Abstract
Background
The diagnosis of ischemic cerebellar stroke is challenging because of nonspecific symptoms and very limited accuracy of commonly applied computed tomography (CT) imaging. Advances in CT perfusion imaging provide increasing value in the detection of posterior circulation stroke, but the prognostic value remains unclear. We aimed to identify imaging parameters that predict morphologic outcome in cerebellar stroke patients using advanced CT including whole‐brain CT perfusion (WB‐CTP).
Methods and Results
We selected all subjects with cerebellar WB‐CTP perfusion deficits and follow‐up‐confirmed cerebellar infarction from a consecutive cohort with suspected stroke who underwent WB‐CTP. Posterior‐circulation‐Acute‐Stroke‐Prognosis‐Early‐CT‐Score (pc‐ASPECTS) was determined on noncontrast CT, CT angiography source images, and on parametric WB‐CTP maps. Cerebellar perfusion deficit volumes on all maps and the final infarction volume on follow‐up imaging were quantified. Uni‐ and multivariate regression analyses were performed. Sixty patients fulfilled the inclusion criteria. pc‐ASPECTS on CT angiography source images (ß, −9.239; 95% CI, −14.220 to −4.259; P<0.001) and cerebral blood flow deficit volume (ß, 0.886; 95% CI, 0.684 to 1.089; P<0.001) were significantly associated with final infarction volume in univariate linear regression analysis. The association of cerebral blood flow deficit volume (ß, 0.830; 95% CI, 0.605–1.055; P<0.001) was confirmed in a multivariate linear regression model adjusted for age, sex, pc‐ASPECTS on noncontrast CT, and CT angiography source images and the National Institutes of Health Stroke Scale score on admission. No other clinical or imaging parameters were associated with cerebellar stroke final infarction volume (P>0.05).
Conclusions
In contrast to noncontrast CT and CT angiography, WB‐CTP imaging contains prognostic information for morphologic outcome in patients with acute cerebellar stroke.
Keywords: CT perfusion imaging, ischemic stroke, perfusion imaging, posterior circulation
Subject Categories: Ischemic Stroke, Computerized Tomography (CT)
Clinical Perspective
What Is New?
In acute cerebellar stroke, perfusion deficit volumes in whole‐brain computed tomography perfusion imaging are associated with final cerebellar infarction volumes.
On the other hand, established imaging parameters derived from noncontrast computed tomography or computed tomography angiography did not show any associations with the size of the final cerebellar infarction.
What Are the Clinical Implications?
For stroke centers applying computed tomography in acute settings, whole‐brain computed tomography perfusion contains prognostic information for morphological outcome estimation in cerebellar stroke.
Further studies may be warranted to assess whether whole‐brain computed tomography perfusion can support treatment decision‐making based on the identification of true ischemic target lesions.
Cerebellar stroke accounts for 3% of all acute ischemic strokes.1 It is a challenging clinical diagnosis because of nonspecific and heterogeneous symptoms. Therefore, accurate diagnosis of acute cerebellar stroke depends highly on clinical experience, and misdiagnosis is common. Triaging patients only based on the clinical presentation has been shown to be inadequate.2 In the acute setting of cerebellar stroke, standard applied computed tomography (CT) imaging, including noncontrast CT (NCCT) and CT angiography (CTA), rules out differentials such as hemorrhage or basilar artery occlusion. Yet, it lacks diagnostic sensitivity and fails to provide prognostic information for isolated cerebellar stroke.1
Magnetic resonance imaging with diffusion‐weighted imaging of the brain represents the criterion standard test for cerebellar infarction but is not widely available, especially after hours. Advances in CT perfusion imaging enable the entire brain to be covered (whole‐brain CT perfusion, WB‐CTP), including the posterior fossa.3 WB‐CTP provides incremental value compared with NCCT and CTA alone in the detection of posterior circulation stroke.4, 5, 6
WB‐CTP imaging parameters have been shown to enable outcome prediction in patients with anterior circulation stroke7, 8, 9, 10, 11 and may guide therapeutic decision making by providing key information about ischemic brain regions that can potentially be rescued.12, 13, 14 On the other hand, the value of WB‐CTP for outcome prediction in cerebellar stroke has not yet been evaluated. Recently, the predictive value of WB‐CTP deficit volumes regarding the development of malignant cerebellar edema was shown.15 In this study, we aimed to assess the value of WB‐CTP for morphologic outcome prediction in acute cerebellar stroke.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Population
Our initial cohort consisted of 3648 consecutive patients with suspected ischemic stroke who underwent WB‐CTP between April 2009 and January 2018.
Out of this cohort, we included all patients with the following:
significant cerebellar perfusion deficits on WB‐CTP and
follow‐up‐confirmed cerebellar infarction in the superior cerebellar artery, anterior inferior cerebellar artery or posterior inferior cerebellar artery territory, or any combination thereof.
We excluded patients with the following:
evidence of basilar artery occlusion,
evidence of vertebral artery hypoplasia or crossed cerebellar diaschisis,
crossed cerebellar diaschisis as potential nonischemic causes for the cerebellar perfusion deficits,
incomplete coverage of the cerebellum, or
nondiagnostic quality of WB‐CTP.
A significant cerebellar perfusion deficit was defined as the presence of a focal abnormality in any color‐coded CTP map, namely, a focal decrease of cerebellar blood flow (CBF) or cerebellar blood volume, or a focal increase of time to maximum of the residue function, mean transit time (MTT) or time to drain compared with the contralateral side on at least 2 adjacent slices. Because cerebellar perfusion deficits are also attributed to causes unrelated to cerebellar ischemia such as vertebral artery hypoplasia16 and crossed cerebellar diaschisis,17 we excluded these patients from the study cohort (Figure 1). The institutional review board of the LMU Munich (Ethikkommission der Medizinischen Fakultät der Ludwig‐Maximilians‐Universität München) approved this retrospective study, which was conducted according to the Helsinki Declaration of 2013, and waived requirement for informed consent.
Figure 1.
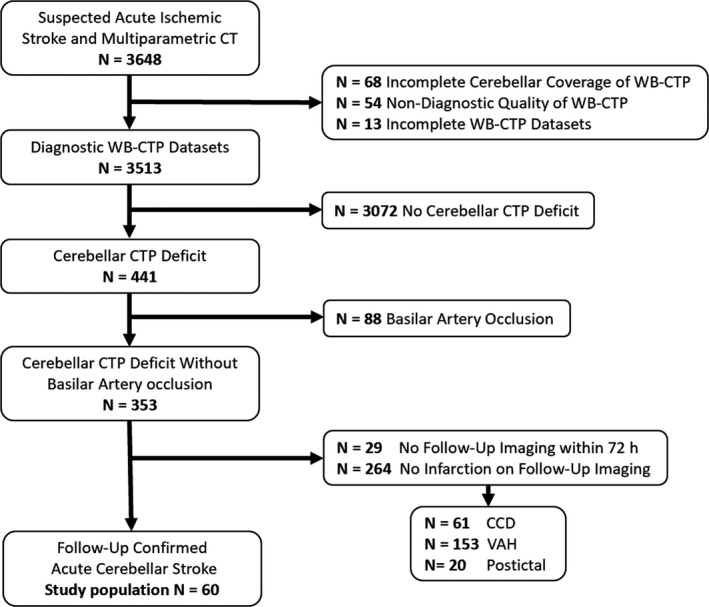
Flow chart of patient selection. CCD indicates crossed cerebellar diaschisis; CT, computed tomography; VAH, vertebral artery hypoplasia; WB‐CTP, whole‐brain CT perfusion.
Multiparametric CT Imaging and Analysis
All patients underwent a standardized multiparametric CT protocol consisting of NCCT, CTA, and WB‐CTP with a coverage of 10 cm in the z‐axis. The acquisition protocol has been described in detail before.18 NCCT follow‐up within 72 hours was available in 70%, and magnetic resonance imaging follow‐up in 73% of all patients. Both modalities were available in 43% of all patients. The assessment of all qualitative and quantitative imaging parameters was performed by 2 independent readers who were blinded to all clinical and follow‐up imaging data. In case of disagreement, a consensus was reached in a separate session.
Early ischemic changes were assessed using the 11‐point posterior circulation Acute Stroke Prognosis Early CT Score (pc‐ASPECTS)19 on NCCT, CTA source images, and on parametric WB‐CTP maps of CBF, cerebellar blood volume, mean transit time, time to drain, and time to maximum of the residue function. Vessel occlusions were documented. Cerebellar perfusion deficits on all CTP maps and follow‐up infarction were volumetrically assessed using OsiriX v.8.0.2 (Pixmeo; Bernex, Switzerland) as previously described.18, 20 On magnetic resonance imaging, follow‐up diffusion‐weighted imaging was used. Relative infarction growth was computed as final infarction volume (FIV) divided by CBF deficit volume (FIV/CBF).
Functional and Clinical Data
The National Institutes of Health Stroke Scale (NIHSS) score21 was determined upon hospital admission. The modified Rankin Scale score22 was assessed at admission (stroke severity in the emergency department), upon discharge, and 90 days after the stroke event. In addition, the premorbid modified Rankin Scale score was evaluated. Further clinical parameters included time from symptom onset, cardiovascular risk factors, and the cause of stroke according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification.23
Statistical Analysis
Statistical analysis was performed using SPSS (SPSS 23; IBM, Armonk/NY). Proportion tests for categorical variables were performed using the Fisher exact test, and nonparametric tests for ordinal and continuous variables were performed using the Mann–Whitney U test. Uni‐ and multivariate linear regression analysis were performed to assess associations between parameters. The regression coefficients (ß) and their 95% CI are reported. Categorical variables are presented as count (percentages). Ordinal and continuous variables are presented as median (interquartile range). Subgroup analyses were performed for the full study population stratified by treatment (intravenous thrombolysis [IVT] versus supportive care) and for the subgroup of patients with CTA‐detectable occlusions stratified by treatment. P values <0.05 were considered to indicate statistical significance.
Results
Patient Characteristics
Sixty patients matched the inclusion criteria. Detailed patient characteristics are shown in Table 1. Mean age was 73 (interquartile range: 55–81) years. Twenty‐three (38.3%) patients were female. Twenty‐nine (48.3%) had a vessel occlusion on CTA, most often of the vertebral artery (18; 30.0%). Twenty‐one (35.0%) patients had an additional brainstem infarction, 27 (45.0%) an additional supratentorial infarction at follow‐up imaging.
Table 1.
Patient Characteristic
| Overall (N=60) | |
|---|---|
| Patient data | |
| Age, y | 73 (55–81) |
| Male sex | 37 (61.7%) |
| Time from symptom onset (min)a | 130 (105–208) |
| NIHSS on admissiona | 3 (2–6) |
| NCCT imaging data | |
| pc‐ASPECTS NCCT | 10 (9–10) |
| Cerebellar atrophy | 22 (26.7%) |
| CTA imaging data | |
| pc‐ASPECTS CTA‐SI | 9 (9–10) |
| VA occlusion | 18 (30.0%) |
| PICA occlusion | 3 (5.0%) |
| AICA occlusion | 1 (1.7%) |
| SCA occlusion | 7 (11.7%) |
| WB‐CTP imaging data | |
| Bilateral ischemia | 17 (28.3%) |
| PICA ischemia | 43 (71.7%) |
| AICA ischemia | 6 (10.0%) |
| Ischemia | 27 (45.0%) |
| Brainstem ischemia | 22 (36.7%) |
| Supratentorial ischemia | 28 (46.7%) |
| pc‐ASPECTS CBF | 8 (6–9) |
| pc‐ASPECTS CBV | 9 (8–9) |
| pc‐ASPECTS MTT | 8 (7–9) |
| pc‐ASPECTS TTD | 8 (7–9) |
| pc‐ASPECTS Tmax | 8 (7–9) |
| CBF deficit volume, mL | 14 (5–22) |
| CBV deficit volume, mL | 4 (1–12) |
| MTT deficit volume, mL | 10 (4–18) |
| TTD deficit volume, mL | 10 (4–17) |
| Tmax deficit volume, mL | 11 (5–18) |
| CBF‐CBV mismatch, % | 61 (40–80) |
| Treatment data | |
| IVTa | 27 (45.0%) |
| Follow‐up imaging data | |
| FIV, mL | 5 (2–23) |
| PICA infarction | 44 (73.3%) |
| ICA infarction | 8 (13.3%) |
| SCA infarction | 27 (45.0%) |
| Brainstem infarction | 21 (35.0%) |
| Supratentorial infarction | 27 (45.0%) |
| FIV/CBF | 0.7 (0.3–1.5) |
| Functional data | |
| Premorbid mRSa | 0 (0–1) |
| Admission mRSa | 3 (2–4) |
| Discharge mRSa | 2 (1–4) |
| 90‐Day mRSa | 2 (1–6) |
Values presented are count (percentage) for categorical and median (interquartile range) for ordinal and continuous variables. AICA indicates anterior inferior cerebellar artery; CBF/CBV, cerebral blood flow/volume; CTA‐SI, CT angiography source images; FIV, final infarction volume; FIV/CBF, relative infarction growth; ICA, internal carotid artery; IVT, intravenous thrombolysis; mRS, modified Rankin Scale; MTT, mean transit time; NIHSS, National Institutes of Health Stroke Scale; pc‐ASPECTS, posterior circulation‐Alberta Stroke Program Early CT Score; PICA, posterior inferior cerebellar artery; SCA, superior cerebellar artery; Tmax, time to maximum; TTD, time to drain; VA, vertebral artery; WB‐CTP, whole brain CT perfusion.
Missing values: time from symptom onset, 22/60; IVT, 1/60; admission NIHSS, 11/60; premorbid mRS, 2/60; admission mRS (stroke severity in the emergency department), 4/60; discharge mRS 3/60; 90‐day mRS, 31/60.
Association of Imaging Parameters With Morphologic Outcome
Linear regression analyses were performed to predict FIV with baseline variables. NCCT parameters and admission NIHSS score showed no significant association with FIV (all P>0.05). Perfusion deficit volume on all WB‐CTP maps (CBF, cerebellar blood volume, time to drain, mean transit time, and time to maximum of the residue function; all P<0.001) as well as CTA source images imaging parameters (P<0.001) were significantly associated with FIV in univariate analysis. Among the perfusion parameters, CBF was chosen for further multivariate analysis, as it had the strongest association in univariate analysis and is commonly used in the clinical routine. In a multivariate linear regression model, adjusted for age, sex, pc‐ASPECTS on NCCT and CTA source images, and NIHSS score, only the association of CBF deficit volume (ß, 0.830; 95% CI, 0.605–1.055; P<0.001) remained significantly associated with FIV (Table 2). Case examples are shown in Figure 2.
Table 2.
Predictors of Final Infarction Volume
| FIV | ||||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| Independent variables | ß | P Value | ß | P Value |
| Age | 0.008 | 0.969 | −0.070 | 0.594 |
| Sex | −5.019 | 0.408 | −6.240 | 0.141 |
| NIHSS on admission | 0.555 | 0.253 | 0.416 | 0.404 |
| pc‐ASPECTS NCCT | −5.974 | 0.076 | 1.936 | 0.602 |
| pc‐ASPECTS CTA‐SI | −9.239 | <0.001a | −4.256 | 0.212 |
| CBF deficit volume | 0.754 | <0.001a | 0.830 | <0.001a |
Uni‐ and multivariate linear regression analysis were performed for the indicated FIV parameters for the complete study population of 60 patients. CBF/CBV indicates cerebral blood flow/volume; CTA‐SI, computed tomography angiography source images; FIV, final infarction volume; NCCT, noncontrast computed tomography; NIHSS, National Institutes of Health Stroke Scale; pc‐ASPECTS; posterior circulation Alberta Stroke Program Early CT Score.
P values indicate statistical significance.
Figure 2.
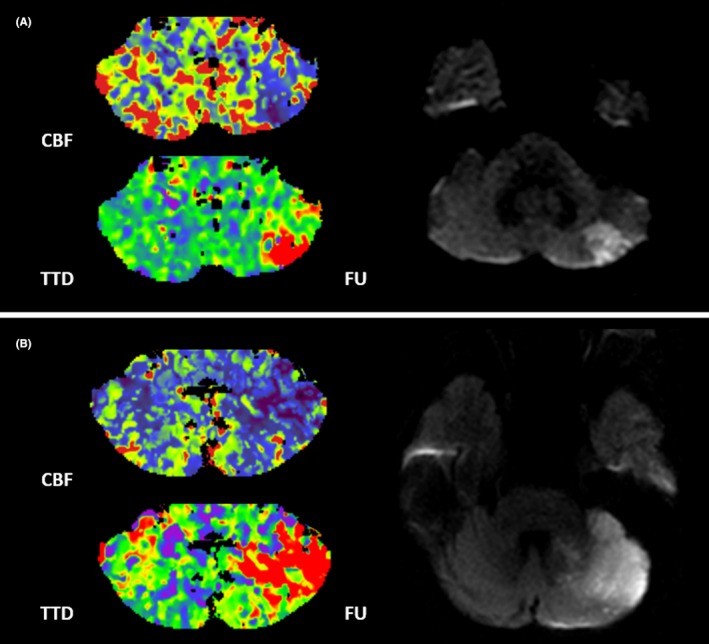
Case examples. Patient examples of acute cerebellar stroke. Patient A is a 77‐year‐old man with an initial CBF deficit volume of 5 mL of the left cerebellar hemisphere. On follow‐up MRI, the patient had a PICA territory infarction with a FIV of 2 mL. Patient B is a 67‐year‐old woman with an initial CBF deficit of 21 mL of the left cerebellar hemisphere. On follow‐up MRI, the patient had a PICA and SCA territory infarction with a total FIV of 22 mL. Both patients received IVT. CBF indicates cerebellar blood flow; FIV, final infarction volume; FU, follow‐up MRI; IVT, intravenous thrombolysis; PICA, posterior inferior cerebellar artery; SCA, superior cerebellar artery; TTD, time to drain.
Cerebellar Infarction Growth and Impact of Thrombolysis
Overall median FIV/CBF (interquartile range) was 0.68 (0.28–1.48). IVT was administered to 27 (45.0%) patients. There was no significant difference in FIV/CBF between the IVT and non‐IVT group (P=0.475, Table 3). Also, in subgroup analyses regarding the presence of a CTA‐detected vessel occlusion or the different vessel territories, IVT had no significant impact on FIV/CBF.
Table 3.
Clinical and Imaging Characteristics in Patients by Treatment Status
| IVT (n=27) | Non‐IVT (n=32) | P Value | |
|---|---|---|---|
| CTA‐detected occlusion | 16 (59.3%) | 15 (46.9%) | 0.435 |
| CBF deficit volume | 12 (5–23) | 15 (4–21) | 0.796 |
| CBV deficit volume | 4 (1–10) | 5 (2–12) | 0.692 |
| MTT deficit volume | 10 (5–19) | 7 (3–16) | 0.229 |
| TTD deficit volume | 13 (5–17) | 8 (2–17) | 0.186 |
| Tmax deficit volume | 12 (5–18) | 9 (4–17) | 0.356 |
| FIV | 5 (2–19) | 5 (2–28) | 0.687 |
| FIV/CBF | 0.64 (0.25–1.48) | 0.75 (0.25–1.69) | 0.475 |
| NIHSS on admission | 4 (3–7) | 3 (2–5) | 0.291 |
| Admission mRS | 3 (2–4) | 3 (2–4) | 0.604 |
| Discharge mRS | 2 (1–4) | 3 (1–6) | 0.325 |
Values presented are count (percentage) for categorical and median (interquartile range) for ordinal and continuous variables. Proportion analysis tests for categorical variables were performed using the Fisher exact test. Nonparametric tests for ordinal and continuous variables were performed using the Mann–Whitney U test. CBF indicates cerebral blood flow/volume; CBV, cerebral blood volume; CTA, computed tomography angiography; FIV, final infarction volume; FIV/CBF, relative infarction growth; IVT, intravenous thrombolysis; mRS, modified Rankin Scale; MTT, mean transit time; NIHSS, National Institutes of Health Stroke Scale; Tmax, time to maximum; TTD, time to drain.
Discussion
In our study on acute cerebellar stroke, baseline WB‐CTP imaging provided prognostic information on the FIV. We did observe a strong correlation with FIV, while absolute estimates in certain perfusion parameter maps yielded overestimation of the FIV. The regression analysis showed that for every additional 1 mL of cerebellar CBF deficit volume, the final cerebellar infarction volume increased by ≈0.8 mL. In our sample, we found no association between baseline clinical parameters (eg, NIHSS on admission) and morphologic outcome.
Because of the relevant frequencies of concomitant brainstem and supratentorial involvement, clinical outcome evaluation was not feasible because significant bias by these cofounders would have impacted the analysis. However, an association of FIV and clinical outcome has been shown for anterior circulation strokes24 as well as for cerebellar strokes.25 Furthermore, a recent study identified an independent predictive value of CT perfusion parameters for clinical outcome in patients with vertebral or basilar artery occlusion.26
The management of acute cerebellar stroke is still a matter of debate because no dedicated randomized trials have been conducted, because of the relatively low disease incidence. Extrapolating evidence from the IVT trials since 1995, IVT is indicated within 4.5 hours if stroke symptoms are considered disabling and/or amount to an NIHSS >5.14 However, since successful real‐world IVT application has only been reported in a few cases27, 28 and many cerebellar strokes present with minor neurological deficits of NIHSS 0 to 5 (this was the case in 36 patients [60.0%] of our study population), the efficacy of cerebellar stroke thrombolysis is still obscure.
The recently published PRISMS (A Study of the Efficacy and Safety of Alteplase in Participants With Mild Stroke) trial was unable to show a clinical benefit of IVT, yet early termination precludes definitive conclusions.29 Further ongoing trials such as the TEMPO‐2 trial (A Randomized Controlled Trial of TNK‐tPA Versus Standard of Care for Minor Ischemic Stroke With Proven Occlusion; NCT02398656) may help in understanding the role of IVT in patients with low NIHSS. In our study, we could not demonstrate a significant impact of IVT administration on the development of the acute ischemic lesion size from the time point of WB‐CTP to the final infarct lesion on follow‐up imaging. However, the sample size is too small to draw any definitive conclusions. Furthermore, data on recanalization after IVT was not available because there was no clinical indication according to guidelines to perform repeated noninvasive angiography. Yet, it is recognized that the recanalization rate is an important variable in the prediction of the final infarct.
Because clinical assessment of the severity of cerebellar stroke is often difficult and limited, more objective imaging parameters such as WB‐CTP might potentially support clinical decision‐making, and hence facilitate clinical trial design by identifying real ischemic target lesions and excluding stroke mimics, which may otherwise confound results in the low NIHSS clinical trial setting.
There are limitations to this study that need to be considered when interpreting the results. First, this is a single‐center retrospective study with a limited number of patients, reflecting the relatively low incidence of cerebellar compared with supratentorial infarction. This may limit adequate correlation between CBF and magnetic resonance imaging. Second, based on the study design and the selection criteria, only patients who had undergone additional WB‐CTP were included, which may represent a selection bias. However, all enrolled patients were recruited from a prospective stroke registry and standardized stroke protocols were applied to minimize this potential bias. Third, this is a volume‐based analysis rather than a voxel‐based analysis, which might be more accurate, though, to our knowledge, there are no automated, commercial solutions for perfusion analysis in posterior circulation stroke. Fourth, aside from the CBF map, other perfusion imaging maps also correlated with FIV. To avoid issues with multicollinearity, we only included CBF‐determined lesion volumes in the multivariate analysis.
In conclusion, WB‐CTP imaging, in contrast to NCCT and/or CTA imaging, contains prognostic information for morphologic outcome prediction in patients with acute cerebellar stroke.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e013069 DOI: 10.1161/JAHA.119.013069.)
References
- 1. Edlow JA, Newman‐Toker DE, Savitz SI. Diagnosis and initial management of cerebellar infarction. Lancet Neurol. 2008;7:951–964. [DOI] [PubMed] [Google Scholar]
- 2. Neugebauer H, Witsch J, Zweckberger K, Juttler E. Space‐occupying cerebellar infarction: complications, treatment, and outcome. Neurosurg Focus. 2013;34:E8. [DOI] [PubMed] [Google Scholar]
- 3. Morhard D, Wirth CD, Fesl G, Schmidt C, Reiser MF, Becker CR, Ertl‐Wagner B. Advantages of extended brain perfusion computed tomography: 9.6 cm coverage with time resolved computed tomography‐angiography in comparison to standard stroke‐computed tomography. Invest Radiol. 2010;45:363–369. [DOI] [PubMed] [Google Scholar]
- 4. van der Hoeven EJ, Dankbaar JW, Algra A, Vos JA, Niesten JM, van Seeters T, van der Schaaf IC, Schonewille WJ, Kappelle LJ, Velthuis BK; Investigators D . Additional diagnostic value of computed tomography perfusion for detection of acute ischemic stroke in the posterior circulation. Stroke. 2015;46:1113–1115. [DOI] [PubMed] [Google Scholar]
- 5. Bollwein C, Plate A, Sommer WH, Thierfelder KM, Janssen H, Reiser MF, Straube A, von Baumgarten L. Diagnostic accuracy of whole‐brain ct perfusion in the detection of acute infratentorial infarctions. Neuroradiology. 2016;58:1077–1085. [DOI] [PubMed] [Google Scholar]
- 6. Sporns P, Schmidt R, Minnerup J, Dziewas R, Kemmling A, Dittrich R, Zoubi T, Heermann P, Cnyrim C, Schwindt W, Heindel W, Niederstadt T, Hanning U. Computed tomography perfusion improves diagnostic accuracy in acute posterior circulation stroke. Cerebrovasc Dis. 2016;41:242–247. [DOI] [PubMed] [Google Scholar]
- 7. Parsons MW, Pepper EM, Chan V, Siddique S, Rajaratnam S, Bateman GA, Levi CR. Perfusion computed tomography: prediction of final infarct extent and stroke outcome. Ann Neurol. 2005;58:672–679. [DOI] [PubMed] [Google Scholar]
- 8. Minnerup J, Wersching H, Ringelstein EB, Heindel W, Niederstadt T, Schilling M, Schabitz WR, Kemmling A. Prediction of malignant middle cerebral artery infarction using computed tomography‐based intracranial volume reserve measurements. Stroke. 2011;42:3403–3409. [DOI] [PubMed] [Google Scholar]
- 9. Psychogios MN, Schramm P, Frolich AM, Kallenberg K, Wasser K, Reinhardt L, Kreusch AS, Jung K, Knauth M. Alberta stroke program early CT scale evaluation of multimodal computed tomography in predicting clinical outcomes of stroke patients treated with aspiration thrombectomy. Stroke. 2013;44:2188–2193. [DOI] [PubMed] [Google Scholar]
- 10. Horsch AD, Dankbaar JW, Stemerdink TA, Bennink E, van Seeters T, Kappelle LJ, Hofmeijer J, de Jong HW, van der Graaf Y, Velthuis BK; investigators D . Imaging findings associated with space‐occupying edema in patients with large middle cerebral artery infarcts. Am J Neuroradiol. 2016;37:831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Seeters T, Biessels GJ, Kappelle LJ, van der Schaaf IC, Dankbaar JW, Horsch AD, Niesten JM, Luitse MJ, Majoie CB, Vos JA, Schonewille WJ, van Walderveen MA, Wermer MJ, Duijm LE, Keizer K, Bot JC, Visser MC, van der Lugt A, Dippel DW, Kesselring FO, Hofmeijer J, Lycklama ANGJ, Boiten J, van Rooij WJ, de Kort PL, Roos YB, Meijer FJ, Pleiter CC, Mali WP, van der Graaf Y, Velthuis BK. Ct angiography and ct perfusion improve prediction of infarct volume in patients with anterior circulation stroke. Neuroradiology. 2016;58:327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega‐Gutierrez S, McTaggart RA, Torbey MT, Kim‐Tenser M, Leslie‐Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG; Investigators DT . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 14. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie‐Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 15. Fabritius MP, Thierfelder KM, Meinel FG, Othman AE, Dorn F, Sabel BO, Scheffler P, Ertl‐Wagner B, Sommer WH, Kunz WG. Early imaging prediction of malignant cerebellar edema development in acute ischemic stroke. Stroke. 2017;48:2597–2600. [DOI] [PubMed] [Google Scholar]
- 16. Thierfelder KM, Baumann AB, Sommer WH, Armbruster M, Opherk C, Janssen H, Reiser MF, Straube A, von Baumgarten L. Vertebral artery hypoplasia: frequency and effect on cerebellar blood flow characteristics. Stroke. 2014;45:1363–1368. [DOI] [PubMed] [Google Scholar]
- 17. Kunz WG, Sommer WH, Hohne C, Fabritius MP, Schuler F, Dorn F, Othman AE, Meinel FG, von Baumgarten L, Reiser MF, Ertl‐Wagner B, Thierfelder KM. Crossed cerebellar diaschisis in acute ischemic stroke: impact on morphologic and functional outcome. J Cereb Blood Flow Metab. 2017;37:3615–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kunz WG, Sommer WH, Havla L, Dorn F, Meinel FG, Dietrich O, Buchholz G, Ertl‐Wagner B, Thierfelder KM. Detection of single‐phase CTA occult vessel occlusions in acute ischemic stroke using CT perfusion‐based wavelet‐transformed angiography. Eur Radiol. 2017;27:2657–2664. [DOI] [PubMed] [Google Scholar]
- 19. Puetz V, Sylaja PN, Coutts SB, Hill MD, Dzialowski I, Mueller P, Becker U, Urban G, O'Reilly C, Barber PA, Sharma P, Goyal M, Gahn G, von Kummer R, Demchuk AM. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke. 2008;39:2485–2490. [DOI] [PubMed] [Google Scholar]
- 20. Thierfelder KM, Sommer WH, Baumann AB, Klotz E, Meinel FG, Strobl FF, Nikolaou K, Reiser MF, von Baumgarten L. Whole‐brain CT perfusion: reliability and reproducibility of volumetric perfusion deficit assessment in patients with acute ischemic stroke. Neuroradiology. 2013;55:827–835. [DOI] [PubMed] [Google Scholar]
- 21. Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, Rorick M, Moonmaw CJ, Walker M. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. [DOI] [PubMed] [Google Scholar]
- 22. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. [DOI] [PubMed] [Google Scholar]
- 23. Adams HP, Bendixen BH Jr, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 24. Zaidi SF, Aghaebrahim A, Urra X, Jumaa MA, Jankowitz B, Hammer M, Nogueira R, Horowitz M, Reddy V, Jovin TG. Final infarct volume is a stronger predictor of outcome than recanalization in patients with proximal middle cerebral artery occlusion treated with endovascular therapy. Stroke. 2012;43:3238–3244. [DOI] [PubMed] [Google Scholar]
- 25. Calic Z, Cappelen‐Smith C, Cuganesan R, Anderson CS, Welgampola M, Cordato DJ. Frequency, aetiology, and outcome of small cerebellar infarction. Cerebrovasc Dis Extra. 2017;7:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alemseged F, Shah DG, Bivard A, Kleinig TJ, Yassi N, Diomedi M, Di Giuliano F, Sharma G, Drew R, Yan B, Dowling RJ, Bush S, Sallustio F, Caltagirone C, Mercuri NB, Floris R, Parsons MW, Levi CR, Mitchell PJ, Davis SM, Campbell BC. Cerebral blood volume lesion extent predicts functional outcome in patients with vertebral and basilar artery occlusion. Int J Stroke. 2019;14:540–547. [DOI] [PubMed] [Google Scholar]
- 27. Kohrmann M, Sauer R, Huttner HB, Engelhorn T, Doerfler A, Schellinger PD. Mri mismatch‐based intravenous thrombolysis for isolated cerebellar infarction. Stroke. 2009;40:1897–1899. [DOI] [PubMed] [Google Scholar]
- 28. Gobert F, Cho TH, Desilles JP, Hermier M, Mechtouff L, Derex L, Nighoghossian N. Magnetic resonance imaging‐based intravenous thrombolysis 6 hours after onset of minor cerebellar stroke. Arch Neurol. 2011;68:678. [DOI] [PubMed] [Google Scholar]
- 29. Khatri P, Kleindorfer DO, Devlin T, Sawyer RN Jr, Starr M, Mejilla J, Broderick J, Chatterjee A, Jauch EC, Levine SR, Romano JG, Saver JL, Vagal A, Purdon B, Devenport J, Pavlov A, Yeatts SD; PRISMS Investigators . Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits: the prisms randomized clinical trial. JAMA. 2018;320:156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]


