Abstract
Background
Maternal smoking during pregnancy has been associated with higher blood pressure and autonomic imbalance in the offspring. However, it has been difficult to determine the selective prenatal and postnatal contributions as children frequently have been exposed to smoking both before and after birth. The specific role of nicotine is also unclear. We aimed to determine whether exclusive prenatal exposure to nicotine from maternal use of smokeless tobacco (Swedish snus) in pregnancy was associated with blood pressure and autonomic heart rate control in their children.
Methods and Results
We measured oscillometric blood pressures in forty 5‐ to 6‐year‐old children with snus exposure in fetal life (n=21) and in tobacco‐free controls (n=19). Taking the child′s age and height into account, snus‐exposed children had 4.2 (95% CI, 0.2–8.1) mm Hg higher systolic blood pressure than controls (P=0.038). The corresponding sex‐, age‐, and height‐standardized systolic blood pressure centiles were 61 and 46 (95% CI of the difference, 2–28) (P=0.029). Heart rate variability was tested in 30 of the children. The spectral heart rate variability variable low‐frequency/high‐frequency ratio was higher (median, 0.69; interquartile range, 0.45–1.21) in snus‐exposed children than in controls (median, 0.21; interquartile range, 0.32–0.57; P=0.034).
Conclusions
Prenatal snus exposure was associated with higher systolic blood pressure and altered heart rate variability at 6 years of age. These findings may indicate adverse prenatal programming of nicotine, but implications for cardiovascular health in later life remain to be studied. Meanwhile, women should be recommended to abstain from all types of tobacco and nicotine products during pregnancy.
Keywords: autonomic function, blood pressure, fetal programming, heart rate/heart rate variability, nicotine, pediatric, smokeless tobacco
Subject Categories: High Blood Pressure, Pediatrics, Risk Factors, Pregnancy
Short abstract
See Editorial Watanabe and Parikh
Clinical Perspective
What Is New?
Smoking in pregnancy has been associated with higher blood pressure in the offspring. Continuous exposure to cigarette smoking during childhood has complicated the interpretation of fetal exposure on issues of timing, short‐ or long‐term effects, and the specific role of nicotine.
Studying fetal exposure to maternal intake of oral moist smokeless tobacco and without further nicotine exposure beyond the breastfeeding period, we found a significant association between selective prenatal nicotine exposure and elevated blood pressure in preschool children.
What Are the Clinical Implications?
Prenatal nicotine exposure is a risk factor for higher blood pressure during childhood.
The clinical implications of prenatal nicotine exposure for future cardiovascular health in older children and adult offspring remain to be clarified.
Meanwhile, women should be recommended to avoid all types of nicotine‐containing products during pregnancy.
Introduction
There is increasing evidence of a developmental contribution, beginning already in utero, to hypertension and cardiovascular disease in adult life.1, 2 Environmental stimuli during critical periods of early development are considered to trigger adaptations with lasting physiological effects, a process also known as fetal programming.1 Although malnutrition, hypoxia, and oxidative stress have been most frequently suggested as causes for fetal programming,3, 4, 5 less is known about smoking in pregnancy; however, smoking represents the most common modifiable adverse exposure during fetal life. Although the global prevalence of smoking during pregnancy is low (≈1.7%), it is still prevalent in many countries, such as Ireland (38%), Uruguay (29%), and Bulgaria (29%).6
Nicotine is considered to be the main culprit in cigarette smoke, and nicotine levels are higher in the amniotic fluid and in the placenta than in the corresponding maternal serum.7 However, because cigarette smoke contains thousands of different substances, many of which become circulating and capable of passing the placenta, it has been difficult to separate the role of nicotine from other toxins.
Smoking during pregnancy has been shown to the increase risk of preterm birth, stillbirth, low birth weight, and sudden infant death syndrome.8, 9, 10, 11, 12 Besides affecting perinatal outcomes, exposure to cigarette smoke in fetal life and in childhood has been associated with higher blood pressure and altered autonomic cardiac control,13, 14, 15, 16 suggesting that adverse effects on the offspring may be long lasting. However, it has been difficult to separate prenatal and postnatal effects as most children exposed in utero also will be exposed to continued second‐hand cigarette smoke during childhood. Contemporary second‐hand smoking complicates interpretations even further, as it makes discrimination between long‐ or short‐term effects difficult.
Swedish snus is moist smokeless tobacco for oral use, packed in pouches that are placed under the lip. It delivers high doses of nicotine but without the combustion products found in cigarette smoke. Therefore, snus use during pregnancy exposes the fetus mainly to nicotine. After birth, infants to mothers using snus may be exposed to nicotine and its metabolites via breast milk,17 but thereafter the exposure is ended.
Accordingly, studying children exposed to perinatal snus has the advantage of limiting confounding from long‐term second‐hand smoke exposure and focuses on the effects of nicotine alone. In this study, our aim was to determine if there was an association between maternal use of snus in pregnancy, blood pressure, and autonomic cardiac control, measured by heart rate variability (HRV) in the offspring at the age of 5 to 6 years. Our hypothesis was that fetal exposure to nicotine would be associated with a higher blood pressure and a higher low‐frequency/high‐frequency ratio in spectral analysis of HRV in childhood.
Methods
Study Design
This was a cohort study, with participants originating from a larger study of perinatal snus exposure described previously.18 The women in the original snus study cohort were recruited in Sweden during 2006 to 2011 when signing in at the local maternity center early in pregnancy. All women were healthy before pregnancy. On the basis of their tobacco use when first visiting the maternity center, they were classified as snus users, smokers, or tobacco‐free controls. The women answered questionnaires about their tobacco habits at 3 separate occasions during gestation: early in pregnancy, late in pregnancy (gestational week 32), and after the delivery. Characteristics of their newborn infants were collected after birth. From the original cohort, we selected families in which the mother had been using snus during pregnancy, the infants were born healthy at term with appropriate birth weight, the child had reached an age of 5 to 6 years, and in which the families were residing in the 2 cities (Umeå and Stockholm) in which follow‐up was performed. Using the same inclusion criteria except for snus in pregnancy, we identified a similar number of tobacco‐free control families.
Multiple pregnancy and active or passive smoking during pregnancy and childhood were exclusion criteria for all participants. In total, 56 families were identified (n=28 using snus in pregnancy), 12 were lost to follow‐up (no address), 44 were invited, and 2 declined. Two families made a last‐minute cancellation to their appointment as a result of family illness, rendering a final cohort of 40 children. A flowchart of inclusion is displayed in Figure 1.
Figure 1.
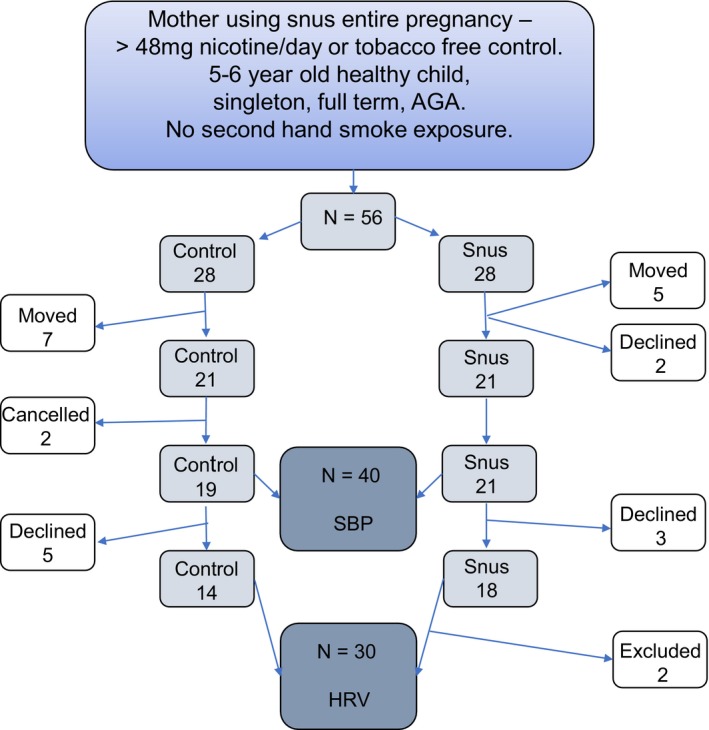
Flowchart of inclusion for smokeless tobacco (Swedish snus)–exposed children and tobacco‐free controls. AGA indicates appropriate for gestational age; HRV, heart rate variability; SBP, systolic blood pressure.
Only children with mothers who had used snus during the whole pregnancy and with a high nicotine dose (≥48 mg/d) were included as snus exposed (n=21). There were no other forms of tobacco reported by the snus using mother from 12 weeks before pregnancy to the study booking. The use of snus after delivery and during breastfeeding was not prospectively quantified, but the duration of breastfeeding (in months) was determined using a questionnaire at the follow‐up. All the controls were completely tobacco free and had not used tobacco 12 weeks before pregnancy, during pregnancy, or during breastfeeding (n=19).
Follow‐up was scheduled during daytime. Height and weight were measured with the child undressed to wearing light clothing. Body mass index (BMI) was calculated for each child as weight (in kilograms)/height (in meters squared), and the BMI percentile was calculated using the World Health Organization reference data from 2007 for ages 5 to 19 years.19
Individual data and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedures because the institutional review board and the informed consent of the parents only permit aggregated data to be publicly available or published. Analytic methods, however, are presented in this article to assist other researchers in reproducing the results or replicating the procedures.
Blood Pressure Measurements
After 15 minutes of calm adaptation to the examination room and with the child sitting down, an oscillometric device (Dinamap; GE Health Care, Fairfield, CT) with an appropriate cuff size was used to measure systolic (SBP) and diastolic (DBP) blood pressure in the right arm. Three consecutive measurements of blood pressure with at least 1 minute in between were obtained by the same trained pediatric nurse, blinded to the study groups. The mean of the 3 consecutive measurements was calculated for all the children. The SBP and DBP values were compared as crude values and as centiles for age, sex, and height, according to a US pediatric reference population.20, 21
A blood pressure >90th percentile was classified as elevated and >99th percentile as hypertension. The 97.7th percentile represents +2 SDs.
Heart Rate Variability
A resting 12‐lead ECG was taken after blood pressure measurements with the child lying down. All children (n=40) were then invited to do a 24‐hour ECG recording at home, and 32 of 40 accepted to wear the recorder. The reason for declining the recorder was either concerns of disturbing the sleep or physical activity. Two recordings were excluded from analysis because of low quality with prevalent artifacts. A total of 30 (14 control and 16 snus‐exposed) high‐quality 24‐hour ECGs were recorded by a 3‐channel portable ECG recorder (LifeCard CF; Spacelabs Healthcare Limited, Hertford, UK). An artifact‐free and manually edited period of 2 hours sleep during nighttime was analyzed in the software program Impressario software, version 7 (Del Mar Reynolds Medical, Hertford, UK) for frequency domain analysis. All editing and HRV analysis were performed by the same analyst (F.N.), blinded to the study groups.
For the analysis of the frequency domain indexes, beat‐to‐beat fluctuations were transformed to the frequency domain by fast Fourier transformation, and the specific measures were computed as the square root of the areas under the power spectrum, expressed as ms2/Hz.
Spectral indexes over 3 standard frequency regions of interest were determined: very low frequency (VLF; 0.017–0.05 Hz), low frequency (LF; 0.05–0.150 Hz), and high frequency (HF; 0.15–0.5 Hz). Total power (all frequencies between 0.017–0.5 Hz) was also determined as well as the LF/HF ratio. The spectral analysis was made in accordance with the standards of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology.22 Our primary HRV outcome was the LF/HF, based on earlier studies on prenatal tobacco exposure and HRV in infants.23, 24
Questionnaires at Follow‐up
While the child was tested, the mother answered questions, including those about child and parental health and in particular questions related to blood pressure, medications, duration of breastfeeding, and exposure to second‐hand smoke. The parents reported any history of cardiovascular disease and were specifically asked about history of hypertension with or without medical treatment.
Questionnaires during Pregnancy
The original questionnaires answered during pregnancy included questions about maternal use of medications, alcohol, and drugs, as well as the type and pattern of tobacco use during gestation. All women denied use of illicit substances. The reported average daily snus intake (0–1, 2–3, 4–5, 6–7, or >7 pouches) was converted to a mean nicotine dose (mg/d), taking the nicotine content of different brands (4–8 mg per pouch) into account. The calculation was based on the highest amount of each category, and >7 pouches was estimated to 56 mg. In this study, only women using ≥6 pouches were included.
Ethical Approval
This study was approved by the regional ethical review board in Stockholm, and written consent was obtained from all parents.
Statistical Analysis
Data are described as means (SDs) and 95% CIs, medians (ranges), or numbers (proportions). Group differences for normally distributed variables (all except LF/HF ratio) were compared with Student's t test. Group differences in LF/HF ratio were compared with Mann–Whitney U test because of skewed data. Proportions of categorical variables were analyzed with a χ2 test.
Besides differences in exposure to perinatal snus, the following covariates were compared between the 2 groups: city of residence (site); a parental history of hypertension; maternal age, weight, height, and parity; duration of breastfeeding; and the child′s birth weight, sex, current age, weight, height, and BMI. Group differences with a P<0.2 were entered into multiple linear regression models to evaluate selective contributions to blood pressures. P<0.05 was considered statistically significant.
To attain 80% power with a 5% significance level and considering a mean difference in SBP of ≥4 mm Hg (SD=6 mm Hg) as clinically significant, we estimated that 40 subjects would be sufficient, with equal sizes of the 2 groups. All statistical analyses were performed using SPSS for Windows, version 25 (IBM Corp, Armonk, NY).
Results
Study Cohort
Four children in the snus group and none in the control group had parents reporting high blood pressure (P=0.07). There were no statistically significant differences found between snus using mothers and tobacco‐free control mothers in age, weight, height, or BMI. The mean duration of breastfeeding was not statistically different between the groups, but breastfeeding <6 months was more common in the snus group (67%) than in controls (32%; P=0.027). There were no statistically significant differences between children of snus using mothers and tobacco‐free control children on age, weight, or height and BMI at follow‐up. There were 3 individuals in each group with a BMI >90th percentile (Table 1.
Table 1.
Parental, Maternal, and Infant Characteristics, and for the child at Follow‐Up
| Total (n=40) | Controls (n=19) | Snus‐Exposed Children (n=21) | P Value |
|---|---|---|---|
| Parental high blood pressure | 0 | 4 (19) | 0.07 |
| Maternal age, y | 29.7 (5.8) | 32.5 (4.1) | 0.09 |
| Maternal height, cm | 166.6 (4.0) | 165.9 (4.6) | 0.67 |
| Prepregnancy weight, kg | 66.1 (10.1) | 73.0 (16.7) | 0.14 |
| Prepregnancy BMI, kg/m2 | 25.0 (3.62) | 26.6 (5.78) | 0.33 |
| Infancy | |||
| Girls | 10 (52) | 9 (43) | 0.54 |
| Birth weight, g | 3695 (314) | 3497 (590) | 0.20 |
| Breastfeeding duration, mo | 8.3 (4.7) | 5.7 (4.1) | 0.08 |
| Breastfeeding duration <6 mo | 6 (32) | 14 (67) | 0.03a |
| Follow‐up at 5–6 y | |||
| Age, mo | 70 (8.2) | 73.6 (7.6) | 0.16 |
| Height, cm | 115.4 (7) | 119.2 (7.5) | 0.11 |
| Weight, median, kg | 21.0 (10.7–17) | 23.3 (16.7–34) | 0.11 |
| BMI, kg/m2 | 16.0 (1.1) | 16.8 (1.8) | 0.09 |
| BMI, percentile | 62.4 (24.6) | 69.6 (22) | 0.33 |
Data are proportions (percentages) or mean (SD) values, if not stated otherwise. BMI indicates body mass index; Snus, smokeless tobacco.
Significant.
Systolic Blood Pressure
Children prenatally exposed to snus had a mean SBP of 99 (SD, 7.1) mm Hg, which was higher than in control children (93 [SD, 5.8] mm Hg; P=0.013) (Table 2. The corresponding SBP centiles for sex, age, and height were 61st (SD, 21st) for children exposed to snus and 46th (SD, 20th) for unexposed controls (P=0.029) (Figure 2.
Table 2.
BP in Controls and Snus‐Exposed Children
| Total (n=40) | Controls (n=19) | Snus‐Exposed Children (n=21) | P Value |
|---|---|---|---|
| BP systolic, mm Hg | 93.3 (5.8) | 98.7 (7.1) | 0.013a |
| BP diastolic, mm Hg | 54.5 (4.5) | 56.1 (5.2) | 0.33 |
| BP systolic, percentile | 46.1 (20) | 60.7 (20.6) | 0.029a |
| BP diastolic, percentile | 44.9 (16.1) | 48.6 (19.9) | 0.53 |
| Heart rate, beats/min | 91 (11) | 90 (12) | 0.81 |
Data are given as mean (SD) values. BP indicates blood pressure; Snus, smokeless tobacco.
Significant.
Figure 2.
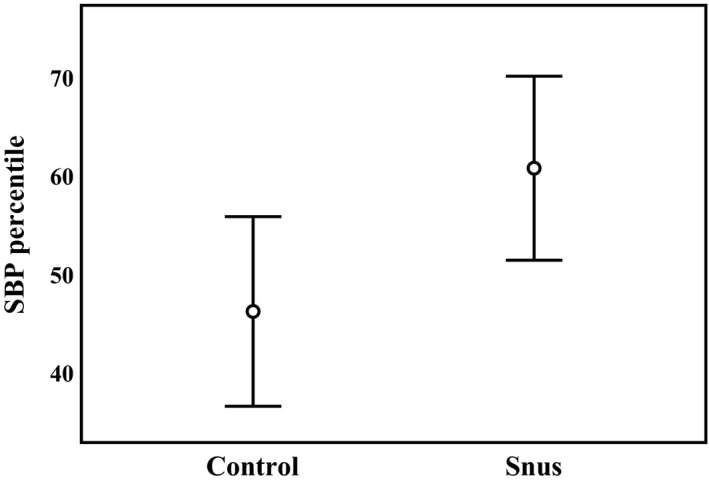
Systolic blood pressure (SBP) percentiles (for sex, age, and height) in controls and smokeless tobacco (Swedish snus)–exposed children. Mean and 95% CI are given. P value for group difference=0.029.
Two children from the snus group (none in the control group) had an SBP >90th percentile and were classified as having blood pressure in the hypertensive range.
Among maternal/parental characteristics, 4 variables (parental hypertension, maternal age, maternal prepregnancy weight, and breastfeeding duration) had a P<0.20 for a group difference between the snus and control groups. In a multiple regression model, none of these variables was significantly associated with SBP. Among the characteristics of the participating children, 3 variables (age, weight, and height) have a P<0.20 for a group difference between the snus and control groups, and all were associated with SBP.
Two final multiple regression models with SBP as outcome were executed: one with perinatal snus exposure and the child′s age and height as exposures, and the other exchanging current height for weight. In the first model, SBP was unassociated to age (β=−0.3 mm Hg; 95% CI, −0.7 to 0.1 mm Hg; P=0.095) but significantly associated with height (β=0.6 mm Hg; 95% CI, 0.2–1.0 mm Hg; P=0.003) and perinatal snus exposure (β=4.2 mm Hg; 95% CI, 0.2–8.1 mm Hg; P=0.038). In the second model, SBP was associated with weight (β=1.1 mm Hg; 95% CI, 0.6–1.6 mm Hg; P<0.001) but not significantly associated with perinatal snus exposure (β=3.1 mm Hg; 95% CI, −0.6 to 6.7 mm Hg; P=0.10) or age (β=−0.2 mm Hg; 95% CI, −0.5 to 0.1 mm Hg; P=0.12).
Diastolic Blood Pressure
There were no statistically significant differences in mean DBP or in age‐, sex‐, and height‐adjusted DBP centiles between children in the snus‐exposed and control groups (Table 2.
Heart Rate and RR Intervals
There were no group differences in heart rate. Three children had supraventricular extrasystoles, and one child had an AV‐block II during nighttime sleep; these were all children in the tobacco‐free control group. There were no other arrhythmias or abnormalities in any of the 12‐lead ECGs. The 3‐lead ECG recording measuring RR intervals showed no significant differences in maximum, minimum, or mean RR intervals between snus‐exposed and control groups (Table 3.
Table 3.
HRV in Snus‐Exposed and Control Groups
| HRV (n=30) | Controls (n=14) | Snus‐Exposed Group (n=16) | P Value |
|---|---|---|---|
| RR interval maximum, ms | 1209 (88) | 1238 (136) | 0.49 |
| RR interval minimum, ms | 401 (81) | 449 (89) | 0.13 |
| RR interval mean, ms | 782 (84) | 789 (78) | 0.80 |
| HRV SD, ms | 102 (37) | 98 (35) | 0.75 |
| Very low frequency, ms2/Hz | 1128 (766) | 1315 (710) | 0.49 |
| Low frequency, ms2/Hz | 1596 (992) | 1384 (823) | 0.53 |
| High frequency, ms2/Hz | 3895 (3107) | 3494 (4831) | 0.79 |
| Total power, ms2/Hz | 6783 (4375) | 6168 (5573) | 0.75 |
| Low‐frequency/high‐frequency ratio, median (range) | 0.487 (0.325–0.572) | 0.694 (0.448–1.21) | 0.034a |
Data are given as mean (SD) values, if not stated otherwise. HRV indicates heart rate variability; Snus, smokeless tobacco.
Significant.
Heart Rate Variability
The LF/HF ratio was higher in snus‐exposed children, with a median of 0.69 (interquartile range, 0.45–1.21) versus 0.49 (interquartile range, 0.32–0.57) in controls (P=0.034) (Figure 3. The frequency domain HRV did not differ between the snus group and controls in any of the power spectrum very LF, LF, or HF bands, or total power (Table 3.
Figure 3.
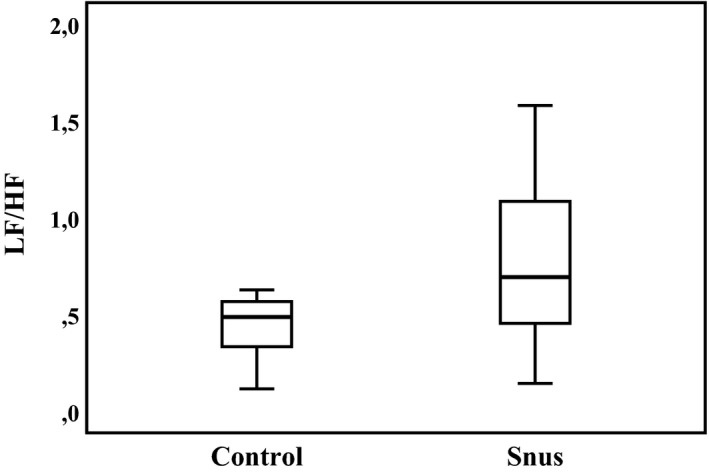
Heart rate variability low‐frequency/high‐frequency (LF/HF) ratio in controls and smokeless tobacco (Swedish snus)–exposed children. Median and interquartile range are given. P value for group difference=0.034.
Discussion
This cohort study demonstrates an association between exposure to smokeless tobacco in fetal life and higher SBP at 5 to 6 years of age. Cigarette smoking during pregnancy has been linked to higher blood pressure in exposed children, but data have been inconsistent and continued exposure to secondhand smoke during childhood has complicated interpretations.13, 14, 25, 26, 27 By studying exposure to snus during pregnancy and breastfeeding, we were able to exclude both the influence of combustion toxins from cigarette smoke and that of secondhand smoke exposure during childhood. Our results suggest that prenatal nicotine exposure per se may be associated with a higher blood pressure in the offspring.
The difference of 4.2 mm Hg in SBP between snus‐exposed children and controls is larger than the difference reported in studies of maternal smoking, commonly amounting to 1 to 3 mm Hg.13, 16, 28 If this reflects a dose‐response relationship needs further exploration; in our study, only high‐dose nicotine exposure was studied.
Blood pressure follows a trajectory, and a higher blood pressure during childhood may be a risk factor of becoming hypertensive as an adult.29, 30, 31 However, although we tried to control for some potentially confounding factors, our data do not provide evidence for a causal relationship or long‐standing effects beyond early childhood.
In addition to the higher SBP, we also found an association with prenatal snus exposure and higher LF/HF ratio in preschool children. The higher ratio of LF/HF indicates an altered balance between the parasympathetic and sympathetic tone. Although activity in the HF band is attributed to parasympathetic tone, the LF band is more complex and is generally assumed to constitute a combination of sympathetic and parasympathetic tone.22 A higher LF/HF ratio in infants to mothers using snus during pregnancy has been reported earlier by our group.23 In addition, a higher LF/HF ratio has also been reported in infants to smokers born preterm but has not been shown in preschool children before.24 Thus, the altered autonomic balance demonstrated in earlier studies of tobacco‐exposed infants and in 1‐year‐old toddlers15, 23 seems to persist also in preschool children and needs to be studied further.
The mechanism behind increased blood pressure and altered autonomic cardiac control after prenatal nicotine exposure is not fully understood, but an involvement of the baroreflex loop, impairing the regulation of SBP, is possible.32, 33 However, the connection between the autonomic nervous system and blood pressure regulation is complex, and other pathways via the renin‐angiotensin‐aldosterone system may also be involved.34 Animal studies show that rats exposed to nicotine during fetal life have an increased response in SBP to angiotensin II.33 Structural changes have also been implicated, such as smaller kidneys in the offspring after smoking during pregnancy, with a potential subsequent dysfunction of the renin‐angiotensin‐aldosterone system.35, 36 Other possible mechanisms of nicotinic effects on the developing cardiovascular system include direct changes in vessel structure.33, 37, 38, 39 As nicotine is a potent mediator in many organs and regulatory systems during development, a combination of many mechanisms may be involved.
The children in this study were all appropriate for gestational age at birth, and there was no significant difference in birth weight, although the snus‐exposed newborns weighed 200 g less than controls. The effect from nicotine exposure on fetal growth is smaller than that seen from combustion products in smoking and is more likely associated with preterm birth.40 There was no significant difference in weight between the preschool children exposed to snus and tobacco‐free controls, but there was a trend toward higher weight in the snus‐exposed children. The association between prenatal tobacco exposure and childhood overweight41 and the association between SBP and weight have been reported earlier.42
Cigarette smoking is decreasing all over the world but is still a major concern for worldwide health. In Sweden, the prevalence of smoking is lower than in many other European countries, but there is instead a tradition of using oral moist tobacco, known as Swedish snus. The total burden of tobacco is thus similar to other countries. Snus is most frequently used by men, but its use among women is increasing; and in 2016, as many as 4.7% of Swedish women reported using snus 12 weeks before pregnancy.43 The effects of maternal snus use have not been described as extensively as those after maternal smoking, but studies showing increased risk of stillbirth, preterm birth, neonatal apnea, and altered autonomic cardiac control have all been reported.18, 23, 40, 44, 45, 46
Snus use delivers high doses of nicotine and metabolites to the fetus, but the combustion products and many other toxins that are abundant in cigarette smoke are absent. The findings in studies of prenatal and perinatal snus exposure are, therefore, explained as mainly mediated by nicotine, which also is supported by numerous animal studies.47 Maternal snus during pregnancy may, therefore, represent a suitable model for studying nicotinic effects in humans; and the findings are likely to be applicable for all sources of nicotine. This is of importance as the uses of e‐cigarettes, hookahs, oral nicotine pouches, nasal nicotine spray, nicotine‐mediated transdermal patches, and nicotine gums are all likely to have the potential of affecting the fetus if used during pregnancy.
The main limitation of this study is the relatively small number of children, which may have influenced the possibility of finding smaller differences. Thus, the observed tendencies of heavier mothers and children in snus‐exposed groups may be significant in a larger study cohort. Likewise, it cannot be excluded that DBP may differ in larger cohorts. However, many studies of smoking during pregnancy report higher SBP without changes in DBP,13, 14, 48, 49 which is in congruence with our results. Only a few studies have reported changes in DBP after maternal smoking.27, 48 The limited number of infants also complicates control of confounding factors that may be interpreted as associations. The relatively large differences in SBP warrant further studies to corroborate these findings. It is possible that snus‐using mothers represent a less healthy group than the tobacco‐free controls and that there may be other factors influencing associations in addition to use of alcohol and other drugs, which were asked for.
In contrast, the prospective approach of this study, with prenatal and postnatal data collection and repeated questionnaires during pregnancy about tobacco use and exposure, is a major strength, minimizing the risk of recall bias. The strict inclusion criteria, the study design, and a follow‐up for >5 to 6 years add strengths to the study.
In conclusion, our findings of increased SBP and altered HRV in preschool children, associated with exposure to snus in fetal life, may indicate adverse prenatal programming of nicotine, but the implications for cardiovascular health in later life remain to be studied. Meanwhile, women should be recommended to abstain from all types of tobacco and nicotine products during pregnancy.
Sources of Funding
This work was supported by the Swedish Medical Research Council (521‐2009‐4884), the Swedish Council for Working Life and Social Research (209‐1619), the Samaritan Foundation, Odd Fellows Grand Lodge Sweden and the Childhood Foundation of the Swedish Order of Freemasons.
Disclosures
None.
Acknowledgments
We would like to thank all parents and their children for participating in this study and the pediatric cardiology unit in Umeå.
(J Am Heart Assoc. 2019;8:e012629 DOI: 10.1161/JAHA.119.012629.)
References
- 1. Barker DJ. In utero programming of chronic disease. Clin Sci (London). 1998;95:115–128. [PubMed] [Google Scholar]
- 2. de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta‐analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander BT, Dasinger JH, Intapad S. Fetal programming and cardiovascular pathology. Compr Physiol. 2015;5:997–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thompson LP, Al‐Hasan Y. Impact of oxidative stress in fetal programming. J Pregnancy. 2012;2012:582748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morton JS, Cooke CL, Davidge ST. In utero origins of hypertension: mechanisms and targets for therapy. Physiol Rev. 2016;96:549–603. [DOI] [PubMed] [Google Scholar]
- 6. Lange S, Probst C, Rehm J, Popova S. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta‐analysis. Lancet Glob Health. 2018;6:e769–e776. [DOI] [PubMed] [Google Scholar]
- 7. Luck W, Nau H, Hansen R, Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther. 1985;8:384–395. [DOI] [PubMed] [Google Scholar]
- 8. Mei‐Dan E, Walfisch A, Weisz B, Hallak M, Brown R, Shrim A. The unborn smoker: association between smoking during pregnancy and adverse perinatal outcomes. J Perinat Med. 2015;43:553–558. [DOI] [PubMed] [Google Scholar]
- 9. Adams SM, Good MW, Defranco GM. Sudden infant death syndrome. Am Fam Physician. 2009;79:870–874. [PubMed] [Google Scholar]
- 10. Baba S, Wikstrom AK, Stephansson O, Cnattingius S. Influence of smoking and snuff cessation on risk of preterm birth. Eur J Epidemiol. 2012;27:297–304. [DOI] [PubMed] [Google Scholar]
- 11. Erickson AC, Arbour LT. Heavy smoking during pregnancy as a marker for other risk factors of adverse birth outcomes: a population‐based study in British Columbia, Canada. BMC Public Health. 2012;12:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chong DS, Yip PS, Karlberg J. Maternal smoking: an increasing unique risk factor for sudden infant death syndrome in Sweden. Acta Paediatr. 2004;93:471–478. [DOI] [PubMed] [Google Scholar]
- 13. Blake KV, Gurrin LC, Evans SF, Beilin LJ, Landau LI, Stanley FJ, Newnham JP. Maternal cigarette smoking during pregnancy, low birth weight and subsequent blood pressure in early childhood. Early Hum Dev. 2000;57:137–147. [DOI] [PubMed] [Google Scholar]
- 14. Cabral M, Fonseca MJ, Gonzalez‐Beiras C, Santos AC, Correia‐Costa L, Barros H. Maternal smoking: a life course blood pressure determinant? Nicotine Tob Res. 2018;20:674–680. [DOI] [PubMed] [Google Scholar]
- 15. Cohen G, Jeffery H, Lagercrantz H, Katz‐Salamon M. Long‐term reprogramming of cardiovascular function in infants of active smokers. Hypertension. 2010;55:722–728. [DOI] [PubMed] [Google Scholar]
- 16. Simonetti GD, Schwertz R, Klett M, Hoffmann GF, Schaefer F, Wuhl E. Determinants of blood pressure in preschool children: the role of parental smoking. Circulation. 2011;123:292–298. [DOI] [PubMed] [Google Scholar]
- 17. Nordenstam F, Lundell B, Edstedt Bonamy AK, Raaschou P, Wickstrom R. Snus users had high levels of nicotine, cotinine and 3‐hydroxycotinine in their breastmilk, and the clearance was slower than in smoking mothers. Acta Paediatr. 2019;108:1250–1255. [DOI] [PubMed] [Google Scholar]
- 18. Gunnerbeck A, Raaschou P, Cnattingius S, Edstedt Bonamy AK, Wickstrom R. Maternal snuff use and cotinine in late pregnancy: a validation study. Acta Obstet Gynecol Scand. 2018;97:1373–1380. [DOI] [PubMed] [Google Scholar]
- 19. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school‐aged children and adolescents. Bull World Health Organ. 2007;85:660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 21. Flynn JT, Kaelber DC, Baker‐Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. [DOI] [PubMed] [Google Scholar]
- 22. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 23. Nordenstam F, Lundell B, Cohen G, Tessma MK, Raaschou P, Wickstrom R. Prenatal exposure to snus alters heart rate variability in the infant. Nicotine Tob Res. 2017;19:797–803. [DOI] [PubMed] [Google Scholar]
- 24. Stephan‐Blanchard E, Chardon K, Djeddi DD, Leke A, Delanaud S, Bach V, Telliez F. The dynamics of cardiac autonomic control in sleeping preterm neonates exposed in utero to smoking. Clin Neurophysiol. 2016;127:2871–2877. [DOI] [PubMed] [Google Scholar]
- 25. Oken E, Huh SY, Taveras EM, Rich‐Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res. 2005;13:2021–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brion MJ, Leary SD, Smith GD, Ness AR. Similar associations of parental prenatal smoking suggest child blood pressure is not influenced by intrauterine effects. Hypertension. 2007;49:1422–1428. [DOI] [PubMed] [Google Scholar]
- 27. Hogberg L, Cnattingius S, Lundholm C, D'Onofrio BM, Langstrom N, Iliadou AN. Effects of maternal smoking during pregnancy on offspring blood pressure in late adolescence. J Hypertens. 2012;30:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morley R, Leeson Payne C, Lister G, Lucas A. Maternal smoking and blood pressure in 7.5 to 8 year old offspring. Arch Dis Child. 1995;72:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yong LC, Kuller LH, Rutan G, Bunker C. Longitudinal study of blood pressure: changes and determinants from adolescence to middle age: the Dormont High School follow‐up study, 1957–1963 to 1989–1990. Am J Epidemiol. 1993;138:973–983. [DOI] [PubMed] [Google Scholar]
- 30. Nelson MJ, Ragland DR, Syme SL. Longitudinal prediction of adult blood pressure from juvenile blood pressure levels. Am J Epidemiol. 1992;136:633–645. [DOI] [PubMed] [Google Scholar]
- 31. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta‐regression analysis. Circulation. 2008;117:3171–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cohen G, Vella S, Jeffery H, Lagercrantz H, Katz‐Salamon M. Cardiovascular stress hyperreactivity in babies of smokers and in babies born preterm. Circulation. 2008;118:1848–1853. [DOI] [PubMed] [Google Scholar]
- 33. Xiao D, Xu Z, Huang X, Longo LD, Yang S, Zhang L. Prenatal gender‐related nicotine exposure increases blood pressure response to angiotensin II in adult offspring. Hypertension. 2008;51:1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kooijman MN, Bakker H, Franco OH, Hofman A, Taal HR, Jaddoe VW. Fetal smoke exposure and kidney outcomes in school‐aged children. Am J Kidney Dis. 2015;66:412–420. [DOI] [PubMed] [Google Scholar]
- 36. Taal HR, Geelhoed JJ, Steegers EA, Hofman A, Moll HA, Lequin M, van der Heijden AJ, Jaddoe VW. Maternal smoking during pregnancy and kidney volume in the offspring: the Generation R Study. Pediatr Nephrol. 2011;26:1275–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Geerts CC, Bots ML, Grobbee DE, Uiterwaal CS. Parental smoking and vascular damage in young adult offspring: is early life exposure critical? The atherosclerosis risk in young adults study. Arterioscler Thromb Vasc Biol. 2008;28:2296–2302. [DOI] [PubMed] [Google Scholar]
- 38. Asmussen I, Kjeldsen K. Intimal ultrastructure of human umbilical arteries: observations on arteries from newborn children of smoking and nonsmoking mothers. Circ Res. 1975;36:579–589. [DOI] [PubMed] [Google Scholar]
- 39. Xiao D, Huang X, Yang S, Zhang L. Antenatal nicotine induces heightened oxidative stress and vascular dysfunction in rat offspring. Br J Pharmacol. 2011;164:1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baba S, Wikstrom AK, Stephansson O, Cnattingius S. Changes in snuff and smoking habits in Swedish pregnant women and risk for small for gestational age births. BJOG. 2013;120:456–462. [DOI] [PubMed] [Google Scholar]
- 41. Riedel C, Schonberger K, Yang S, Koshy G, Chen YC, Gopinath B, Ziebarth S, von Kries R. Parental smoking and childhood obesity: higher effect estimates for maternal smoking in pregnancy compared with paternal smoking—a meta‐analysis. Int J Epidemiol. 2014;43:1593–1606. [DOI] [PubMed] [Google Scholar]
- 42. McGavock JM, Torrance B, McGuire KA, Wozny P, Lewanczuk RZ. The relationship between weight gain and blood pressure in children and adolescents. Am J Hypertens. 2007;20:1038–1044. [DOI] [PubMed] [Google Scholar]
- 43. The Swedish National Board of Health and Welfare . Statistics on pregnancies, deliveries and newborn infants 2016. 2018:4. Available at: https://sdb.socialstyrelsen.se/if_mfr_004/resultat.aspx. Accessed March 12, 2019.
- 44. Baba S, Wikstrom AK, Stephansson O, Cnattingius S. Influence of snuff and smoking habits in early pregnancy on risks for stillbirth and early neonatal mortality. Nicotine Tob Res. 2014;16:78–83. [DOI] [PubMed] [Google Scholar]
- 45. England LJ, Levine RJ, Mills JL, Klebanoff MA, Yu KF, Cnattingius S. Adverse pregnancy outcomes in snuff users. Am J Obstet Gynecol. 2003;189:939–943. [DOI] [PubMed] [Google Scholar]
- 46. Dahlin S, Gunnerbeck A, Wikstrom AK, Cnattingius S, Edstedt Bonamy AK. Maternal tobacco use and extremely premature birth—a population‐based cohort study. BJOG. 2016;123:1938–1946. [DOI] [PubMed] [Google Scholar]
- 47. Wickstrom R. Effects of nicotine during pregnancy: human and experimental evidence. Curr Neuropharmacol. 2007;5:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Taal HR, de Jonge LL, van Osch‐Gevers L, Steegers EA, Hofman A, Helbing WA, van der Heijden AJ, Jaddoe VW. Parental smoking during pregnancy and cardiovascular structures and function in childhood: the Generation R Study. Int J Epidemiol. 2013;42:1371–1380. [DOI] [PubMed] [Google Scholar]
- 49. Lawlor DA, Najman JM, Sterne J, Williams GM, Ebrahim S, Davey Smith G. Associations of parental, birth, and early life characteristics with systolic blood pressure at 5 years of age: findings from the Mater‐University study of pregnancy and its outcomes. Circulation. 2004;110:2417–2423. [DOI] [PubMed] [Google Scholar]


