Abstract
Background
An increased risk of acute ischemic stroke is recognized among patients with cancer. However, the mechanism behind cancer‐related stroke is unclear. In this study, we determined the presence of associated venous thromboembolism and arterial thromboembolism and their clinical impact on patients with cancer‐related stroke.
Methods and Results
Patients with embolic stroke of undetermined source with or without cancer were evaluated for venous thromboembolism (deep vein thrombosis [DVT] and/or pulmonary embolism) and arterial thromboembolism by using Doppler sonography to determine the presence of lower‐extremity DVT and the microembolic signal of the symptomatic cerebral circulation, respectively. Infarct volume was determined by diffusion‐weighted magnetic resonance imaging. The multivariable linear regression and Cox proportional hazard analysis were used to investigate the effect of DVT and microembolic signal on infarct volume and 1‐year survival, respectively. Of 142 screened patients, 118 were included (37 with, 81 without cancer). Those with cancer had a higher prevalence of DVT or microembolic signal than did the noncancer group (62.2% versus 19.8%; P<0.001). Among patients with cancer‐related stroke, DVT was associated with a greater infarct volume in magnetic resonance imaging (beta, 13.14; 95% CI, 1.62–24.66; P=0.028). Presence of DVT (hazard ratio, 16.79; 95% CI, 2.05–137.75; P=0.009) and microembolic signal (hazard ratio, 8.16; 95% CI, 1.36–48.85; P=0.022) were independent predictors of poor 1‐year survival.
Conclusions
Patients with cancer‐associated embolic stroke of undetermined source have an elevated risk of associated venous thromboembolism and arterial thromboembolism, both of which have a significant negative impact on 1‐year survival. The results of this study may enhance our understanding of cancer‐associated stroke and improve risk stratification of patients with this disease.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/.Unique identifier: NCT02212496
Keywords: cancer and stroke, deep vein thrombosis, microembolic signal, thromboembolism
Subject Categories: Ischemic Stroke, Mortality/Survival, Clinical Studies
Clinical Perspective
What Is New?
Venous and arterial thromboembolism are found in almost two‐thirds of embolic stroke of undetermined source patients with cancer.
Venous and arterial thromboembolism contribute differently to infarct volume, although they are independently associated with poor 1‐year survival.
Hypercoagulability, rather than consumptive coagulopathy, may be the more‐contributory mechanism in cancer‐related stroke.
What Are the Clinical Implications?
Screening for evidence of venous and arterial thromboembolism in embolic stroke of undetermined source patients with cancer may aid in the diagnosis and prognostication of cancer‐related stroke.
Further pathophysiological studies on clot types (venous versus arterial thromboembolism) will contribute to tailored antithrombotic therapy for cancer‐related stroke patients.
Both cancer and stroke are leading causes of death worldwide. Systemic cancer is associated with increased risk of ischemic stroke1, 2, 3 Cryptogenic stroke can be a clue suggesting hidden malignancy.4 Recent studies have consistently reported on poor survival in patients with cancer and stroke, especially in those with a cryptogenic subtype and hypercoagulable states5, 6, 7
To date, several hypotheses on the mechanisms underlying the cancer‐stroke association have been suggested. Cancer cells can produce procoagulating factors, leading to a thrombotic tendency.8, 9 Consequent hypercoagulability is now regarded as a hallmark of cancer‐related stroke.10, 11 However, the mode of clot delivery to the cerebral circulation is still under debate. Traditionally, nonbacterial thrombotic endocarditis was considered the cause of stroke in patients with cancer.12 However, it is infrequently detected in clinical settings.13 Rather, given that systemic cancer is a well‐known risk factor of venous thromboembolism, venous thrombosis and paradoxical embolism has been suggested as a mechanism for stroke.14 In addition, the microembolic signal (MES) is relatively common in patients with cancer and cryptogenic stroke.11 More recently, the risk of arterial thromboembolism has increased in patients with systemic cancer.3 Taken together, venous or arterial thromboembolism may be common in patients with cancer and play a role in the pathophysiology of cancer‐related stroke.
In this prospective study, we investigated the presence of venous or arterial thromboembolism associated with embolic stroke of undetermined source (ESUS) in patients with and without active cancer and its impact on infarct volume and survival.
Methods
Subjects
Consecutive patients with ESUS were prospectively recruited between August 2014 and March 2018 at the Samsung Medical Center, which is a university hospital with a comprehensive stroke and cancer center. During the study period, patients who: (1) had acute ischemic stroke diagnosed within 7 days after symptom onset; (2) had embolic infarction outside the perforator territory as revealed by diffusion‐weighted imaging; (3) lacked conventional stroke mechanisms as revealed by ECG and intra‐ and extracranial vessel imaging; and (4) agreed to participate in this study were included. After initial inclusion, we undertook a thorough evaluation (described in the following section) of conventional stroke mechanisms defined as evident causes of stroke included in the Causative Classification System (http://ccs.mgh.harvard.edu/ccs_title.php).15 If large‐artery atherosclerosis, aortogenic or cardiogenic embolism, or other mechanisms were suspected as a cause of the index stroke, then we excluded those patients. Exclusion criteria included: (1) age <18 years; (2) single subcortical infarction; (3) inability to perform comprehensive studies; and (4) refusal or withdrawal of consent. This study was preregistered at Clinicaltrials.gov (identifier: NCT02212496). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Group Definition
The cancer group included patients with a diagnosis of or cancer treatment during the 6 months preceding the stroke diagnosis or the presence of recurrent or metastatic cancer.11 Those without cancer were included in the noncancer group. In patients with cancer, data on cancer type, histology (adenocarcinoma), and metastasis were collected. Among the 142 eligible participants, other evident etiologies were documented by thorough investigations in 21 patients (atrial fibrillation in 10, aortic atheroma in 2, left ventricular thrombus in 1, left ventricular aneurysm in 1, complex atheroma in the parent artery of <50% stenosis in 5, Takayasu arteritis in 1, pulmonary arteriovenous malformation in 1, and infective endocarditis in 1). Three patients withdrew their consent because they wanted an early discharge from the hospital before the workup was completed. Thus, a total of 118 patients with ESUS (37 in the cancer group and 81 in the noncancer group) were included in the study.
Investigations
Workup for venous or arterial thromboembolism
All included patients were evaluated to reveal evidence of venous or arterial thromboembolism. Venous thromboembolism (VTE) was investigated primarily by Doppler sonography for proximal (popliteal, femoral, and iliac) and distal (peroneal, posterior and anterior tibial, and muscular veins) lower‐extremity veins and/or computed tomography pulmonary angiography combined with venous phase computed tomography angiography of the lower extremities. Among patients with deep vein thrombosis (DVT), concomitant pulmonary embolisms were sought in those who had undergone additional pulmonary computed tomography angiography because of respiratory symptoms or as part of the routine workup for DVT. Among patients with symptomatic DVT, chronicity was defined as acute (<14 days from symptom onset), intermediate (14–28 days from symptom onset), or chronic (>28 days from symptom onset). Doppler ultrasound findings of vascular wall thickening were also used to differentiate chronic thrombi. The MES was considered a surrogate marker for arterial thromboembolism. Patients underwent transcranial Doppler sonography (Pioneer TC 8080; Nicolet Vascular, Madison, WI). Both of the middle cerebral arteries were monitored by a transtemporal window by two 2‐MHz probes (insonation depth, 40–60 mm; Marc 500; Spencer Technologies, Northborough, MA) fixed with a head frame.11 Basilar arteries were assessed using the same apparatus rotated 90 degrees by the suboccipital window (insonation depth, 80–100 mm).16 Doppler recordings were performed for 30 minutes in the supine position. Patients with ≥1 MES during the transcranial Doppler sonography recording were classified as MES positive.11
Evaluation of the cardiogenic and arteriogenic embolic source
Transesophageal echocardiography (TEE) was performed as a part of the etiological investigation. The presence of a right‐to‐left shunt was demonstrated by the appearance of microbubbles in the left atrium following intravenous injection of agitated saline that occurred spontaneously and/or after a Valsalva maneuver.17, 18 The presence of nonbacterial thrombotic endocarditis and aortic arch atheroma was also sought. When TEE was difficult to perform, transthoracic echocardiography was performed to exclude potential cardioembolic sources. Regarding the right‐to‐left shunt and nonbacterial thrombotic endocarditis, only the data from patients who underwent TEE were analyzed. To detect any arrhythmia that could act as a cardioembolic source, all participants underwent a single routine ECG and 24‐hour cardiac rhythm monitoring or 72‐hour inpatient telemonitoring. Patients with possible large artery atherosclerosis with stenoses <50% underwent an additional evaluation, either a carotid Doppler ultrasound or contrast computed tomography angiography was used to determine the vulnerability of the plaque.
Evaluation of the coagulation profile
We measured platelet count (×109/L), prothrombin time (seconds), activated partial thromboplastin time (seconds), fibrinogen (mg/dL), D‐dimer (μg/mL), and fibrinogen degradation products (μg/mL) in each patient's blood to investigate the presence of increased D‐dimer concentration and overt disseminated intravascular coagulopathy (DIC). A D‐dimer level of 3.00 μg/mL was used as a cut‐off value to determine elevation.19 Overt DIC was defined by The International Society for Thrombosis and Haemostasis DIC scoring system20: (1) platelet count (×109/L); >100=0, <100=1, or <50=2; (2) fibrin‐related markers (ie, D‐dimer and fibrinogen degradation products; μg/mL); strong increase=3, moderate increase=2, or no increase=0; (3) prothrombin time (seconds); <3=0, >3 but <6=2, or >6=3; and (4) fibrinogen concentration (g/dL); <1=1 or >1=0. If the composite of these scores summed to ≥5 points, the participant was considered to have overt DIC.
Measurement of infarct volume
Infarct volumes were measured by diffusion‐weighted imaging and apparent diffusion coefficients calculated from the imaging data. Magnetic resonance imaging postprocessing was performed by the investigators (B.‐y.P. and H.P.) who were blinded to the clinical data. Using diffusion‐weighted imaging, the mean intensity value of each hemisphere was calculated. The hemisphere with the lower value was considered as the normal hemisphere. A histogram of the intensity values of the normal hemisphere was constructed, and voxels with an intensity value that did not belong to 95% of the histogram were considered as outliers and removed. The intensity values of the remaining voxels were transformed into z‐scores. Potential infarcts were segmented by using a threshold z‐value >2. Segmented lesions were confirmed by visual inspection and further edited as necessary. Of these lesions, voxels with an apparent diffusion coefficient <650 were considered as infarct lesions. Clinical investigators reviewed all segmented voxels and excluded false‐positive lesions. The infarct volume was calculated by multiplying the number of voxels by their physical voxel dimensions.
Statistical Analysis
Based on the assumption that VTE may account for 25% of patients from the cancer group and 5% of patients from the noncancer group, our sample size calculation yielded 37 and 87 patients in the cancer and noncancer groups, respectively, to reach 80% power with an alpha of 5%. Sample size was calculated to find a significant difference in DVT prevalence between the cancer and noncancer groups.
Statistical analyses were performed using the Statistical Package for the Social Sciences software (ver. 24.0; IBM Corp., Armonk, NY). The differences in demographics, presence of venous or arterial thromboembolism, plasma D‐dimer concentration, the rate of elevated D‐dimer level (>3.0 μg/mL) or overt DIC, the number of patients with nonbacterial thrombotic endocarditis, and right‐to‐left shunts were compared between groups using the Student t test or chi‐square test. A subgroup analysis was performed for a comparison between premorbid anticoagulant users and drug‐naïve patients. Univariable and multivariable linear regression analyses were performed to evaluate factors contributing to infarct volume adjusted for age, sex, and presence of overt DIC. Univariable and multivariable Cox proportional hazard analyses were performed to evaluate the potential association between venous or arterial thromboembolism and 1‐year survival adjusted for age, sex, National Institutes of Health Stroke Scale score, presence of overt DIC, infarct volume, metastasis, and histology type (adenocarcinoma). The impact of VTE and arterial thromboembolism was analyzed 3 ways: any versus none, the presence versus absence of VTE, and the presence versus absence of arterial thromboembolism. The linear regression coefficient beta, 95% CIs, and 2‐tailed P values were obtained. P<0.05 was considered significant.
Standard Protocol Approvals, Registrations, and Patient Consent
The Samsung Medical Center Institutional Review Board approved this study. All patients and controls provided written informed consent before participation.
Results
Study Subjects
Baseline characteristics of patients from the noncancer (n=81) and cancer (n=37) groups are listed in Table 1. Patients from the noncancer group were significantly younger than those from the cancer group (P=0.003). Furthermore, the National Institutes of Health Stroke Scale score was significantly lower (P=0.011) and hyperlipidemia was more commonly observed in the noncancer group (P=0.020). No other significant differences regarding sex, comorbidities (diabetes mellitus, hypertension, or ischemic heart disease), or the use of tobacco and alcohol were observed between the 2 groups. Among patients with cancer, metastasis was observed in 29 (78.4%) patients, and 22 (59.5%) patients had adenocarcinoma.
Table 1.
Demographics and Baseline Characteristics of Included Subjects
| Baseline Measures | Noncancer (n=81) | Cancer (n=37) | P Value |
|---|---|---|---|
| Age, y | 57.7±15.84 | 66.6±11.28 | 0.003 |
| Female sex | 33 (40.7%) | 14 (37.8%) | 0.765 |
| NIHSS score (IQR) | 1 (0–3) | 3 (0.5–6) | 0.011 |
| Cardiovascular risk factors | |||
| Hypertension | 37 (45.7%) | 19 (51.4%) | 0.567 |
| Diabetes mellitus | 14 (17.3%) | 8 (21.6%) | 0.575 |
| Hyperlipidemia | 45 (55.6%) | 12 (32.4%) | 0.020 |
| Ischemic heart disease | 8 (9.9%) | 1 (2.7%) | 0.270 |
| Smoking history | 28 (34.6%) | 10 (27.0%) | 0.416 |
| Cancer characteristics | |||
| Cancer types | |||
| Stomach/esophagus | 4 (10.8%) | ||
| Pancreatic | 1 (2.7%) | ||
| Colorectal | 0 (0.0%) | ||
| Urological | 3 (8.1%) | ||
| Hepatobiliary | 5 (13.5%) | ||
| Lung | 15 (40.5%) | ||
| Gynecological | 4 (10.8%) | ||
| Breast | 0 (0.0%) | ||
| Others | 4 (10.8%) | ||
| Adenocarcinoma | 22 (59.5%) | ||
| Metastasis | 29 (78.4%) | ||
| Previous usage of anticoagulants | 5 (13.5%) | ||
| Concomitant chemotherapy | 15 (40.5%) | ||
Data are presented as mean±SD or number (%), unless specified. IQR indicates interquartile range; NIHSS, National Institutes of Health Stroke Scale.
Presence of Venous or Arterial Thromboembolism and Comorbid Conditions
MES monitoring was conducted for a median of 2.5 days (interquartile range, 1.00–4.25) from symptom onset, and screening for DVT was conducted for a median of 2.6 days (interquartile range, 1.00–4.25) from symptom onset. Compared with the noncancer group, the cancer group had a significantly higher prevalence of DVT and MES (62.2% versus 19.8%; P<0.001; Figure). Patients in the cancer group showed a higher prevalence of both DVT and MES (18.9% versus 2.5%), DVT only (16.2% versus 6.2%), and MES only (27.0% versus 11.1%) compared with patients in the noncancer group, respectively. The results of the venous and arterial thromboembolism assessment, TEE, plasma D‐dimer concentration measurement, and overt DIC scoring are summarized in Table 2. Prevalence of DVT and the MES was significantly higher in the cancer group (35.1% [95% CI, 20.2–52.5] and 45.9% [95% CI, 29.5–63.1], respectively) compared with the noncancer group (P<0.001 for both). Patients with cancer had higher plasma D‐dimer concentrations than those without cancer (P<0.001), showing an elevation in D‐dimer concentration (>3.00 μg/mL) in 24 (64.9%) patients (P<0.001). Four (10.8%) patients from the cancer group had overt DIC, which was not statistically different from the noncancer group (P=0.203). Nonbacterial thrombotic endocarditis was infrequent in our cohort of patients. In patients who underwent TEE, no significant differences were detected in prevalence of right‐to‐left shunts, or the type, amount, or activity of the shunt (P=0.892, 0.976, 0.882, and 0.082, respectively; Table 2).
Figure 1.
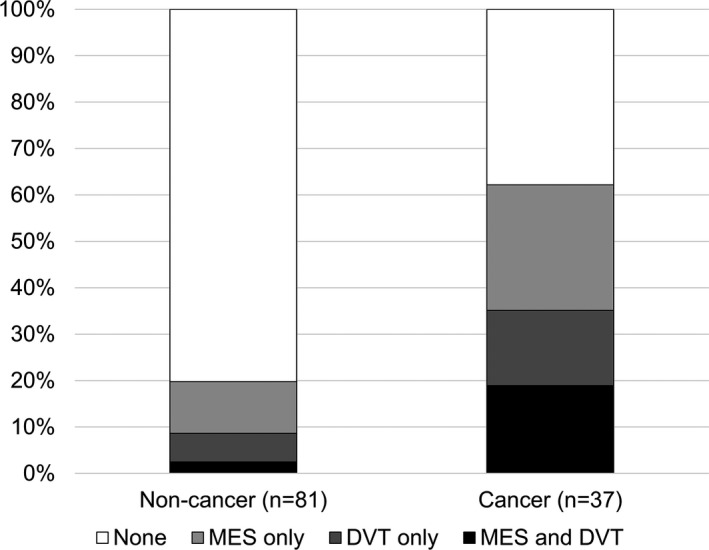
Presence of DVT and MES in patients with ESUS. The proportion of patients with DVT or MES is shown with colored bars. The total number in the noncancer group was 81 and 37 in the cancer group. For the noncancer group, no DVT or MES positive=65 (80.2%), MES positive only=9 (11.1%), DVT positive only=5 (6.2%), and DVT and MES positive=2 (2.5%). For the cancer group, no DVT or MES positive=14 (37.8%), MES positive only=10 (27.0%), DVT positive only=6 (16.2%), and DVT and MES positive=7 (18.9%). DVT indicates deep venous thrombosis; MES, microembolic signal.
Table 2.
Presence of DVT, MES, Right‐to‐Left Shunt, Nonbacterial Thrombotic Endocarditis, Elevated Plasma D‐Dimer Concentration, and Overt DIC in Patients
| Etiological Workup | Noncancer (n=81) | Cancer (n=37) | P Value |
|---|---|---|---|
| DVT | 7 (8.6%) | 13 (35.1%) | <0.001 |
| Locationa | |||
| Proximal | 1 (14.3%) | 11 (84.6%) | 0.002 |
| Distal | 6 (85.7%) | 7 (53.8%) | |
| Proximal and distal | 0 (0.0%) | 5 (38.5%) | |
| Concomitant PEa | |||
| Absent | 7 (100.0%) | 11 (84.6%) | |
| Present | 0 (0.0%) | 2 (15.4%) | 0.521 |
| Symptomatica | 0 (0.0%) | 6 (46.2%) | 0.051 |
| MES | 11 (13.6%) | 17 (45.9%) | <0.001 |
| Right‐to‐left shuntb | 68 (86.8%) | 28 (85.7%) | 0.892 |
| Amount, mbc , b | |||
| 1 to 10 | 20 (29.4%) | 8 (28.6%) | 0.882 |
| 11 to 20 | 19 (27.9%) | 10 (35.7%) | |
| 21 to 30 | 19 (27.9%) | 4 (14.3%) | |
| >30 | 1 (1.5%) | 2 (7.1%) | |
| Type of shuntc | 0.976 | ||
| PFO (IAS) | 40 (58.8%) | 15 (53.6%) | 0.638 |
| Pulmonary AVF | 30 (44.1%) | 13 (46.4%) | 0.837 |
| PFO+AVF | 11 (16.2%) | 4 (14.3%) | 1.000 |
| Active shuntc | 49 (72.1%) | 15 (53.6%) | 0.082 |
| NBTEb | 0 (0.0%) | 1 (2.9%) | 0.304 |
| Plasma D‐dimer concentration, μg/mL | 2.6 (8.31) | 12.2 (13.90) | <0.001 |
| Elevated D‐dimer concentration | 12 (14.8%) | 24 (64.9%) | <0.001 |
| Overt DIC | 3 (3.7%) | 4 (10.8%) | 0.203 |
Data are presented as number (%) or mean±SD, unless specified. AVF indicates arteriovenous fistula; DIC, disseminated intravascular coagulation; DVT, deep venous thrombosis; IAS, interatrial shunt; mb, microbubbles; MES, microembolic signal; NBTE, nonbacterial thrombotic endocarditis; PE, pulmonary embolism; PFO, patent foramen ovale.
Calculated in 7 noncancer and 13 cancer patients with DVT.
Only patients who completed transesophageal echocardiography were included in these analyses (78 patients without cancer and 34 patients with cancer).
Measured in 59 patients without cancer and 24 with cancer.
Among patients with cancer, 4 (30.8%) had chronic DVT based on Doppler sonography findings (vascular wall thickening). Most patients in the cancer group had proximal DVT, whereas patients without cancer had distal DVTs (P=0.002) more frequently. There was no difference in concomitant pulmonary embolism or symptomaticity between the cancer and noncancer groups (P=0.521 and P=0.051; Table 2). In the cancer group, 5 patients were already using anticoagulants at enrollment and 6 had started anticoagulants for the index stroke before data collection (median, −1.5 days) began. There was no difference in demographics or patient characteristics between anticoagulant users versus drug‐naïve patients (Table S1). DVT and MES were more frequently observed in patients taking anticoagulants (P=0.028 and P=0.069, respectively; Table S2), suggesting that premorbid hypercoagulability affected the development of stroke when venous or arterial thromboembolism was not controlled by anticoagulant use.
Impact on Infarct Volume
In patients with cancer, the overall presence of DVT and MES was not associated with increased infarct volume (Table 3). However, when considered separately, DVT was independently associated with an increased infarct volume (adjusted P=0.028, Table 3). Being female was also independently associated with increased infarct volume (adjusted P=0.030; Table 3).
Table 3.
Univariable and Multivariable Regression Analyses of Infarct Volume in Patients With Cancer
| Variables | Univariable | Multivariablea | ||
|---|---|---|---|---|
| Beta (95% CI) | P Value | Beta (95% CI) | P Value | |
| Age | 0.05 (−0.46 to 0.56) | 0.840 | 0.18 (−0.34 to 0.69) | 0.478 |
| Female sex | 8.97 (−1.43 to 19.36) | 0.087 | 11.59 (1.25 to 21.93) | 0.030 |
| DIC | 11.01 (−2.52 to 24.53) | 0.105 | 11.79 (−26.50 to 2.92) | 0.109 |
| Venous or arterial thromboembolism | ||||
| Any | 5.55 (−5.50 to 16.60) | 0.307 | 3.11 (−8.99 to 15.21) | 0.595 |
| DVT | 14.93 (3.58 to 26.28) | 0.013 | 13.14 (1.62 to 24.66) | 0.028 |
| MES | −3.33 (−14.43 to 7.77) | 0.538 | −4.35 (−15.45 to 6.76) | 0.420 |
DIC indicates disseminated intravascular coagulation; DVT, deep venous thrombosis; MES, microembolic signal.
Each multivariable regression model included age, sex, and disseminated intravascular coagulation as covariates.
Impact on Survival
Survival analysis was performed to test the impact of venous and arterial thromboembolism in the cancer group. Presence of DVT or MES was associated with poor 1‐year survival (univariable hazard ratio=3.56, 95% CI=1.45–8.73, P=0.006; adjusted hazard ratio=12.21, 95% CI=1.94–76.76, P=0.008; Table 4). Presence of DVT (hazard ratio=16.79; 95% CI=2.05–137.75; P=0.009) and MES (hazard ratio=8.16; 95% CI=1.36–48.85; P =0.022) were independently associated with poor 1‐year survival. Older age and overt DIC were also associated with poor 1‐year survival (P=0.021 and 0.017, respectively; Table 4).
Table 4.
Univariable and Multivariable Cox Proportional Hazard Model for 1‐Year Mortality in Patients With Cancer
| Variables | Univariable | Multivariablea | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age | 1.02 (0.99 to 1.05) | 0.251 | 1.08 (1.01 to 1.16) | 0.021 |
| Female sex | −1.63 (‐3.70 to −0.72) | 0.239 | −0.78 (−4.63 to −0.13) | 0.786 |
| NIHSS score | 1.01 (0.92 to 1.11) | 0.897 | 0.95 (0.73 to 1.22) | 0.665 |
| Infarct volume, mm3 | 1.0001 (1.00001 to 1.0001) | 0.018 | 1.0003 (0.99996 to 1.0001) | 0.483 |
| Overt DIC | 16.47 (3.57 to 76.03) | <0.001 | 21.75 (1.74 to 271.58) | 0.017 |
| Metastasis | 1.67 (0.57 to 4.90) | 0.348 | 1.13 (0.25 to 5.13) | 0.874 |
| Adenocarcinoma | 0.364 (0.156 to 0.850) | 0.020 | 0.23 (0.03 to 1.63) | 0.140 |
| Venous or arterial thromboembolism | ||||
| Any | 3.56 (1.45 to 8.73) | 0.006 | 12.21 (1.94 to 76.76) | 0.008 |
| DVT | 3.72 (1.55 to 8.96) | 0.004 | 16.79 (2.05 to 137.75) | 0.009 |
| MES | 2.51 (1.13 to 5.56) | 0.023 | 8.16 (1.36 to 48.85) | 0.022 |
DIC indicates disseminated intravascular coagulation; DVT, deep venous thrombosis; HR, hazard ratio; MES, microembolic signal; NIHSS, National Institutes of Health Stroke Scale.
Each multivariate regression model included age, sex, NIHSS score, infarct volume, metastasis, histology (adenocarcinoma), and disseminated intravascular coagulation as covariates.
Discussion
In this study, we found that: (1) DVT or MES were observed in two‐thirds of ESUS patients with cancer; (2) DVT and MES had different impact on infarct volume; and (3) both outcomes had an independent impact on 1‐year survival.
We found direct evidence of arterial or venous thromboembolism and their clinical impact on patients with cancer and ESUS. A previous study reported that 12% of patients with cancer who were hospitalized for stroke had experienced past venous thromboembolism.21 Our data show a higher prevalence of DVT (35.1%) and MES (45.9%) among patients with extensive malignancy (78% metastatic and 40% on chemotherapy) and associated stroke. Although DVT may cause stroke through paradoxical embolism, our data suggest that its high prevalence, together with the MES, reflects a hypercoagulable state in patients with cancer, considering that: (1) not all patients with DVT had a right‐to‐left shunt; (2) there was no difference in the prevalence and severity of right‐to‐left shunts between patients with versus without cancer; and (3) DVT was associated with a greater infarct volume and both DVT and MES were associated with reduced 1‐year survival in patients with cancer. Whereas previous studies indicate that the MES was found mostly in large‐artery disease or cardioembolism,22, 23 our data show an unexpectedly high prevalence of the MES in patients with cancer. Thus, we considered the MES as evidence of an increase in the formation of clots in cancer‐related stroke, presumably occurring in situ in the arterial circulation. Previously, our group demonstrated a higher frequency of increased plasma D‐dimer concentrations in patients with stroke and cancer, especially when conventional stroke mechanisms were lacking, and suggested the diagnostic and prognostic role of increased plasma D‐dimer concentrations in this patient population.4, 5, 11, 24 Although these results have been externally validated by subsequent studies,6, 10 the following question remains: Is an elevated D‐dimer level related to increased clot formation or increased clot lysis, given that D‐dimer is a by‐product of fibrin degradation? In the present study, we demonstrated an increased prevalence of venous and arterial thromboembolism, but a low prevalence of overt DIC, in patients with ESUS and cancer, suggesting that hypercoagulability may be a more‐important mechanism than consumptive coagulopathy in cancer‐related stroke. Our recent report that cancer‐cell–derived extracellular vesicles cause coagulopathy through a tissue factor‐independent way in patients with active cancer and ischemic stroke further supports this hypothesis.9
This study also demonstrated the impact of venous and arterial thromboembolism on infarct volume and 1‐year survival in patients with ESUS and cancer. Based on all currently available classification systems, stroke in patients with cancer is classified as cryptogenic unless a conventional stroke mechanism has been detected.15, 25, 26 Previous studies have shown that cryptogenic stroke in patients with cancer is associated with an atypical presentation and a poor 1‐year survival, and that D‐dimer levels could serve as a prognostic biomarker.5, 7, 27 This, taken together with our present study results, indicates that cancer‐related stroke might be a biologically distinct entity with increased hypercoagulability as a key feature. We suggest that active cancer should be considered an independent etiological mechanism of stroke, warranting a specific treatment strategy to reduce stroke recurrence and death.1, 5, 28 We are currently conducting a prospective study on the optimal anticoagulation therapy in patients with cancer and stroke (ClinicalTrials.gov identifier: NCT02743052).
The impact of arterial and venous thromboembolism on infarct volume differed in our study. VTE was associated with greater infarct volume in magnetic resonance imaging than arterial thromboembolism. Because of slower flow rate, clots would be of greater size in cases with VTE than in cases with arterial thromboembolism. However, clot size may not be the only factor to explain a greater infarct volume, unless patients had a right‐to‐left shunt large enough to allow clot passage. Moreover, 14.3% of patients with ESUS and cancer and 2 patients with DVT lacked the right‐to‐left shunt in this study. Therefore, we suggest the role of DVT and the MES as surrogate markers of increased clot formation attributable to cancer‐related hypercoagulability. Arterial and venous thromboembolism may have different clot formation pathophysiologies, that is, different contributions from tissue factors, circulating microparticles, mucins, and anticancer therapies.29, 30, 31, 32 Further investigation is necessary to uncover the pathophysiology and clinical presentation according to clot types to enable tailored antithrombotic therapy.
Our study has several strengths. First, this is the first comprehensive study to reveal direct evidence of hypercoagulability in ESUS patients with cancer. Second, imaging data and clinical outcomes were prospectively evaluated. Third, we have suggested a distinct mechanism of cancer‐related stroke and discussed the therapeutic implication of our results. However, this study is not without limitations. First, it was an observational, single‐center investigation of patients of a single ethnicity (ie, Koreans). Furthermore, our study population was younger than other ESUS cohorts.33, 34 We believe the younger ages of patients in our cohort reflect that we thoroughly evaluated any potential sources of embolism in all ESUS patients. As a result, older patients were further excluded because they had more hidden atrial fibrillation or aortic arch pathologies. Second, the prevalence of nonbacterial thrombotic endocarditis was lower in this study, compared with previous studies. Third, the impact of different anticancer and antithrombotic treatments on survival was not investigated. Fourth, patients with cancer‐related stroke were not stratified according to their stage and type of cancer because not all cancers share the same staging system and histological classification. Nevertheless, most patients with stroke and cancer had metastasis at the time of stroke diagnosis. Fifth, survival dates were calculated by subtracting the admission date from the death date; therefore, we measured survival date from the first cancer‐related stroke diagnosis rather than survival date from the cancer diagnosis. Thus, the survival date used in the study may not reflect the natural course of each cancer, given that some patients would develop stroke in the very last stage of disease, whereas others may develop it earlier in the disease course. The fourth and fifth limitations may be resolved by taking into consideration the different stage and type of cancer, and the patient's place in the natural time course of the cancer in subsequent studies. Sixth, the MES is best detected within 6 hours from the onset of stroke symptoms, and its sensitivity decreases as time passes. Although our inclusion criteria of ischemic stroke within 7 days after onset was relatively long, our MES monitoring was conducted for a median of 2.5 days (interquartile range, 1.00–4.25) from symptom onset. Seventh, the sample size for this study was calculated for the comparison of DVT between 2 groups. Therefore, the number of study participants might not have been sufficient for the secondary outcomes, such as infarct volume and 1‐year survival, which may cause type 2 errors. Moreover, the CIs for prevalence of DVT and the MES was wide and probably attributable to a small sample size. Finally, we did not measure other biomarkers, such as creatine kinase‐myocardial band, B‐type natriuretic peptide, and carbohydrate antigen 125, in cancer‐related stroke patients.35 These potential biomarkers should be incorporated in future studies.
In conclusion, venous and arterial thromboembolism are common in patients with ESUS and cancer. We suggest a distinct stroke mechanism through increased clot formation in patients with cancer. Given that venous and arterial thromboembolism have a significant impact on 1‐year survival, early recognition of cancer‐related stroke and optimal antithrombotic therapy may be necessary.
Sources of Funding
This study was supported by the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C1521) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP; 2017R1A2B2009086 and 2017R1A2B4007254).
Disclosures
None.
Supporting information
Table S1. Demographic Comparison Between Anticoagulant Users and Drug‐Naïve Patients Among Patients With Cancer
Table S2. Presence of DVT, MES, Right‐to‐Left Shunt, Nonbacterial Thrombotic Endocarditis, Elevated Plasma D‐Dimer Concentration, and Overt DIC in Anticoagulant Users Versus Drug‐Naïve Patients Among Cancer Patients
Acknowledgments
The authors wish to thank all of the patients who participated in this study.
(J Am Heart Assoc. 2019;8:e013215 DOI: 10.1161/JAHA.119.013215.)
References
- 1. Cestari DM, Weine DM, Panageas KS, Segal AZ, De Angelis LM. Stroke in patients with cancer: incidence and etiology. Neurology. 2004;62:2025–2030. [DOI] [PubMed] [Google Scholar]
- 2. Navi BB, Reiner AS, Kamel H, Iadecola C, Elkind MS, Panageas KS, DeAngelis LM. Association between incident cancer and subsequent stroke. Ann Neurol. 2015;77:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, Panageas KS, DeAngelis LM. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim SJ, Park JH, Lee MJ, Park YG, Ahn MJ, Bang OY. Clues to occult cancer in patients with ischemic stroke. PLoS One. 2012;7:e44959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee MJ, Chung JW, Ahn MJ, Kim S, Seok JM, Jang HM, Kim GM, Chung CS, Lee KH, Bang OY. Hypercoagulability and mortality of patients with stroke and active cancer: the Oasis‐cancer study. J Stroke. 2017;19:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nam KW, Kim CK, Kim TJ, An SJ, Oh K, Mo H, Kang MK, Han MK, Demchuk AM, Ko SB, Yoon BW. Predictors of 30‐day mortality and the risk of recurrent systemic thromboembolism in cancer patients suffering acute ischemic stroke. PLoS One. 2017;12:e0172793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Navi BB, Singer S, Merkler AE, Cheng NT, Stone JB, Kamel H, Iadecola C, Elkind MS, DeAngelis LM. Cryptogenic subtype predicts reduced survival among cancer patients with ischemic stroke. Stroke. 2014;45:2292–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yeh ETH, Chang HM. Cancer and clot: between a rock and a hard place. J Am Coll Cardiol. 2017;70:939–941. [DOI] [PubMed] [Google Scholar]
- 9. Bang OY, Chung JW, Lee MJ, Kim SJ, Cho YH, Kim GM, Chung CS, Lee KH, Ahn MJ, Moon GJ. Cancer cell‐derived extracellular vesicles are associated with coagulopathy causing ischemic stroke via tissue factor‐independent way: the Oasis‐cancer study. PLoS One. 2016;11:e0159170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwarzbach CJ, Schaefer A, Ebert A, Held V, Bolognese M, Kablau M, Hennerici MG, Fatar M. Stroke and cancer: the importance of cancer‐associated hypercoagulation as a possible stroke etiology. Stroke. 2012;43:3029–3034. [DOI] [PubMed] [Google Scholar]
- 11. Seok JM, Kim SG, Kim JW, Chung CS, Kim GM, Lee KH, Bang OY. Coagulopathy and embolic signal in cancer patients with ischemic stroke. Ann Neurol. 2010;68:213–219. [DOI] [PubMed] [Google Scholar]
- 12. Kooiker JC, MacLean JM, Sumi SM. Cerebral embolism, marantic endocarditis, and cancer. Arch Neurol. 1976;33:260–264. [DOI] [PubMed] [Google Scholar]
- 13. Merkler AE, Navi BB, Singer S, Cheng NT, Stone JB, Kamel H, Iadecola C, Elkind MS, DeAngelis LM. Diagnostic yield of echocardiography in cancer patients with ischemic stroke. J Neurooncol. 2015;123:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iguchi Y, Kimura K, Kobayashi K, Ueno Y, Inoue T. Ischaemic stroke with malignancy may often be caused by paradoxical embolism. J Neurol Neurosurg Psychiatry. 2006;77:1336–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ay H, Benner T, Arsava EM, Furie KL, Singhal AB, Jensen MB, Ayata C, Towfighi A, Smith EE, Chong JY, Koroshetz WJ, Sorensen AG. A computerized algorithm for etiologic classification of ischemic stroke: the causative classification of stroke system. Stroke. 2007;38:2979–2984. [DOI] [PubMed] [Google Scholar]
- 16. Hwang J, Kim SJ, Hong JM, Bang OY, Chung CS, Lee KH, Kim GM. Microembolic signals in acute posterior circulation cerebral ischemia: sources and consequences. Stroke. 2012;43:747–752. [DOI] [PubMed] [Google Scholar]
- 17. Kim SJ, Shin HY, Ha YS, Kim JW, Kang KW, Na DL, Bang OY. Paradoxical embolism as a cause of silent brain infarctions in healthy subjects: the icons study (identification of the cause of silent cerebral infarction in healthy subjects). Eur J Neurol. 2013;20:353–360. [DOI] [PubMed] [Google Scholar]
- 18. Lee MJ, Park SJ, Yoon CH, Hwang JW, Ryoo S, Kim SJ, Kim GM, Chung CS, Lee KH, Bang OY. Association of left atrial enlargement with cortical infarction in subjects with patent foramen ovale. J Stroke. 2016;18:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toh CH, Hoots WK. The scoring system of the scientific and standardisation committee on disseminated intravascular coagulation of the international society on thrombosis and haemostasis: a 5‐year overview. J Thromb Haemost. 2007;5:604–606. [DOI] [PubMed] [Google Scholar]
- 20. Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24–33. [DOI] [PubMed] [Google Scholar]
- 21. Zhang YY, Chan DK, Cordato D, Shen Q, Sheng AZ. Stroke risk factor, pattern and outcome in patients with cancer. Acta Neurol Scand. 2006;114:378–383. [DOI] [PubMed] [Google Scholar]
- 22. Kaposzta Z, Young E, Bath Philip MW, Markus Hugh S. Clinical application of asymptomatic embolic signal detection in acute stroke. Stroke. 1999;30:1814–1818. [DOI] [PubMed] [Google Scholar]
- 23. Poppert H, Sadikovic S, Sander K, Wolf O, Sander D. Embolic signals in unselected stroke patients: prevalence and diagnostic benefit. Stroke. 2006;37:2039–2043. [DOI] [PubMed] [Google Scholar]
- 24. Kim SG, Hong JM, Kim HY, Lee J, Chung PW, Park KY, Kim GM, Lee KH, Chung CS, Bang OY. Ischemic stroke in cancer patients with and without conventional mechanisms: a multicenter study in Korea. Stroke. 2010;41:798–801. [DOI] [PubMed] [Google Scholar]
- 25. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 26. Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence‐based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58:688–697. [DOI] [PubMed] [Google Scholar]
- 27. Seok JM, Kim SJ, Song P, Chung CS, Kim GM, Lee KH, Bang OY. Clinical presentation and ischemic zone on MRI in cancer patients with acute ischemic stroke. Eur Neurol. 2012;68:368–376. [DOI] [PubMed] [Google Scholar]
- 28. Navi BB, Singer S, Merkler AE, Cheng NT, Stone JB, Kamel H, Iadecola C, Elkind MS, DeAngelis LM. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology. 2014;83:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wahrenbrock M, Borsig L, Le D, Varki N, Varki A. Selectin‐mucin interactions as a probable molecular explanation for the association of trousseau syndrome with mucinous adenocarcinomas. J Clin Invest. 2003;112:853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muhsin‐Sharafaldine MR, Saunderson SC, Dunn AC, Faed JM, Kleffmann T, McLellan AD. Procoagulant and immunogenic properties of melanoma exosomes, microvesicles and apoptotic vesicles. Oncotarget. 2016;7:56279–56294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rak J, Milsom C, May L, Klement P, Yu J. Tissue factor in cancer and angiogenesis: the molecular link between genetic tumor progression, tumor neovascularization, and cancer coagulopathy. Semin Thromb Hemost. 2006;32:54–70. [DOI] [PubMed] [Google Scholar]
- 32. Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375:1457–1467. [DOI] [PubMed] [Google Scholar]
- 33. Perera KS, Vanassche T, Bosch J, Giruparajah M, Swaminathan B, Mattina KR, Berkowitz SD, Arauz A, O'Donnell MJ, Ameriso SF, Hankey GJ, Yoon BW, Lavallee P, Cunha L, Shamalov N, Brouns R, Gagliardi RJ, Kasner SE, Pieroni A, Vermehren P, Kitagawa K, Wang Y, Muir K, Coutinho J, Vastagh I, Connolly SJ, Hart RG. Embolic strokes of undetermined source: prevalence and patient features in the ESUS Global Registry. Int J Stroke. 2016;11:526–533. [DOI] [PubMed] [Google Scholar]
- 34. Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic stroke of undetermined source: a systematic review and clinical update. Stroke. 2017;48:867–872. [DOI] [PubMed] [Google Scholar]
- 35. Santamarina E, Penalba A, Garcia‐Berrocoso T, Delgado P, Quintana M, Gonzalez‐Alujas T, Ribo M, Maisterra O, Molina CA, Evangelista A, Alvarez‐Sabin J, Montaner J. Biomarker level improves the diagnosis of embolic source in ischemic stroke of unknown origin. J Neurol. 2012;259:2538–2545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographic Comparison Between Anticoagulant Users and Drug‐Naïve Patients Among Patients With Cancer
Table S2. Presence of DVT, MES, Right‐to‐Left Shunt, Nonbacterial Thrombotic Endocarditis, Elevated Plasma D‐Dimer Concentration, and Overt DIC in Anticoagulant Users Versus Drug‐Naïve Patients Among Cancer Patients


