groups, and the numbers of AAAs in some subgroups were low. The risk estimates were therefore imprecise, and neither of the interaction terms reached statistical significance or added information to the main model (range, P interaction: 0.22–0.61), partly because of low power of the test. This limits the generalizability of the results from the subgroup analyses. In our study, however, the association between depressive symptoms and AAA was slightly more pronounced in women than men (HR of 1.56 versus 1.24), and among those with high cholesterol and reported hypertension. In contrast, the association between depressive symptoms and AAA was somewhat lower in subgroups of smokers, and among people with lower BMI, reported CHD, and diabetes mellitus.
Table 4.
Subgroup Analysis and Tests for Interactions: HR with 95% CI for Incident AAA in People With Versus Without Depressive Symptoms, in Subgroups Defined by Other Risk Factorsa
| No. of AAA | HR (95% CI) | ||
|---|---|---|---|
| HADS‐D <8 | HADS‐D ≥8 | ≥8 vs <8 | |
| Sex | |||
| Men | 384 | 32 | 1.24 (0.9–1.6) |
| Women | 129 | 77 | 1.56 (1.1–2.3) |
| Age at measured HADS‐D, y | |||
| <60 | 131 | 35 | 1.88 (1.2–3.0) |
| 60–70 | 211 | 37 | 1.35 (0.9–1.9) |
| 70–80 | 153 | 32 | 1.15 (0.9–1.5)b |
| ≥80 | 18 | 5 | |
| CHD | |||
| Yes | 131 | 31 | 1.17 (0.8–1.7) |
| No | 382 | 78 | 1.40 (1.1–1.8) |
| Diabetes mellitus | |||
| Yes | 17 | 4 | 0.90 (0.4–2.2) |
| No | 496 | 105 | 1.35 (1.1–1.7) |
| Hypertension | |||
| Yes | 372 | 76 | 1.3 (1.1–1.6) |
| No | 141 | 33 | 1.13 (0.7–1.7) |
| Smoking | |||
| Never | 50 | 11 | 1.67 (0.9–3.1) |
| Past | 170 | 34 | 1.40 (1.0–1.9) |
| Current | 293 | 64 | 1.22 (0.9–1.6) |
| BMI | |||
| <25 | 158 | 38 | 1.54 (1.1–2.2) |
| 25–29 | 251 | 54 | 1.28 (0.9–1.7) |
| 30–35 | 91 | 12 | 1.11 (0.7–1.7)b |
| ≥35 | 13 | 5 | |
| Cholesterol, mmol/L | |||
| <6.5 | 284 | 56 | 1.16 (0.9–1.6) |
| ≥6.5 | 221 | 61 | 1.51 (1.1–2.0) |
AAA indicates abdominal aortic aneurysm; BMI, body mass index; CHD, coronary heart disease; HADS‐D, Hospital Anxiety and Depression Scale–Depression; HR, hazard ratio.
Results based on Cox proportional hazard regression model adjusted for all factors, and with interaction terms (dichotomized score and each risk factor) included 1 at a time.
Last 2 categories combined. P for interaction, range: 0.22 to 0.61. Cholesterol dichotomized at 6.5 mmol/L, based on clinically used values.
Sensitivity Analyses
In the sensitivity analyses where systolic and diastolic blood pressure, respectively, replaced the dichotomized hypertension variable based on combined values, the HR for HADS‐D did not change notably (systolic blood pressure: HR=1.32 [1.1–1.6], diastolic blood pressure: HR=1.33 [1.1–1.6]). Only diastolic blood pressure was significantly associated with AAA (HR, diastolic blood pressure=1.02 [1.01–1.02]). In a sensitivity analysis where all the categorized variables were treated as continuous variables (BMI, systolic or diastolic blood pressure), the HR for HADS‐D remained unchanged (HR=1.32 [1.1–1.6]). Analyses based on data with multiple imputation for missing values (n=59 136, n=742 AAA) produced results similar to those based on complete cases (Table S2). An unadjusted HR for the association between HADS‐D≥8 and AAA was 1.45 (95% CI, 1.2–1.8, P<0.001) after multiple imputation, whereas the multivariate adjusted HR was 1.30 (95% CI, 1.07–1.59, P=0.009).
Discussion
In this large, prospective population‐based study, individuals with symptoms of depression had ≈30% higher risk of incident AAA than individuals without depressive symptoms. The association remained significant after adjustment for established risk factors for AAA, and the importance of smoking, male sex, CHD, hypertension, and increasing levels of cholesterol was confirmed.
An association between depressive symptoms and risk of AAA has only been reported in 1 previous study. In a population‐based study evaluating 12 different disease outcomes, depression was associated with subsequent cardiac, cerebrovascular, and peripheral arterial disease, as well as AAA.12 However, the follow‐up time in the present study was more than twice as long, and the association between depressive symptoms and AAA was stronger than the previously reported 12% increase in risk. The lower risk may be related to a different assessment of depressive symptoms (diagnosed by general practitioner).
Although the risk of AAA was higher among individuals with depressive symptoms defined by a HADS‐D ≥8, no difference was found between individuals with severe (HADS‐D ≥11) and less severe symptoms (HADS‐D 8–11). The number of individuals with severe depressive symptoms was low in our study population, which might result from recruitment bias. Individuals with depression, particularly severe depression, tend to not attend screening programs or population‐based studies upon invitation.29, 30, 31 Only 5 of 622 AAA individuals had a HADS‐D score ≥15. It is possible that this may have led to an underestimation of the association between depressive symptoms and AAA (selection bias). Moreover, we found no marked difference between the effect of intermittent and persistent symptoms of depression in the subgroup with repeated measurements of HADS‐D, but the results from these analyses were imprecise.
Depression is associated with several negative behavioral factors such as smoking, lower rate of smoking cessation,32 alcohol and drug abuse, less physical activity, poor diet, impaired self‐care, and low adherence to prescribed drugs.33, 34 Indisputably, smoking is the major risk factor for AAA,4, 5, 35, 36, 37 which our data also support. Otherwise, there have been no consistently reported associations between these factors and risk of AAA. A recent study reported a reduced risk of AAA among individuals who walked or bicycled >40/min per day.36 Another study reported lower risk of AAA among individuals adhering to a diet aiming to prevent hypertension.38 Although we did not adjust for these 2 factors, the adjustments made for BMI, diabetes mellitus, and cholesterol levels may indirectly reflect the effect of physical activity and diet. Thus, it is unlikely that any of the abovementioned factors would lead to severe bias in the risk estimates for HADS‐D because of confounding. There are other lifestyle factors that may also be considered to influence the risk of AAA, such as lower level of education39, 40, 41 and low socioeconomic status.42, 43, 44, 45 We did not have these data in the present study, and cannot exclude that this may have resulted in an overestimation of the association between depressive symptoms and AAA. On the other hand, depressive symptoms were not considered as an explanatory factor in the previous studies. In summary, apart from family history, the present study has performed multivariable adjustments for all other established risk factors for AAA.
Some studies suggest that the elevated risk of CVD among individuals with depression is more pronounced among women than men,46 although the results are inconsistent.10, 13, 47 There was no significant difference in the association between depression and AAA in women compared with men, although the relative risk estimates were slightly higher for women. The lack of statistical significance may be because of low power in the interaction tests. The study by Daskalopoulou et al reported somewhat similar risk estimates for both men and women (HR, men 1.15 versus HR, women 1.09).12
The risk of depression has been reported to be higher among those with established CVD,48, 49 but depression is also a risk factor for development of CVD,10, 11, 13, 47 indicating a bidirectional effect. These observations are based on contributions from different studies. A similar bidirectional relationship between depression and AAA has not yet been established. Depression has been found to be more prevalent among patients diagnosed with AAA than in the general population.50, 51 To our knowledge, only 1 previous study has shown that depression might be a risk factor for AAA.12 Thus, our study adds evidence supporting that a bidirectional relationship may also exist between depression and AAA.
Strengths and Limitations
Our study has some major strengths. The prospective design, the size of the study population, and the duration of follow‐up made it possible to address the association between depression and the risk of AAA, adjusted for a comprehensive range of potential confounding factors. The access to repeated measurements of risk factors made it possible to account for changes in the exposure levels during follow‐up (defined as time‐varying covariates). The numbers at risk within a particular risk group are then more correctly defined, thereby reducing potential bias in the risk estimates because of misclassification of the exposure variable. All diagnoses of AAA were ICD‐based and verified manually from patient charts. The association with HADS‐D was consistently observed across subgroups, although the magnitude of risk estimates varied somewhat. The results based on the analyses that used sets of imputed values for missing values were nearly identical to the results from complete case analyses.
However, there are also some limitations that should be addressed. The evaluation of depression and other risk factors was largely based on self‐reported questionnaires, potentially leading to information bias.28, 52 Symptoms of depression may vary over time, and we did not have repeated measures on all individuals in our cohort. Thus, potential misclassification might have occurred, despite the use of time‐dependent covariates. However, possible misclassification is likely to have been random, most probably resulting in an underestimation of relative risk estimates. There was no information about use of antidepressants, family history of AAA or physical activity, and unmeasured unknown confounding is always a limitation. The amount of missing data was highest for HADS‐D, particularly among the oldest age groups, for which reason the occurrence of depression may potentially have been underestimated. Moreover, it is likely that asymptomatic AAA was underdiagnosed. The observed 1.2% occurrence of incident AAA in our study was consistent with the expected rate,53 even though it was based on identification of diagnosed AAA, since ultrasound examination of the abdominal aorta was not part of the HUNT study.
Implications
Our study provides significant new support to include assessment of depression in the evaluation of risk of AAA, which could potentially be especially important when screening subgroups at increased risk of this disease.
Conclusions
Depression has previously been linked to the risk of CHD and stroke. In this large population‐based, prospective study, individuals with symptoms of depression had significantly higher risk of AAA, even after adjustment for established risk factors.
Sources of Funding
This work received funding from the Department of Circulation and Medical Imaging at the Norwegian University of Science and Technology (NTNU). This research was also supported by the Swedish Heart‐Lung Foundation (20160266, Hultgren). The funding sources played no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosures
None.
Supporting information
Table S1. Distribution of Missing Values for HADS for Each of the Covariates
Table S2. Adjusted Hazard Ratios (HR) With 95% Confidence Intervals (CI) for the Association Between HADS‐D and †AAA Development, Based on Results After Multiple Imputation (n=50 data sets)
Acknowledgments
The Nord‐Trøndelag Health Study (The HUNT Study) is a collaboration between the HUNT Research Centre (Faculty of Medicine and Health Sciences, NTNU‐ Norwegian University of Science and Technology), the Nord‐Trøndelag County Council, the Central Norway Regional Health Authority, and the Norwegian Institute of Public Health. We thank The Swedish Connection LLC for revising the manuscript for grammar and syntax.
(J Am Heart Assoc. 2019;8:e012535 DOI: 10.1161/JAHA.119.012535.)
References
- 1. Svensjo S, Bjorck M, Gurtelschmid M, Djavani Gidlund K, Hellberg A, Wanhainen A. Low prevalence of abdominal aortic aneurysm among 65‐year‐old swedish men indicates a change in the epidemiology of the disease. Circulation. 2011;124:1118–1123. [DOI] [PubMed] [Google Scholar]
- 2. Wanhainen A, Hultgren R, Linne A, Holst J, Gottsater A, Langenskiold M, Smidfelt K, Bjorck M, Svensjo S. Outcome of the swedish nationwide abdominal aortic aneurysm screening program. Circulation. 2016;134:1141–1148. [DOI] [PubMed] [Google Scholar]
- 3. Oliver‐Williams C, Sweeting MJ, Turton G, Parkin D, Cooper D, Rodd C, Thompson SG, Earnshaw JJ. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25‐year ultrasound population screening programme. Br J Surg. 2018;105:68–74. [DOI] [PubMed] [Google Scholar]
- 4. Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7‐year prospective study: the tromso study, 1994‐2001. Circulation. 2009;119:2202–2208. [DOI] [PubMed] [Google Scholar]
- 5. Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, Gelijns AC, Greco G. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539–548. [DOI] [PubMed] [Google Scholar]
- 6. Larsson E, Granath F, Swedenborg J, Hultgren R. A population‐based case‐control study of the familial risk of abdominal aortic aneurysm. J Vasc Surg. 2009;49:47–50; discussion 51. [DOI] [PubMed] [Google Scholar]
- 7. De Rango P, Farchioni L, Fiorucci B, Lenti M. Diabetes and abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2014;47:243–261. [DOI] [PubMed] [Google Scholar]
- 8. Sakalihasan N, Michel JB, Katsargyris A, Kuivaniemi H, Defraigne JO, Nchimi A, Powell JT, Yoshimura K, Hultgren R. Abdominal aortic aneurysms. Nat Rev Dis Primers. 2018;4:34. [DOI] [PubMed] [Google Scholar]
- 9. Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta‐analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763–2774. [DOI] [PubMed] [Google Scholar]
- 10. Gan Y, Gong Y, Tong X, Sun H, Cong Y, Dong X, Wang Y, Xu X, Yin X, Deng J, Li L, Cao S, Lu Z. Depression and the risk of coronary heart disease: a meta‐analysis of prospective cohort studies. BMC Psychiatry. 2014;14:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grenon SM, Hiramoto J, Smolderen KG, Vittinghoff E, Whooley MA, Cohen BE. Association between depression and peripheral artery disease: insights from the heart and soul study. J Am Heart Assoc. 2012;1:e002667 DOI: 10.1161/JAHA.112.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daskalopoulou M, George J, Walters K, Osborn DP, Batty GD, Stogiannis D, Rapsomaniki E, Pujades‐Rodriguez M, Denaxas S, Udumyan R, Kivimaki M, Hemingway H. Depression as a risk factor for the initial presentation of twelve cardiac, cerebrovascular, and peripheral arterial diseases: data linkage study of 1.9 million women and men. PLoS One. 2016;11:e0153838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gustad LT, Laugsand LE, Janszky I, Dalen H, Bjerkeset O. Symptoms of anxiety and depression and risk of acute myocardial infarction: the hunt 2 study. Eur Heart J. 2014;35:1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meyer FA, Hugentobler E, Stauber S, Wilhelm M, Znoj H, von Kanel R. Depressive symptoms at discharge from rehabilitation predict future cardiovascular‐related hospitalizations. Cardiology. 2015;131:80–85. [DOI] [PubMed] [Google Scholar]
- 15. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 16. Kohler O, Krogh J, Mors O, Benros ME. Inflammation in depression and the potential for anti‐inflammatory treatment. Curr Neuropharmacol. 2016;14:732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayley S, Poulter MO, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor‐ and cytokine‐induced alterations of neuroplasticity. Neuroscience. 2005;135:659–678. [DOI] [PubMed] [Google Scholar]
- 18. Howren MB, Lamkin DM, Suls J. Associations of depression with c‐reactive protein, il‐1, and il‐6: a meta‐analysis. Psychosom Med. 2009;71:171–186. [DOI] [PubMed] [Google Scholar]
- 19. Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment‐resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pikhart H, Hubacek JA, Kubinova R, Nicholson A, Peasey A, Capkova N, Poledne R, Bobak M. Depressive symptoms and levels of c‐reactive protein: a population‐based study. Soc Psychiatry Psychiatr Epidemiol. 2009;44:217–222. [DOI] [PubMed] [Google Scholar]
- 21. Kuivaniemi H, Ryer EJ, Elmore JR, Tromp G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert Rev Cardiovasc Ther. 2015;13:975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michel JB, Martin‐Ventura JL, Egido J, Sakalihasan N, Treska V, Lindholt J, Allaire E, Thorsteinsdottir U, Cockerill G, Swedenborg J. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc Res. 2011;90:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eagleton MJ. Inflammation in abdominal aortic aneurysms: cellular infiltrate and cytokine profiles. Vascular. 2012;20:278–283. [DOI] [PubMed] [Google Scholar]
- 24.The hunt study—a longitudinal population health study in norway. Available at: http://www.ntnu.edu/hunt/data. Accessed August 20, 2019.
- 25. Krokstad S, Langhammer A, Hveem K, Holmen TL, Midthjell K, Stene TR, Bratberg G, Heggland J, Holmen J. Cohort profile: the Hunt study, Norway. Int J Epidemiol. 2013;42:968–977. [DOI] [PubMed] [Google Scholar]
- 26. Holmen J, Midthjell K, Krüger Ø, Langhammer A, Lingaas Holmen TL, Bratberg GH, Vatten L, Lund‐Larsen PG. The nord‐trøndelag health study 1995‐97 (hunt 2): objectives, contents, methods and participation. Norsk Epidemiologi. 2003;13:19–32. [Google Scholar]
- 27. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res. 2002;52:69–77. [DOI] [PubMed] [Google Scholar]
- 28. Myrtveit SM, Ariansen AM, Wilhelmsen I, Krokstad S, Mykletun A. A population based validation study of self‐reported pensions and benefits: the nord‐trondelag health study (hunt). BMC Res Notes. 2013;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Torvik FA, Rognmo K, Tambs K. Alcohol use and mental distress as predictors of non‐response in a general population health survey: the hunt study. Soc Psychiatry Psychiatr Epidemiol. 2012;47:805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holmen J, Midthjell K, Forsen L, Skjerve K, Gorseth M, Oseland A. [a health survey in nord‐trondelag 1984‐86. Participation and comparison of attendants and non‐attendants]. Tidsskrift for den Norske laegeforening : tidsskrift for praktisk medicin, ny raekke. 1990;110:1973–1977. [Article in Norwegian]. [PubMed] [Google Scholar]
- 31. Munkhaugen J, Sverre E, Peersen K, Egge O, Gjertsen Eikeseth C, Gjertsen E, Gullestad L, Erik Otterstad J, Husebye E, Dammen T. Patient characteristics and risk factors of participants and non‐participants in the nor‐cor study. Scand Cardiovasc J. 2016;50:317–322. [DOI] [PubMed] [Google Scholar]
- 32. Covey LS, Glassman AH, Stetner F. Cigarette smoking and major depression. J Addict Dis. 1998;17:35–46. [DOI] [PubMed] [Google Scholar]
- 33. Velten J, Lavallee KL, Scholten S, Meyer AH, Zhang XC, Schneider S, Margraf J. Lifestyle choices and mental health: a representative population survey. BMC Psychol. 2014;2:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta‐analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. [DOI] [PubMed] [Google Scholar]
- 35. Aune D, Schlesinger S, Norat T, Riboli E. Tobacco smoking and the risk of abdominal aortic aneurysm: a systematic review and meta‐analysis of prospective studies. Sci Rep. 2018;8:14786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stackelberg O, Wolk A, Eliasson K, Hellberg A, Bersztel A, Larsson SC, Orsini N, Wanhainen A, Bjorck M. Lifestyle and risk of screening‐detected abdominal aortic aneurysm in men. J Am Heart Assoc. 2017;6:e004725 DOI: 10.1161/JAHA.116.004725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tang W, Yao L, Roetker NS, Alonso A, Lutsey PL, Steenson CC, Lederle FA, Hunter DW, Bengtson LG, Guan W, Missov E, Folsom AR. Lifetime risk and risk factors for abdominal aortic aneurysm in a 24‐year prospective study: the ARIC study (atherosclerosis risk in communities). Arterioscler Thromb Vasc Biol. 2016;36:2468–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haring B, Selvin E, He X, Coresh J, Steffen LM, Folsom AR, Tang W, Rebholz CM. Adherence to the dietary approaches to stop hypertension dietary pattern and risk of abdominal aortic aneurysm: results from the ARIC study. J Am Heart Assoc. 2018;7:e009340 DOI: 10.1161/JAHA.118.009340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Iribarren C, Darbinian JA, Go AS, Fireman BH, Lee CD, Grey DP. Traditional and novel risk factors for clinically diagnosed abdominal aortic aneurysm: the kaiser multiphasic health checkup cohort study. Ann Epidemiol. 2007;17:669–678. [DOI] [PubMed] [Google Scholar]
- 40. Zarrouk M, Holst J, Malina M, Lindblad B, Wann‐Hansson C, Rosvall M, Gottsater A. The importance of socioeconomic factors for compliance and outcome at screening for abdominal aortic aneurysm in 65‐year‐old men. J Vasc Surg. 2013;58:50–55. [DOI] [PubMed] [Google Scholar]
- 41. Jahangir E, Lipworth L, Edwards TL, Kabagambe EK, Mumma MT, Mensah GA, Fazio S, Blot WJ, Sampson UK. Smoking, sex, risk factors and abdominal aortic aneurysms: a prospective study of 18 782 persons aged above 65 years in the southern community cohort study. J Epidemiol Community Health. 2015;69:481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zommorodi S, Leander K, Roy J, Steuer J, Hultgren R. Understanding abdominal aortic aneurysm epidemiology: socioeconomic position affects outcome. J Epidemiol Community Health. 2018;72:904–910. [DOI] [PubMed] [Google Scholar]
- 43. Linne A, Leander K, Lindstrom D, Tornberg S, Hultgren R. Reasons for non‐participation in population‐based abdominal aortic aneurysm screening. Br J Surg. 2014;101:481–487. [DOI] [PubMed] [Google Scholar]
- 44. Jacomelli J, Summers L, Stevenson A, Lees T, Earnshaw JJ. Editor's choice—inequalities in abdominal aortic aneurysm screening in england: effects of social deprivation and ethnicity. Eur J Vasc Endovasc Surg. 2017;53:837–843. [DOI] [PubMed] [Google Scholar]
- 45. Faulds J, Bell NJ, Harrington DM, Novick TV, Harris JR, DeRose G, Forbes TL. Socioeconomic and geographic disparities in access to endovascular abdominal aortic aneurysm repair. Ann Vasc Surg. 2013;27:1061–1067. [DOI] [PubMed] [Google Scholar]
- 46. Moller‐Leimkuhler AM. Gender differences in cardiovascular disease and comorbid depression. Dialogues Clin Neurosci. 2007;9:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample. Circulation. 1996;93:1976–1980. [DOI] [PubMed] [Google Scholar]
- 48. Celano CM, Huffman JC. Depression and cardiac disease: a review. Cardiol Rev. 2011;19:130–142. [DOI] [PubMed] [Google Scholar]
- 49. Stenman M, Holzmann MJ, Sartipy U. Association between preoperative depression and long‐term survival following coronary artery bypass surgery—a systematic review and meta‐analysis. Int J Cardiol. 2016;222:462–466. [DOI] [PubMed] [Google Scholar]
- 50. Liberzon I, Abelson JL, Amdur RL, King AP, Cardneau JD, Henke P, Graham LM. Increased psychiatric morbidity after abdominal aortic surgery: risk factors for stress‐related disorders. J Vasc Surg. 2006;43:929–934. [DOI] [PubMed] [Google Scholar]
- 51. Bath MF, Sidloff D, Saratzis A, Bown MJ. Impact of abdominal aortic aneurysm screening on quality of life. Br J Surg. 2018;105:203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Drieling RL, LaCroix AZ, Beresford SA, Boudreau DM, Kooperberg C, Heckbert SR. Validity of self‐reported medication use compared with pharmacy records in a cohort of older women: findings from the women's health initiative. Am J Epidemiol. 2016;184:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ulug P, Powell JT, Sweeting MJ, Bown MJ, Thompson SG. Meta‐analysis of the current prevalence of screen‐detected abdominal aortic aneurysm in women. Br J Surg. 2016;103:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Distribution of Missing Values for HADS for Each of the Covariates
Table S2. Adjusted Hazard Ratios (HR) With 95% Confidence Intervals (CI) for the Association Between HADS‐D and †AAA Development, Based on Results After Multiple Imputation (n=50 data sets)


