Abstract
Background
The 1‐hour glucose challenge test (GCT) is routinely performed in pregnancy to screen for gestational diabetes mellitus. Remarkably, it has recently emerged that the GCT can also predict a woman's future risk of cardiovascular disease, although the mechanistic basis of this relationship is unclear. In this context we hypothesized that a higher GCT may identify women with an otherwise unrecognized adverse cardiovascular phenotype. Thus, we sought to evaluate the relationship between the antepartum GCT and subsequent postpartum cardiovascular risk factor profile.
Methods and Results
In this study 503 women completed a screening GCT in late second trimester and then underwent cardiometabolic characterization at 3 months postpartum, whereupon traditional (blood pressure, glucose, lipids) and nontraditional (apolipoprotein B, C‐reactive protein, adiponectin) cardiovascular risk factors were compared across GCT tertiles. At 3 months postpartum, each of the following risk factors progressively worsened from the lowest to middle to highest GCT tertile: fasting glucose (P=0.0002), 2‐hour glucose (P<0.0001), total cholesterol:high‐density lipoprotein cholesterol (P=0.0004), high‐density lipoprotein cholesterol (P=0.004), triglycerides (P=0.001), apolipoprotein B (P=0.001), and adiponectin (P=0.02). On multiple linear regression analyses, the GCT emerged as a significant independent predictor of higher fasting glucose (P=0.0006), 2‐hour glucose (P<0.0001), total cholesterol: high‐density lipoprotein cholesterol (P=0.0004), triglycerides (P=0.001), low‐density lipoprotein cholesterol (P=0.01), and apolipoprotein B (P=0.004) and of lower high‐density lipoprotein cholesterol (P=0.02) and adiponectin (P=0.0099). Moreover, these independent associations persisted after excluding women who had gestational diabetes mellitus.
Conclusions
The antepartum GCT can identify women with an adverse underlying cardiovascular risk factor phenotype.
Keywords: cardiovascular disease, glucose challenge test, lipids, risk factors, women
Subject Categories: Pregnancy, Risk Factors, Women
Clinical Perspective
What Is New?
The glucose challenge test in pregnancy is a significant independent predictor of higher postpartum fasting glucose, 2‐hour glucose, total cholesterol: high‐density lipoprotein cholesterol ratio, triglycerides, low‐density lipoprotein cholesterol, apolipoprotein B, and apolipoprotein‐B:apolipoprotein‐A1, in addition to lower high‐density lipoprotein cholesterol and adiponectin.
The screening glucose challenge test that is routinely performed in pregnancy thus can identify women with an adverse underlying cardiovascular phenotype.
What Are the Clinical Implications?
Coupled with recent recognition of the cardiovascular risk prediction capacity of the glucose challenge test, these data have identified risk factors that now warrant further study to determine their possible relationship with future cardiovascular risk potential.
Introduction
Screening for gestational diabetes mellitus (GDM) is the only situation in clinical practice in which population screening for diabetes mellitus is undertaken. It holds this unique distinction despite the fact that the screening protocols with which it is performed vary across jurisdictions.1 Although the International Association of Diabetes and Pregnancy Study Groups attempted to achieve consensus in 2010 by proposing universal 1‐step screening of all pregnant women with a 2‐hour 75‐g oral glucose tolerance test (OGTT),2 the clinical uptake of this recommendation has been uneven, partly due to concerns around the resultant testing burden and the implications thereof.1, 3 Indeed, many authoritative bodies (including a National Institutes of Health Consensus Panel)4 continue to recommend a traditional 2‐step screening strategy consisting of a 50‐g glucose challenge test (GCT) in all pregnant women, followed by a diagnostic OGTT only in those in whom the 1‐hour postchallenge glucose value on the GCT exceeds a defined threshold (typically 7.8 mmol/L). With this approach, the screening test that is performed in all pregnant women is the GCT rather than the OGTT.
Interestingly, it has recently emerged that the GCT can predict a woman's future risk of cardiovascular disease (CVD) in the years after her pregnancy.5 Indeed, in a population‐based study involving over 250 000 women, we have demonstrated that each 1 mmol/L increment in the 1‐hour postchallenge glucose value on the GCT is associated with a 13% higher future risk of CVD (myocardial infarction, acute coronary syndrome, stroke, coronary artery bypass grafting, percutaneous coronary intervention, or carotid endarterectomy) in both the general obstetrical population and women who do not have GDM.5 Moreover, this relationship is apparent within the first decade postpartum (ie, median follow‐up 3.9 years after delivery) and is not dependent on intercurrent progression to type 2 diabetes mellitus (T2DM).5 This demonstrated capacity to identify cardiovascular risk in the otherwise low‐risk patient population of young women of reproductive age is suggestive of the potency of the GCT as a predictor of CVD. However, the mechanistic basis by which the GCT is capturing this future cardiovascular risk potential is unclear. In this context we hypothesized that a higher GCT may be identifying women with an otherwise unrecognized adverse underlying cardiovascular phenotype. To test this hypothesis, our objective in the current study was to evaluate the relationship between the antepartum GCT and the subsequent maternal cardiovascular risk factor profile at 3 months postpartum.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request. This analysis was conducted in the setting of a prospective observational cohort study of early events in the natural history of T2DM in which a cohort of women recruited at the time of antepartum screening for GDM is undergoing longitudinal metabolic characterization in pregnancy and the postpartum period. The study protocol has been described in detail previously.6 In brief, at our institution, the clinical protocol for GDM screening has consisted of all pregnant women undergoing a 50‐g GCT in late second trimester. The GCT is performed in the casual state (ie, fasting not required), with women ingesting 50 g of glucose followed by measurement of venous blood glucose 1‐hour later. If this 1‐hour postchallenge blood glucose measurement is ≥7.8 mmol/L, the women proceed to a diagnostic OGTT (which is performed after overnight fasting). As per standard obstetrical care at our institution, any women diagnosed with GDM are referred to the diabetes mellitus in pregnancy clinic for clinical management, where they receive glucose‐lowering treatment in pregnancy, consisting of dietary/lifestyle counseling±antepartum insulin therapy.
For this study, women without diabetes mellitus were recruited either before or after their clinical GCT, and all participants then completed a 3‐hour 100‐g OGTT (even if the GCT was normal). The OGTT was performed after an overnight fast and included venous blood glucose measurements at fasting and at 1, 2, and 3 hours following ingestion of the 100‐g glucose load. As previously described,6 the recruitment of women after an abnormal GCT served to enrich the study population for those with GDM. At 3 months postpartum, participants returned to the clinical investigation unit for cardiometabolic characterization. The current analysis was restricted to the first 503 women in whom complete cardiometabolic characterization was performed. The protocol has been approved by the Mount Sinai Hospital Research Ethics Board, and all women provided written informed consent for their participation.
Cardiometabolic Characterization at 3 Months Postpartum
At 3 months postpartum, participants attended the clinical investigation unit after an overnight fast. At this study visit, interviewer‐administered questionnaires were completed, and physical examination was performed, including measurement of blood pressure (measured twice 5 minutes apart by automatic sphygmomanometer [Dinamap Pro 100‐400, Critikon, Tampa, FL]), weight, and waist circumference. As previously described,6 participants underwent a 2‐hour 75‐g OGTT, with serum samples collected for the measurement of traditional and nontraditional cardiovascular risk factors.
Total cholesterol (Roche CHOL2 reagent), high‐density lipoprotein (HDL) cholesterol (Roche direct HDL reagent, HDLC3), and triglycerides (Roche TRIGL reagent) were measured from fasting serum, using the Roche Cobas 6000 c 501 analyzer (Roche Diagnostics, Laval, Quebec, Canada). Lipid measurements were standardized by the Centers for Disease Control and Prevention Lipid Standardization Program (Atlanta, GA). Low‐density lipoprotein (LDL) cholesterol (calculated by Friedewald formula) and total cholesterol:HDL ratio were determined from these measurements. Apolipoprotein B (apoB), apolipoprotein A1 (apoA1), and high‐sensitivity C‐reactive protein (N high‐sensitivity C‐reactive protein reagent) were measured using the Siemens Healthcare Diagnostics BN ProSpec (Siemens Healthcare Diagnostics, Mississauga, Ontario, Canada). Total adiponectin was measured by enzyme‐linked immunosorbent assay (Kit #EZHADP‐61, EMD Millipore, St. Charles, MO).
Statistical Analyses
All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). Continuous variables were tested for normality of distribution, and natural log transformations of skewed variables were used, where necessary, in subsequent analyses. The study participants were first stratified into tertiles based on their GCT in pregnancy, enabling comparison of their postpartum clinical and cardiometabolic characteristics across these 3 groups in Table 1. Continuous variables were compared by ANOVA, and categorical variables were compared by chi‐squared or Fisher Exact test. In Table 2, a series of multiple linear regression analyses were performed to determine if the 1‐hour postchallenge glucose value on the GCT in pregnancy could predict the following cardiovascular risk factors at 3 months postpartum: systolic blood pressure, diastolic blood pressure, fasting glucose, 2‐hour glucose, total cholesterol, total cholesterol:HDL ratio, HDL cholesterol, triglycerides, LDL cholesterol, apoB, apoB:apoA1 ratio, CRP, and adiponectin. The models in Table 2 were first adjusted for the following clinical risk factors relevant to diabetes mellitus: age, ethnicity, family history of diabetes mellitus, and breastfeeding (model I). To address the potential impact of current weight on the relationships between the GCT and these cardiovascular risk factors, each model was then further adjusted for body mass index (BMI) at 3 months postpartum (model II). To exclude the potential impact of current glucose intolerance or GDM on these relationships, the multiple linear regression analyses were performed similarly in Tables 3 and 4 after restriction of the study population to (1) women who had normal glucose tolerance at 3 months (Table 3) or (2) those who did not have GDM in the recent pregnancy (Table 4). To determine if glucose measurements from the antepartum OGTT can also predict postpartum cardiovascular risk factors, the multiple linear regression analyses were also similarly repeated with area under the glucose curve on the OGTT and fasting glucose, respectively, as predictors in place of the GCT (Table 5).
Table 1.
Clinical Characteristics and Postpartum Cardiovascular Risk Factor Profile of Study Participants Stratified Into Tertiles Based on Their Glucose Challenge Test in Pregnancy
| Tertile 1 | Tertile 2 | Tertile 3 | P Value | |
|---|---|---|---|---|
| GCT ≤7.9 mmol/L | GCT 8.0‐8.7 mmol/L | GCT ≥8.8 mmol/L | ||
| (n=162) | (n=167) | (n=174) | ||
| Age, y | 34.8 [4.4] | 34.5 [4.5] | 34.6 [4.2] | 0.80 |
| Ethnicity | ||||
| White, % | 75.3 | 73.7 | 70.7 | 0.87 |
| Asian, % | 9.3 | 11.4 | 11.5 | |
| Other, % | 15.4 | 15.0 | 17.8 | |
| Family history of T2DM, % | 58.4 | 55.7 | 63.8 | 0.30 |
| Parity | ||||
| 1, % | 61.1 | 53.3 | 50.6 | 0.34 |
| 2, % | 30.9 | 34.7 | 37.4 | |
| >2, % | 8.0 | 12.0 | 12.1 | |
| OGTT in index pregnancy | ||||
| Fasting glucose, mmol/L | 4.4 [0.5] | 4.5 [0.5] | 4.7 [0.6] | <0.0001 |
| AUCglucose | 21.1 [3.2] | 23.5 [3.3] | 25.1 [3.8] | <0.0001 |
| GDM, % | 9.3 | 28.1 | 46.0 | <0.0001 |
| Breastfeeding, % | 94.4 | 92.8 | 92.5 | 0.76 |
| BMI, kg/m2 | 24.9 [22.5‐28.6] | 26.2 [23.7‐30.0] | 26.1 [23.3‐30.4] | 0.09 |
| Waist circumference, cm | 87.2 [10.5] | 89.5 [12.7] | 88.3 [11.8] | 0.23 |
| Cardivascular risk factors at 3 months | ||||
| Current smoking, % | 4.3 | 3.4 | 4.6 | 0.89 |
| Systolic BP, mm Hg | 109 [10] | 110 [12] | 111 [11] | 0.17 |
| Diastolic BP, mm Hg | 65 [9] | 66 [10] | 66 [9] | 0.51 |
| Fasting glucose, mmol/L | 4.5 [0.4] | 4.6 [0.5] | 4.7 [0.5] | 0.0002 |
| 2‐hour glucose, mmol/L | 5.7 [1.2] | 6.2 [1.5] | 6.9 [2.0] | <0.0001 |
| Total cholesterol, mmol/L | 5.00 [0.96] | 5.16 [0.96] | 5.25 [0.99] | 0.07 |
| Total cholesterol:HDL ratio | 3.45 [2.82‐4.18] | 3.62 [3.01‐4.43] | 3.88 [3.15‐4.71] | 0.0004 |
| HDL cholesterol, mmol/L | 1.46 [0.32] | 1.42 [0.34] | 1.35 [0.30] | 0.004 |
| Triglycerides, mmol/L | 0.85 [0.63‐1.26] | 0.94 [0.69‐1.45] | 1.05 [0.75‐1.55] | 0.001 |
| LDL cholesterol, mmol/L | 3.07 [0.87] | 3.22 [0.89] | 3.30 [0.87] | 0.07 |
| apoB, g/L | 0.83 [0.71‐1.03] | 0.92 [0.75‐1.04] | 0.94 [0.80‐1.10] | 0.001 |
| apoB:apoA1 ratio | 0.55 [0.46‐0.64] | 0.58 [0.47‐0.69] | 0.63 [0.50‐0.74] | 0.003 |
| CRP, mg/L | 1.6 [1.0‐4.2] | 2.2 [0.9‐4.3] | 2.3 [1.1‐4.1] | 0.27 |
| Adiponectin, μg/mL | 9.2 [3.3] | 8.4 [2.9] | 8.3 [3.2] | 0.02 |
Continuous variables are shown as median followed by interquartile range (if skewed) or mean followed by standard deviation (if normally distributed). P values refer to overall comparison across the 3 groups by ANOVA (for continuous variables) and either chi‐squared or Fisher Exact test (for categorical variables). apoA1 indicates apolipoprotein A1; apoB, apolipoprotein B; AUCglucose, area under the glucose curve; BMI, body mass index; BP, blood pressure; CRP, C‐reactive protein; GCT, glucose challenge test; GDM, gestational diabetes mellitus; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; OGTT, oral glucose tolerance test; T2DM, type 2 diabetes mellitus.
Table 2.
Glucose Challenge Test in Pregnancy as a Predictor of the Indicated Cardiovascular Risk Factors at 3 Months Postpartum in the Study Population (n=503) After Adjustment for Various Covariates
| Model | GCT as Predictor | Outcome | ||
|---|---|---|---|---|
| Estimate | t | P Value | Cardiovascular Risk Factor | |
| Model I | 0.426752 | 1.24 | 0.21 | Systolic blood pressure |
| Model II | 0.223891 | 0.68 | 0.50 | Systolic blood pressure |
| Model I | 0.021299 | 0.08 | 0.94 | Diastolic blood pressure |
| Model II | −0.092280 | −0.34 | 0.73 | Diastolic blood pressure |
| Model I | 0.055846 | 3.87 | 0.0001 | Fasting glucose |
| Model II | 0.048769 | 3.48 | 0.0006 | Fasting glucose |
| Model I | 0.330778 | 6.85 | <0.0001 | 2‐hour glucose |
| Model II | 0.330325 | 6.72 | <0.0001 | 2‐hour glucose |
| Model I | 0.074689 | 2.50 | 0.01 | Total cholesterol |
| Model II | 0.076391 | 2.51 | 0.01 | Total cholesterol |
| Model I | 0.034725 | 3.97 | <0.0001 | Log total chol:HDL ratio |
| Model II | 0.029802 | 3.58 | 0.0004 | Log total chol:HDL ratio |
| Model I | −0.028589 | −2.95 | 0.003 | HDL cholesterol |
| Model II | −0.021198 | −2.32 | 0.02 | HDL cholesterol |
| Model I | 0.056164 | 3.73 | 0.0002 | Log triglycerides |
| Model II | 0.045582 | 3.22 | 0.001 | Log triglycerides |
| Model I | 0.070809 | 2.59 | 0.01 | LDL cholesterol |
| Model II | 0.070585 | 2.56 | 0.01 | LDL cholesterol |
| Model I | 0.028379 | 3.77 | 0.0002 | Log apoB |
| Model II | 0.026447 | 3.54 | 0.0004 | Log apoB |
| Model I | 0.028119 | 3.19 | 0.002 | Log apoB:apoA1 ratio |
| Model II | 0.024234 | 2.83 | 0.005 | Log apoB:apoA1 ratio |
| Model I | 0.048308 | 1.51 | 0.13 | Log CRP |
| Model II | 0.019470 | 0.68 | 0.50 | Log CRP |
| Model I | −0.263163 | −2.87 | 0.004 | Adiponectin |
| Model II | −0.235611 | −2.59 | 0.0099 | Adiponectin |
Model I: Adjusted for age, ethnicity, family history of diabetes mellitus, and breastfeeding. Model II: Model I further adjusted for BMI at 3 months postpartum. The estimate for the GCT as predictor in each model is presented per 1‐mmol/L increase in GCT. apoA1 indicates apolipoprotein A1; apoB, apolipoprotein B; BMI, body mass index; chol, cholesterol; CRP, C‐reactive protein; GCT, glucose challenge test; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Table 3.
Glucose Challenge Test in Pregnancy as a Predictor of the Indicated Cardiovascular Risk Factors at 3 Months Postpartum in Women With Normal Glucose Tolerance at the Time (n=414) After Adjustment for Various Covariates
| Model | GCT as Predictor | Outcome | ||
|---|---|---|---|---|
| Estimate | t | P Value | Cardiovascular Risk Factor | |
| Model I | 0.352248 | 0.95 | 0.34 | Systolic blood pressure |
| Model II | 0.180916 | 0.51 | 0.61 | Systolic blood pressure |
| Model I | 0.022006 | 0.07 | 0.94 | Diastolic blood pressure |
| Model II | −0.081053 | −0.27 | 0.78 | Diastolic blood pressure |
| Model I | 0.055442 | 4.11 | <0.0001 | Fasting glucose |
| Model II | 0.049547 | 3.82 | 0.0002 | Fasting glucose |
| Model I | 0.137528 | 3.87 | 0.0001 | 2‐hour glucose |
| Model II | 0.140063 | 3.89 | 0.0001 | 2‐hour glucose |
| Model I | 0.059377 | 1.88 | 0.06 | Total cholesterol |
| Model II | 0.063457 | 1.98 | 0.048 | Total cholesterol |
| Model I | 0.033859 | 3.64 | 0.0003 | Log total chol:HDL ratio |
| Model II | 0.030055 | 3.41 | 0.0007 | Log total chol:HDL ratio |
| Model I | −0.028589 | −2.95 | 0.003 | HDL cholesterol |
| Model II | −0.023559 | −2.40 | 0.017 | HDL cholesterol |
| Model I | 0.055798 | 3.42 | 0.0007 | Log triglycerides |
| Model II | 0.045293 | 2.98 | 0.003 | Log triglycerides |
| Model I | 0.057132 | 2.01 | 0.045 | LDL cholesterol |
| Model II | 0.060075 | 2.09 | 0.037 | LDL cholesterol |
| Model I | 0.026910 | 3.32 | 0.001 | Log apoB |
| Model II | 0.025750 | 3.21 | 0.002 | Log apoB |
| Model I | 0.027654 | 2.96 | 0.003 | Log apoB:apoA1 ratio |
| Model II | 0.024805 | 2.75 | 0.006 | Log apoB:apoA1 ratio |
| Model I | 0.014375 | 0.42 | 0.68 | Log CRP |
| Model II | −0.014595 | −0.47 | 0.64 | Log CRP |
| Model I | −0.246081 | −2.39 | 0.02 | Adiponectin |
| Model II | −0.217711 | −2.17 | 0.03 | Adiponectin |
Model I: Adjusted for age, ethnicity, family history of diabetes mellitus, and breastfeeding. Model II: Model I further adjusted for BMI at 3 months postpartum. The estimate for the GCT as predictor in each model is presented per 1‐mmol/L increase in GCT. apoA1 indicates apolipoprotein A1; apoB, apolipoprotein B; BMI, body mass index; chol, cholesterol; CRP, C‐reactive protein; GCT, glucose challenge test; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Table 4.
Glucose Challenge Test in Pregnancy as a Predictor of the Indicated Cardiovascular Risk Factors at 3 Months Postpartum in Women Who Did Not Have Gestational Diabetes Mellitus (n=361) After Adjustment for Various Covariates
| Model | GCT as Predictor | Outcome | ||
|---|---|---|---|---|
| Estimate | t | P Value | Cardiovascular Risk Factor | |
| Model I | 0.296064 | 0.78 | 0.43 | Systolic blood pressure |
| Model II | 0.126313 | 0.35 | 0.73 | Systolic blood pressure |
| Model I | −0.316240 | −1.01 | 0.31 | Diastolic blood pressure |
| Model II | −0.405116 | −1.32 | 0.19 | Diastolic blood pressure |
| Model I | 0.046180 | 2.80 | 0.006 | Fasting glucose |
| Model II | 0.039011 | 2.42 | 0.02 | Fasting glucose |
| Model I | 0.218079 | 4.36 | <0.0001 | 2‐hour glucose |
| Model II | 0.222994 | 4.37 | <0.0001 | 2‐hour glucose |
| Model I | 0.051111 | 1.50 | 0.13 | Total cholesterol |
| Model II | 0.056133 | 1.62 | 0.11 | Total cholesterol |
| Model I | 0.030083 | 2.99 | 0.003 | Log total chol:HDL ratio |
| Model II | 0.025928 | 2.73 | 0.007 | Log total chol:HDL ratio |
| Model I | −0.028866 | −2.56 | 0.01 | HDL cholesterol |
| Model II | −0.021721 | −2.07 | 0.04 | HDL cholesterol |
| Model I | 0.049798 | 3.02 | 0.003 | Log triglycerides |
| Model II | 0.039410 | 2.55 | 0.01 | Log triglycerides |
| Model I | 0.054111 | 1.72 | 0.09 | LDL cholesterol |
| Model II | 0.057281 | 1.81 | 0.07 | LDL cholesterol |
| Model I | 0.021929 | 2.78 | 0.006 | Log apoB |
| Model II | 0.020998 | 2.66 | 0.008 | Log apoB |
| Model I | 0.014938 | 2.4 | 0.02 | Log apoB:apoA1 ratio |
| Model II | 0.012993 | 2.18 | 0.03 | Log apoB:apoA1 ratio |
| Model I | 0.006306 | 0.17 | 0.86 | Log CRP |
| Model II | −0.021097 | −0.63 | 0.53 | Log CRP |
| Model I | −0.236161 | −2.10 | 0.04 | Adiponectin |
| Model II | −0.211561 | −1.90 | 0.06 | Adiponectin |
Model I: Adjusted for age, ethnicity, family history of diabetes mellitus, and breastfeeding. Model II: Model I further adjusted for BMI at 3‐months postpartum. The estimate for the GCT as predictor in each model is presented per 1‐mmol/L increase in GCT. apoA1 indicates apolipoprotein A1; apoB, apolipoprotein B; BMI, body mass index; chol, cholesterol; CRP, C‐reactive protein; GCT, glucose challenge test; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Table 5.
AUCglucose and Fasting Glucose on the Antepartum OGTT as Predictors of the Indicated Cardiovascular Risk Factors at 3‐Months Postpartum in the Study Population (n=503), After Adjustment for Various Covariates
| AUCglucose on OGTT as Predictor | Fasting Glucose on OGTT as Predictor | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model I | Model II | Model I | Model II | |||||||||
| Estimate | t | P Value | Estimate | T | P Value | Estimate | t | P Value | Estimate | t | P Value | |
| Fasting glucose | 0.038548 | 7.24 | <0.0001 | 0.033196 | 6.34 | <0.0001 | 0.353579 | 10.0 | <0.0001 | 0.295093 | 7.97 | <0.0001 |
| 2‐hour glucose | 0.141123 | 7.75 | <0.0001 | 0.139349 | 7.48 | <0.0001 | 0.348254 | 2.64 | 0.009 | 0.299615 | 2.12 | 0.03 |
| Total cholesterol | 0.040505 | 3.57 | 0.0004 | 0.038962 | 3.37 | 0.0008 | 0.122735 | 1.56 | 0.12 | 0.086648 | 1.03 | 0.31 |
| Log total chol:HDL | 0.017354 | 5.27 | <0.0001 | 0.013268 | 4.19 | <0.0001 | 0.12088 | 5.32 | <0.0001 | 0.062639 | 2.71 | 0.007 |
| HDL cholesterol | −0.01339 | −3.65 | 0.0003 | −0.00820 | −2.35 | 0.02 | −0.129932 | −5.20 | <0.0001 | −0.06027 | −2.39 | 0.02 |
| Log triglycerides | 0.025225 | 4.43 | <0.0001 | 0.017269 | 3.20 | 0.002 | 0.230281 | 5.95 | <0.0001 | 0.124167 | 3.18 | 0.002 |
| LDL cholesterol | 0.039992 | 3.86 | 0.0001 | 0.037293 | 3.54 | 0.0004 | 0.114203 | 1.58 | 0.11 | 0.059988 | 0.78 | 0.44 |
| Log apoB | 0.014163 | 4.97 | <0.0001 | 0.012057 | 4.24 | <0.0001 | 0.074301 | 3.76 | 0.0002 | 0.043089 | 2.07 | 0.04 |
| Log apoB:apoA1 | 0.015362 | 4.62 | <0.0001 | 0.011876 | 3.65 | 0.0003 | 0.101752 | 4.44 | <0.0001 | 0.051238 | 2.16 | 0.03 |
| Log CRP | 0.04841 | 4.02 | <0.0001 | 0.026824 | 2.47 | 0.01 | 0.45001 | 5.49 | <0.0001 | 0.14967 | 1.91 | 0.057 |
| Adiponectin | −0.12032 | −3.44 | 0.0006 | −0.09265 | −2.66 | 0.008 | −0.795221 | −3.30 | 0.001 | −0.38400 | −4.77 | <0.0001 |
Model I: Adjusted for age, ethnicity, family history of diabetes mellitus, and breastfeeding. Model II: Model I further adjusted for BMI at 3 months postpartum. apoA1 indicates apolipoprotein A1; apoB, apolipoprotein B; AUCglucose, area under the glucose curve; BMI, body mass index; chol, cholesterol; CRP, C‐reactive protein; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; OGTT, oral glucose tolerance test.
Results
GCT Tertiles and Postpartum Cardiovascular Risk Factors
Table 1 shows the demographic, clinical and cardiometabolic characteristics of the study population (n=503) at 3 months postpartum, with the women stratified into tertiles based on their 1‐hour postchallenge glucose result on the GCT in pregnancy. The lowest tertile was comprised of women with GCT≤7.9 mmol/L, those in the middle tertile had 1‐hour glucose value between 8.0 and 8.7 mmol/L inclusive, and the highest tertile consisted of women with GCT ≥8.8 mmol/L. As would be expected, the likelihood that the women developed GDM in the recent pregnancy progressively increased across the GCT tertiles (P<0.0001). Otherwise, the groups did not differ with respect to age, ethnicity, family history of T2DM, parity, current breastfeeding, BMI, or waist circumference at 3 months postpartum. However, there were clear differences among the 3 groups in their respective cardiovascular risk factor profiles. Specifically, at 3 months postpartum, each of the following risk factors progressively increased from the lowest to the middle to the highest GCT tertile: fasting glucose (P=0.0002), 2‐hour glucose (P<0.0001), total cholesterol:HDL ratio (P=0.0004), triglycerides (P=0.001), apoB (P=0.001), and apoB:apoA1 ratio (P=0.003); these gradients were mirrored by stepwise decrements in HDL (P=0.004) and adiponectin (P=0.02).
Independent Associations of GCT With Postpartum Cardiovascular Risk Factors
We next performed multiple linear regression analyses to determine whether the GCT was independently associated with cardiovascular risk factors at 3 months postpartum (Table 2). After adjustment for potential confounding covariates (age, ethnicity, family history of diabetes mellitus, breastfeeding), the GCT remained a significant independent predictor of higher postpartum fasting glucose (P=0.0001), 2‐hour glucose (P<0.0001), total cholesterol (P=0.01), total cholesterol:HDL ratio (P<0.0001), triglycerides (P=0.0002), LDL cholesterol (P=0.01), apoB (P=0.0002), and apoB:apoA1 ratio (P=0.002) as well as lower HDL (P=0.003) and adiponectin (P=0.004). Furthermore, these significant independent associations were unchanged on further adjustment for current BMI at 3 months postpartum (Table 2). We also performed sensitivity analyses in which all of the multiple linear regression models were repeated after exclusion of any women with glucose intolerance at 3‐months postpartum. These analyses confirmed that all of the significant independent associations of the GCT with cardiovascular risk factors observed in Table 2 were maintained in women who had normal glucose tolerance at 3 months postpartum (Table 3). Thus, the single 1‐hour postchallenge glucose value on the GCT emerges as an independent predictor of a specific adverse postpartum cardiovascular risk factor profile even after exclusion of women with glucose intolerance.
Independent Associations of GCT Categories With Postpartum Cardiovascular Risk Factors
In clinical practice the GCT is a screening test that is interpreted as either abnormal or normal around a threshold value that thereby defines categorical strata. Thus, to characterize the observed relationships from a clinical perspective, we next determined the mean adjusted levels of clinical cardiovascular risk factors within each GCT tertile after covariate adjustment (Figure). This analysis showed that, in moving from the lowest to the middle to the highest GCT tertile, there was a progressive stepwise increase in mean adjusted fasting glucose (P=0.0003), 2‐hour glucose (P<0.0001), total cholesterol (P=0.06), total cholesterol:HDL ratio (P=0.0003), triglycerides (P=0.0008), LDL cholesterol (P=0.059), and apoB (P=0.0008), coupled with a stepwise decrease in HDL cholesterol (P=0.008) (Figure). Thus, categorical classification of the GCT identifies gradients of postpartum cardiovascular risk factors, thereby mirroring the gradient of future CVD risk that GCT categories have been recently shown to predict.5
Figure 1.
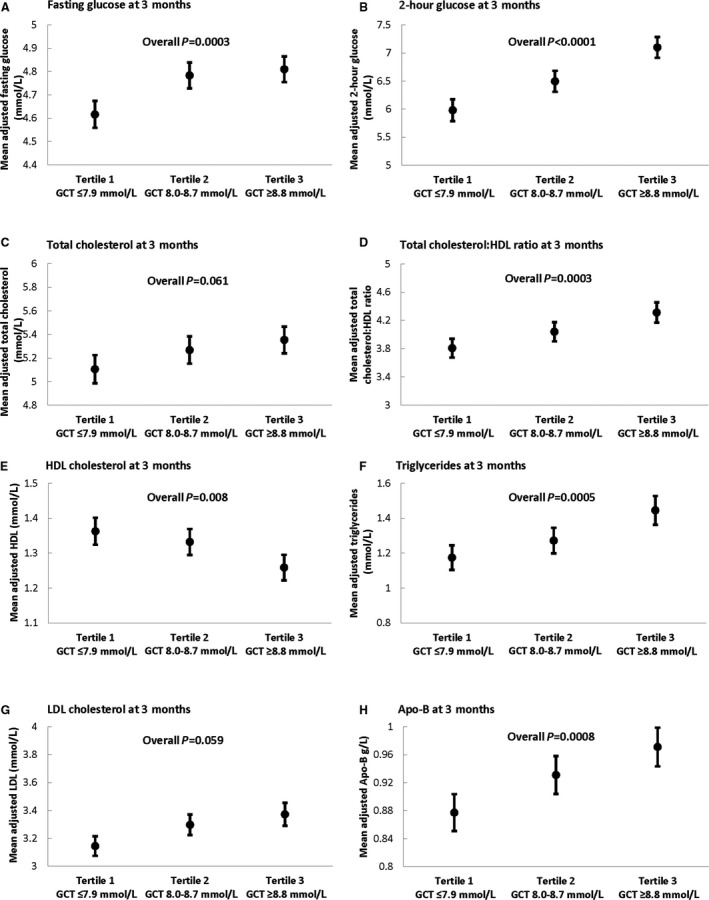
Comparison of mean adjusted levels of the following cardiovascular risk factors at 3 months postpartum between glucose challenge test (GCT) tertiles: (A) fasting glucose; (B) 2‐hour glucose; (C) total cholesterol; (D) total cholesterol:HDL ratio; (E) HDL cholesterol; (F) triglycerides; (G) LDL cholesterol; and (H) apolipoprotein B. For each cardiovascular risk factor, adjusted mean (and standard error) are shown, after adjustment for age, ethnicity, family history of diabetes mellitus, and breastfeeding. Apo‐B indicates apolipoprotein B; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
GCT and Postpartum Cardiovascular Risk Factors in Women Without GDM
Because the GCT identifies maternal risk of GDM, which itself predicts future risk of CVD,7 we next sought to evaluate the independent associations between the GCT and postpartum cardiovascular risk factors after excluding those women who had GDM (Table 4). Indeed, in the women who did not have GDM (n=361), the GCT remained a significant independent predictor of higher fasting glucose (P=0.006), 2‐hour glucose (P<0.0001), total cholesterol:HDL (P=0.003), triglycerides (P=0.003), apoB (P=0.006), and apoB:apoA1 ratio (P=0.02) as well as lower HDL (P=0.01) and adiponectin (P=0.04). Although the GCT was not significantly associated with LDL on this analysis (P=0.09), it should be noted that it remained a significant predictor of apoB (P=0.006). The observed significant independent associations were unchanged with further adjustment for current BMI at 3 months postpartum with the exception of that for adiponectin, which was attenuated to borderline significance (P=0.06) (Table 4). Thus, both in the entire study population and when restricted to women without GDM, the GCT emerges as an independent predictor of a characteristic postpartum cardiovascular risk factor profile.
Glycemia on the OGTT and Postpartum Cardiovascular Risk Factors
Because some jurisdictions apply the OGTT for glucose screening in all pregnant women without a preceding GCT, we next sought to determine if glycemic parameters from the antepartum OGTT also predict cardiovascular risk factors at 3 months postpartum. As shown in Table 5, both area under the glucose curve on the antepartum OGTT and fasting glucose are indeed independent predictors of postpartum cardiovascular risk factors. Of note, area under the glucose curve (which incorporates postchallenge glycemia in contrast to fasting glucose) largely mirrored the GCT in the postpartum cardiovascular risk factors that it predicted, whereas fasting glucose was not significantly associated with total and LDL cholesterol. It thus emerges that the cardiovascular risk factor relationships demonstrated for the GCT extend to glycemia on the antepartum OGTT, particularly postchallenge glycemia.
Discussion
In this study we demonstrate that the 1‐hour post‐challenge glucose value on the screening GCT in pregnancy provides insight into a woman's cardiovascular risk factor profile at 3‐months postpartum. Specifically, the GCT is a significant independent predictor of higher postpartum fasting glucose, 2‐hour glucose, total cholesterol:HDL ratio, triglycerides, LDL, apoB, and apoB:apoA1 in addition to lower HDL and adiponectin. It thus emerges that the screening GCT that is routinely performed in pregnancy can identify women with an otherwise unrecognized adverse cardiovascular phenotype. The clinical relevance of this insight is underscored by recent recognition5 that the GCT can predict a woman's future risk of clinical CVD events in the years to come after her pregnancy.
Although it has been known for over 50 years that GDM identifies women at risk of developing T2DM, it has only become apparent in the past decade that this diagnosis also predicts future cardiovascular risk.8 A recent meta‐analysis involving over 5 million women revealed that those who develop GDM have a 2‐fold higher risk of CVD events as compared with their peers.7 Moreover, this analysis yielded 3 key insights relevant to the broader relationship between gestational glycemia and CVD. First, the increased risk of CVD in women with GDM was apparent within the first decade postpartum, suggesting a degree of potency as a risk predictor.7 Second, this risk was not dependent on postpartum progression to T2DM (ie, women with GDM have a higher incidence of CVD than their peers even if they do not develop T2DM).7 Third, the studies comprising this literature consistently link GDM with future risk of CVD, irrespective of differences in the screening protocol or the diagnostic criteria by which GDM was diagnosed.9, 10, 11, 12, 13, 14, 15, 16, 17 Indeed, the resultant heterogeneity in the severity of dysglycemia that is classified as GDM led to the hypothesis that a continuous relationship between gestational glycemia and subsequent CVD likely extends into the nondiagnostic (ie, non‐GDM) range.5 We recently confirmed this hypothesis using the GCT as a continuous measure of gestational glycemia.5 Furthermore, mirroring the earlier findings seen with GDM, we showed that this risk was not dependent on postpartum progression to T2DM,5 thereby raising the question of how gestational glycemia may be capturing a woman's future cardiovascular risk.
The current study was designed to address this question and has yielded 4 key insights in this regard. First, the GCT predicted a specific postpartum cardiovascular risk phenotype that is characterized by higher glucose levels, poorer lipid profile (higher LDL and apoB coupled with higher triglycerides and lower HDL), and lower adiponectin. Although the relationship with LDL was at borderline significance in Figure (P=0.059) and not significant (P=0.09) in women without GDM (Table 4), it should be recognized that the GCT remained significantly associated with apoB on both analyses. In contrast, the GCT did not relate to blood pressure or C‐reactive protein, suggesting that these factors may not be components of the risk phenotype at this very early point in the natural history of cardiovascular disease in young women. Second, the relationship of the GCT with the indicated postpartum risk factor profile was not solely mediated by maternal weight (as evident on adjustment for BMI in the multiple linear regression analyses), postpartum glucose intolerance (as shown in Table 3), or presence of GDM (as shown in Table 4). Third, the presence of clear differences in the implicated risk factors between GCT strata by as early as 3 months postpartum suggests that these gradients likely preceded the pregnancy. Fourth, as shown in Figure, the absolute levels of these risk factors are not of a magnitude where clinical treatment would be necessarily indicated in young postpartum women. Rather, it is more likely that long‐term exposure to these cardiovascular risk factor gradients ultimately contributes to the differential CVD event rates that have been shown for GCT strata.5 Accordingly, the concept emerging from these data is that, beyond its implications in pregnancy, gestational glycemia can identify latent underlying cardiovascular risk factor gradients that are otherwise unrecognized in young women but that may mediate their future risk of CVD that the GCT predicts.
This concept can hold clinical implications for cardiovascular risk reduction in women. Indeed, since glucose screening is routinely performed in pregnant women (whether by GCT or OGTT), a measure of gestational glycemia is readily available in current clinical practice. One such measure (1‐hour glucose on the GCT) has been shown to predict the risk of CVD events in the years thereafter5; however, this capacity likely extends to other measures of maternal glycemia such as the OGTT (although a demonstration is needed). Against this background, the current study now links this single glucose value on the GCT to gradients of specific cardiovascular risk factors in the early postpartum. Thus, we now have putative mediators of the cardiovascular risk potential that is identified by the GCT and hence factors that warrant longitudinal study over time to determine their relationships to incident CVD in women. Ultimately, such research may inform the development of clinical strategies for using the prognostic insight offered by current population glucose screening in pregnancy to guide risk factor surveillance and modification toward the goal of preventing CVD (which remains the leading cause of mortality in women).18, 19 Although such strategies remain to be developed, it is becoming apparent that glucose screening in pregnancy and its implications for future cardiovascular risk provide an opportunity for preventive care that fully aligns with a recent call from the American College of Obstetricians and Gynecologists for a new paradigm of individualized postpartum care to improve long‐term health.20, 21 Indeed, the opportunity for prevention of heart disease stands at the forefront of this paradigm.22
Whereas the relationship with glucose measurements at 3 months postpartum is understandable, the pathophysiologic basis for why the 1‐hour glucose value on the GCT relates to other elements of the implicated cardiovascular phenotype (LDL, apoB, triglycerides, lower HDL, lower adiponectin) remains to be elucidated. Nevertheless, it is apparent that this single glucose measurement from clinical screening in pregnancy tracks with both a characteristic set of risk factors (demonstrated herein) and future risk of CVD (demonstrated previously5). A limitation of the current study is the absence of pregravid assessment of cardiovascular risk factors, which could otherwise indicate whether the postpartum gradients associated with the GCT exist before pregnancy. Another important limitation to note is that this study does not directly link specific risk factors to subsequent cardiovascular events. Accordingly, we cannot conclude from these data that the indicated factors are mediators of future cardiovascular risk potential. Rather, in identifying a postpartum cardiometabolic phenotype that is predicted by the GCT, these data have provided a set of cardiovascular risk factors that now warrant further longitudinal study in relation to incident CVD in women.
In conclusion, the 1‐hour postchallenge glucose value on the screening GCT in pregnancy can provide insight into a woman's postpartum cardiovascular risk factor profile. Notably, the GCT is a significant independent predictor of higher postpartum fasting glucose, 2‐hour glucose, total cholesterol:HDL ratio, triglycerides, LDL, apoB, and apoB:apoA1 in addition to lower HDL and adiponectin. Thus, a screening test that is routinely performed in pregnancy can identify women with an adverse underlying cardiovascular phenotype. Coupled with the cardiovascular risk prediction capacity of the GCT,5 these data have identified risk factors that now warrant further study to determine their possible relationship with future cardiovascular risk potential. Such research ultimately may inform strategies for cardiovascular risk reduction in women.
Sources of Funding
This study was supported by operating grants from the Canadian Institutes of Health Research (CIHR) (MOP‐84206 and PJT‐156286) and Diabetes Canada (CDA‐OG‐3‐15‐4924‐RR). Retnakaran holds the Boehringer Ingelheim Chair in Beta‐Cell Preservation, Function and Regeneration at Mount Sinai Hospital, and his research program is supported by the Sun Life Financial Program to Prevent Diabetes in Women. Zinman is the Stephen and Suzie Pustil Diabetes Research Scientist at Mount Sinai Hospital.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e014231 DOI: 10.1161/JAHA.119.014231.)
References
- 1. Donovan L, Hartling L, Muise M, Guthrie A, Vandermeer B, Dryden DM. Screening tests for gestational diabetes: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159:115–122. [DOI] [PubMed] [Google Scholar]
- 2. International Association of Diabetes and Pregnancy Study Groups Consensus Panel , Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PM, Damm P, Dyer AR, Hod M, Kitzmiller JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y. International Association of Diabetes and Pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McIntyre HD, Jensen DM, Jensen RC, Kyhl HB, Jensen TK, Glintborg D, Andersen M. Gestational diabetes mellitus: does one size fit all? A challenge to uniform worldwide diagnostic thresholds. Diabetes Care. 2018;41:1339–1342. [DOI] [PubMed] [Google Scholar]
- 4. Vandorsten JP, Dodson WC, Espeland MA, Grobman WA, Guise JM, Mercer BM, Minkoff HL, Poindexter B, Prosser LA, Sawaya GF, Scott JR, Silver RM, Smith L, Thomas A, Tita AT. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013;29:1–31. [PubMed] [Google Scholar]
- 5. Retnakaran R, Shah BR. Glucose screening in pregnancy and future risk of cardiovascular disease in women: a retrospective, population‐based cohort study. Lancet Diabetes Endocrinol. 2019;7:378–384. [DOI] [PubMed] [Google Scholar]
- 6. Retnakaran R, Ye C, Kramer CK, Connelly PW, Hanley AJ, Sermer M, Zinman B. Maternal serum prolactin and prediction of postpartum beta‐cell function and risk of prediabetes/diabetes. Diabetes Care. 2016;39:1250–1258. [DOI] [PubMed] [Google Scholar]
- 7. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta‐analysis. Diabetologia. 2019;62:905–914. [DOI] [PubMed] [Google Scholar]
- 8. Retnakaran R. Hyperglycemia in pregnancy and its implications for a woman's future risk of cardiovascular disease. Diabetes Res Clin Pract. 2018;145:193–199. [DOI] [PubMed] [Google Scholar]
- 9. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes. Diabetes Care. 2008;31:1668–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kessous R, Shoham‐Vardi I, Pariente G, Sherf M, Sheiner E. An association between gestational diabetes mellitus and long‐term maternal cardiovascular morbidity. Heart. 2013;99:1118–1121. [DOI] [PubMed] [Google Scholar]
- 11. Fadl H, Magnuson A, Östlund I, Montgomery S, Hanson U, Schwarcz E. Gestational diabetes mellitus and later cardiovascular disease: a Swedish population‐based case‐control study. Br J Obst Gynaecol. 2014;121:1530–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaul P, Savu A, Nerenberg KA, Donovan LE, Chik CL, Ryan EA, Johnson JA. Impact of gestational diabetes mellitus and high maternal weight on the development of diabetes, hypertension and cardiovascular disease: a population‐level analysis. Diabet Med. 2015;32:164–173. [DOI] [PubMed] [Google Scholar]
- 13. Goueslard K, Cottenet J, Mariet AS, Giroud M, Cottin Y, Petit JM, Quantin C. Early cardiovascular events in women with a history of gestational diabetes mellitus. Cardiovasc Diabetol. 2016;15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Retnakaran R, Shah BR. Role of type 2 diabetes in determining retinal, renal and cardiovascular outcomes in women with previous gestational diabetes. Diabetes Care. 2017;40:101–108. [DOI] [PubMed] [Google Scholar]
- 15. Tobias DK, Stuart JJ, Li S, Chavarro J, Rimm EB, Rich‐Edwards J, Hu FB, Manson JE, Zhang C. Association of history of gestational diabetes with long‐term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med. 2017;177:1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daly B, Toulis KA, Thomas N, Gokhale K, Martin J, Webber J, Keerthy D, Jolly K, Saravanan P, Nirantharakumar K. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: a population‐based cohort study. PLoS Med. 2018;15:e1002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McKenzie‐Sampson S, Paradis G, Healy‐Profitós J, St‐Pierre F, Auger N. Gestational diabetes and risk of cardiovascular disease up to 25 years after pregnancy: a retrospective cohort study. Acta Diabetol. 2018;55:315–322. [DOI] [PubMed] [Google Scholar]
- 18. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 19. Hauspurg A, Ying W, Hubel CA, Michos ED, Ouyang P. Adverse pregnancy outcomes and future maternal cardiovascular disease. Clin Cardiol. 2018;41:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American College of Obstetricians and Gynecologists Committee . Opinion no. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140–e150. [DOI] [PubMed] [Google Scholar]
- 21. Murray Horwitz ME, Molina RL, Snowden JM. Postpartum care in the United States—New policies for a new paradigm. N Engl J Med. 2018;379:1691–1693. [DOI] [PubMed] [Google Scholar]
- 22. Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, Smith GN, Gore GC, Ray JG, Nerenberg K, Platt RW. Cardiovascular disease‐related morbidity and mortality in women with a history of pregnancy complications. Circulation. 2019;139:1069–1079. [DOI] [PubMed] [Google Scholar]


