Abstract
Background
To evaluate whether blood markers of lead, cadmium, and mercury can improve prediction for cardiovascular disease (CVD) mortality when added individually, jointly, or as an integrative index/Environmental Risk Score (ERS), in a model with established risk factors.
Methods and Results
Our study sample comprised 16 028 adults aged ≥40 years who were enrolled in the National Health and Nutrition Examination Survey 1999–2012 and followed up through December 31, 2015. The study sample was randomly split into training for the ERS construction (n=8043) and testing for the evaluation of prediction performance (n=7985). ERS was computed using elastic‐net penalized Cox's model based on the selected metal predictors predicting CVD mortality. During median follow‐up of 7.2 years, 517 died from CVD. In the training set, linear terms of cadmium and mercury, squared terms of lead and mercury, and all 3 pairwise interactions were selected by elastic‐net for ERS construction. In the testing set, the C‐statistic increased from 0.845 when only established CVD risk factors were in the model to 0.854 when the ERS was additionally added to the model. Addition of all linear, squared, and pairwise interaction terms of blood metals to the Cox's models improved C‐statistic from 0.845 to 0.857. The improvement remained significant when it was assessed by net reclassification improvement and integrated discrimination improvement.
Conclusions
Our findings suggest that blood markers of toxic metals can improve CVD risk prediction over the established risk factors and highlight their potential utility for CVD risk assessment, prevention, and precision health.
Keywords: cardiovascular mortality, risk prediction, blood lead, blood cadmium, blood mercury
Subject Categories: Cardiovascular Disease, Primary Prevention, Risk Factors, Mortality/Survival
Clinical Perspective
What Is New?
For the first time, the predictive value of blood markers of toxic metals, including lead, cadmium, and mercury, for cardiovascular disease mortality was assessed in a representative sample of US adults aged ≥40 years.
Addition of multiple predictors of blood lead, cadmium, and mercury to the established risk factors significantly improved risk discrimination and risk reclassification of cardiovascular disease mortality.
What Are the Clinical Implications?
Assessment of blood toxic metals may be useful for identifying subpopulations that may benefit most from additional blood tests for predicting cardiovascular disease mortality.
Our findings highlight a potential utility of assessment of exposure to environmental toxicants for cardiovascular disease risk assessment and prevention.
Introduction
Cardiovascular disease (CVD) remains the leading cause of death in the United States as well as globally.1, 2 Accurate assessment of CVD risk as an essential step toward disease prevention is an important public health goal. Scoring algorithms such as the Framingham Risk Score and the Pooled Cohort Equations (PCE) for the atherosclerotic CVD risk,3, 4 which combines the established risk factors, for example, systolic blood pressure, serum lipid levels, smoking, obesity, and diabetes mellitus, have been widely used for cardiovascular risk assessment in the general population. However, the accuracy of such algorithms has been questioned.5 Attempts to improve risk prediction algorithms have been made by incorporating additional risk factors, including novel biomarkers, subclinical measures, and genetic variations.6, 7, 8, 9, 10, 11, 12, 13, 14, 15 However, the incremental information with regard to the prediction of cardiovascular events and death added by those risk markers beyond that of the established risk factors were mostly small or inconclusive.6, 7, 8, 16, 17, 18, 19
Exposure to environmental toxicants, such as lead, cadmium, and mercury, are known to have lasting adverse effects on the cardiovascular system, for example, hypertension, coronary heart disease, peripheral arterial disease, stroke, as well as mortality.20, 21, 22, 23, 24, 25, 26 Although associations between blood lead, cadmium, and mercury and risk of CVD‐related morbidity and mortality have been extensively reported,20, 25, 27 the predictive value of blood measures of these toxic metals has never been examined.
The present study attempted to evaluate whether blood markers of toxic metals (lead, cadmium, and mercury) can improve prediction for CVD mortality when added individually, jointly, or as an integrative index—the Environmental Risk Score (ERS), in a model with established CVD risk factors. The ERS has been suggested as a summary measure of health risk of exposure to multiple toxicants in epidemiological research.28, 29 Data from the National Health and Nutrition Examination Survey (NHANES), that represents the general US population, were used to develop and validate our model.
Methods
Study Population
The study sample consists of 7 continuous cycles of the NHANES between 1999 and 2012, which used a stratified, multistage probability cluster design, with oversampling of selected subpopulations, to obtain a representative sample of the civilian, noninstitutionalized US population.30 All data and materials have been made publicly available at the National Center for Health Statistics website (https://www.cdc.gov/nchs/nhanes/index.htm). The protocols for NHANES were approved by the National Center for Health Statistics of the Centers for Disease Control and Prevention Institutional Review Board, and informed consents were obtained from all participants.
Blood metal concentrations were measured in the entire NHANES samples through all cycles between 1999 and 2012 except for total blood mercury, which was available among participants aged 1 to 5 years and females aged 16 to 49 years in 2 earlier cycles (1999–2000 and 2001–2002).30 Because individuals aged <40 years have very low cardiovascular risk,4, 31 we restricted our analysis using individuals aged ≥40 years. Among a total of 17 284 men and women aged ≥40 years who completed blood sample collections for lead, cadmium, and total mercury assessments in NHANES 1999–2012, 17 260 participants were linked with the mortality data available in the National Death Index. We excluded participants with missing information on systolic blood pressure (n=752), current smoking status (n=14), total cholesterol and high‐density lipoprotein (HDL) cholesterol levels (n=258), and body mass index (n=322; the numbers are not mutually exclusive), leaving a total of 16 028 participants for the current study. We randomly split our study by a ratio of 1:1 into the training set (n=8043) for construction of the CVD death‐related ERS of blood metals, and the testing set (n=7985) for evaluation of performances of blood metals, including the constructed ERS, for predicting CVD mortality in addition to the established risk factors. An overview of our methodology is depicted in Figure S1.
Blood Metal Measurements
Blood lead, cadmium, and total mercury were measured at the Environmental Health Sciences Laboratory of the Centers for Disease Control and Prevention National Center for Environmental Health following extensive quality‐control procedures.30 The limit of detection (LOD) for blood lead was 0.3 μg/dL (system of units conversion, multiply by 0.0483 for μmol/L) in NHANES 1999–2004 and 0.25 μg/dL in 2005–2012.30 For blood cadmium, the LOD was 0.3 μg/L (system of units conversion, multiply by 8.897 for nmol/L) in 1999–2002 and 0.2 μg/L in 2003–2012.30 LODs for total blood mercury varied according to cycle and batch, ranging between 0.14 and 0.33 μg/L (system of units, multiply by 4.99 for nmol/L).30 Of all participants in our analysis, 0.1%, 11.8%, and 8.6% had blood lead, cadmium, and mercury concentrations below LODs, respectively. Metal concentrations below LODs were assigned with the LOD divided by the square root of 2.
Outcomes
The public use NHANES linked mortality data, which linked NHANES records with death certificates from the National Death Index using probabilistic matching algorithms, was used to determine the vital status and cause of death. A detailed description of the matching criteria and calibration is available from the National Center for Health Statistics.32 All participants were followed up through December 31, 2015. The International Classification of Diseases, Tenth Revision (ICD‐10) was used to determine the underlying cause of death. Death from cardiovascular causes (ICD‐10 codes I00–I09, I11, I13, I20–I51, and I60–I69) were identified.
Other Covariates
Information on age, sex, race/ethnicity (Hispanic including Mexican American and other Hispanic, non‐Hispanic white, non‐Hispanic black, and other), and current smoking status was collected using self‐administered questionnaires.30 Blood pressures were measured 3 consecutive times (sometimes 4 times) with each participant in a seated position by using a standardized protocol following the American Heart Association guidelines.33 We calculated means of systolic blood pressure (mm Hg) by averaging up to 3 measures after disregarding the first reading. Serum total cholesterol (mg/dL) was measured using enzymatic methods.30 Serum HDL cholesterol (mg/dL) was measured by heparin‐manganese precipitation methods.30 Body mass index was calculated as weight in kilograms divided by the square of height in meters. Diabetes mellitus was defined as self‐reported physician diagnosis of diabetes mellitus, use of self‐reported antidiabetes medication, or hemoglobin A1c level ≥6.5%. Serum CRP (C‐reactive protein) was analyzed by high‐sensitivity latex‐enhanced nephelometry.30 Family history of CVD was determined based on response (yes or no) to the following NHANES question: “Including living and deceased, were any of your close biological that is, blood relatives including father, mother, sisters or brothers, ever told by health professional that they had a heart attack or angina before the age of 50?”30
Statistical Analysis
Logarithmic transformations with natural base were applied to blood lead, cadmium, and mercury concentrations given the highly skewed distributions of metals. Cox's proportional hazards models were performed to examine the associations between blood metals and CVD mortality. We report effect estimates (hazards ratios and 95% CIs) comparing the 75th versus the 25th percentile of blood metal concentrations. All the models were adjusted for the following predictors used in the PCE, including age, sex, race/ethnicity, current smoking status, use of antihypertensive medications, total cholesterol level, HDL cholesterol level, diabetes mellitus, and body mass index.
Performance of blood metals for predicting CVD mortality was assessed progressively. Initially, the linear term of each log‐transformed metal concentration was individually evaluated in the model for CVD mortality with adjustment for the established risk factors (predictors used in the PCE). We then examined whether the addition of a “full combination” of blood metals improved the predictive performance by incorporating all 3 linear terms, 3 squared terms, and 3 pairwise interactions of blood lead, cadmium, and mercury, a total of 9 predictors, simultaneously in the model adjusting for the established risk factors. The squared terms accounted for nonlinear associations between metals and CVD mortality, and interaction terms implied potential departures from additive effects. Finally, we created a parsimonious, rather than comprehensive, index—the ERS, for integrating CVD death risk of exposure to metals only based on the most important metal predictors necessary to predict CVD death. The underlying idea behind the ERS is to build a predictive risk score as a weighted sum of the toxicant concentrations from simultaneous assessment of multiple toxicants.34 Weights are determined by the magnitudes of the associations of each toxicant, as well as toxicant‐toxicant interactions, with the outcome of interest from the regression model.28, 29 To achieve this goal, first we utilized the elastic net (ENET) penalized Cox's regression,35 as a machine learning algorithm designed for variable selection, to identify a parsimonious set of blood metal predictors associated with CVD mortality in the training set. Cox's proportional hazards model is the most popular survival model for studying the relationship between survival time and predictor variables. However, this conventional method does not work well in the presence of potentially high‐dimensional predictors or when predictors are highly correlated (multicollinearity).36 To combat these issues, the ENET penalized model, as one of the sparse penalized Cox's model, has been introduced, which was proved satisfactory in both handling correlated covariates and prediction performance.35 ENET shrinks coefficients of “unimportant” predictors toward exact zeroes and thus promises to be a useful tool for variable selection.35, 37 In our analysis, 3 linear terms, 3 squared terms, and 3 pairwise interactions of blood lead, cadmium, and mercury, which were all included in the model in our initial step, served as predictors to be selected in the ENET. Age, sex, smoking, and race/ethnicity were always adjusted for (“forced”) in the model selection as confounders. Other established CVD risk factors, including systolic blood pressure, use of antihypertensive medications, total cholesterol, HDL cholesterol, diabetes mellitus, and body mass index, were not forced because of their potential role of intermediates on the pathways between blood metals and CVD death.29, 38, 39 The ENET penalized parameters were ascertained based on a 5‐fold cross‐validation for minimal prediction errors. We also repeated this procedure based on 10‐fold cross‐validation, and the results were consistent. The R package “fastcox” was used to implement ENET penalized Cox's model.35 We also used another R package that supports ENET Cox regression models, “coxnet,” and obtained consistent results.40 The ERS was then computed in the testing set as a weighted sum of nonzero predictors selected from ENET penalized Cox's model by
| (1) |
where (j=1, …, p) is the log‐transformed concentration of the j‐th metal in the testing set; is the beta coefficient of the j‐th metal in ENET; is the beta coefficient of interaction between metals k and l; and is the coefficient of the squared term of the m‐th metal. All beta coefficients were estimated in the training set. Note that coefficients of less‐important predictor terms were shrunk to zero by ENET. The sample R code for construction of the ERS using ENET is available at https://github.com/um-mpeg/Environmental-Risk-Score. We then investigated whether the incorporation of the ERS in the Cox's model with established risk factors improved the prediction of CVD mortality.
The following metrics were then calculated to assess the improvement of inclusion of the blood metal predictors in risk prediction models in the testing set: (1) C‐statistic, to quantify the concordance in predicted and observed survival times between subjects41; (2) net reclassification improvement (NRI) with prespecified risk categories (0–5%, 5–10%, 10–20%, and >20%); (3) continuous NRI, which does not require discrete risk categories, to quantify the proportions of cases correctly assigned a higher predictive probability and noncases correctly assigned a lower probability by inclusion of additional predictors42; and (4) integrated discrimination improvement (IDI), to quantify the improved sensitivity without affecting specificity in updated prediction models.42
In a sensitivity analysis, we additionally adjusted for the following potential confounding factors in the Cox models, including the NHANES survey cycles, intensity of current exposure to tobacco smoke using serum cotinine levels,23 cumulative smoking using number of pack‐years,23 and seafood intake using the total amount of omega‐3 fatty acid intake (sum of eicosapentaenoic acid and docosahexaenoic acid) based on 24‐hour dietary recall,43 which were not used as predictors in the PCE. For comparison purpose, we also fitted a Cox model including CRP (log‐transformed), as 1 of the most studied inflammatory biomarkers in association with CVD,6, 7, 9, 44 in addition to the established risk factors. This analysis was performed in a subpopulation of the testing set excluding the participants from NHANES 2011–2012, in which CRP data were not available. A similar comparison was also made by fitting a Cox model including family history of CVD in addition to the established risk factors. All analyses were conducted by R aoftware (version 3.4.0; www.R-project.org).
Results
Characteristics of the study population in both training and testing sets are summarized in Table 1. In general, characteristics were similar in the 2 data sets. In the training set, participants had a mean age of 59.4 years, ranging from 40 to 85 years. During the follow‐up period (median, 7.1 years; range, 0.1–16.8), 1211 participants died; 261 deaths were from CVD. In the testing set, mean age at baseline was 59.3 years (range, 40–85). During the follow‐up (median, 7.2 years; range, 0.2–16.7), 1207 participants died, among which 256 were from CVD. Median concentrations of blood lead and cadmium were 1.62 μg/dL and 0.40 μg/L, respectively, in both training and testing sets. Median concentration of blood mercury was 1.00 μg/L in the training set and 0.97 μg/L in the testing set.
Table 1.
Baseline Characteristics of the Study Cohort (NHANES 1999–2012)
| Characteristic | Training Set (n=8043) | Testing Set (n=7985) |
|---|---|---|
| No. (%) death of all causes | 1211 (15.1) | 1207 (15.1) |
| No. (%) death of CVD | 261 (3.2) | 256 (3.2) |
| Follow‐up, median (range), y | 7.1 (0.1–16.8) | 7.2 (0.2–16.7) |
| Age, mean (SD), y | 59.4 (12.9) | 59.3 (12.8) |
| No. (%) female | 4169 (51.8) | 4239 (53.1) |
| No. (%) racial/ethnical groups | ||
| White | 4072 (50.6) | 3990 (50.0) |
| Black | 1600 (19.9) | 1631 (20.4) |
| Hispanica | 1910 (23.8) | 1904 (23.8) |
| Other race | 462 (5.7) | 461 (5.8) |
| No. (%) current smoker | 1519 (18.9) | 1580 (19.8) |
| SBP, mean (SD), mm Hg | 129 (20) | 129 (20) |
| Total cholesterol, mean (SD), mg/dL | 202.14 (42.32) | 201.52 (42.62) |
| HDL cholesterol, mean (SD), mg/dL | 53.71 (16.56) | 53.61 (16.14) |
| No. (%) diabetes mellitus | 1540 (19.1) | 1641 (20.6) |
| BMIb, mean (SD), kg/m2 | 29.2 (6.5) | 29.2 (6.4) |
| Blood lead, median (IQR), μg/dL | 1.62 (1.10–2.49) | 1.62 (1.10–2.44) |
| Blood cadmium, median (IQR), μg/L | 0.40 (0.26–0.66) | 0.40 (0.26–0.67) |
| Total blood mercury, median (IQR), μg/L | 1.00 (0.52–1.93) | 0.97 (0.50–1.92) |
SI conversion factor: To convert values for blood lead concentrations to μmol/L, multiply by 0.0483; to convert values for blood cadmium concentrations to nmol/L, multiply by 8.897; to convert values for blood mercury concentrations to nmol/L, multiply by 4.99. BMI indicates body mass index; CVD, cardiovascular disease; HDL, high‐density lipoprotein; IQR, interquartile range; NHANES, National Health and Nutrition Examination Survey; SBP, systolic blood pressure; SI, system of units.
Combined from Mexican American and other Hispanic in NHANES.
Calculated as weight in kilograms divided by the square of height in meters.
In ENET penalized Cox model fitted using the training data, a total of 7 blood metal predictors were retained (after shrinkage) as nonzero predictors of CVD death: 2 linear terms (blood cadmium, β=0.13; blood mercury, β=−0.09); 2 squared terms (blood lead, β=0.01; blood mercury, β=0.001); and all 3 pairwise linear interactions (between blood lead and cadmium, β=0.03; between blood lead and mercury, β=0.01; between blood cadmium and mercury, β=0.04; Table 2). All other predictor terms were shrunk to zero. These beta coefficients were then incorporated as the weights into the construction of the ERS in the testing set according to the formula (1). The ERS ranged from −0.96 to 0.35 with a mean (SD) equal to −0.11 (0.16) in the testing set. Higher values in ERS indicate more susceptibility to CVD death in relation to exposure to multiple metals. The multivariable adjusted hazard ratio of CVD death comparing the 75th to 25th percentile of ERS was 1.84 (95% CI, 1.48, 2.27; Figure).
Table 2.
Selected Blood Metal Predictorsa in Elastic‐Net Penalized Cox's Regressionb for Cardiovascular Death in the Training Set
| Selected Nonzero Blood Metal Predictor | β for Log‐Transformed Metal Concentrationsc |
|---|---|
| Linear terms | |
| Cadmium | 0.13 |
| Mercury | −0.09 |
| Squared terms | |
| Lead | 0.01 |
| Mercury | 0.001 |
| Pairwise interactions | |
| Lead×cadmium | 0.03 |
| Lead×mercury | 0.01 |
| Cadmium×mercury | 0.04 |
Logarithmic transformations with natural base were applied to all the blood metal concentrations.
Age, sex, race/ethnicity (white vs black vs Hispanic vs other races), and current smoking status were forced in the model during variable selection. Other established risk factors for cardiovascular disease were not forced in model selection because of to their potential role of intermediates on the pathways between blood metals and cardiovascular death.
Beta coefficients of selected predictors were used as weights in the following construction of the environmental risk score.
Figure 1.
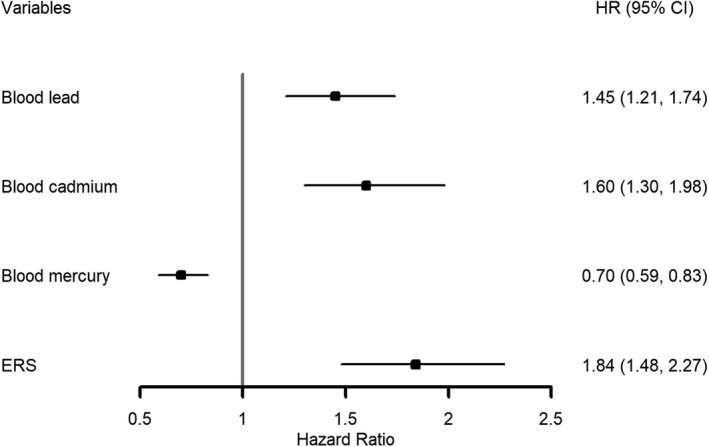
Hazard ratios for death from cardiovascular disease, according to individual blood metal concentrations and the Environmental Risk Score in the testing set. Hazard ratio (95% CI) comparing the 75th vs the 25th percentile of each variable. Blood lead, blood cadmium, and blood mercury were log transformed. Each variable was included separately in each Cox's model. All models were adjusted age, sex, race/ethnicity, current smoking status, systolic blood pressure, use of antihypertensive medications, total cholesterol level, high‐density lipoprotein cholesterol level, diabetes mellitus, and body mass index. ERS indicates environmental risk score; HR, hazard ratio.
An increase in C‐statistic for the prediction of CVD mortality was observed when predictors of blood metals were incorporated into a model with established risk factors (Table 3). The C‐statistic estimate was highest for the addition of the full combination of metal predictors, including all 3 linear term, 3 squared term, and 3 pairwise interactions, improving C‐statistic from 0.845 (95% CI, 0.822, 0.868) to 0.857 (95% CI, 0.835, 0.879) in the testing set. Beta coefficients (95% CI) of the full combination of blood metal predictors in Cox's models are shown in Table S1. Addition of the ERS to the model with established risk factors also improved the C‐statistic to 0.854 (95% CI, 0.831, 0.876).
Table 3.
C‐Statistics for Cox's Regression Models Predicting Death From Cardiovascular Disease in the Testing Set
| Risk Factors | C‐Statistics for CVD Death (95% CI) |
|---|---|
| Established risk factorsa | 0.845 (0.822, 0.868) |
| Established+blood lead (linear term) | 0.849 (0.826, 0.872) |
| Established+blood cadmium (linear term) | 0.851 (0.829, 0.874) |
| Established+blood mercury (linear term) | 0.847 (0.824, 0.871) |
| Established+3 linear term+3 squared term+3 pairwise interactions of blood lead, cadmium, and mercury | 0.857 (0.835, 0.879) |
| Established+ERS | 0.854 (0.831, 0.876) |
CVD indicates cardiovascular disease; ERS, environmental risk score.
Established risk factors include age, sex, race/ethnicity, current smoking status, systolic blood pressure, use of antihypertensive medications, total cholesterol level, high‐density lipoprotein cholesterol level, diabetes mellitus, and body mass index.
Reclassification for participants who died from CVD and for those who were alive in the testing set is summarized in Table 4. The addition of the full combination of blood metal predictors to the established risk factors led to a significant improvement in risk reclassification with an NRI of 0.08 (95% CI, 0.01, 0.14), a continuous NRI of 0.36 (95% CI, 0.24, 0.48), and an IDI of 0.011 (95% CI, 0.006, 0.016). The addition of the ERS in the model also significantly improved the risk reclassification with an NRI of 0.07 (95% CI, 0.01, 0.13), a continuous NRI of 0.27 (95% CI, 0.15, 0.40), and an IDI of 0.006 (95% CI, 0.002, 0.010; Table S2).
Table 4.
Reclassification of Participants Who Died From Cardiovascular Causes or Who Were Alive When All Blood Metal Predictors Were Added to the Established Risk Factors in the Testing Set
| Model With Established Risk Factorsa | Model With Established Risk Factors and All Linear, Squared, and Pairwise Interaction Terms of Blood Lead, Cadmium, and Mercuryb | ||||
|---|---|---|---|---|---|
| <5% Risk | 5% to 10% Risk | 10% to 20% Risk | ≥20% Risk | Total No. | |
| Participants who died from cardiovascular disease | |||||
| <5% risk | 55 | 8 | 0 | 0 | 63 |
| 5% to 10% risk | 7 | 56 | 21 | 3 | 87 |
| 10% to 20% risk | 1 | 13 | 60 | 12 | 86 |
| ≥20% risk | 0 | 0 | 5 | 15 | 20 |
| Total no. | 63 | 77 | 86 | 30 | 256 |
| Participants who did not die | |||||
| <5% risk | 5690 | 172 | 0 | 0 | 5862 |
| 5% to 10% risk | 325 | 788 | 176 | 1 | 1290 |
| 10% to 20% risk | 6 | 116 | 312 | 73 | 507 |
| ≥20% risk | 0 | 3 | 29 | 38 | 70 |
| Total no. | 6021 | 1079 | 517 | 112 | 7729 |
Established risk factors include age, sex, race/ethnicity, current smoking status, systolic blood pressure, use of antihypertensive medications, total cholesterol level, high‐density lipoprotein cholesterol level, diabetes mellitus, and body mass index.
The net reclassification improvement equals to 0.08 (95% CI, 0.01, 0.14).
In a sensitivity analysis, additional adjustment for the NHANES survey cycles, serum cotinine level, number of pack‐years of tobacco smoking, and omega‐3 fatty acid intake did not alter our findings significantly, in terms of both associations between metal predictors/ERS and CVD mortality (Figure S2) and improvements in C‐statistics (Table S3). In addition, a sensitivity analysis was performed with the CRP and family history of CVD included in the models, separately, based on 6535 participants in the testing set in which the information on CRP was available. In this subpopulation, a similar association between ERS and CVD mortality was observed as in the primary analysis, which was stronger than the associations of both CRP and family history of CVD with CVD mortality (Figure S3). In the same subpopulation, greater improvement in risk prediction for CVD death was observed when either ERS or the full combination of metal predictors were added, compared with addition of CRP or family history, in the model adjusting for the established risk factors, as evidenced by greater increases in C‐statistics (Table S4), as well as greater NRI and IDI (data not shown).
Discussion
We examined blood markers of toxic metals, including lead, cadmium, and mercury, for predicting the risk of CVD death above and beyond the established CVD risk factors, in a representative sample of US adults aged ≥40 years. The use of multiple blood metal predictors, by either incorporating a full combination of metal predictors including all linear terms, squared terms, and pairwise interactions simultaneously or adding the ERS as an integrated measure of cardiovascular health effects of exposure to blood metal mixtures, significantly improved risk prediction for CVD death, as evidenced by the increase in C‐statistics, as well as the significant NRI, continuous NRI, and IDI, in a validation data set.
To the best of our knowledge, this is the first study to investigate the improved risk prediction of CVD death by adding the information on exposure to environmental toxicants. We selected blood lead, blood cadmium, and total blood mercury as representative measures of environmental cardiovascular risk factors, on the basis of scientific evidence and data availability. Multiple disease pathways including impaired renal function, mitochondrial dysfunction, HDL dysfunction, systemic oxidative stress, inflammation, and epigenetic and endocrine disruption mechanisms have been suggested as potential mechanisms underlying the association between exposure to toxic metals and CVD risks.25, 45, 46, 47, 48, 49 In humans, an increased cardiovascular mortality has been attributed to elevated blood lead concentrations in the US general population22, 50 Meta‐analyses and systematic reviews have demonstrated that there is sufficient evidence of the association of lead exposure with hypertension.20, 38 Blood cadmium has also been shown to be an important determinant of all‐cause and CVD mortality, increased risk of peripheral arterial disease, and elevated blood pressure.23, 51, 52 Mercury was suggested to contribute to the development of CVD, though data regarding the association between blood mercury and cardiovascular end points were inconsistent.43, 53, 54 It is important to note that this association may be confounded by seafood consumption, which raises mercury concentrations but lowers cardiovascular risk.55 In our study, an inverse association between blood mercury and CVD mortality was observed, even after further adjustment for omega‐3 fatty acid intake (Figure S2). Higher mercury exposures have also been associated with lower risks of cardiovascular disease and hypertension in previous studies.43, 53 However, to the best of our knowledge, there is no biological evidence supporting that mercury itself would induce cardiovascular benefits. The observed inverse association may reflect that blood concentrations of mercury are a surrogate measure of dietary seafood consumption and thus probably provide independent information on to what extent each individual consumes seafood.53 It should also be noted that the aim of the current study is to assess the predictive performance of blood metal predictors, rather than to investigate causes of disease. More potential confounding factors and complex interactions between mercury exposure and nutrients should be considered in studies where the association between mercury exposure and cardiovascular outcomes is the primary goal of investigation.
In our study, the improvement in risk prediction for either incorporation of the full combination of metal predictors or the ERS was stronger than that for CRP and family history of CVD. CRP has been investigated extensively as a marker of inflammatory response that is useful in cardiovascular risk predictions.6, 7, 9, 44 The strong associations of family history with CVD risks suggests the role of genetic predisposition as a cardiovascular risk predictor.56, 57 Our finding is in line with the rationale of using multiple biomarkers involved in multiple disease pathways to improve the risk prediction of death from cardiovascular causes.9
This study suggests that the assessment of blood metals may be useful for identifying subpopulations that may benefit most from additional blood tests. Blood tests are commonly used for exposure assessment of environmental toxicants. Concentrations of lead, cadmium, and mercury in whole blood have gained wide acceptance as the most useful tool for screening and diagnostic testing.58 Blood lead concentrations in adults have also been closely monitored and served as a useful public health tool over the past 3 decades in the United States.59 Many established CVD risk factors are downstream effects of environmental exposures and may mediate the effect of toxic metals on CVD risk.29, 38, 39 This supports the role of blood metals as a potential upstream modifiable risk factor to prevent the development of other established risk factors initially, rather than treating risk factors only when they become elevated for which a restoration is difficult to achieve.60 Adding novel predictors, including lifestyle and environmental factors, to the conventional prediction models is also closely tied to the concept of precision medicine, an emerging approach for disease treatment and prevention based on patient characteristics such as genetics and lifestyle factors.61, 62 Current CVD prevention in clinical setting is majorly through pharmacological treatment in an attempt to modify the elevated clinical risk factors, yet assessing environmental exposures contributing to the elevated CVD risks has received little attention by the medical community. Our finding may facilitate the additional blood test for a limited number of toxicants for better CVD risk assessment, particularly among patients particularly classified as “low risk” by clinically used risk models such as Framingham Risk Score and PCE. Furthermore, the risk prediction tool incorporating environmental toxicants may highlight the importance to explore novel approaches for preventing cardiovascular outcomes in a timely manner through interventions on underlying environmental toxicants in our human bodies. A recent prospective cohort study of middle‐aged to elderly men reported that a “prudent” dietary pattern, characterized by high intake of fruit, vegetables, legumes, tomatoes, poultry, and seafood, might reduce the risk of development of coronary heart disease in relation to bone lead, suggesting that benefits from dietary interventions on CVD could be achieved by shifting to diets with a combination of natural antagonists to metals’ toxicity.21, 63 The toxic metal chelation has also been proposed recently as a secondary prevention of atherosclerotic disease by mobilizing lead and cadmium from their chronic tissue storage compartments and facilitating their excretion from human bodies.64, 65, 66 This is supported by a recent large, double‐blinded, placebo‐controlled, randomized trial of patients with previous myocardial infarction, the Trial to Assess Chelation Therapy, that a repeated edetate disodium chelation treatment, compared with placebo, significantly reduced the risk of adverse cardiovascular outcomes, including recurrent myocardial infarction, stroke, coronary revascularization, hospitalization for angina, and mortality.65, 67 Nevertheless, we acknowledge that feasibility and effectiveness of these blood metals in CVD risk assessment and prevention when incorporated into the clinical care setting, particular in combination with established clinically based risk models, should be addressed in more research in the future. From the point of view of population health and taking the principles of precision public health into account, our findings may also encourage the implementation of a targeted blood toxicant screening program, especially in areas where environmental contamination is high,68 for providing more‐efficient and ‐effective prevention and treatment strategies and potentially reducing the cost of care.62
In our study, a better prediction performance was offered by addition of the full combination of metal predictors or the ERS than that by the adjustment of linear terms of blood metals individually. This is because not only linear effects of single metals (ie, linear dose responses), but also squared terms as well as all the combinations of pairwise interactions (ie, nonlinear dose responses) of multiple metals were considered. Although the highest C‐statistics were observed for the addition of the full combination of metal predictors in our analysis, the same strategy may not be the optimal one in some other settings. For study of environmental toxicants, the potential predictors may include a large number of exposures of interest, and the relationship between these exposures and the health outcome can be complex, including nonlinear and ‐additive associations. Even with just a few exposures of interest, the combination of nonlinear and ‐additive associations can lead to a high‐dimensional exposure‐response relationship. As illustrated in our analysis, we considered all the linear terms, squared terms, and pairwise interactions of metals, yielding a total of 9 predictors to be included in the model given 3 blood metals of interests. However, if the number of exposure goes up, this would result in a model with 65 parameters in the case of 10 exposures, 230 parameters in the case of 20 exposures, and more generally, parameters in the case of p exposures. Thus, in the setting of a high‐dimensional environmental exposures, fitting a Cox model including all parameters as illustrated above is challenging and can lead to problems with overfitting. Our ERS powered by the advanced machine learning algorithms (ENET) has been proven satisfactory in analyzing high‐dimensional data while handling statistical challenges, including complex correlation structures among metals, nonlinear relationships, and confounding by copollutants.28, 29 Thus, our proposed ERS is not limited to blood metals, but can be expanded to a broader range (potentially high dimensional) of cardiovascular risk factors encompassing other environmental toxicants, pathophysiological biomarkers, lifestyle and psychosocial factors, as well as their interactions depending on investigator's points of view and research questions, as a broader public health tool for risk assessment.
Our study has several limitations. First, we selected only 3 blood metals as environmental cardiovascular risk factors because of data availability in a large sample that can allow development and validation of ERS. Other environmental chemicals not measured in every NHANES cycle (eg, dioxins)69 or in the entire NHANES sample (eg, urinary metals) might have provided additional information to better predict CVD mortality. Second, we did not predict the risk of incident cardiovascular events because of the cross‐sectional nature of the NHANES study design. Our findings require confirmations in other longitudinal cohort studies where well‐defined longitudinal cardiovascular events are available. Third, the ENET penalized Cox's regression, as a shrinkage‐based variable selection method, provides biased effect estimates, and CIs and test statistics are difficult to compute. Finally, sex‐specific models were not performed because of the limited number of CVD death providing inadequate power for sex‐stratified analyses. Sex‐stratified analyses would provide more‐accurate CVD risk predictions given sex differences in the toxicity of metals.70
Conclusions
The use of multiple predictors of exposure to blood lead, cadmium, and mercury improves risk prediction performance for death from cardiovascular causes over the established risk factors. Our results require confirmations in other longitudinal cohort studies with incident events, but have important public health implications, given the high burden of CVD morbidity and mortality. These findings highlight a potential utility of blood markers of toxic metals for CVD risk assessment, prevention, and precision health.
Sources of Funding
This study was supported by grants from the National Institute of Environmental Health Sciences (NIEHS) R01‐ES026578, R01‐ES026964, and P30‐ES017885 and by the Centers for Disease Control and Prevention (CDC)/National Institute for Occupational Safety and Health (NIOSH) grant T42‐OH008455.
Disclosures
None.
Supporting information
Table S1. Beta Coefficients* of Linear Terms, Squared Terms, and Pairwise Interactions of Blood Metals for Cardiovascular Disease Mortality in Cox's Regression† in the Testing Set
Table S2. Reclassification of Participants Who Died From Cardiovascular Disease or Who Were Alive When the Environmental Risk Score Was Added to the Established Risk Factors in the Testing Set
Table S3. C‐Statistics for Cox's Regression Models Predicting Death From Cardiovascular Disease in the Testing Set Adjusting for Additional Covariates
Table S4. C‐Statistics for Cox's Regression Models Predicting Death From Cardiovascular Disease in the Subpopulation of Testing Set*
Figure S1. Schematic diagram of study methodology in NHANES 1999–2012. CVD indicates cardiovascular disease; ENET, elastic net; ERS, Environmental Risk Score; HDL, high‐density lipoprotein; NHANES, National Health and Nutrition Examination Survey.
Figure S2. Hazard ratios for death from cardiovascular disease, according to individual blood metal concentrations and the Environmental Risk Score in the testing set, after adjusting for additional covariates. *Hazard ratio (95% CI) comparing the 75th vs the 25th percentile of each variable. Blood lead, blood cadmium, and blood mercury were log transformed. Each variable was included separately in each Cox's model. All models were adjusted for age, sex, race/ethnicity, current smoking status, systolic blood pressure, use of antihypertensive medications, total cholesterol level, high‐density lipoprotein cholesterol level, diabetes mellitus, and body mass index and additionally adjusted for NHANES survey cycles, serum cotinine, pack‐year of cigarette smoking, and omega‐3 fatty acids from dietary intake. ERS indicates environmental risk score; NHANES, National Health and Nutrition Examination Survey.
Figure S3. Hazard ratios for death from cardiovascular disease, according to individual blood metal concentration, the Environmental Risk Score, C‐reactive protein, and family history of cardiovascular disease in the subpopulation of testing set. *Hazard ratio (95% CI) comparing the 75th vs the 25th percentile of each variable, except for family history of CVD (comparing participants with family history of CVD with those without family history). Blood lead, blood cadmium, and blood mercury were log transformed. Each covariate was included separately in each Cox's model. All models were adjusted for age, sex, race/ethnicity, current smoking status, systolic blood pressure, use of antihypertensive medications, total cholesterol level, high‐density lipoprotein cholesterol level, diabetes mellitus, and body mass index. All models were performed in the testing set excluding the participants enrolled in the NHANES 2011–2012 cycle, in which information on CRP is not available. CVD indicates cardiovascular disease; CRP, C‐reactive protein; ERS, Environmental Risk Score; NHANES, National Health and Nutrition Examination Survey.
(J Am Heart Assoc. 2019;8:e013571 DOI: 10.1161/JAHA.119.013571.)
References
- 1. Xu J, Murphy SL, Kochanek KD, Bastian B, Arias E. Deaths: final data for 2016. Natl Vital Stat Rep. 2018;67:1–76. [PubMed] [Google Scholar]
- 2. World Health Organization . The top 10 causes of death. Geneva Switzerland: World Health Organization ; 2018.
- 3. Wilson PWF, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 4. Goff DC, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Sorlie P, Stone NJ, Wilson PWF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 5. Brindle P, Beswick A, Fahey T, Ebrahim S. Accuracy and impact of risk assessment in the primary prevention of cardiovascular disease: a systematic review. Heart. 2006;92:1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Folsom AR, Chambless LE, Ballantyne CM, Coresh J, Heiss G, Wu KK, Boerwinkle E, Mosley TH, Sorlie P, Diao G, Sharrett AR. An assessment of incremental coronary risk prediction using C‐reactive protein and other novel risk markers. Arch Intern Med. 2006;166:1368. [DOI] [PubMed] [Google Scholar]
- 7. Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton‐Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D'Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. [DOI] [PubMed] [Google Scholar]
- 8. Rothenbacher D, Koenig W, Brenner H. Comparison of N‐terminal pro–B‐natriuretic peptide, C‐reactive protein, and creatinine clearance for prognosis in patients with known coronary heart disease. Arch Intern Med. 2006;166:2455. [DOI] [PubMed] [Google Scholar]
- 9. Zethelius B, Berglund L, Sundström J, Ingelsson E, Basu S, Larsson A, Venge P, Ärnlöv J. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. [DOI] [PubMed] [Google Scholar]
- 10. Parikh RH, Seliger SL, Christenson R, Gottdiener JS, Psaty BM, deFilippi CR. Soluble ST2 for prediction of heart failure and cardiovascular death in an elderly, community‐dwelling population. J Am Heart Assoc. 2016;5:e003188 DOI: 10.1161/JAHA.115.003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ankle Brachial Index Collaboration , Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton‐Tyrrell K, Fowkes FG, Lee AJ, Price JF, d'Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodríguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality. JAMA. 2008; 300:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fowkes F, Murray G, Butcher I, Folsom A, Hirsch A, Couper D, DeBacker G, Kornitzer M, Newman A, Sutton‐Tyrrell K, Cushman M, Lee A, Price J, D'Agostino R, Murabito J, Norman P, Masaki K, Bouter L, Heine R, Stehouwer C, McDermott M, Stoffers H, Knottnerus J, Ogren M, Hedblad B, Koenig W, Meisinger C, Cauley J, Franco O, Hunink M, Hofman A, Witteman J, Criqui M, Langer R, Hiatt W, Hamman R; Ankle Brachial Index Collaboration. Development and validation of an ankle brachial index risk model for the prediction of cardiovascular events. Eur J Prev Cardiol. 2014;21:310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morrison AC, Bare LA, Chambless LE, Ellis SG, Malloy M, Kane JP, Pankow JS, Devlin JJ, Willerson JT, Boerwinkle E. Prediction of coronary heart disease risk using a genetic risk score: the atherosclerosis risk in communities study. Am J Epidemiol. 2007;166:28–35. [DOI] [PubMed] [Google Scholar]
- 14. Hughes MF, Saarela O, Stritzke J, Kee F, Silander K, Klopp N, Kontto J, Karvanen J, Willenborg C, Salomaa V, Virtamo J, Amouyel P, Arveiler D, Ferrières J, Wiklund P‐G, Baumert J, Thorand B, Diemert P, Trégouët DA, Hengstenberg C, Peters A, Evans A, Koenig W, Erdmann J, Samani NJ, Kuulasmaa K, Schunkert H. Genetic markers enhance coronary risk prediction in men: the MORGAM prospective cohorts. PLoS ONE. 2012;7:e40922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Talmud PJ, Cooper JA, Palmen J, Lovering R, Drenos F, Hingorani AD, Humphries SE. Chromosome 9p21.3 coronary heart disease locus genotype and prospective risk of CHD in healthy middle‐aged men. Clin Chem. 2008;54:467–474. [DOI] [PubMed] [Google Scholar]
- 16. Clarke R, Emberson JR, Parish S, Palmer A, Shipley M, Linksted P, Sherliker P, Clark S, Armitage J, Fletcher A, Collins R. Cholesterol fractions and apolipoproteins as risk factors for heart disease mortality in older men. Arch Intern Med. 2007;167:1373–1378. [DOI] [PubMed] [Google Scholar]
- 17. Homocysteine Studies Collaboration . Homocysteine and risk of ischemic heart disease and stroke: a meta‐analysis. JAMA. 2002;288:2015–2022. [DOI] [PubMed] [Google Scholar]
- 18. Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GDO, Pepys MB, Gudnason V. C‐reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. [DOI] [PubMed] [Google Scholar]
- 19. Nygård O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337:230–237. [DOI] [PubMed] [Google Scholar]
- 20. Navas‐Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease: a systematic review. Environ Health Perspect. 2007;115:472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ding N, Wang X, Tucker KL, Weisskopf MG, Sparrow D, Hu H, Park SK. Dietary patterns, bone lead and incident coronary heart disease among middle‐aged to elderly men. Environ Res. 2018;168:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lanphear BP, Rauch S, Auinger P, Allen RW, Hornung RW. Low‐level lead exposure and mortality in US adults: a population‐based cohort study. Lancet Public Heal. 2018;3:e177–e184. [DOI] [PubMed] [Google Scholar]
- 23. Tellez‐Plaza M, Navas‐Acien A, Menke A, Crainiceanu CM, Pastor‐Barriuso R, Guallar E. Cadmium exposure and all‐cause and cardiovascular mortality in the U.S. general population. Environ Health Perspect. 2012;120:1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tellez‐Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, Silbergeld EK, Devereux RB, Navas‐Acien A. Cadmium exposure and incident cardiovascular disease. Epidemiology. 2013;24:421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Genchi G, Sinicropi MS, Carocci A, Lauria G, Catalano A. Mercury exposure and heart diseases. Int J Environ Res Public Health. 2017;14:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Virtanen JK, Rissanen TH, Voutilainen S, Tuomainen TP. Mercury as a risk factor for cardiovascular diseases. J Nutr Biochem. 2007;18:75–85. [DOI] [PubMed] [Google Scholar]
- 27. Tellez‐Plaza M, Jones MR, Dominguez‐Lucas A, Guallar E, Navas‐Acien A. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr Atheroscler Rep. 2013;15:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park SK, Zhao Z, Mukherjee B. Construction of environmental risk score beyond standard linear models using machine learning methods: application to metal mixtures, oxidative stress and cardiovascular disease in NHANES. Environ Health. 2017;16:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang X, Mukherjee B, Park SK. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003–2014. Environ Int. 2018;121:683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. CDC/NCHS . NHANES—Questionnaires, Datasets, and Related Documentation. Hyattsville, MD: National Center for Health Statistics; 2018.
- 31. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2013 update. Circulation. 2013;127:e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. NCHS (National Center for Health Statistics) . NHANES (1999–2010) Linked Mortality Files: Public‐Use Data. Hyattsville, MD: NCHS (National Center for Health Statistics); 2015.
- 33. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome. Circulation. 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 34. Park SK, Tao Y, Meeker JD, Harlow SD, Mukherjee B. Environmental risk score as a new tool to examine multi‐pollutants in epidemiologic research: an example from the NHANES study using serum lipid levels. PLoS ONE. 2014;9:e98632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang Y, Zou H. A cocktail algorithm for solving the elastic net penalized Cox's regression in high dimensions. Stat Interface. 2013;6:167–173. [Google Scholar]
- 36. Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16:385–395. [DOI] [PubMed] [Google Scholar]
- 37. Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Ser. 2005;67(pt 2):301–320. [Google Scholar]
- 38. Nawrot TS, Thijs L, Den Hond EM, Roels HA, Staessen JA. An epidemiological re‐appraisal of the association between blood pressure and blood lead: a meta‐analysis. J Hum Hypertens. 2002;16:123–131. [DOI] [PubMed] [Google Scholar]
- 39. Khan AR, Awan FR. Metals in the pathogenesis of type 2 diabetes. J Diabetes Metab Disord. 2014;13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simon N, Friedman J, Hastie T, Tibshirani R. Regularization paths for Cox's proportional hazards model via coordinate descent. J Stat Softw. 2011;39:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. [DOI] [PubMed] [Google Scholar]
- 42. Pencina MJ, D'Agostino RB, Steyerberg EW Sr. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park SK, Lee S, Basu N, Franzblau A. Associations of blood and urinary mercury with hypertension in U.S. Adults: the NHANES 2003–2006. Environ Res. 2013;123:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ridker PM, Hennekens CH, Buring JE, Rifai N. C‐reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. [DOI] [PubMed] [Google Scholar]
- 45. Solenkova NV, Newman JD, Berger JS, Thurston G, Hochman JS, Lamas GA. Metal pollutants and cardiovascular disease: mechanisms and consequences of exposure. Am Heart J. 2014;168:812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang B, Li Y, Shao C, Tan Y, Cai L. Cadmium and its epigenetic effects. Curr Med Chem. 2012;19:2611–2620. [DOI] [PubMed] [Google Scholar]
- 47. Alissa EM, Ferns GA. Heavy metal poisoning and cardiovascular disease. J Toxicol. 2011;2011:870125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iavicoli I, Fontana L, Bergamaschi A. The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev. 2009;12:206–223. [DOI] [PubMed] [Google Scholar]
- 49. Ruiz‐Hernandez A, Kuo CC, Rentero‐Garrido P, Tang WY, Redon J, Ordovas JM, Navas‐Acien A, Tellez‐Plaza M. Environmental chemicals and DNA methylation in adults: a systematic review of the epidemiologic evidence. Clin Epigenetics. 2015;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lustberg M, Silbergeld E. Blood lead levels and mortality. Arch Intern Med. 2002;162:2443–2449. [DOI] [PubMed] [Google Scholar]
- 51. Tellez‐Plaza M, Navas‐Acien A, Crainiceanu CM, Sharrett AR, Guallar E. Cadmium and peripheral arterial disease: gender differences in the 1999–2004 US National Health and Nutrition Examination Survey. Am J Epidemiol. 2010;172:671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tellez‐Plaza M, Navas‐Acien A, Crainiceanu CM, Guallar E. Cadmium exposure and hypertension in the 1999–2004 National Health and Nutrition Examination Survey (NHANES). Environ Health Perspect. 2008;116:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mozaffarian D, Shi P, Morris JS, Spiegelman D, Grandjean P, Siscovick DS, Willett WC, Rimm EB. Mercury exposure and risk of cardiovascular disease in two U.S. cohorts. N Engl J Med. 2011;364:1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Larsen TJ, Jørgensen ME, Larsen CVL, Dahl‐Petersen IK, Rønn PF, Bjerregaard P, Byberg S. Whole blood mercury and the risk of cardiovascular disease among the Greenlandic population. Environ Res. 2018;164:310–315. [DOI] [PubMed] [Google Scholar]
- 55. Kris‐Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega‐3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. [DOI] [PubMed] [Google Scholar]
- 56. Valerio L, Peters RJ, Zwinderman AH, Pinto‐Sietsma SJ. Association of family history with cardiovascular disease in hypertensive individuals in a multiethnic population. J Am Heart Assoc. 2016;5:e004260 DOI: 10.1161/JAHA.116.004260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Folsom AR. Classical and novel biomarkers for cardiovascular risk prediction in the United States. J Epidemiol. 2013;23:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Barbosa F, Tanus‐Santos JE, Gerlach RF, Parsons PJ, Parsons PJ. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ Health Perspect. 2005;113:1669–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Centers for Disease Control and Prevention . Adult Blood Lead Epidemiology and Surveillance (ABLES). Cincinnati, OH: The National Institute for Occupational Safety and Health (NIOSH); 2009.
- 60. Liu K, Colangelo LA, Daviglus ML, Goff DC, Pletcher M, Schreiner PJ, Sibley CT, Burke GL, Post WS, Michos ED, Lloyd‐Jones DM. Can antihypertensive treatment restore the risk of cardiovascular disease to ideal levels? J Am Heart Assoc. 2015;4:e002275 DOI: 10.1161/JAHA.115.002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chiuve SE, Cook NR, Shay CM, Rexrode KM, Albert CM, Manson JE, Willett WC, Rimm EB. Lifestyle‐based prediction model for the prevention of CVD: the Healthy Heart Score. J Am Heart Assoc. 2014;3:e000954 DOI: 10.1161/JAHA.114.000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Khoury MJ, Galea S. Will precision medicine improve population health? JAMA. 2016;316:1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang X, Ding N, Tucker KL, Weisskopf M, Sparrow D, Hu H, Park SK. A western diet pattern is associated with higher concentrations of blood and bone lead among middle‐aged and elderly men. J Nutr. 2017;147:1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Aneni EC, Escolar E, Lamas GA. Chronic toxic metal exposure and cardiovascular disease: mechanisms of risk and emerging role of chelation therapy. Curr Atheroscler Rep. 2016;18:81. [DOI] [PubMed] [Google Scholar]
- 65. Lamas GA, Navas‐Acien A, Mark DB, Lee KL. Heavy metals, cardiovascular disease, and the unexpected benefits of chelation therapy. J Am Coll Cardiol. 2016;67:2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Waters RS, Bryden NA, Patterson KY, Veillon C, Anderson RA. EDTA chelation effects on urinary losses of cadmium, calcium, chromium, cobalt, copper, lead, magnesium, and zinc. Biol Trace Elem Res. 2001;83:207–221. [DOI] [PubMed] [Google Scholar]
- 67. Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, Lindblad L, Lewis EF, Drisko J, Lee KL; TACT Investigators . Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013;309:1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hanna‐Attisha M, LaChance J, Sadler RC, Champney Schnepp A. Elevated blood lead levels in children associated with the flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. 2016;106:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Humblet O, Birnbaum L, Rimm E, Mittleman MA, Hauser R. Dioxins and cardiovascular disease mortality. Environ Health Perspect. 2008;116:1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vahter M, Åkesson A, Lidén C, Ceccatelli S, Berglund M. Gender differences in the disposition and toxicity of metals. Environ Res. 2007;104:85–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Beta Coefficients* of Linear Terms, Squared Terms, and Pairwise Interactions of Blood Metals for Cardiovascular Disease Mortality in Cox's Regression† in the Testing Set
Table S2. Reclassification of Participants Who Died From Cardiovascular Disease or Who Were Alive When the Environmental Risk Score Was Added to the Established Risk Factors in the Testing Set
Table S3. C‐Statistics for Cox's Regression Models Predicting Death From Cardiovascular Disease in the Testing Set Adjusting for Additional Covariates
Table S4. C‐Statistics for Cox's Regression Models Predicting Death From Cardiovascular Disease in the Subpopulation of Testing Set*
Figure S1. Schematic diagram of study methodology in NHANES 1999–2012. CVD indicates cardiovascular disease; ENET, elastic net; ERS, Environmental Risk Score; HDL, high‐density lipoprotein; NHANES, National Health and Nutrition Examination Survey.
Figure S2. Hazard ratios for death from cardiovascular disease, according to individual blood metal concentrations and the Environmental Risk Score in the testing set, after adjusting for additional covariates. *Hazard ratio (95% CI) comparing the 75th vs the 25th percentile of each variable. Blood lead, blood cadmium, and blood mercury were log transformed. Each variable was included separately in each Cox's model. All models were adjusted for age, sex, race/ethnicity, current smoking status, systolic blood pressure, use of antihypertensive medications, total cholesterol level, high‐density lipoprotein cholesterol level, diabetes mellitus, and body mass index and additionally adjusted for NHANES survey cycles, serum cotinine, pack‐year of cigarette smoking, and omega‐3 fatty acids from dietary intake. ERS indicates environmental risk score; NHANES, National Health and Nutrition Examination Survey.
Figure S3. Hazard ratios for death from cardiovascular disease, according to individual blood metal concentration, the Environmental Risk Score, C‐reactive protein, and family history of cardiovascular disease in the subpopulation of testing set. *Hazard ratio (95% CI) comparing the 75th vs the 25th percentile of each variable, except for family history of CVD (comparing participants with family history of CVD with those without family history). Blood lead, blood cadmium, and blood mercury were log transformed. Each covariate was included separately in each Cox's model. All models were adjusted for age, sex, race/ethnicity, current smoking status, systolic blood pressure, use of antihypertensive medications, total cholesterol level, high‐density lipoprotein cholesterol level, diabetes mellitus, and body mass index. All models were performed in the testing set excluding the participants enrolled in the NHANES 2011–2012 cycle, in which information on CRP is not available. CVD indicates cardiovascular disease; CRP, C‐reactive protein; ERS, Environmental Risk Score; NHANES, National Health and Nutrition Examination Survey.


