Abstract
Background
Existing data on predictors of late mortality and prevention of sudden cardiac death after atrial switch repair surgery for D‐transposition of the great arteries (D‐TGA) are heterogeneous and limited by statistical power.
Methods and Results
We conducted a systematic review and meta‐analysis of 29 observational studies, comprising 5035 patients, that reported mortality after atrial switch repair with a minimum follow‐up of 10 years. We also examined 4 additional studies comprising 105 patients who reported rates of implantable cardioverter‐defibrillator therapy in this population. Average survival dropped to 65% at 40 years after atrial switch repair, with sudden cardiac death accounting for 45% of all reported deaths. Mortality was significantly lower in cohorts that were more recent and operated on younger patients. Patient‐level risk factors for late mortality were history of supraventricular tachycardia (odds ratio [OR] 3.8, 95% CI 1.4–10.7), Mustard procedure compared with Senning (OR 2.9, 95% CI 1.9–4.5) and complex D‐TGA compared with simple D‐TGA (OR 4.4, 95% CI 2.2–8.8). Significant risk factors for sudden cardiac death were history of supraventricular tachycardia (OR 4.7, 95% CI 2.2–9.8), Mustard procedure (OR 2.2, 95% CI 1.1–4.1), and complex D‐TGA (OR 5.7, 95% CI 1.8–18.0). Out of a total 124 implantable cardioverter‐defibrillator discharges over 330 patient‐years in patients with implantable cardioverter‐defibrillators for primary prevention, only 8% were appropriate.
Conclusions
Patient‐level risk of both mortality and sudden cardiac death after atrial switch repair are significantly increased by history of supraventricular tachycardia, Mustard procedure, and complex D‐TGA. This knowledge may help refine current selection practices for primary prevention implantable cardioverter‐defibrillator implantation, given disproportionately high rates of inappropriate discharges.
Keywords: atrial switch, D‐transposition of the great arteries, long‐term outcomes, mustard, senning, sudden cardiac death
Subject Categories: Meta Analysis, Mortality/Survival, Cardiovascular Surgery, Sudden Cardiac Death, Congenital Heart Disease
Clinical Perspective
What Is New?
Sudden cardiac death is the leading cause of late mortality in patients who have undergone atrial switch repair for D‐transposition of the great arteries) and is responsible for 45% of long‐term deaths reported in pooled data from the current literature.
Patient‐level risk of both late mortality and sudden cardiac death are significantly increased by a history of supraventricular tachycardia, complex D‐transposition of the great arteries, and Mustard procedure
Patients who have received implantable cardioverter‐defibrillators for primary prevention of sudden cardiac death after atrial switch repair suffer from high rates of inappropriate discharges with comparatively low rates of appropriate discharges.
What Are the Clinical Implications?
Current selection algorithms for implantable cardioverter‐defibrillator implantation for primary prevention of sudden cardiac death (SCD) after atrial switch repair are in need of improvement, and our identification of three unique risk factors for SCD not routinely considered in the selection process may help in its revision.
Our finding that a history of supraventricular tachyarrhythmias confers a 5‐fold risk of SCD carries important implications for SCD prevention.
It is possible that aggressive upfront management of supraventricular tachyarrhythmias using catheter ablation and/or antiarrhythmic pharmacotherapy might mitigate SCD risk, and additional primary data are required to investigate this hypothesis.
Introduction
Surgical management of D‐transposition of the great arteries (D‐TGA) has evolved since the era of palliative shunting. The atrial switch repair (ASR), using native tissue as described by Senning in 19591 or Gore‐tex patch material as described by Mustard in 1964,2 was the procedure of choice until the late 1980s, when it was largely supplanted by the arterial switch operation initially described by Jatene et al.3 In the present day, there remains a large cohort of adult D‐TGA patients who underwent ASR in childhood. These individuals are at risk of ASR‐specific complications of the atrial baffles and the systemic right ventricle (RV).4, 5, 6, 7 The current literature consists of numerous observational, mostly single‐center cohort studies, with marked heterogeneity in mortality rates as well as rates of adverse outcomes, including congestive heart failure (CHF), baffle obstruction and valvular dysfunction. Identifying risk factors for long‐term mortality is of paramount importance to risk stratify patients for better surveillance and treatment.
Sudden cardiac death (SCD) has been reported as a leading cause of mortality in patients after ASR,6, 8, 9 with a reported lifetime incidence as high as 15%.10 Proposed risk factors for SCD11 include supraventricular tachyarrhythmias (SVT),6, 10, 12 right ventricular dysfunction (RVD),10, 12 atrioventricular block,13 tricuspid regurgitation (TR),9, 12 and QTc dispersion on electrocardiography.14
Current ASR publications are limited by population size and low absolute event rates. There is at present no uniform risk stratification scheme that predicts risk of SCD in the ASR population. Furthermore, current practice guidelines for implantable cardioverter‐defibrillator (ICD) placement in adults with systemic right ventricles are based on scant data,15 with devices being placed on a case‐by‐case basis by extrapolating from guidelines for adults with ischemic cardiomyopathy.16, 17 As such, the therapeutic benefit of ICD implantation in ASR patients remains unclear.11 This has been recognized by the 2016 joint report by the National Heart, Lung and Blood Institute and the Adult Congenital Heart Association as a high‐impact and priority area for further research.18 The most recent guideline for the management of adults with congenital heart disease also recognizes the lack of research in this area and notes that RVD on its own is not adequate to guide ICD implantation in these patients.19
To better understand this adult ASR population, we conducted a systematic review and meta‐analysis of the published literature with the following goals:
examine the rates of long‐term mortality, SCD and morbidity outcomes among patients who had an ASR with either the Mustard or Senning procedure;
identify risk factors for long‐term mortality and SCD; and
estimate the efficacy and risks of ICDs for SCD prevention in this population.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Prospective Registration
This systematic review was conducted in adherence with guidelines stipulated by the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement20 and the Meta‐analysis Of Observational Studies in Epidemiology checklist.21
The study protocol was registered on the PROSPERO international prospective register of systematic reviews (CRD42016045556) and can be accessed at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=45556.
Eligibility Criteria
All studies describing mortality of patients with D‐TGA after ASR with a mean or median follow‐up period of at least 10 years were eligible. These included patients with complex D‐TGA, most commonly defined in the literature as D‐TGA with a concomitant ventricular septal defect, pulmonic stenosis, or other left ventricular outflow obstruction. Also eligible were studies describing incidence of ICD discharges in patients with D‐TGA and ASR who received an ICD for prevention of SCD. Given the absence of randomized controlled trials, we included cohort studies as the best available evidence. The most recent search of the literature for eligible studies was conducted in November 2018; all studies published up until November 2018 were hence eligible for consideration.
Exclusion criteria were as follows:
Operative cohort containing patients with concomitant complex congenital lesions (with the exception of left ventricular outflow obstruction), such as Tetralogy of Fallot or single ventricle anatomy.
ASR done as part of a double‐switch procedure for congenitally corrected transposition of the great arteries.
ASR done for palliation of cyanosis in patients with large ventricular septal defect and severe pulmonic stenosis rendering their D‐TGA irreparable (ie, palliative atrial switch).
Study including patients from a center from where there was another study meeting inclusion criteria. In this case, the study describing a larger cohort of patients and/or containing additional individual patient‐level outcomes data was included.
Fewer than 5 patients in the study.
Study written in a language other than English for which an interpreter was not available to perform data extraction.
Search Strategy
We searched multiple databases including Cochrane, MEDLINE, OvidSP, Embase, and Web of Science Core Collection. Additional records were identified by use of the Cited Reference Search in Web of Science and by review of conference proceedings, which included unpublished data. We placed no language or date restrictions on the search strategy. All titles dated November 2018 or earlier were included in the search. We searched for studies examining long‐term outcomes after ASR as well as outcomes after ICD implantation in the post‐ASR population. The complete list of databases examined, and detailed search strategy are available in Data S1.
Screening and Data Extraction
Both screening and data extraction were done by 2 independent reviewers (PV, RAP) with conflicts being resolved by a third independent reviewer (HSS). Assistance from native speakers was sought for studies published in a language other than English. If no native speaker was available and an English language abstract was available, then the abstract was used for screening and data extraction.
Screening was done in a 2‐stage process. The first stage involved screening the titles and abstracts for the definitive presence of any of the aforementioned exclusion criteria. Citations lacking this were carried forward to the second stage, where full‐text review of each study was conducted. Studies fulfilling all eligibility criteria were then included for data extraction.
We extracted data using a pre‐specified data collection schema on study design characteristics, baseline characteristics of the operative cohort, degree of loss to follow‐up, and patient outcomes.
The primary outcome examined was late mortality, defined uniformly in the available literature as death occurring at least 30 days after ASR. Secondary outcomes included SCD, SVT, ventricular tachyarrhythmias, sinus node dysfunction (SND), RVD, incidence of CHF, reintervention, baffle obstruction, and TR.
For studies describing efficacy of ICDs in the post‐ASR population, the primary outcome of interest was the rate of appropriate shock, while secondary outcomes included rate of inappropriate shock, lead fracture or dislodgment, and ICD‐related infections. Studies were also evaluated for analysis of risk of late mortality or SCD and when reported, all data pertaining to risk analysis including but not limited to odds ratios, risk ratios, hazard ratios and log‐rank statistics were extracted.
Quality Assessment
Study quality and risk of bias were assessed independently by 2 reviewers (PV and RAP) based on a modified version of the Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies devised by the National Heart, Lung, and Blood Institute.22 The tool was modified to address the common sources of bias and potential flaws specific to these survival studies. The major components of the quality assessment tool addressed consecutive enrollment of participants, absence of age cut‐offs at enrollment, which may adversely influence survival outcome (eg, retrospective enrollment of adults of a certain age), and the consideration of confounding factors such as the era of surgery during statistical analysis. The complete questionnaire used is detailed in Table S1.
Statistical Analysis
To account for heterogeneity across published cohorts, aggregate event rates for primary and secondary outcomes were reported as median values with interquartile ranges. Means and standard deviations were reported only when there was no significant heterogeneity across studies.
Cohort risk assessment of late mortality or SCD was performed using univariate linear regression, which was weighted by study size given significant heterogeneity in this regard, such that larger studies contributed greater weight to the regression. Sensitivity analysis was performed if the data being analyzed included an outlier. Available patient level data were entered for risk factors of late mortality and SCD into a random effects meta‐analysis model, and a pooled summary estimate was obtained for each variable after odds ratios for mortality and/or SCD were individually calculated. Fixed effects meta‐analysis was also used for comparison. Studies with insufficient primary patient‐level data to permit calculation of odds ratios were not used in the model. In addition, we analyzed the strength of association for each risk factor with the outcomes of late mortality and SCD across the different studies, using both the Cochran Mantel‐Haenszel test and binary logistic regression with Firth correction to correct for low event rates.
Statistical analyses were performed using SPSS Statistics for Mac, Version 25.0 (IBM Corp, Armonk, New York, USA), Stata 14 (StataCorp, College Station, Texas, USA) and Review Manager (RevMan) Version 5.3 (The Cochrane Collaboration, Copenhagen, Denmark).
Results
Selection of Studies
Our initial search yielded 6508 citations describing long‐term mortality and 135 citations potentially describing outcomes after ICD implantation. Of the 6643 citations, 312 met primary inclusion criteria and underwent full text screening. In the secondary review, 278 studies were excluded, primarily because of inadequate follow‐up or a lack of mortality outcomes data. At the end of the screening process, 29 studies4, 6, 7, 9, 13, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 met criteria for long‐term mortality, and 4 additional studies8, 47, 48, 49 met criteria for ICD outcomes (Figure 1). One study met criteria for both long‐term mortality and ICD outcomes.9 One study was only available as a conference abstract,37 while the rest were obtained in manuscript form. Successful translation was performed for the 2 non‐English language studies that met inclusion criteria.26, 28
Figure 1.
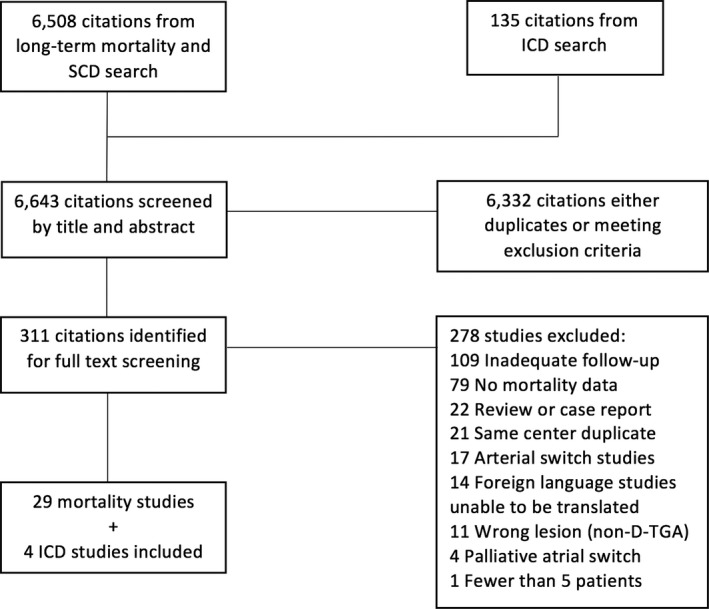
Flow diagram summarizing selection process for inclusion of studies. D‐TGA indicates D‐transposition of the great arteries; ICD, implantable cardioverter‐defibrillator; SCD, Sudden cardiac death.
Study Characteristics
The 29 studies with data on mortality included 5035 patients, of which 4588 (91%) survived beyond 30 days after ASR. There were 4409 patients with available long‐term follow‐up for the primary study end point of mortality. The studies were conducted in 18 different countries across 3 continents, with 20 studies conducted in Europe. All were retrospective observational cohort studies, except for a single prospective cohort study.29 Only 4 studies4, 30, 44, 46 involved more than one center.
The studies varied in population size, proportion of complex D‐TGA patients in the operative cohort, age of patients at ASR, era during which ASR was performed, preferred type of ASR, and duration of follow‐up (Table 1). Study sample sizes ranged from 8 to 534 with a median of 109 (interquartile range [IQR] 85–222). The median follow‐up time reported by each study ranged from 10 years (the minimum to be eligible for inclusion) to 35 years after ASR (Table 1).
Table 1.
Baseline Characteristics of Included Studies Reporting Late Mortality Data
| Study | Year Published | Number of Patients With Complete Follow‐Up | Time Period of Surgeries | Single‐ or Multi‐Center | Cardio‐Plegia Use | Mean Age of Cohort at Surgery (mo) | Proportion of Patients With Mustard Procedure (%) | Proportion of Patients With Complex D‐TGA (%) | Follow‐Up Duration (mean or median) (y) | Loss to Follow‐Up for Late Mortality (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ashraf et al24 | 1986 | 106 | 1967 to 1976 | S | No | 12 | 100 | 48.1 | 10.9 | 0 |
| Turley et al43 | 1988 | 36 | 1975 to 1980 | S | No | 1.5 | 100 | 0 | 10 | 0 |
| Merlo et al39 | 1991 | 104 | 1971 to 1978 | S | N/A | 16.8 | 83.7 | 15.4 | 12 | 0 |
| Helbing et al35 | 1994 | 112 | 1961 to 1987 | S | Yes | N/A | 49.2 | 27.1 | 16 | 8.2 |
| Myridakis40 | 1994 | 74 | 1971 to 1985 | S | Yes | 17.5 | 100 | 26.3 | N/A | 2.6 |
| Gelatt13, 33 | 1997 | 478 | 1963 to 1993 | S | Yes | 15.6 | 100 | 34.1 | 11.5 | 0.8 |
| Birnie et al25 | 1998 | 93 | 1972 to 1988 | S | N/A | N/A | 76.8 | N/A | 10 | 4.2 |
| Wilson et al7 | 1998 | 113 | 1964 to 1982 | S | Yes | 13 | 100 | 0 | 19.7 | 0 |
| Sarkar6 | 1999 | 358 | 1965 to 1992 | S | Yes | 19.2 | 63.1 | 0 | 12.3 | 0 |
| Genoni et al32 | 1999 | 228 | 1962 to 1987 | S | Yes | 46 | 0 | 49.4 | 13.7 | 4.6 |
| Kirjavainen et al36 | 1999 | 98 | 1978 to 1988 | S | No | 7 | 0 | 25 | 12.8 | 0 |
| Carrel and Pfammatter27 | 2000 | 189 | 1970 to 1993 | S | N/A | N/A | N/A | N/A | 16 | 0 |
| Moons et al4 | 2004 | 283 | 1970 to 1998 | M | N/A | 9.7 | 36.6 | 28.0 | 17.1 | 0 |
| Agnetti et al23 | 2004 | 70 | 1978 to 1987 | S | No | 7 | 0 | 7.1 | 19 | 0 |
| Borowicka et al26 | 2004 | 8 | N/A | S | N/A | 10 | 0 | N/A | 12.6 | 0 |
| Dos et al31 | 2005 | 137 | 1973 to 1997 | S | N/A | 14 | N/A | 13.9 | 16.7 | 20.8 |
| Lange et al38 | 2006 | 374 | 1974 to 2001 | S | N/A | 14.8 | 20.1 | 36.5 | 19.1 | 5.3 |
| Chaloupecky et al28 | 2006 | 168 | 1984 to 1997 | S | N/A | 6 | 0 | 20.8 | 14 | 0 |
| Rekhraj and Freeman41 | 2007 | 20 | N/A | S | N/A | N/A | 70 | 50 | 29 | 9.1 |
| Ebenroth 32 | 2007 | 44 | 1970 to 1986 | S | N/A | N/A | 100 | N/A | 24 | 2.2 |
| Gorler et al34 | 2010 | 215 | 1973 to N/A | S | N/A | 17 | 96.4 | 35.6 | 16 | 0 |
| Roubertie 42 | 2011 | 125 | 1977 to 2004 | S | Yes | 11.6 | 0 | 20.8 | 19.5 | 0 |
| Knez37 | 2011 | 79 | N/A | S | N/A | N/A | 41.8 | N/A | 17.6 | N/A |
| Dobson et al30 | 2013 | 92 | N/A | M | N/A | N/A | 82.5 | N/A | 28.8 | 5.2 |
| Cuypers et al29 | 2014 | 69 | 1973 to 1980 | S | Yes | 22.3 | 100 | 39.6 | 35 | 19.8 |
| Wheeler et al9 | 2014 | 78 | N/A | S | N/A | 14 | 68.0 | 26.9 | 30 | 12.4 |
| Vejlstrup et al44 | 2015 | 371 | 1967 to 2003 | M | N/A | 22.8 | 66.2 | 32.1 | 26.1 | 1.1 |
| Dennis et al45 | 2018 | 83 | N/A | S | N/A | 17 | N/A | N/A | 10.1 | 0 |
| Kiener et al46 | 2018 | 257 | 1982 to 1991 | M | Yes | 5.6 | 38.9 | 25.3 | 26.5 | 20.6 |
D‐TGA indicates D‐transposition of the great arteries; M, multicenter; N/A, data not available or not applicable; S, single center.
The definition of complex D‐TGA, while not uniform across studies, was stated in most studies as D‐TGA with either a concomitant ventricular septal defect or left ventricular outflow obstruction, including pulmonic stenosis. Four studies4, 25, 41, 46 expanded the definition to include patients with coarctation of the aorta, while one study24 included atrial septal defect and ventricular septal aneurysm. Three studies only included patients with simple (defined as non‐complex) D‐TGA.6, 7, 43
The median time interval between the first and last surgery in each cohort was 17 years (IQR 9–26). Changes in surgical technique and patient selection during the ASR era occurred in many cohorts. Cold cardioplegia was adopted in the mid‐late 1970s, with 6 centers reporting on undergoing the transition from hypothermic circulatory arrest to cold cardioplegia.13, 29, 33, 35, 40, 42 Three centers reported on changing their ASR procedure of choice,4, 6, 31 while others exclusively performed the Mustard (7 centers) or the Senning (6 centers) operation (Table 1). The mean age of patients at the time of ASR also varied among studies (Table 1), with a significant trend towards earlier age at ASR in later decades (r=0.65, P=0.004, Figure S1). Of note, when a sensitivity analysis was performed excluding the study by Genoni et al, which was a clear outlier with a mean age of 46 months,33 the trend remained significant (r=0.62, P=0.01).
Quality Assessment
Assessment of study quality and risk of bias is detailed in Table S2. While most studies enrolled consecutive patients undergoing ASR, 4 retrospective studies9, 30, 41, 45 mandatorily recruited only adult patients instead of all post‐ASR cases, thus creating selection bias. Only 16 (55%) of the 29 studies offered data that allowed statistical analysis of risk factors for adverse outcome. Loss to follow‐up for the primary outcome of mortality was low for most studies (median 0.4%, IQR 0–5.2). The majority of studies (15 of 29) reported additional loss to follow‐up for secondary outcomes, including arrhythmia development, RVD and TR. Mean loss to follow‐up for arrhythmia assessment per cohort was 6% (median 0%, IQR 0–4), while mean loss to follow‐up for echocardiographic assessment was 10% (median 0%, IQR 0–10).
Mortality
Late mortality ranged widely across studies from 3% to 24%, with a median of 11% (IQR 9–17). The median annual incidence of late mortality was 0.7% (IQR 0.5–1.1). When averaged across studies, survival post‐ASR was 91% at 10 years, 86% at 20 years, 76% at 30 years, and 65% at 40 years.
Mean age at time of death varied across cohorts from 7 to 34 years. For most studies where the data were available, the median age at time of death was over 10 years.6, 7, 25, 31, 32, 45
Out of the 4409 patients followed up across the 29 studies, there were 642 (14%) reported deaths. Data on etiologies of individual cases of late mortality were available in 25 studies describing a total of 413 deaths (64% of total reported deaths). Overall, cardiac causes accounted for 89% of all late mortality. SCD, uniformly defined as deaths that were either definitively or presumably arrhythmogenic with no autopsy data to suggest a non‐arrhythmogenic cause, was the most common cause of late mortality, responsible for 45% of total late deaths (Figure 2A). Median annual incidence of SCD was 0.2% (IQR 0.17–0.38) with cumulative cohort incidence ranging from 2% to 13% (Table S3).6 Data from autopsies of SCD cases were described in 2 manuscripts6, 7 and were unrevealing, apart from individual examples of RV fibroelastosis and pulmonary hypertension changes.
Figure 2.
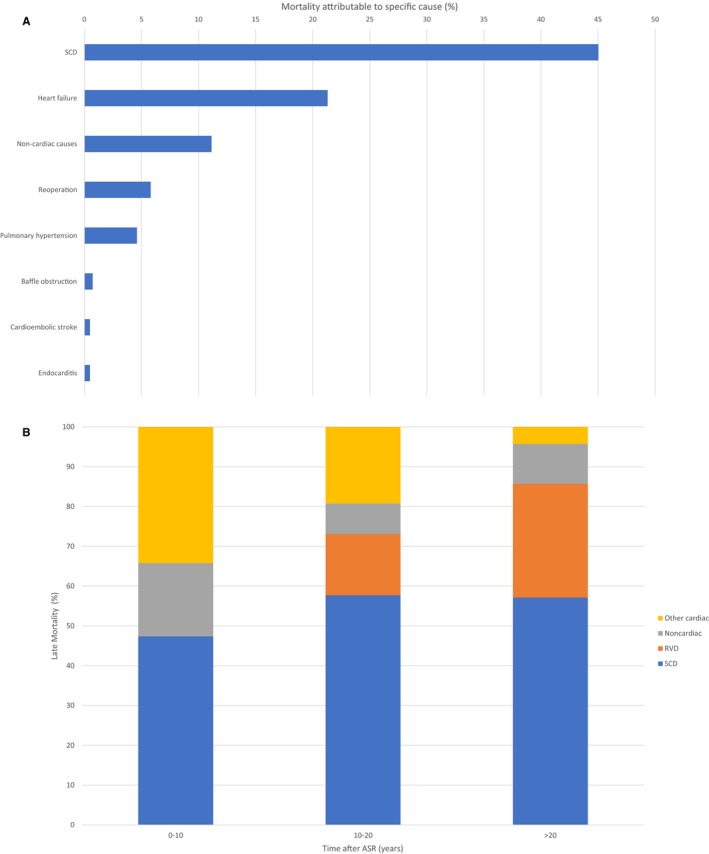
A, Causes of late mortality from pooled patient‐level data across included studies (n=413 deaths). SCD indicates sudden cardiac death. B, Causes of late mortality from patient‐level data stratified by time after atrial switch repair (n=78). Other cardiac etiologies include baffle obstruction, reoperation, pulmonary hypertension, cardioembolic stroke, and endocarditis. ASR indicates atrial switch repair; RVD, right ventricular dysfunction; SCD, sudden cardiac death.
Congestive heart failure from RVD was the second most common cause of mortality, responsible for 21% of deaths. Other reported causes of late mortality included pulmonary hypertension, complications from reoperation, and venous baffle obstruction (Figure 2A). SCD was also the most common cause of death in each postoperative decade. RVD did not cause any deaths in the first postoperative decade but was responsible for progressively more deaths as time after ASR elapsed. In contrast, other cardiac etiologies of death, including baffle obstruction and reoperation, accounted for a greater proportion of fatalities in the first decade after ASR, but progressively lower proportion in the second and third post‐surgical decades (Figure 2B). Non‐cardiac etiologies accounted for 11% of the reported mortality (Figure 2A).
Arrhythmias
All but 3 of the 29 studies27, 44, 46 reported development of arrhythmias during the follow‐up period. The median arrhythmia‐free survival at 10 years after ASR was 58% (IQR 46–61) and at 20 years after ASR was 40% (IQR 38–63). Non‐sinus rhythm was commonly reported with a median incidence per cohort of 54% (IQR 28–61). SND had a median incidence of 30% (IQR 24–60) and junctional rhythm of 20% (IQR 13–33). Third degree atrioventricular‐nodal heart block was rare, occurring in a median of 1% of patients per cohort (IQR 0.8–1.5). Permanent pacemakers were implanted in a median of 10% of patients per cohort (IQR 6–17), with SND being the most common indication.
SVT events were reported in a median of 14% of patients per cohort (range 0–47%, IQR 8–22) (Table S3). Cumulative patient‐level data from 2143 patients showed a similar SVT incidence of 14%, with atrial flutter accounting for 83% of SVT. Atrial fibrillation and junctional tachycardia were reported less frequently. Data regarding ventricular tachycardia (VT) was only provided by 9 studies; median incidence throughout the follow‐up period was 3% (IQR 0–4).
Systemic Right Ventricle
Function of the systemic RV late after ASR was documented in 25 of 29 studies. Transthoracic echocardiography was the standard means of assessing RV function, with only 3 studies reporting magnetic resonance imaging data.29, 30, 42 Cardiac catheterization and radionuclide ventriculography were used as supplementary modalities in 313, 23, 40 and 54, 7, 13, 32, 42 studies, respectively.
The incidence of RVD during the follow‐up time ranged from 7% to 98%, with a median of 19% per cohort (IQR 11–58). Among aggregated patient‐level data, RVD was documented during follow‐up in 356 (33%) of 1070 patients. The severity of RVD among these 356 patients was considered mild in 37%, moderate in 44%, and severe in 19%. The median incidence of heart failure with New York Heart Association functional class III or IV was only 4% per cohort (IQR 1–5).39
The reported incidence of moderate or severe TR ranged from 2% to 35% (Table S3), with median of 9% (IQR 6–20). Median incidence of severe TR was 3% (IQR 2–8).
Reinterventions
27 studies, comprising 3990 patients, reported data on 433 reinterventions, with a median reintervention incidence of 8% per cohort (IQR 3–12). Out of these, only 33 (8%) were percutaneous interventions, with the rest being surgical. 56% of reinterventions were baffle repairs for baffle obstruction (80% of baffle complications) or less commonly baffle leak (20% of baffle complications), with tricuspid valve surgery being the next most common indication, accounting for 7% of reinterventions. Notably, 24 PA band placements, 9 arterial switch conversions, and 14 heart transplants were conducted across cohorts (Figure S2). Reintervention mortality was 6% (29 of 433).
Predictors of Mortality and Sudden Cardiac Death
Among the studies that assessed for risk factors of late mortality, the significant predictors of mortality included operating at an earlier surgical era, presence of complex lesions or need for ventricular septal defect closure, history of SVT, and RVD or CHF (Table S4). Only 3 studies assessed for risk factors of SCD.6, 9, 13 The presence of atrial tachyarrhythmias at any point during follow‐up was found to significantly increase SCD risk from 4‐fold to 21‐fold in all 3 studies. Additionally, Gelatt et al reported increased SCD risk with smaller infant size at operation and permanent heart block,13 while Wheeler et al reported older age at surgery, presence of at least moderate TR, and history of CHF as risk factors for SCD.9
Annual late mortality was found to be significantly lower for more recent cohorts (Figure 3A), and for cohorts with a younger mean age at time of ASR (Figure 3B), which positively correlated with borderline significance. When examining the relationship of late mortality to mean age at the time of ASR, the study by Genoni 33 was a clear outlier (Figure 3B). After dropping this study as part of a sensitivity analysis, there was a significant correlation of mean age at ASR and annual late mortality (Figure 3C). Annual incidence of SCD was found to be significantly lower for more recent operative cohorts (Figure 3D). No other cohort‐level factors showed significant correlation with incidence of late mortality or SCD (Table S5).
Figure 3.
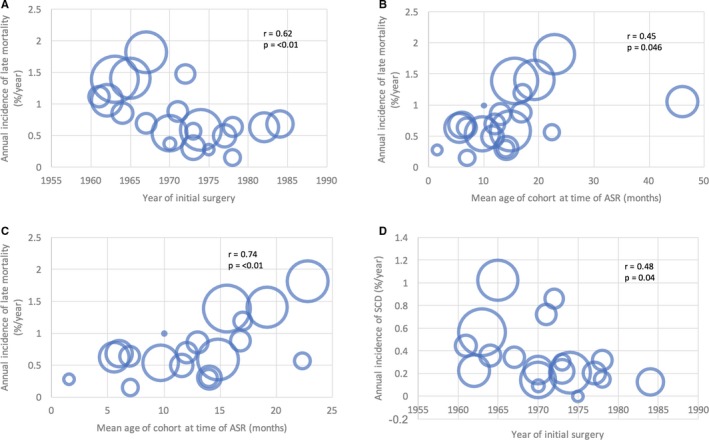
Scatterplots showing correlations between annual incidence of late mortality and (A) year of initial ASR surgery, (B) mean age of cohort at ASR and (C) mean age of cohort at ASR with exclusion of Genoni et al. (D) demonstrates correlation between annual incidence of SCD and year of initial surgery. Size of bubbles represent sample size of individual studies. P values and r values were obtained by linear regression weighted by study size. ASR indicates atrial switch repair; SCD, sudden cardiac death.
From our random‐effects meta‐analysis model, the odds of late mortality were increased by history of SVT (odds ratio [OR] 3.8, 95% CI 1.4–10.7, I2 0%), Mustard procedure compared with Senning (OR 2.9, 95% CI 1.9–4.5, I2 13) and complex D‐TGA compared with simple D‐TGA (OR 4.4, 95% CI 2.2–8.8, I2 31%) (Figure 4). Significant risk factors for SCD were history of SVT (OR 4.7, 95% CI 2.2–9.8, I2 0%), Mustard procedure (OR 2.2, 95% CI 1.1–4.1, I2 0%), and complex D‐TGA (OR 5.7, 95% CI 1.8–18.0, I2 0%) (Figure 5). Fixed effects meta‐analysis computations revealed similar results and are provided in the appendix (Figures S3 and S4). Cochran Mantel‐Haenszel testing showed the presence of the same significant associations as described in Figures 4 and 5, while the association between RVD and SCD was not statistically significant (Table S6). Additionally, binary logistic regression performed for each individual relationship of risk factor to outcome showed that after adjusting for the study cohort, odds of both late mortality and SCD were increased by history of SVT, Mustard procedure, and complex D‐TGA, while once again, RVD did not significantly predict SCD (Table S7).
Figure 4.
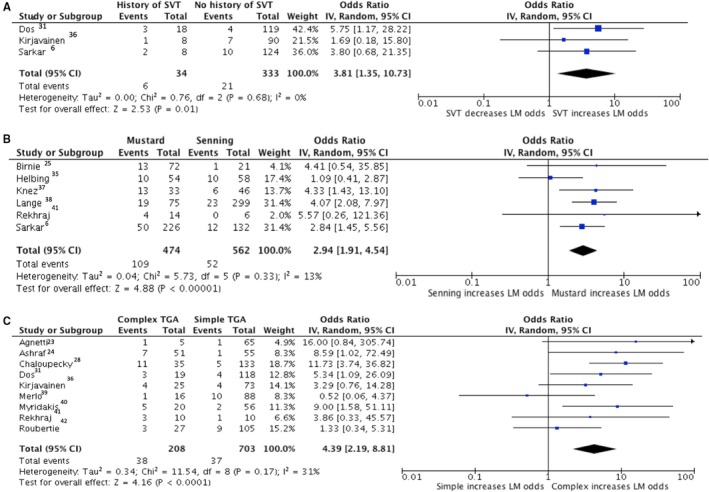
Forest plots showing pooled odds ratios of SVT (A), Mustard procedure (B) and complex D‐TGA (C) for late mortality using a random effects meta‐analysis approach. D‐TGA indicates D‐transposition of the great arteries; LM, late mortality; SVT, supraventricular tachycardia.
Figure 5.
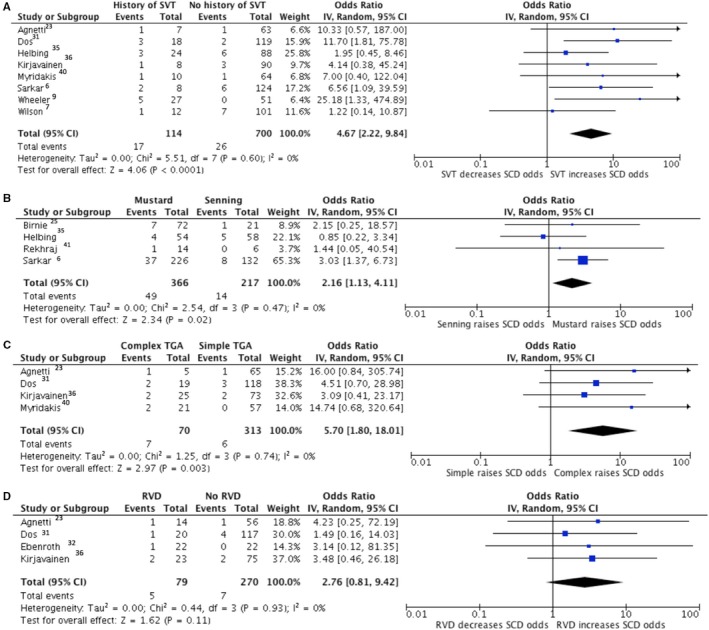
Forest plots showing pooled odds ratios of SVT (A), Mustard procedure (B), Complex D‐TGA (C) and RVD (D) for SCD using a random effects meta‐analysis approach. D‐TGA indicates D‐transposition of the great arteries; SCD, sudden cardiac death; SVT, supraventricular tachycardia; RVD, right ventricular dysfunction.
Implantable Cardioverter‐Defibrillator
Five studies, comprising a total of 105 patients, met inclusion criteria for evaluating ICD outcomes.8, 9, 47, 48, 49 One of the 105 patients died immediately after ICD placement; 104 were followed to assess outcomes for an average follow‐up time of about 4 years. ICDs were implanted at a mean age range of 26 to 31 years, with 79% (83 devices) implanted for primary prevention of SCD. Among those who received ICDs for primary prevention, 48% had severe RVD, 40% non‐sustained VT on Holter monitoring, 25% inducible VT on electrophysiological study, and 26% a history of syncope (some patients had more than one indication). Moderate‐to‐severe TR was present in 28% of the primary prevention patients, while the incidence of SVT in this subgroup varied among cohorts from 33% to 80%. Mean QRS ranged from 115 to 160 ms and mean QTc interval ranged from 442 to 487 ms. Of note, a variable proportion (44%–100%) were treated with a beta‐blocker, while fewer (17%–34%) were taking an additional antiarrhythmic drug at the time of ICD placement.
Table 2 summarizes the main outcomes of all 83 patients who underwent ICD implantation for primary prevention. Over the follow‐up period of 330 patient‐years, 124 total ICD discharges occurred, with some patients receiving multiple discharges. Only 8% (9 discharges) of the total discharges were considered appropriate, while the remaining 92% occurred primarily because of inappropriate sensing of SVT with rapid ventricular conduction or farfield sensing, though some occurred because of over‐sensing from lead failure or fracture. ICD complications included lead fractures, lead dislodgment, endocarditis, and device infections as detailed in Table 2. Three deaths occurred during the follow‐up period; the only one directly related to ICD implantation occurred from cardiac arrest a few hours after implantation, following defibrillation testing.48
Table 2.
Outcomes After Implantation of ICDs for Primary Prevention of Sudden Cardiac Death (n=83)
| Study | Year of Publication | Number of ICDs | Follow‐Up Duration (y) | Number of Appropriate Discharges | Annual rate of Appropriate Discharges | Number of Inappropriate Discharges | Annual Rate of Inappropriate Discharges | Number of Lead Fractures/Dislodgments | Number of Infectious Complications |
|---|---|---|---|---|---|---|---|---|---|
| Khairy et al8 | 2008 | 23 | 3.6 | 1 | 0.3 | 67 | 18.6 | 0 | 0 |
| Wheeler et al9 | 2014 | 5 | 4.6 | 0 | 0 | 2 | 0.4 | 0 | 0 |
| Bouzeman et al48 | 2014 | 8 | 1.6 | 0 | 0 | 1 | 0.6 | 2 | 0 |
| Backhoff et al47 | 2016 | 29 | 4.8 | 7 | 1.5 | 12 | 2.5 | 5 | 2 (ICD infections) |
| Buber et al49 | 2016 | 18 | 4.0 | 1 | 0.3 | 33 | 8.3 | 5 | 1 (endocarditis) |
ICD indicates implantable cardioverter‐defibrillator.
Two studies reported detailed patient‐level data on ICD discharges.8, 47 While the study by Backhoff et al did not have a sufficiently high event rate to permit additional analysis, it reported the highest rate of appropriate discharges (1.5 per year)—7 out of the 9 total appropriate discharges across the studies were in this cohort (Table 2). While event rates were too low to confirm any predictors of appropriate discharges by linear regression (data not shown), this cohort did contain the greatest proportion of patients with complex D‐TGA (45%).47 Khairy et al analyzed intracardiac electrograms and found SVT rhythm to precede or coexist with VT in 50% of cases of appropriate therapy.8 In their study, while the presence of non‐sustained VT on Holter was an indication for primary prevention ICD in 44% patients, none of the patients with secondary prevention ICDs had a history of documented non‐sustained VT on routine Holter monitoring before the inciting event. Multivariate analysis revealed the only risk factors for appropriate discharge as being a secondary prevention indication (hazard ratio [HR] 18, 95% CI 1.2–261) and the absence of beta‐blocker therapy (HR 17, 95% CI 1.3–185).8
Discussion
This is the largest systematic review and meta‐analysis of post‐surgical outcomes in D‐TGA patients, and only the second to examine long‐term outcomes. This is also the first systematic review to examine risk factors for SCD and data on ICD efficacy.
Our study has shown that survival after ASR drops to 65% by the fourth postoperative decade. This underscores the urgent need to better delineate modifiable risk factors for mortality in this aging population. Also, the fact that SCD accounts for nearly half the number of late deaths reaffirms risk assessment and prevention of SCD as prime targets in the attempt to improve long‐term outcomes.
Our predictors of increased late mortality at the cohort level included non‐modifiable risk factors of the year of initial ASR surgery and mean age at surgery, the former also conferring increased risk of SCD. It is intuitive that the earlier the surgical era, the steeper the surgical learning curve, greater the usage of hypothermic circulatory arrest, and hence the higher the risk of intraoperative myocardial ischemia and subsequent scar formation, which may be contributing to late postoperative SCD and mortality.42 Similarly, earlier surgical cohorts performed ASR at older age than later decades. It is reasonable to hypothesize that subjecting the myocardium to longer duration of chronic hypoxemia before repair may result in greater long‐term complications including mortality.13, 29, 42
We found that the Mustard procedure increased the risk of SCD and late mortality, in contrast to the meta‐analysis by Khairy et al,50 which demonstrated a trend towards improved survival after Mustard procedure compared with Senning. Their study, however, did not investigate the rates of SCD or SVT in the pooled cohorts and included fewer patients with a much shorter follow‐up duration.50 Given our finding that the contribution of SCD to late mortality increases beyond the first postoperative decade, inadequate follow‐up time in the studies included by Khairy et al might explain the contradictory findings. Possible mechanisms for increased risk with Mustard surgery include higher incidence of SVT and other malignant arrhythmias attributable to use of foreign material to construct the baffles and consequent increased amount of atrial suture lines, higher risk of baffle obstruction, and consequent need for reoperation.
We also identified complex D‐TGA as a risk factor of SCD. Complex D‐TGA may result in a delayed age of ASR and thus increased duration of chronic hypoxemia and higher technical challenges in surgery. These factors may lead to increased chances of myocardial ischemia and scar, iatrogenic right bundle branch block, and recurrence of shunt needing reoperation, ultimately leading to increased risk of future RVD and/or SVT.13, 38 This finding may also potentially explain why the highest rates of appropriate ICD discharges occurred in the study by Backhoff 47 which enrolled the highest proportion of patients with complex D‐TGA, though this observation could not be further investigated because of low event rates and lack of sufficient patient‐level data.
Unexpectedly, RVD was not a statistically significant predictor of late mortality or SCD (Figure 5D). This was most likely because of suboptimal characterization of systemic RV function by echocardiography, which lacks standardization and is consequently fraught with high inter‐observer variability.4, 13, 29, 39, 51 This makes it an inferior test to magnetic resonance52, 53, 54—a modality that was used by only 3 studies. It is therefore probable that many patients without a truly failing RV had evidence of RVD on echocardiography, thus creating the observed discrepancy between imaging evidence of RVD and clinical CHF and blunting the effect of RVD as a potential risk factor of SCD. Additionally, the predictive power of RV systolic function for mortality is variable. While some studies found RVD to be a significant risk factor,30, 32, 36, 45others have pointed out discrepancy between RV systolic function and functional capacity or symptomatic heart failure.29, 31, 35, 39 Potential reasons for this include the contribution of RV diastolic function—a parameter not easily quantified by imaging—to the heart failure syndrome,31 as well as the presence of unique compensatory mechanisms of the systemic RV to stress that may have little to do with the appearance of RV systolic function on imaging.39 Clinical RV failure, not adequately reported at the individual patient level in the included cohorts, might thus prove to be a better predictor of mortality and SCD.
In our analysis, the only modifiable risk factor for late mortality and SCD was a history of SVT, which conferred nearly 5‐fold increased odds of SCD. SVT has been implicated in the pathogenesis of SCD in the post‐ASR population in several ways, including 1:1 conduction through a healthy atrio‐ventricular node,23, 31 rapid preload reduction (particularly in settings of exercise, occult baffle obstruction, or pre‐existing RV failure),31, 35, 42 and ischemia in an often already diseased systemic RV with a single arterial supply from the right coronary artery. SVT may also be contributing as a marker for adverse RV remodeling that may cause SCD via ventricular arrhythmias.8
SVT may also represent a surrogate marker for progressive heart failure, as a poorly functioning, dilated RV with significant TR can in turn result in atrial dilation leading to SVTs. However, given the low reported incidence of New York Heart Association class III or IV CHF in our systematic review (median incidence 4%), it is unlikely that the mortality risk conferred by SVT is solely attributable to death by heart failure.
The link between SVT and SCD suggests that the presence of SVT alone should be enough of a “red flag” to warrant aggressive investigation and/or treatment in ASR patients. SVT may trigger greater rhythm monitoring such as 1‐ to 4‐week event monitors, regular exercise testing, implantable loop recorders, and electrophysiology studies. The impact of anti‐arrhythmic therapy and catheter ablation for SVT in SCD prevention remains to be fully determined. Although Kammeraad et al reported no benefit of anti‐arrhythmics in their cohort of SCD patients,10 that conclusion is limited because digoxin was the most commonly used anti‐arrhythmic therapy. Khairy 8 found that beta blockade is protective against ventricular tachyarrhythmias. Catheter ablation in patients with ASR has an acute success rate for treating SVT of 73% to 100%,55 making it an attractive option for drug‐refractory and/or malignant SVT. There is, however, a 33% recurrence rate within 1.5 years of initial ablation in centers of excellence,56, 57 and the effect of this procedure on mitigating SCD risk is yet to be validated in large prospective studies.
Despite these gaps in knowledge, we strongly advocate individualized consideration of ICD implantation for primary prevention in the ASR population given our demonstration of SCD being the leading cause of death. While the presence of frequent non‐sustained VT on monitoring or significant RVD should engender an ICD discussion, the exceedingly low rate of appropriate ICD discharges especially in the primary prevention subgroup compared with the high rate of inappropriate discharges suggests that our current selection process for primary prevention ICDs warrants revision. While our study does not directly identify patients most likely to benefit from primary prevention ICDs, the strong link between SVT and SCD is valuable information in this regard. Admittedly, the link between SVT and SCD is challenging in the context of ICD implantation, since a high SVT burden may intuitively increase the likelihood of inappropriate shocks. On the other hand, not only is SVT prevalent in the community, but the concept of SVT begetting VT is both conceivable and demonstrable. It can be hypothesized that aggressive treatment of SVTs with early initiation of antiarrhythmic agents including beta‐blockade and possibly catheter ablation may be beneficial in this subset of patients. Additionally, while the specter of inappropriate ICD shocks must be discussed with the individual, a more progressive strategy of ICD implantation may be beneficial to this unique population.
A meta‐analysis of primarily retrospective cohort studies is subject to bias, confounding, and the ecological fallacy. There is considerable heterogeneity across studies on multiple levels, including differences in surgical skill, surgical procedure, postoperative care, patient age, and socioeconomic characteristics, patient comorbidities, follow‐up time, intensity of follow‐up surveillance, frequency, and quality of follow‐up clinical care and non‐random missing data—many of these factors cannot be quantitatively evaluated given available data. We were unable to uniformly assign a standard definition of complex D‐TGA across the studies to reduce the heterogeneity in our sample because of the lack of sufficient patient level data. Our results summarizing secondary outcomes are prone to the most error because valid estimates depend on the assumption of universal screening by echocardiography, electrocardiography, and other modalities during follow‐up, regardless of patient‐reported symptoms, which is only feasible for studies that are planned prospectively. Our outcomes data in the ICD studies were limited by low event rates. This precluded analysis of risk for appropriate and inappropriate discharge and limited our ability to explain why the study by Backhoff et al appeared to select patients better than others for primary prevention ICDs. Additionally, our evaluation of mortality and SCD risk did not include additional variables such as QT dispersion, pulmonary hypertension, and cardioprotective medical therapy, which were not reported adequately.
Conclusions
Our systematic review and meta‐analysis, the largest in the D‐TGA literature, reveals several important findings on the long‐term outcomes after ASR and establishes presence of SVT, Mustard procedure, and complex D‐TGA as important patient‐level risk factors for both long‐term mortality and SCD. The data also establish the unsatisfactory outcome of patients with ICDs for primary prevention of SCD, and advocate for a more refined risk stratification scheme for ICD implantation. There remain many questions about the long‐term outcomes of the post‐ASR patient that cannot be answered by the existing pool of data, and additional multicenter patient‐level studies are required to further investigate SCD risk and guide the management of this complex patient population.
Disclosures
None.
Supporting information
Data S1. Search Strategy.
Table S1. Seven‐Point Scale Used for Quality Assessment of Included Studies
Table S2. Quality Assessment of Included Studies Reporting Late Mortality
Table S3. Primary and Key Secondary Outcomes of Included Studies Reporting Late Mortality
Table S4. Significant Risk Factors for Late Mortality After ASR as Reported by Individual Cohorts
Table S5. Linear Regression Data of Late Mortality and SCD With Multiple Cohort Level Covariates
Table S6. Results of Cochran Mantel‐Haenszel Test Results for Each Association of Predictor Variable and Outcome Examined Using Available Patient‐Level Data
Table S7. Results of Binary Logistic Regression Results With Firth Correction, for Each Association of Predictor Variable and Outcome Examined Using Available Patient‐Level Data, After Adjusting for the Study
Figure S1. Scatterplot depicting significant negative correlation of mean age of study patients at the time of atrial switch repair (ASR) with year when the initial surgery was performed in a cohort.
Figure S2. Indications for reintervention after ASR using pooled cohort data; n=433 reinterventions. ASR indicates atrial switch repair, LVOT, left ventricular outflow tract, PA, pulmonary artery; PDA, patent ductus arteriosus; TR, tricuspid regurgitation; VSD, ventricular septal defect.
Figure S3. Forest plots showing fixed effects pooled odds ratios of SVT (A), Mustard procedure (B) and complex D‐TGA (C) for late mortality (LM). D‐TGA indicates D‐transposition of the great arteries; SVT, supraventricular tachycardia.
Figure S4. Forest plots showing fixed effects pooled odds ratios of SVT (A), Mustard procedure (B), Complex D‐TGA (C) and RVD (D) for SCD. D‐TGA indicates D‐transposition of the great arteries; RVD, right ventricular dysfunction; SVT, supraventricular tachycardia.
Acknowledgments
The authors thank Ehete Bahiru, Irfan Minhas, Kana Fujikura, Michael Zullo, Georges Ephrem, and Agata Bielska for their contributions.
(J Am Heart Assoc. 2019;8:e012932 DOI: 10.1161/JAHA.119.012932.)
References
- 1. Senning A. Surgical correction of transposition of the great vessels. Surgery. 1959;45:966–980. [PubMed] [Google Scholar]
- 2. Mustard WT. Successful Two‐Stage Correction of Transposition of the Great Vessels. Surgery. 1964;55:469–472. [PubMed] [Google Scholar]
- 3. Jatene AD, Fontes VF, Paulista PP, Souza LC, Neger F, Galantier M, Sousa JE. Anatomic correction of transposition of the great vessels. J Thorac Cardiovasc Surg. 1976;72:364–370. [PubMed] [Google Scholar]
- 4. Moons P, Gewillig M, Sluysmans T, Verhaaren H, Viart P, Massin M, Suys B, Budts W, Pasquet A, De Wolf D, Vliers A. Long term outcome up to 30 years after the Mustard or Senning operation: a nationwide multicentre study in Belgium. Heart. 2004;90:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oechslin E, Jenni R. 40 years after the first atrial switch procedure in patients with transposition of the great arteries: long‐term results in Toronto and Zurich. Thorac Cardiovasc Surg. 2000;48:233–237. [DOI] [PubMed] [Google Scholar]
- 6. Sarkar D, Bull C, Yates R, Wright D, Cullen S, Gewillig M, Clayton R, Tunstill A, Deanfield J. Comparison of long‐term outcomes of atrial repair of simple transposition with implications for a late arterial switch strategy. Circulation. 1999;100:II176–II181. [DOI] [PubMed] [Google Scholar]
- 7. Wilson NJ, Clarkson PM, Barratt‐Boyes BG, Calder AL, Whitlock RM, Easthope RN, Neutze JM. Long‐term outcome after the mustard repair for simple transposition of the great arteries. 28‐year follow‐up. J Am Coll Cardiol. 1998;32:758–765. [DOI] [PubMed] [Google Scholar]
- 8. Khairy P, Harris L, Landzberg MJ, Fernandes SM, Barlow A, Mercier LA, Viswanathan S, Chetaille P, Gordon E, Dore A, Cecchin F. Sudden death and defibrillators in transposition of the great arteries with intra‐atrial baffles: a multicenter study. Circ Arrhythm Electrophysiol. 2008;1:250–257. [DOI] [PubMed] [Google Scholar]
- 9. Wheeler M, Grigg L, Zentner D. Can we predict sudden cardiac death in long‐term survivors of atrial switch surgery for transposition of the great arteries? Congenit Heart Dis. 2014;9:326–332. [DOI] [PubMed] [Google Scholar]
- 10. Kammeraad JA, van Deurzen CH, Sreeram N, Bink‐Boelkens MT, Ottenkamp J, Helbing WA, Lam J, Sobotka‐Plojhar MA, Daniels O, Balaji S. Predictors of sudden cardiac death after Mustard or Senning repair for transposition of the great arteries. J Am Coll Cardiol. 2004;44:1095–1102. [DOI] [PubMed] [Google Scholar]
- 11. Sodhi SS, Cedars AM. Primary prevention of sudden cardiac death in adults with transposition of the great arteries: a review of implantable cardioverter‐defibrillator placement. Tex Heart Inst J. 2015;42:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Janousek J, Paul T, Luhmer I, Wilken M, Hruda J, Kallfelz HC. Atrial baffle procedures for complete transposition of the great arteries: natural course of sinus node dysfunction and risk factors for dysrhythmias and sudden death. Z Kardiol. 1994;83:933–938. [PubMed] [Google Scholar]
- 13. Gelatt M, Hamilton RM, McCrindle BW, Connelly M, Davis A, Harris L, Gow RM, Williams WG, Trusler GA, Freedom RM. Arrhythmia and mortality after the Mustard procedure: a 30‐year single‐center experience. J Am Coll Cardiol. 1997;29:194–201. [DOI] [PubMed] [Google Scholar]
- 14. Schwerzmann M, Salehian O, Harris L, Siu SC, Williams WG, Webb GD, Colman JM, Redington A, Silversides CK. Ventricular arrhythmias and sudden death in adults after a Mustard operation for transposition of the great arteries. Eur Heart J. 2009;30:1873–1879. [DOI] [PubMed] [Google Scholar]
- 15. Khairy P, Van Hare GF, Balaji S, Berul CI, Cecchin F, Cohen MI, Daniels CJ, Deal BJ, Dearani JA, Groot N, Dubin AM, Harris L, Janousek J, Kanter RJ, Karpawich PP, Perry JC, Seslar SP, Shah MJ, Silka MJ, Triedman JK, Walsh EP, Warnes CA. PACES/HRS Expert Consensus Statement on the Recognition and Management of Arrhythmias in Adult Congenital Heart Disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Heart Rhythm. 2014;11:e102–e165. [DOI] [PubMed] [Google Scholar]
- 16. Al‐Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15:e190–e252. [DOI] [PubMed] [Google Scholar]
- 17. Vehmeijer JT, Brouwer TF, Limpens J, Knops RE, Bouma BJ, Mulder BJ, de Groot JR. Implantable cardioverter‐defibrillators in adults with congenital heart disease: a systematic review and meta‐analysis. Eur Heart J. 2016;37:1439–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gurvitz M, Burns KM, Brindis R, Broberg CS, Daniels CJ, Fuller SM, Honein MA, Khairy P, Kuehl KS, Landzberg MJ, Mahle WT, Mann DL, Marelli A, Newburger JW, Pearson GD, Starling RC, Tringali GR, Valente AM, Wu JC, Califf RM. Emerging Research directions in adult congenital heart disease: a report from an NHLBI/ACHA Working Group. J Am Coll Cardiol. 2016;67:1956–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e698–e800. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 22. National Heart L, and Blood Institute . Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies. 2014.
- 23. Agnetti A, Carano N, Cavalli C, Tchana B, Bini M, Squarcia U, Frigiola A. Long‐term outcome after senning operation for transposition of the great arteries. Clin Cardiol. 2004;27:611–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ashraf MH, Cotroneo J, DiMarco D, Subramanian S. Fate of long‐term survivors of Mustard procedure (inflow repair) for simple and complex transposition of the great arteries. Ann Thorac Surg. 1986;42:385–389. [DOI] [PubMed] [Google Scholar]
- 25. Birnie D, Tometzki A, Curzio J, Houston A, Hood S, Swan L, Doig W, Wilson N, Jamieson M, Pollock J, Hillis WS. Outcomes of transposition of the great arteries in the ear of atrial inflow correction. Heart. 1998;80:170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borowicka E, Smolenska‐Petelenz J, Dukalska M, Markiewicz‐Loskot G, Szydlowski L. [Long‐term results of valvular condition and clinical state in patients operated with the Senning method]. Przegl Lek. 2004;61:653–655. [PubMed] [Google Scholar]
- 27. Carrel T, Pfammatter JP. Complete transposition of the great arteries: surgical concepts for patients with systemic right ventricular failure following intraatrial repair. Thorac Cardiovasc Surg. 2000;48:224–227. [DOI] [PubMed] [Google Scholar]
- 28. Chaloupecky VT, Bartakova H, Svobodova I, Reich O, Tomek V, Janousek J, Gebauer R, Radvansky J. Long‐term results of Senning procedure for transposition of the great arteries. Cor Vasa. 2006;48:90–97. [Google Scholar]
- 29. Cuypers JA, Eindhoven JA, Slager MA, Opic P, Utens EM, Helbing WA, Witsenburg M, van den Bosch AE, Ouhlous M, van Domburg RT, Rizopoulos D, Meijboom FJ, Bogers AJ, Roos‐Hesselink JW. The natural and unnatural history of the Mustard procedure: long‐term outcome up to 40 years. Eur Heart J. 2014;35:1666–1674. [DOI] [PubMed] [Google Scholar]
- 30. Dobson R, Danton M, Nicola W, Hamish W. The natural and unnatural history of the systemic right ventricle in adult survivors. J Thorac Cardiovasc Surg. 2013;145:1493–1501; discussion 1501–1503. [DOI] [PubMed] [Google Scholar]
- 31. Dos L, Teruel L, Ferreira IJ, Rodriguez‐Larrea J, Miro L, Girona J, Albert DC, Goncalves A, Murtra M, Casaldaliga J. Late outcome of Senning and Mustard procedures for correction of transposition of the great arteries. Heart. 2005;91:652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ebenroth ES, Hurwitz RA. Long‐term functional outcome of patients following the mustard procedure: the next decade of follow‐up. Congenit Heart Dis. 2007;2:235–241. [DOI] [PubMed] [Google Scholar]
- 33. Genoni M, Vogt P, von Segesser L, Seifert B, Arbenz U, Jenni R, Turina M. Extended follow‐up after atrial repair for transposition of the great arteries: a younger age at surgery improves late survival. J Card Surg. 1999;14:246–251. [DOI] [PubMed] [Google Scholar]
- 34. Gorler H, Ono M, Thies A, Lunkewitz E, Westhoff‐Bleck M, Haverich A, Breymann T, Boethig D. Long‐term morbidity and quality of life after surgical repair of transposition of the great arteries: atrial versus arterial switch operation. Interact Cardiovasc Thorac Surg. 2011;12:569–574. [DOI] [PubMed] [Google Scholar]
- 35. Helbing WA, Hansen B, Ottenkamp J, Rohmer J, Chin JG, Brom AG, Quaegebeur JM. Long‐term results of atrial correction for transposition of the great arteries. Comparison of Mustard and Senning operations. J Thorac Cardiovasc Surg. 1994;108:363–372. [PubMed] [Google Scholar]
- 36. Kirjavainen M, Happonen JM, Louhimo I. Late results of Senning operation. J Thorac Cardiovasc Surg. 1999;117:488–495. [DOI] [PubMed] [Google Scholar]
- 37. Knez IO, Schweiger M, Dacar D, Huber K, Gamillscheg A, Tscheiliessnigg K. Senning and mustard atrial switch procedures: long term follow‐up, morbidity and complications. Thorac Cardiovasc Surg. 2011;59:(V197). Available at: https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0030-1269239. [Google Scholar]
- 38. Lange R, Horer J, Kostolny M, Cleuziou J, Vogt M, Busch R, Holper K, Meisner H, Hess J, Schreiber C. Presence of a ventricular septal defect and the Mustard operation are risk factors for late mortality after the atrial switch operation: thirty years of follow‐up in 417 patients at a single center. Circulation. 2006;114:1905–1913. [DOI] [PubMed] [Google Scholar]
- 39. Merlo M, de Tommasi SM, Brunelli F, Abbruzzese PA, Crupi G, Ghidoni I, Casari A, Piti A, Mamprin F, Parenzan L. Long‐term results after atrial correction of complete transposition of the great arteries. Ann Thorac Surg. 1991;51:227–231. [DOI] [PubMed] [Google Scholar]
- 40. Myridakis DJ, Ehlers KH, Engle MA. Late follow‐up after venous switch operation (Mustard procedure) for simple and complex transposition of the great arteries. Am J Cardiol. 1994;74:1030–1036. [DOI] [PubMed] [Google Scholar]
- 41. Rekhraj SF, Freeman LJ. Outcome of atrial repair procedures in patients with transposition of the great arteries followed up in a district general hospital. Br J Cardiol. 2007;14:19–22. [Google Scholar]
- 42. Roubertie F, Thambo JB, Bretonneau A, Iriart X, Laborde N, Baudet E, Roques X. Late outcome of 132 Senning procedures after 20 years of follow‐up. Ann Thorac Surg. 2011;92:2206–2213; discussion 2213–2214. [DOI] [PubMed] [Google Scholar]
- 43. Turley K, Hanley FL, Verrier ED, Merrick SH, Ebert PA. The Mustard procedure in infants (less than 100 days of age). Ten‐year follow‐up. J Thorac Cardiovasc Surg. 1988;96:849–853. [PubMed] [Google Scholar]
- 44. Vejlstrup N, Sorensen K, Mattsson E, Thilen U, Kvidal P, Johansson B, Iversen K, Sondergaard L, Dellborg M, Eriksson P. Long‐term outcome of mustard/senning correction for transposition of the great arteries in Sweden and Denmark. Circulation. 2015;132:633–638. [DOI] [PubMed] [Google Scholar]
- 45. Dennis M, Kotchetkova I, Cordina R, Celermajer DS. Long‐term follow‐up of adults following the atrial switch operation for transposition of the great arteries—a contemporary cohort. Heart Lung Circ. 2018;27:1011–1017. [DOI] [PubMed] [Google Scholar]
- 46. Kiener A, Kelleman M, McCracken C, Kochilas L, St. Louis JD, Oster ME. long‐term survival after arterial versus atrial switch in d‐transposition of the great arteries. Ann Thorac Surg. 2018;106:1827–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Backhoff D, Kerst G, Peters A, M LU, Frische C, Horndasch M, Hessling G, Paul T, Krause U. Internal cardioverter defibrillator indications and therapies after atrial baffle procedure for d‐transposition of the great arteries: a multicenter analysis. Pacing Clin Electrophysiol. 2016;39:1070–1076. [DOI] [PubMed] [Google Scholar]
- 48. Bouzeman A, Marijon E, de Guillebon M, Ladouceur M, Duthoit G, Amet D, Martins R, Otmani A, Lavergne T, Bordachar P, Celermajer DS, Thambo JB, Iserin L, Combes N. Implantable cardiac defibrillator among adults with transposition of the great arteries and atrial switch operation: case series and review of literature. Int J Cardiol. 2014;177:301–306. [DOI] [PubMed] [Google Scholar]
- 49. Buber J, Ackley TJ, Daniels CJ, Roble SL, Mah ML, Kamp AN, Kertesz NJ. Outcomes following the implantation of cardioverter‐defibrillator for primary prevention in transposition of the great arteries after intra‐atrial baffle repair: a single‐centre experience. Europace. 2016;18:1016–1022. [DOI] [PubMed] [Google Scholar]
- 50. Khairy P, Landzberg MJ, Lambert J, O'Donnell CP. Long‐term outcomes after the atrial switch for surgical correction of transposition: a meta‐analysis comparing the Mustard and Senning procedures. Cardiol Young. 2004;14:284–292. [DOI] [PubMed] [Google Scholar]
- 51. Srinivasan C, Sachdeva R, Morrow WR, Greenberg SB, Vyas HV. Limitations of standard echocardiographic methods for quantification of right ventricular size and function in children and young adults. J Ultrasound Med. 2011;30:487–493. [DOI] [PubMed] [Google Scholar]
- 52. Crean AM, Maredia N, Ballard G, Menezes R, Wharton G, Forster J, Greenwood JP, Thomson JD. 3D Echo systematically underestimates right ventricular volumes compared to cardiovascular magnetic resonance in adult congenital heart disease patients with moderate or severe RV dilatation. J Cardiovasc Magn Reson. 2011;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iriart X, Horovitz A, van Geldorp IE, Barnetche T, Lederlin M, De Guillebon M, Reant P, Lafitte S, Thambo JB. The role of echocardiography in the assessment of right ventricular systolic function in patients with transposition of the great arteries and atrial redirection. Arch Cardiovasc Dis. 2012;105:432–441. [DOI] [PubMed] [Google Scholar]
- 54. Puchalski MD, Williams RV, Askovich B, Minich LL, Mart C, Tani LY. Assessment of right ventricular size and function: echo versus magnetic resonance imaging. Congenit Heart Dis. 2007;2:27–31. [DOI] [PubMed] [Google Scholar]
- 55. Houck CA, Teuwen CP, Bogers AJ, de Groot NM. Atrial tachyarrhythmias after atrial switch operation for transposition of the great arteries: treating old surgery with new catheters. Heart Rhythm. 2016;13:1731–1738. [DOI] [PubMed] [Google Scholar]
- 56. Gallotti RG, Madnawat H, Shannon KM, Aboulhosn JA, Nik‐Ahd F, Moore JP. Mechanisms and predictors of recurrent tachycardia after catheter ablation for d‐transposition of the great arteries after the Mustard or Senning operation. Heart Rhythm. 2017;14:350–356. [DOI] [PubMed] [Google Scholar]
- 57. Wu J, Deisenhofer I, Ammar S, Fichtner S, Reents T, Zhu P, Jilek C, Kolb C, Hess J, Hessling G. Acute and long‐term outcome after catheter ablation of supraventricular tachycardia in patients after the Mustard or Senning operation for D‐transposition of the great arteries. Europace. 2013;15:886–891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Search Strategy.
Table S1. Seven‐Point Scale Used for Quality Assessment of Included Studies
Table S2. Quality Assessment of Included Studies Reporting Late Mortality
Table S3. Primary and Key Secondary Outcomes of Included Studies Reporting Late Mortality
Table S4. Significant Risk Factors for Late Mortality After ASR as Reported by Individual Cohorts
Table S5. Linear Regression Data of Late Mortality and SCD With Multiple Cohort Level Covariates
Table S6. Results of Cochran Mantel‐Haenszel Test Results for Each Association of Predictor Variable and Outcome Examined Using Available Patient‐Level Data
Table S7. Results of Binary Logistic Regression Results With Firth Correction, for Each Association of Predictor Variable and Outcome Examined Using Available Patient‐Level Data, After Adjusting for the Study
Figure S1. Scatterplot depicting significant negative correlation of mean age of study patients at the time of atrial switch repair (ASR) with year when the initial surgery was performed in a cohort.
Figure S2. Indications for reintervention after ASR using pooled cohort data; n=433 reinterventions. ASR indicates atrial switch repair, LVOT, left ventricular outflow tract, PA, pulmonary artery; PDA, patent ductus arteriosus; TR, tricuspid regurgitation; VSD, ventricular septal defect.
Figure S3. Forest plots showing fixed effects pooled odds ratios of SVT (A), Mustard procedure (B) and complex D‐TGA (C) for late mortality (LM). D‐TGA indicates D‐transposition of the great arteries; SVT, supraventricular tachycardia.
Figure S4. Forest plots showing fixed effects pooled odds ratios of SVT (A), Mustard procedure (B), Complex D‐TGA (C) and RVD (D) for SCD. D‐TGA indicates D‐transposition of the great arteries; RVD, right ventricular dysfunction; SVT, supraventricular tachycardia.


