Abstract
Background
Low‐grade inflammation, largely mediated by monocyte‐derived macrophages, contributes to atherosclerosis. Sedentary behavior is associated with atherosclerosis and cardiovascular diseases (CVD). We examined whether reducing sedentary behavior and improving walking time improves monocyte inflammatory phenotype in subjects with increased cardiovascular risk.
Methods and Results
Across 2 waves, 16 individuals with increased cardiovascular risk performed a 16‐week intervention study (age 64±6 years, body mass index 29.9±4.3 kg/m2), using a device with vibration feedback to promote physical activity. Before and after intervention, we objectively examined physical activity (ActivPAL), cytokine production capacity after ex vivo stimulation in peripheral blood mononuclear cells, metabolism of peripheral blood mononuclear cells, circulating cytokine concentrations, and monocyte immunophenotype. Overall, no significant increase in walking time was found (1.9±0.7 to 2.2±1.2 h/day, P=0.07). However, strong, inverse correlations were observed between the change in walking time and the change in production of interleukin (IL)‐1β, IL‐6, IL‐8, and IL‐10 after lipopolysaccharide stimulation (r s=−0.655, −0.844, −0.672, and −0.781, respectively, all P<0.05). After intervention optimization based on feedback from wave 1, participants in wave 2 (n=8) showed an increase in walking time (2.2±0.8 to 3.0±1.3 h/day, P=0.001) and attenuated cytokine production of IL‐6, IL‐8, and IL‐10 (all P<0.05). Glycolysis (P=0.08) and maximal OXPHOS (P=0.04) of peripheral blood mononuclear cells decreased after intervention. Lower IL‐6 concentrations (P=0.06) and monocyte percentages (P<0.05), but no changes in monocyte subsets were found.
Conclusions
Successfully improving walking time shifts innate immune function towards a less proinflammatory state, characterized by a lower capacity to produce inflammatory cytokines, in individuals with increased cardiovascular risk.
Clinical Trial Registration Information
URL: http://www.trialregister.nl. Unique identifier: NTR6387.
Keywords: atherosclerosis, cardiovascular disease, innate immunity, physical activity, sedentary behavior
Subject Categories: Inflammation, Cellular Reprogramming, Cardiovascular Disease, Exercise
Clinical Perspective
What Is New?
We showed that effectively reducing sedentary behavior and improving walking time in individuals with increased cardiometabolic risk attenuates the cytokine production capacity of circulating mononuclear cells with a strong inverse correlation between the change in walking time and the change in production of interleukin‐1β, interleukin‐6, interleukin‐8, and interleukin‐10 by mononuclear cells after ex vivo stimulation.
What Are the Clinical Implications?
Our results identify a novel mechanism by which increased physical activity levels and reduced sitting contributes to improved cardiovascular outcomes.
By attenuating cytokine production capacity of circulating innate immune cells, interventions that improve physical activity and reduce sitting could be of benefit in patients with chronic inflammatory diseases.
Atherosclerotic cardiovascular diseases (CVD), including myocardial infarction and stroke, are the major cause of death worldwide.1 Although regular exercise training is a potent strategy to reduce risk for future CVD,2 only 50% to 60% of the Western population meet these guidelines3 with even lower prevalence rates in those at risk for CVD.4 In the past decade, an increasing number of studies explored the role of sedentary behavior (ie, any waking behavior in a sitting, reclining or lying posture with energy expenditure <1.5 metabolic equivalents) in the context of CVD. Interestingly, high levels of sedentary behavior, independent of exercise performance, are associated with an increased risk for all‐cause mortality and CVD.5, 6, 7 Cross‐sectional studies have linked sedentary behavior to higher blood pressure,8 body mass index (BMI),9 waist circumference,9, 10 glucose intolerance,10 and lower high‐density lipoprotein cholesterol.9 Despite these cross‐sectional observations, little work explored potential underlying mechanisms of the detrimental impact of sedentary behavior on development of future CVD.
Atherosclerosis is a low‐grade inflammatory disorder of the arterial wall, in which monocyte‐derived macrophages orchestrate the development of atherosclerotic plaques.11 We and others have recently described that circulating monocytes of individuals with atherosclerosis have a long‐lasting proinflammatory phenotype. This is characterized by an enhanced cytokine production capacity and a switch to an increased glycolytic metabolism,12, 13 suggesting that functional and metabolic alterations in circulating monocytes contribute to atherogenesis. Individuals with increased risk for CVD attributable to dyslipoproteinemia are also characterized by a proinflammatory monocyte phenotype.14 Importantly, a previous study found that a Western type diet in atherosclerosis‐prone mice is capable of inducing a persistent proinflammatory phenotype of circulating monocytes and their bone marrow progenitors.15 Furthermore, pharmacological therapy inhibiting interleukin‐1β (IL‐1β) in the innate immunity pathway successfully reduced the risk of future cardiovascular events in patients with CVD.16 This suggests that interventions, including pharmacological and environmental strategies that impact on innate immune function, may alter the process of atherosclerosis.
Sedentary behavior is associated with markers of chronic low‐grade inflammation (eg, high concentrations of C‐reactive protein, IL‐6, neopterin, leptin, adiponectin, and tumor necrosis factor‐alpha [TNFα]).17, 18 However, no previous study directly examined whether a change in sedentary behavior (or physical activity) results in modulation of the innate immune function. Therefore, we examined the impact of a 16‐week intervention to reduce sedentary time and increase physical activity levels on the innate immune function in subjects with increased risk for CVD. For this purpose, we explored the functional and metabolic phenotype of innate immune cells in terms of ex vivo cytokine production capacity and intracellular metabolism.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Subjects
Individuals with an increased cardiovascular risk were recruited by newspaper and internet advertisement in Nijmegen, The Netherlands. Individuals aged >55 years with at least 40 hours per week of self‐reported sedentary behavior were eligible for participation. Criteria for inclusion were the presence of ≥1 cardiovascular risk factors, consisting of high blood pressure (systolic blood pressure >160 mm Hg, diastolic blood pressure >90 mm Hg), anti‐hypertensive medication use, or BMI >28 kg/m2. Exclusion criteria were presence of diabetes mellitus, autoimmune diseases or autoinflammatory diseases, and daily immunomodulatory drug use. Moreover, individuals were excluded if they were not able to perform light‐intensity physical activity (eg, standing and walking) or when unable or not allowed to provide informed consent. The study protocol was approved by the Institutional Review Board Arnhem/Nijmegen, The Netherlands and registered at the Netherlands Trial Register (NTR6387). All individuals gave written informed consent.
Study Design
Subjects reported to our laboratory on 4 measurement days; 2 test days before and 2 test days after the 16‐week intervention (Figure 1). At each measurement day, participants were instructed to refrain from caffeine and alcohol intake at least 12 hours before the test. Moreover, participants were instructed to refrain from vigorous exercise 24 hours before the measurements. At the first visit, participant characteristics were assessed and blood was drawn for immunological assessment. In the subsequent week, baseline sedentary behavior characteristics of the subjects were measured using an activity monitor combined with an inclinometer (ActivPAL, PAL technologies Ltd, Glasgow, UK). At a second visit, participants handed in the activity monitor, received written and oral information about the reduced sitting intervention, and participants started the 16‐week intervention. Physical activity patterns were assessed during the last week of the intervention and thereafter all other measurements were repeated.
Figure 1.
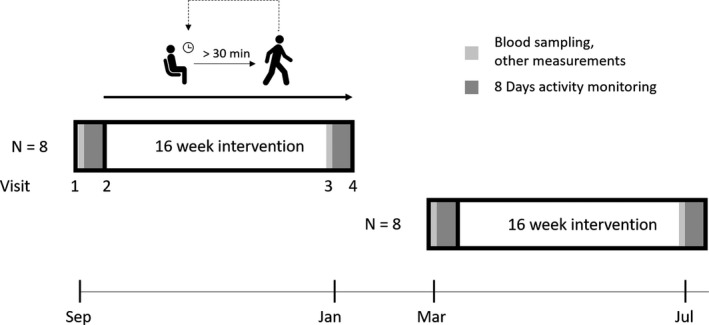
Study design. Measurements were performed across 2 waves (n=8 per wave). Participants visited our laboratory 4 times; 2 test days before and 2 test days after the 16‐week intervention.
Intervention
The 16‐week mHealth reduced sitting intervention involved prevention of prolonged sitting behavior (>30 minutes) throughout the day and promotion of low‐intensity physical activity (eg, walking, standing). Subjects received a pocket pedometer Activ8sit (2M Engineering, Valkenswaard, The Netherlands) which recorded physical activity patterns using an inclinometer and tri‐axial accelerometer. This combination of information allows for recognizing prolonged periods of sedentary behavior and physical activity. A vibrating signal was provided after 30 minutes of sedentary behavior and served as a reminder to break up sitting by low‐ to moderate‐intensity physical activity for at least 2 minutes. Next to direct feedback, participants were able to review their physical activity patterns in a web‐based environment. Furthermore, subjects had a phone meeting with their personal coach periodically to evaluate their participation in the reduced sitting intervention. The intervention study was performed in 2 groups. The intervention for wave 1 was performed in September 2017 to January 2018 (ie, group1.0, n=8) and wave 2 from March to July 2018 (ie, group2.0, n=8). Based on feedback from the participants in group1.0, coaching and support was intensified for subjects in group2.0 to optimize the intervention and to further reduce sedentary behavior.
Measurements
Cardiovascular risk assessment
Medical history, smoking status, medication use, and BMI were assessed in all participants. Capillary blood was used to measure fasting glucose concentrations. Blood pressure was measured twice by a manual sphygmomanometer in sitting position after 5 minutes rest according to American Heart Association guidelines.19
Sedentary behavior
A validated activity monitor (ActivPAL3 micro, PAL technologies, Glasgow, United Kingdom) was used to measure number of steps, sedentary time, standing and walking time.20 The ActivPAL was attached to the ventral side of the right thigh. The monitor was coated in a waterproof sleeve to enable assessment for 8 days continuously. The first day was excluded for data analyses, leaving 7 days of sedentary behavior measurements. ActivPAL data were processed using an analysis script in Matlab R2014b (The Mathworks, Inc, Natick, Massachusetts). This allowed distinguishing between wake times and bed times per day for each subject and calculated sedentary time, sedentary breaks, number of prolonged sedentary bouts (>30 minutes), standing, walking time, and number of steps per day.21
Blood sampling
Directly before and after intervention, non‐fasting blood was collected in EDTA vacutainers. Sample collection was performed at 8.00–9.00 am to avoid interference of circadian rhythms of immune parameters, and sample processing occurred within 2 hours. Plasma and serum were stored at −80°C until further use. Total cholesterol, high‐density lipoprotein cholesterol, and triglycerides were measured in fasting Lithium Heparin plasma using standardized methods, and low‐density lipoprotein cholesterol was calculated with the Friedewald formula. Total blood cell counts were determined with an automated Sysmex‐XN 450 hematology analyzer (Sysmex, Hamburg, Germany).
PBMC isolation and stimulation
Human peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll‐Paque density gradient centrifugation (GE Healthcare, Chicago, IL, USA). Cell composition was evaluated by Sysmex analyzer (Sysmex). PBMCs were concentrated in Roswell Park Memorial Institute 1640 Dutch‐modified culture medium (Life Technologies/Invitrogen, Waltham, MA, USA) supplemented with 2 mmol/L glutamine (Invitrogen), 10 mg/mL gentamicin (Centrafarm, Etten‐Leur, The Netherlands) and 1 mmol/L pyruvate (Invitrogen). To evaluate cytokine production capacity, 5×105 PBMCs per well were stimulated in triplicate for 24 hours in round‐bottom 96‐well plates (Corning, New York, NY, USA) with the following stimuli: Roswell Park Memorial Institute, 10 ng/mL lipopolysaccharide (LPS) from Escherichia coli serotype 055:B522 (Sigma‐Aldrich, St. Louis, MO, USA), 10 μg/mL Pam3CysK4 (P3C) (L2000, EMC microcollections, Tübingen, Germany), 10 μg/mL Resiquimod (R848) (Invivogen, San Diego, CA, USA; Catalog#tlrl‐r848‐5), 50 μMol C16.0 conjugated with Albumin (Sigma‐Aldrich resp. Sanquin, Amsterdam, The Netherlands), and 50 μMol C16.0‐Albumin in combination with 300 μg/mL sonicated monosodium urate crystals (in house). The preparation of C16.0‐Albumin and monosodium urate crystals is previously described.23 Simultaneously, to assess the adaptive immune response, PBMCs were stimulated in triplicate for 7 days in Roswell Park Memorial Institute, 1×106/mL Candida albicans conidia (UC820 strain), and 1×106/mL Staphylococcus aureus (ATCC 29213 strain) both with 10% human pool serum. After the incubation periods of 24 hours and 7 days, supernatants were stored after plate centrifugation at −80°C until cytokine assessment.
Cytokine measurements
In stored supernatants cytokine concentrations were measured using ELISA (Table S1). Circulating cytokine concentrations were determined using Ella cartridges for IL‐1RA, IL‐1β, IL‐6, and IL‐18 on the Ella automated immunoassay platform (Simpleplex, San Jose, CA, USA). Thiobarbituric Acid Reactive Substances were measured in plasma as measure for oxidative stress (OXitek TBARs Assay kit, ZeptoMatrix, New York, NY, USA).
Flow cytometry
Monocyte subpopulations, platelet complexes and expression markers were identified with flow cytometry. Using the lysis‐no‐wash strategy (BD Pharm Lyse lysing buffer, Becton Dickinson, Franklin Lakes, NJ, USA), 50 μL EDTA blood was stained by monoclonal antibodies (CD45 Chrome Orange clone J33 Beckman Coulter, HLA‐DR PE clone immu‐357 Beckman Coulter, CD14 PC7 clone 61D3 Bioscience, CD16 FITC clone CB16 eBioscience, CD3 APC‐750 clone UCTH1 Beckman Coulter, CD56 APC clone N901 Beckman Coulter, CD192 BV421 clone 48607 Becton&Dickinson, CD11b BV785 clone ICRF44 Biolegend, CD41 PC5.5 clone Hip8 Biolegend) and measured with CytoFLEX flow cytometer (Beckman Coulter, Brea, CA, USA). The gating strategy applied is shown in Figure S1, gates were set with the fluorescence minus one method.24, 25 Data were analyzed with Kaluza 3.1 software (Beckman Coulter). Characterization of monocytes subsets is according to current recommendations.24, 25
Metabolic measurements
Lactate levels were measured in unstimulated PBMCs after 24 hours. In a subgroup of subjects, oxygen consumption rate was measured in freshly isolated 5×106 PBMCs collected in Roswell Park Memorial Institute. Oxygen consumption was measured at 37°C using polarographic oxygen sensors in a 2‐chamber Oxygraph (OROBOROS Instruments, Innsbruck, Austria). First, basal oxygen consumption was measured over a period of ≥10 minutes. Then, leak respiration was measured by adding oligomycin A (2.5 μmol/L), a specific inhibitor of mitochondrial complex V. Next, the mitochondrial uncoupler p‐trifluoromethoxy carbonylcyanide phenylhydrazone (FCCP) was added at increasing concentrations (ranging from 0.2 to 1.0 μmol/L final concentration) to determine maximal electron transport chain capacity. Finally, non‐mitochondrial oxygen consumption was established by adding the mitochondrial complex I inhibitor rotenone (0.5 μmol/L) and the mitochondrial complex III inhibitor antimycin A (2.5 μmol/L).
Statistical Analysis
Data are presented as mean±SD for continuous variables, as number (percentage) for categorical variables and as median [interquartile range] for skewed distributed data. All data were analyzed using Wilcoxon signed‐rank tests, unless stated otherwise. A 2‐sided P‐value <0.05 was considered statistically significant. Data were analyzed using Prism version 6.0 (GraphPad software, La Jolla, CA, USA) and SPSS version 21.0 (SPSS Inc, Chicago, IL, USA).
Results
Subject Characteristics
Sixteen participants (age 64±6 years, BMI 29.9±4.3 kg/m2) participated in the study. After 16 weeks, the increase in walking time (1.9±0.7 to 2.2±1.2 h/day, P=0.07) and step count (8864±4138 to 10 656±6821 #/day, P=0.06) did not reach statistical significance. The intervention was performed in 2 waves. Based on feedback from participants in group1.0, coaching and support was intensified for subjects in group2.0 to optimize the intervention. No differences were found between both groups at baseline (Table 1). Importantly, group2.0 (n=8) significantly increased walking time (2.2±0.8 to 3.0±1.3 h/day, P<0.01) and step count (10 659±4930 to 14 909±7321 #/day, P<0.01). No changes were seen in estimated physical fitness. Total cholesterol levels (P=0.05) and systolic blood pressure (P<0.01) decreased in group2.0, whilst group1.0 (n=8) showed a decrease in glucose (P<0.01), an increase in BMI (P=0.01) and in diastolic blood pressure (P=0.01) (Table 1). In addition to analysis of the entire group, sub‐analyses on group2.0 (n=8) were performed to specifically examine the impact of reducing sedentary behavior on innate immune function. All data for group1.0 are reported in Tables S2 through S4.
Table 1.
Participants’ Characteristics
| Baseline Characteristics | Total (n=16) | Group1.0 (n=8) | Group2.0 (n=8) |
|---|---|---|---|
| Sex, % male (n) | 31 (5) | 25 (2) | 38 (3) |
| Age, y | 64±6 | 66±5 | 62±6 |
| Current smoking, % (n) | 13 (2) | 0 (0) | 25 (2) |
| Hypertension, % (n) | 69 (11) | 50 (4) | 88 (7) |
| Lipid lowering therapy, % (n) | 25 (4) | 25 (2) | 25 (2) |
| B‐antagonist use, % (n) | 31 (5) | 38 (3) | 25 (2) |
| Intervention Outcomes | Pre | Post | Pre | Post | Pre | Post |
|---|---|---|---|---|---|---|
| SBP, mmHg | 132±10 | 131±9 | 128±11 | 134±8 | 136±7 | 128±10b |
| DBP, mmHg | 82±7 | 84±7a | 80±7 | 84±6a | 83±7 | 83±8 |
| BMI, kg/m2 | 29.9±4.3 | 30.0±4.2 | 30.2±2.6 | 30.6±2.6a | 29.5±5.7 | 29.4±5.4 |
| Glucose, mmol/L | 6.17±0.77 | 5.78±0.74a | 6.43±0.67 | 5.96±0.72b | 5.91±0.82 | 5.61±0.76 |
| Tchol, mmol/L | 5.11±0.74 | 4.88±0.61 | 5.04±0.76 | 4.94±0.48 | 5.18±0.78 | 4.83±0.75a |
| HDLc, mmol/L | 1.43±0.35 | 1.39±0.32 | 1.37±0.40 | 1.33±0.33 | 1.49±0.31 | 1.45±0.33 |
| LDLc, mmol/L | 2.85±0.72 | 2.77±0.60 | 2.74±0.74 | 2.80±0.64 | 2.96±0.72 | 2.74±0.60 |
| Triglycerides, mmol/L | 1.84±1.11 | 1.63±0.93 | 2.06±1.14 | 1.82±1.00 | 1.62±1.12 | 1.43±0.87 |
| Non‐HDLc, mmol/L | 3.67±0.69 | 3.51±0.52 | 3.66±0.82 | 3.63±0.43 | 3.68±0.60 | 3.40±0.61 |
| Sedentary time, h/d | 10.1±1.3 | 9.8±1.5 | 10.4±1.7 | 10.3±1.5 | 9.9±0.6 | 9.3±1.4 |
| Sitting time >30 min, #/d | 6±2 | 6±2 | 7±2 | 7±1 | 5±1 | 5±1 |
| Walking time, h/d | 1.9±0.7 | 2.2±1.2 | 1.5±0.5 | 1.4±0.4 | 2.2±0.8 | 3.0±1.3b |
| Total step count, #/d | 8864±4138 | 10 656±6821 | 7069±2243 | 6402±2181 | 10 659±4930 | 14 909±7321b |
| Estimated physical fitness, mL·O2/mL/kg | 27.7±7.4 | 27.7±8.8 | 25.3±5.4 | 22.6±4.9 | 30.0±8.6 | 32.8±9.1 |
| Central vascular stiffnessc | 7.8±1.7 | 7.6±2.9 | n.a. | n.a. | 7.8±1.7 | 8.1 ±2.8 |
| Peripheral vascular stiffness‡ | 9.1±1.0 | 9.0±2.4 | n.a. | n.a. | 9.1±1.0 | 9.8 ±1.3 |
Paired samples t‐test, mean, SD, or X2 test for categorical data, mean (n). First and second group were matched (age, sex, BMI, smoking). BMI indicates body mass index; DBP, diastolic blood pressure; HDL, high‐density lipoprotein cholesterol; LDL, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; Tchol, total cholesterol.
P<0.05 between pre and post intervention.
P<0.01.
Data are missing for 8 participants.
Circulating Inflammatory Markers
A trend for a decrease in circulating IL‐6 concentration was observed in group 2.0 (n=8) (3.21 to 2.62 pg/mL, P=0.08) (Figure 2). The concentrations of inflammatory markers C‐reactive protein, IL‐1β, IL‐1RA, and IL‐18 did not change significantly after intervention. In addition, levels of adiponectin and leptin, both adipokines; Thiobarbituric Acid Reactive Substances, a marker of oxidative stress; E‐selectin, and vascular cell adhesion protein 1 (VCAM‐1), markers of endothelial dysfunction; and matrix metalloproteinase‐2, were not affected by the intervention (Table S5).
Figure 2.
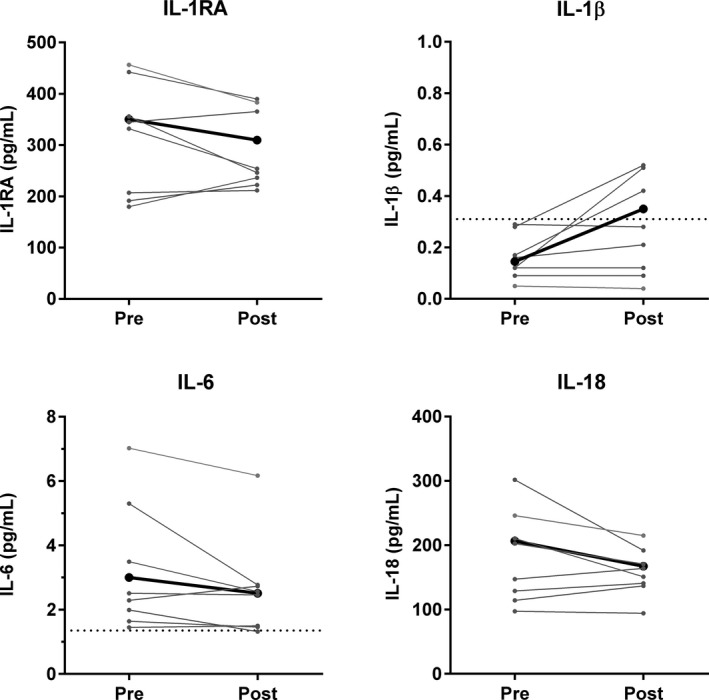
Circulating cytokines. Individual changes in circulating cytokines before (pre) and after (post) intervention (n=8). Mean is represented in black. Lowest detection limit for IL‐1β is 0.31 pg/mL, and for IL‐6 1.35 pg/mL, indicated by the dotted line.
Cytokine Production Capacity of PBMCs
After the intervention a significant reduction in the innate cytokine production capacity of PBMCs was observed in group2.0 (n=8) (Figure 3A). We found an attenuated production of IL‐6, IL‐8, and IL‐10 in PBMCs stimulated for 24 hours with LPS, P3C, and R848 (TLR4, TLR2 and TLR7/8 ligands, respectively) after the 16‐week intervention (all P<0.05). For C16.0 stimulation (ie, a saturated fatty acid) an attenuated production in IL‐1β, IL‐6, and IL‐8 (all P<0.05) was seen after intervention. After C16.0‐MSU, which induces inflammasome activation, IL‐6 and IL‐8 production was significantly attenuated after intervention (all P<0.05). Correction for monocyte count in the PBMC fraction did not alter the results for cytokine production after LPS and R848 stimulation, and IL‐1β production after C16.0 stimulation (see Table S6 for cell percentages in the PBMC fraction). To study the adaptive immune function, 7‐day stimulation of PBMCs with C. albicans and S. aureus was performed. After intervention, a significant increase in the cytokine response of interferon‐gamma (IFNγ) was observed after C. albicans stimulation (P=0.05), and of IFNγ, IL‐22, and IL‐17 after S. aureus stimulation (P<0.05, P=0.08, and P<0.05, respectively) (Figure 3B).
Figure 3.
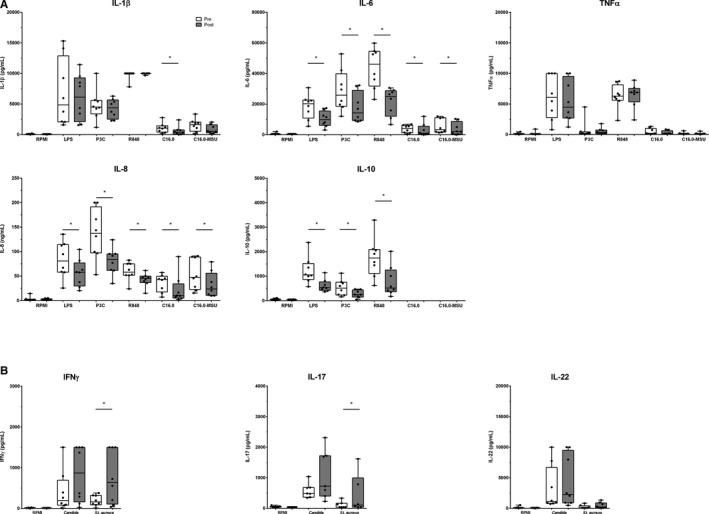
A and B, Cytokine production capacity of PBMCs. A, Innate cytokine production after 24‐hour stimulation (n=8). B, Adaptive cytokine production after 7‐day stimulation (n=8). Data before (white) and after intervention (grey) are shown in boxplots (median±interquartile range). Whiskers represent 95% CI. IL indicates interleukin; LPS, lipopolysaccharide; P3C, Pam3CysK4; R848, Resiquimod; C16.0+MSU, C16.0 with monosodium urate crystals, IFNγ; interferon‐gamma. *P<0.05.
We examined the correlation between the change in walking time and the change in cytokine production capacity during the intervention on the data of both groups (n=16) as all individuals possess data on a change in walking time and consequent changes in cytokine production capacity (Figure 4). This analysis shows a strong, inverse correlation between IL‐1β, IL‐6, IL‐8, and IL‐10 production after stimulation with LPS and R848 versus walking time (all r s> −0.51 and P<0.001, except IL‐8 after LPS P=0.01).
Figure 4.
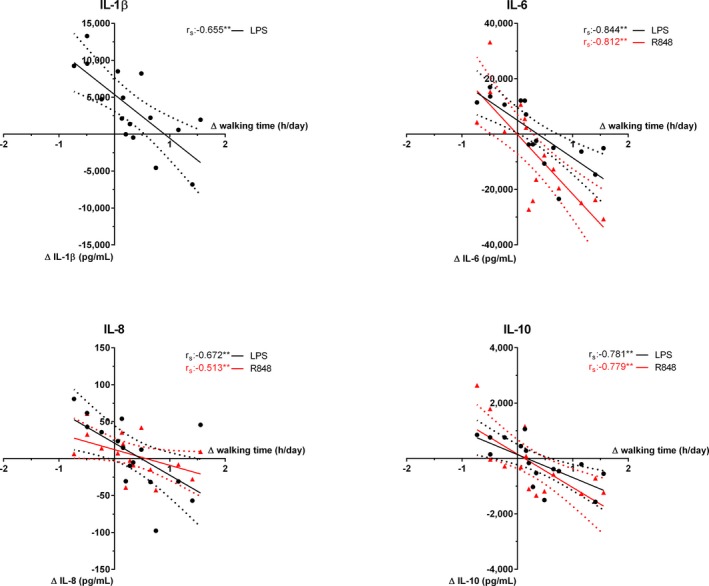
Cytokine production capacity correlated with walking time per day (n=16). The change in cytokine concentration after LPS (black) and R848 (red) stimulation correlated with the change in walking time. Linear regression with 90% CI. IL indicates interleukin; LPS, lipopolysaccharide; R848, Resiquimod. *P<0.05, **P<0.01, r s: Spearman correlation coefficient.
Immunophenotyping of Monocytes
A reduction in the percentage of circulating monocytes (P<0.05) was observed together with an increase in the lymphocyte‐to‐monocyte ratio (P=0.01) after intervention in group2.0 (n=8) (Table 2). The distribution of the monocyte subpopulations, ie, the classical, intermediate, and non‐classical monocytes, did not change during the intervention. Also, CCR2 and CD11b expression in monocytes, and monocyte‐platelet complexes were not altered (Table 2).
Table 2.
Circulating cell counts and immunophenotyping of monocytes (n=8)
| Cell Counts and immunophenotype monocytes | Pre | Post |
|---|---|---|
| WBC, 106/mL | 5.3 [4.4–6.1] | 5.0 [4.5–6.2] |
| Neutrophils, 106/mL | 2.9 [2.6–3.3] | 2.8 [2.4–3.2] |
| Lymphocytes, 106/mL | 1.7 [1.3–2.2] | 1.7 [1.6–2.5] |
| Monocytes, 106/mL | 0.50 [0.37–0.60] | 0.39 [0.27–0.54] |
| Monocytes, % | 9.0 [7.3–10.3] | 7.9 [6.1–8.7]a |
| Lymphocyte monocyte ratio | 3.47 [2.95–4.51] | 4.59 [3.90–6.13]a |
| Neutrophil lymphocyte ratio | 1.54 [1.41–2.46] | 1.61 [1.21–1.83] |
| Monocyte subsets, % total | 7.5 [6.0–7.9] | 5.7 [3.8–6.6] |
| Classical monocytes, % gated | 78.5 [76–81] | 79.0 [75–84] |
| Intermediate monocytes, % gated | 7.3 [4.4–8.1] | 7.0 [4.1–8.0] |
| Non‐classical monocytes, % gated | 14.1 [13.0–16.1] | 12.7 [10.4–18.4] |
| CD41+ monocytes, % gated | 8.0 [7.0–8.7] | 8.5 [8.0–9.2] |
| CCR2+ monocytes, MFI | 5255 [4607–5932] | 4385 [3894–6873] |
| CD11b+ monocytes, MFI | 8872 [6721–10 893] | 10 743 [7924–10 978] |
Median, interquartile range. WBC indicates white blood cell counts; MFI, mean fluorescence intensity.
P<0.05.
Metabolism of PBMCs
A trend for a decrease in lactate production was observed (P=0.08) after 24 hours in unstimulated PBMCs (Figure 5A). Oxygen consumption in freshly isolated PBMCs tended to decrease after intervention for both basal (P=0.06; n=6) and maximal (P<0.05; n=4) respiration (Figure 5B–C). However, no changes were observed for relative and absolute reserve capacity (P>0.20) (Figure 5D–E).
Figure 5.
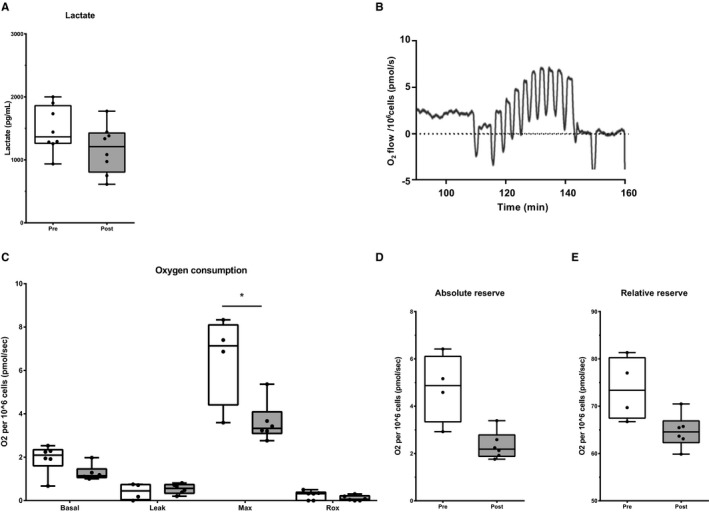
A through E, Metabolism of PBMCs. A, Extracellular lactate production in unstimulated PBMCs (n=8). B, Representative result of the OROBOROS. C, Oxygen consumption is presented as basal respiration (basal) (n=6), proton leak after inhibition of ATP synthase (leak) (n=4), maximal oxygen consumption (max) (n=4), residual oxygen consumption or non‐mitochondrial oxygen consumption (rox) (n=6). D and E, Absolute and relative oxygen consumption reserve (n=4). Data before (white) and after intervention (grey) are shown in boxplots (median±interquartile range). Whiskers represent 90% CI. *P<0.05.
Discussion
Our main finding is that a 16‐week intervention to reduce sedentary behavior and improve walking time alters the innate immune function towards a less proinflammatory state in individuals with increased cardiovascular risk. In support of this observation, we found a strong, inverse correlation between the increase in walking time and attenuated cytokine production capacity that followed a dose‐response like pattern. In other words, individuals who had the highest increase in walking time, demonstrated the strongest reduction in cytokine production capacity of circulating PBMCs. The reduction in inflammatory state after the 16‐week intervention coincided with a trend to decreased glycolysis and oxidative phosphorylation rate. Together, our study provides a mechanistic explanation of the cardiovascular benefits of long‐term reductions in sedentary behavior.
Given the current lack of validated strategies to reduce sedentary behavior, we have co‐developed a physical activity‐monitor with the capacity to provide tactile feedback for the purpose of a “reduce sedentary behavior”‐intervention. During this process, data from group1.0 showed no significant improvement in physical activity patterns. Based on feedback from these participants, we adjusted the intervention, largely through an increased frequency and more detailed feedback to the participants (including weekly online or oral feedback). As a result, successful improvement of physical activity after the 16‐week intervention in participants from group2.0 was achieved, as supported by the increase in walking time of 45 minutes/day. The difficulty of changing daily sedentary behavior is illustrated by previous 3 to 4 month intervention studies, also using mHealth‐devices, that failed to markedly improve walking time26 or sedentary time.27 This emphasizes the importance for (co‐)developing interventions, using participants’ feedback, to successfully alter sedentary behavior patterns. Another important observation in our study was that the reduction in sedentary behavior was achieved through elevation in walking time rather than via increased engagement in exercise training and/or physical fitness. Previous work linked regular exercise training and/or higher fitness to mechanisms of cardiovascular protection and prevention of atherosclerosis.28 Therefore, the absence of changes in physical fitness and/or exercise training, allows us to relate the improved innate immune response to the reduction in sedentary behavior.
The key finding of our study is that reducing sedentary behavior across a 16‐week intervention is associated with a significant reduction in cytokine production capacity of PBMCs (ie, attenuated innate immune response). In previous cross‐sectional studies, sedentary behavior or physical inactivity was found to be associated with several circulating markers of systemic inflammation (C‐reactive protein, IL‐6, TNFα, neopterin),17, 18 but the effect on innate immune cell function was unexplored. Our observations confirm these previous findings in that we found a trend for reduction in circulating IL‐6 concentrations within individuals after the 16‐week intervention.
Next, one could hypothesize about the potential underlying mechanisms driving the attenuated innate immune state. Previous work on the acute impact of sedentary behavior found marked reduction in vascular function, which was linked to a decrease in arterial blood flow and presence of oxidative stress.29 More specifically, vitamin C supplementation prevented the impact of 3‐hour sitting on vascular function. However, the investigators found no changes in the circulating oxidative stress markers.30 In our study we examined a circulating marker of oxidative stress, but neither did we observe significant changes after intervention. Additional markers of antioxidants and oxidative damage are needed to fully exclude a role of oxidative stress. In addition, it is important to note that we cannot exclude the potential presence of local changes of oxidative stress in tissues (eg, active areas).
Another explanation for our observations, might relate to the metabolic effects of sedentary behavior versus low‐intensity physical activity. A previous study found that an acute sedentary bout impaired glucose metabolism.31 Interestingly, we found a trend for decreased glycolysis and oxidative phosphorylation rates in PBMCs after our intervention. Recent studies have shown that the hyper‐responsiveness of circulating monocytes in terms of cytokine production in patients with atherosclerosis coincides with an increased glycolytic metabolism.12, 13 Therefore, metabolic changes during our intervention might contribute to the attenuated inflammatory innate immune function. Moreover, the leptin/adiponectin pathway seems related to the level of physical (in)activity in cross‐sectional observations.17, 32, 33 In contrast to these studies, we found no change in the ratio after the intervention. Together, the decrease in cellular metabolism is one mechanism driving the attenuated immune response after intervention. Still future studies are warranted to better understand our novel observation of the capacity of low‐intensity physical activity to attenuate innate immune responses of PBMCs in humans.
Interestingly, in conjunction with an attenuated innate immune response of PBMCs after the intervention, we observed an enhanced production by the adaptive immune cells, as IFNγ and IL‐17 production after S. aureus stimulation significantly increased after the 16‐week intervention. A similar counter‐regulatory mechanism has been reported previously. A decreased production capacity of IFNγ was found in patients with cerebral small vessel disease in conjunction with an increased innate immune cytokine production.34 This increase in the adaptive immune response may represent a counter‐regulatory mechanism to compensate for the attenuate innate immune response.
Our results are in line with recent studies that have demonstrated that environmental factors can persistently modulate the inflammatory function of circulating monocytes and, consequently, contribute to changes in risk for future CVD.35 For example, sleep fragmentation leads to an increase in proinflammatory monocytes and subsequently to larger atherosclerotic lesions in mice.36 In addition, chronic stress induces monocytosis via accelerated hematopoiesis that subsequently promotes vulnerable plaque lesions.37 Furthermore, a Western‐type diet modulates towards long‐lasting innate immune reprogramming in myeloid cells and progenitor cells in Ldlr‐/‐ mice.15 In agreement with these earlier observations, our results imply that changes in lifestyle may reduce the risk to develop cardiovascular disease through their effects on innate immune function in humans.
Our study may have implications for public health. The ability to successfully reduce sedentary behavior, in a population at risk for CVD and a priori high levels of sedentary behavior, and the associated diminished proinflammatory phenotype of innate immune cells is of potential clinical relevance. More importantly, our work shows that the detrimental effects of sedentary behavior on atherosclerosis and CVD may, at least partly, be explained through the direct effects on the innate immune function. However, some limitations must be considered.
A potential limitation of this study is that, because of the design of our study, we cannot exclude the impact of external modulators of immune function, eg, seasonality. Previously, annual seasonal variability in cytokine production has been described to significantly peak in summer for several cytokines (eg, TNFα, IL‐1β, and IL‐6) after stimulation.38 We observed a decreased cytokine production after the physical activity intervention in July, which is exactly opposite effect of the annual seasonal summer peak. Moreover, no seasonal effect was found for the cytokine production after P3C and LPS stimulation, the stimuli applied in our study. This suggests that the effect of the intervention unlikely can be explained by seasonality per se. Also, the strong correlation between walking time and cytokine production capacity strongly argues for a direct effect of the intervention on cytokine production capacity. Fluctuations of immune cells over time might have influenced our results, however, correction for the monocyte percentage of the PBMC fraction did unalter our strongest findings, namely the cytokine production capacity after LPS and R848 stimulation.
Our relatively small sample size should be considered, although power analysis revealed intermediate (β>0.65) to good (β>0.80) power for most outcomes, except for IL‐8 which is underpowered (see Table S7) and therefore has a larger chance of a type II error (ie, false negative results). Still we were able to detect a significant difference in IL‐8 before and after intervention. Nevertheless, caution should be taken extrapolating our results to other groups and/or physical activity interventions.
To our knowledge, this study is the first to reveal a diminished proinflammatory phenotype of innate immune cells after a successful 16 weeks reduced sitting intervention in subjects with increased cardiovascular risk. These findings, combined with the within‐subject approach of our study, support the presence of a dose‐response relationship between sedentary behavior and innate immune response. Given the central role for activated monocytes in atherosclerosis and development of cardiovascular diseases, our observations may have important clinical implications. These data improve our understanding of the link between sedentary behavior and cardiovascular disease and support the concept that targeting sedentary behavior may be an important approach in preventing atherosclerotic cardiovascular disease.
Sources of Funding
Prof. Riksen, Prof. Netea, and Prof. Joosten received funding from the European Union's Horizon 2020 research and innovation program (No 667837). This study was supported by an IN‐CONTROL CVON grant (CVON2018‐27) to Prof. Riksen, Prof. Netea, and Prof. Joosten. Prof. Netea is further supported by a Netherlands Organization for Scientific Research Spinoza Grant (NWO SPI 94‐212). Prof. Joosten is supported by a Competitiveness Operational Programme grant of the Romanian Ministry of European Funds (HINT, ID P_37_762; MySMIS 103587). Prof. Riksen is a recipient of a grant of the ERA‐CVD Joint Transnational Call 2018, which is supported by the Dutch Heart Foundation (JTC2018, project MEMORY; 2018T093).
Disclosures
Dr. Willems is scientific advisor of Khondrion, Nijmegen, The Netherlands. The remaining authors have no disclosures to report.
Supporting information
Table S1. Product Details of Cytokines and Chemokines Measured With ELISA
Table S2. Circulating Cytokines in Group1.0 (n=8)
Table S3. Cytokine Production Capacity in Group1.0 (n=8)
Table S4. Metabolism: Lactate and Oxygen Consumption in Group1.0 (n=8)
Table S5. Circulating Markers of Fat Tissue, Inflammation, Oxidative Stress and Endothelial Dysfunction (n=8)
Table S6. Cell Types in PBMC Fraction (n=8)
Table S7. Power Analysis of Cytokine Production Capacity
Figure S1. Gating strategy of monocyte subsets and expression markers.
(J Am Heart Assoc. 2019;8:e013764 DOI: 10.1161/JAHA.119.013764.)
References
- 1. Roth GA, Johnson C, Abajobir A, Abd‐Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis‐Guzman N, Amrock S, Ansari H, Arnlov J, Asayesh H, Atey TM, Avila‐Burgos L, Awasthi A, Banerjee A, Barac A, Barnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castaneda‐Orjuela CA, Castillo‐Rivas J, Catala‐Lopez F, Choi JY, Christensen H, Cirillo M, Cooper L Jr, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi‐Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabares‐Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor‐Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blair SN, Jackson AS. Physical fitness and activity as separate heart disease risk factors: a meta‐analysis. Med Sci Sports Exerc. 2001;33:762–764. [DOI] [PubMed] [Google Scholar]
- 3. Marques A, Sarmento H, Martins J, Saboga Nunes L. Prevalence of physical activity in european adults ‐ compliance with the world health organization's physical activity guidelines. Prev Med. 2015;81:333–338. [DOI] [PubMed] [Google Scholar]
- 4. Vasankari V, Husu P, Vaha‐Ypya H, Suni JH, Tokola K, Borodulin K, Wennman H, Halonen J, Hartikainen J, Sievanen H, Vasankari T. Subjects with cardiovascular disease or high disease risk are more sedentary and less active than their healthy peers. BMJ Open Sport Exerc Med. 2018;4:e000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van der Ploeg HP, Chey T, Korda RJ, Banks E, Bauman A. Sitting time and all‐cause mortality risk in 222 497 australian adults. Arch Intern Med. 2012;172:494–500. [DOI] [PubMed] [Google Scholar]
- 6. Chomistek AK, Manson JE, Stefanick ML, Lu B, Sands‐Lincoln M, Going SB, Garcia L, Allison MA, Sims ST, LaMonte MJ, Johnson KC, Eaton CB. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: results from the women's health initiative. J Am Coll Cardiol. 2013;61:2346–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ekelund U, Steene‐Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, Bauman A, Lee IM; Lancet Physical Activity Series 2 Executive Committee; Lancet Sedentary Behaviour Working Group . Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta‐analysis of data from more than 1 million men and women. Lancet. 2016;388:1302–1310. [DOI] [PubMed] [Google Scholar]
- 8. Gerage AM, Benedetti TR, Farah BQ, Santana Fda S, Ohara D, Andersen LB, Ritti‐Dias RM. Sedentary behavior and light physical activity are associated with brachial and central blood pressure in hypertensive patients. PLoS ONE. 2015;10:e0146078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stamatakis E, Hamer M, Tilling K, Lawlor DA. Sedentary time in relation to cardio‐metabolic risk factors: differential associations for self‐report vs accelerometry in working age adults. Int J Epidemiol. 2012;41:1328–1337. [DOI] [PubMed] [Google Scholar]
- 10. Healy GN, Dunstan DW, Salmon J, Shaw JE, Zimmet PZ, Owen N. Television time and continuous metabolic risk in physically active adults. Med Sci Sports Exerc. 2008;40:639–645. [DOI] [PubMed] [Google Scholar]
- 11. Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bekkering S, van den Munckhof I, Nielen T, Lamfers E, Dinarello C, Rutten J, de Graaf J, Joosten LA, Netea MG, Gomes ME, Riksen NP. Innate immune cell activation and epigenetic remodeling in symptomatic and asymptomatic atherosclerosis in humans in vivo. Atherosclerosis. 2016;254:228–236. [DOI] [PubMed] [Google Scholar]
- 13. Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, Tian L, Harrison DG, Giacomini JC, Assimes TL, Goronzy JJ, Weyand CM. The glycolytic enzyme pkm2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. 2016;213:337–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Valk FM, Bekkering S, Kroon J, Yeang C, Van den Bossche J, van Buul JD, Ravandi A, Nederveen AJ, Verberne HJ, Scipione C, Nieuwdorp M, Joosten LA, Netea MG, Koschinsky ML, Witztum JL, Tsimikas S, Riksen NP, Stroes ES. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134:611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Christ A, Gunther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, Bassler K, Klee K, Schulte‐Schrepping J, Ulas T, Moorlag S, Kumar V, Park MH, Joosten LAB, Groh LA, Riksen NP, Espevik T, Schlitzer A, Li Y, Fitzgerald ML, Netea MG, Schultze JL, Latz E. Western diet triggers nlrp3‐dependent innate immune reprogramming. Cell. 2018;172:162–175.e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, CANTOS Trial Group . Relationship of c‐reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the cantos randomised controlled trial. Lancet. 2018;391:319–328. [DOI] [PubMed] [Google Scholar]
- 17. Henson J, Yates T, Edwardson CL, Khunti K, Talbot D, Gray LJ, Leigh TM, Carter P, Davies MJ. Sedentary time and markers of chronic low‐grade inflammation in a high risk population. PLoS One. 2013;8:e78350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Pahor M, Taaffe DR, Brach J, Rubin S, Harris TB. Physical activity, exercise, and inflammatory markers in older adults: findings from the health, aging and body composition study. J Am Geriatr Soc. 2004;52:1098–1104. [DOI] [PubMed] [Google Scholar]
- 19. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, ESC Scientific Document Group . 2018 esc/esh guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 20. Lyden K, Kozey Keadle SL, Staudenmayer JW, Freedson PS. Validity of two wearable monitors to estimate breaks from sedentary time. Med Sci Sports Exerc. 2012;44:2243–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van der Berg JD, Willems PJ, van der Velde JH, Savelberg HH, Schaper NC, Schram MT, Sep SJ, Dagnelie PC, Bosma H, Stehouwer CD, Koster A. Identifying waking time in 24‐h accelerometry data in adults using an automated algorithm. J Sports Sci. 2016;34:1867–1873. [DOI] [PubMed] [Google Scholar]
- 22. Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll‐like receptor 2. J Immunol. 2000;165:618–622. [DOI] [PubMed] [Google Scholar]
- 23. Joosten LA, Netea MG, Mylona E, Koenders MI, Malireddi RK, Oosting M, Stienstra R, van de Veerdonk FL, Stalenhoef AF, Giamarellos‐Bourboulis EJ, Kanneganti TD, van der Meer JW. Engagement of fatty acids with toll‐like receptor 2 drives interleukin‐1beta production via the asc/caspase 1 pathway in monosodium urate monohydrate crystal‐induced gouty arthritis. Arthritis Rheum. 2010;62:3237–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weber C, Shantsila E, Hristov M, Caligiuri G, Guzik T, Heine GH, Hoefer IE, Monaco C, Peter K, Rainger E, Siegbahn A, Steffens S, Wojta J, Lip GY. Role and analysis of monocyte subsets in cardiovascular disease. Joint consensus document of the european society of cardiology (esc) working groups “atherosclerosis & vascular biology” and “thrombosis”. Thromb Haemost. 2016;116:626–637. [DOI] [PubMed] [Google Scholar]
- 25. Ziegler‐Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. [DOI] [PubMed] [Google Scholar]
- 26. Laska MN, Lytle LA, Nanney MS, Moe SG, Linde JA, Hannan PJ. Results of a 2‐year randomized, controlled obesity prevention trial: effects on diet, activity and sleep behaviors in an at‐risk young adult population. Prev Med. 2016;89:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ashe MC, Winters M, Hoppmann CA, Dawes MG, Gardiner PA, Giangregorio LM, Madden KM, McAllister MM, Wong G, Puyat JH, Singer J, Sims‐Gould J, McKay HA. “Not just another walking program”: everyday activity supports you (easy) model‐a randomized pilot study for a parallel randomized controlled trial. Pilot Feasibility Stud. 2015;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev. 2017;97:495–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carter S, Hartman Y, Holder S, Thijssen DH, Hopkins ND. Sedentary behavior and cardiovascular disease risk: mediating mechanisms. Exerc Sport Sci Rev. 2017;45:80–86. [DOI] [PubMed] [Google Scholar]
- 30. Thosar SS, Bielko SL, Wiggins CC, Klaunig JE, Mather KJ, Wallace JP. Antioxidant vitamin c prevents decline in endothelial function during sitting. Med Sci Monit. 2015;21:1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dunstan DW, Kingwell BA, Larsen R, Healy GN, Cerin E, Hamilton MT, Shaw JE, Bertovic DA, Zimmet PZ, Salmon J, Owen N. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35:976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Phillips CM, Dillon CB, Perry IJ. Does replacing sedentary behavior with light or moderate to vigorous physical activity modulate inflammatory status in adults? Int J Behav Nutr Phys Act. 2017;14:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu N, Ruan Y, Gao X, Sun J. Systematic review and meta‐analysis of randomized, controlled trials on the effect of exercise on serum leptin and adiponectin in overweight and obese individuals. Horm Metab Res. 2017;49:164–173. [DOI] [PubMed] [Google Scholar]
- 34. Noz MP, Ter Telgte A, Wiegertjes K, Joosten LAB, Netea MG, de Leeuw FE, Riksen NP. Trained immunity characteristics are associated with progressive cerebral small vessel disease. Stroke; a journal of cerebral circulation. 2018;49:2910–2917. [DOI] [PubMed] [Google Scholar]
- 35. Nahrendorf M, Swirski FK. Lifestyle effects on hematopoiesis and atherosclerosis. Circ Res. 2015;116:884–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McAlpine CS, Kiss MG, Rattik S, He S, Vassalli A, Valet C, Anzai A, Chan CT, Mindur JE, Kahles F, Poller WC, Frodermann V, Fenn AM, Gregory AF, Halle L, Iwamoto Y, Hoyer FF, Binder CJ, Libby P, Tafti M, Scammell TE, Nahrendorf M, Swirski FK. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature. 2019;566:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ter Horst R, Jaeger M, Smeekens SP, Oosting M, Swertz MA, Li Y, Kumar V, Diavatopoulos DA, Jansen AFM, Lemmers H, Toenhake‐Dijkstra H, van Herwaarden AE, Janssen M, van der Molen RG, Joosten I, Sweep F, Smit JW, Netea‐Maier RT, Koenders M, Xavier RJ, van der Meer JWM, Dinarello CA, Pavelka N, Wijmenga C, Notebaart RA, Joosten LAB, Netea MG. Host and environmental factors influencing individual human cytokine responses. Cell. 2016;167:1111–1124.e1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Product Details of Cytokines and Chemokines Measured With ELISA
Table S2. Circulating Cytokines in Group1.0 (n=8)
Table S3. Cytokine Production Capacity in Group1.0 (n=8)
Table S4. Metabolism: Lactate and Oxygen Consumption in Group1.0 (n=8)
Table S5. Circulating Markers of Fat Tissue, Inflammation, Oxidative Stress and Endothelial Dysfunction (n=8)
Table S6. Cell Types in PBMC Fraction (n=8)
Table S7. Power Analysis of Cytokine Production Capacity
Figure S1. Gating strategy of monocyte subsets and expression markers.


