ABSTRACT
Aim
Clinical and radiographic assessment of the regenerative potential of bilateral immature permanent maxillary central incisors with necrotic pulps using blood clot (BC) and platelet-rich plasma (PRP) scaffolds.
Trial design
This is a split mouth double-blinded parallel randomized controlled clinical trial.
Subjects and methods
Randomization and blinding: the study started with 15 patients with bilateral maxillary immature permanent central incisors with necrotic pulp. The two upper bilateral central incisors were randomly assigned to either the control (BC scaffold) group I or examined (PRP scaffold) group II. Participant: 13 patients aged 8–14 years fulfilled the study requirements. A follow-up was done for 3, 6, 9, and 12 months. Standardized radiographs were collected during the follow-up period, and radiographic changes were measured using Image J software. Primary outcome measured were clinical: pain, mobility, swelling, and sinus/fistula. Radiographic outcome included increased root length and increase in root thickness. Secondary outcomes were clinical: discoloration and sensibility test. Radiographic outcome included an increase in bone density measurements and a decrease in apical diameter. Standardized radiographs were collected during the follow-up period, and radiographic changes were measured using Image J software.
Results
All 26 treated teeth survived during the 12-month follow-up period with 100% success rate. PRP-treated teeth showed a statistically significant increase in radiographic root length, width, periapical bone density, and a decrease in apical diameter when compared with BC. At the end of 12 months, all treated teeth did not respond to the sensibility test. BC displayed a significantly higher amount of crown discoloration compared to the PRP group.
Conclusion
For necrotic immature teeth, regenerative endodontic treatment using PRP is a desirable alternative to BC and shows excellent 12-months prognosis.
How to cite this article
Rizk HM, AL-Deen MSS, et al. Regenerative Endodontic Treatment of Bilateral Necrotic Immature Permanent Maxillary Central Incisors with Platelet-rich Plasma versus Blood Clot: A Split Mouth Double-blinded Randomized Controlled Trial. Int J Clin Pediatr Dent 2019;12(4):332–339.
Keywords: Blood clot, Immature permanent necrotic maxillary bilateral central incisors, Platelet-rich plasma, Regenerative endodontic treatment
INTRODUCTION
The pulp of permanent immature teeth may lead to irreversible disease caused by trauma, caries, congenital abnormalities, or consequences of prior dental procedures. The mainstream of such cases are treated by apexification.1,2
The best practice treatment approach is to remove the necrotic pulp tissues and fill its place with regenerated healthy pulp tissues that would continue normal dentinogenesis.3 In recent years, there has been a robust concern in developing such biologically based treatment approach. The treatment modality of dental pulp regeneration has the capability for restoring the vitality of the tooth. In immature teeth, this can permit for continued normal physiologic development.4 The pulp will retain its ability to continue dentin formation, which will increase root thickness and length to prevent fracture and will also result in the development of an apical constriction that will provide adequate seal if root canal treatment is needed.5
Regenerative endodontic procedures (REPs) can be defined as biologically based procedures designed to replace damaged structures, including dentin, root, and cells of the pulp–dentin complex. The basis for regenerative endodontic procedures is the utilization of tissue engineering therapies with the aim to replace, repair, maintain, and/or enhance tissue function.6
It is documented that tissue regeneration requires an interplay of stem cells and growth factors in a bioactive scaffold, without which an empty canal space would not support ingrowth of new tissues from the periapical area. Stimulation of bleeding from the apex is the most commonly used revascularization technique, based on the creation of a natural blood-derived scaffold.7
Platelet-rich plasma (PRP) has been suggested as a possible scaffold for regenerative endodontic treatment. PRP can be defined as a “volume of autologous plasma that has the platelet concentration above the baseline”.8 There is a direct relationship between the number of platelets and the number of growth factors secreted by them, where it aid in the proliferation of stem cells to induce healing and regeneration of tissues. It could be anticipated that if PRP scaffold is used, regenerative endodontic treatment outcomes would improve, as this was the basis of our study.
Furthermore, several case reports and case series have demonstrated control of the infectious process, radiographic thickening of canal walls, and continued root development following pulpal necrosis in teeth with immature apices.9–14 The deficiency of well-designed split mouth randomized controlled clinical trials prevents the widespread application of this promising treatment protocol and also an evidence-based ranking to be established between BC and PRP scaffolds. So we aimed to evaluate and compare the regenerative potential of BC and PRP scaffolds in regeneration of bilateral maxillary immature permanent central incisors with necrotic pulp, clinically and radiographically.
SUBJECTS AND METHODS
Trial Design
The study is a randomized controlled trial (RCT) where two arm parallel groups with an allocation ratio of 1:1 were compared. In addition, it is a double-blinded study in which the child and parents (or the legal guardian) of each participant was blinded. Furthermore, the outcome analyzer and the statistician were blinded.
Sample Size Determination
A total of 30 immature maxillary anterior permanent incisors in 15 subjects of both genders were included, which is determined according to the method described by Pozos-Guillén et al. and Pandis et al.15–17
Study Setting
Subjects were randomly selected from patients seeking treatment from the Outpatient Clinic of Pediatric Dentistry, Faculty of Dentistry, Suez Canal University. Screening of patients continued until the target number to satisfy the inclusion criteria was achieved, which took over a period of 19 months from November 1, 2015 to May 25, 2017 and met the predetermined selection criteria.
Ethical Approval
The (study) research was conducted after it was approved by the postgraduates studies Committee of the Faculty of Dentistry, Suez Canal University in their session held on January 2016.
Eligibility Criteria18,19
The inclusion criteria were as follows: (1) subjects were free from any chronic systemic disease, (2) both gender and age ranged from 8 to 14 years, (3) tooth in question is restorable, (4) bilateral maxillary permanent incisors with incomplete (i.e., immature) root development defined by apical foramen of ≥1.0 mm, and (5) Pulp necrosis, with or without periapical lesions, either due to caries or trauma. The exclusion criteria include: (1) previous allergic response or any patient showed allergic response to ciprofloxacin, metronidazole, or minocycline or against any materials used in the study; (2) subjected teeth have been subjected to fit in exclusion criteria; (3) tooth in question has class III mobility (Miller's classification); (4) radiographic or clinical identification of ankylosis (replacement resorption) or inflammatory (infection-related) root resorption (external or internal); (5) radiographic identification of root fracture or abnormality; (6) uncooperative patients; (7) patients/legal guardian did not consent to participate in the study.
Clinical Diagnostic Procedure
A detailed dental diagnostic chart was utilized to record personal, medical, and dental history; clinical and radiographic examination; treatment plan and follow-up.
Sequence Generation
The study started with 15 patients. Sequence generation was done for the patient's number from 1 to 15 using computer sequence generation (www.random.org).
Allocation Concealment
The two upper central incisors in each group was randomly assigned by a coin toss to either the examined on the head side or the control on the tail side.
Blinding
It was not possible for the operator or the outcome assessor to be blinded owing to the nature of the treatment received.
Informed Consent
An informed consent was obtained from each patient's parents before beginning any procedure, explaining intended treatment, the possible outcomes, complications, follow-up period needed, and sequela of no treatment.
Radiographic Diagnostic Procedure and Standardization
A preoperative radiograph was taken using the standardized paralleling technique by the XCP alignment system (XCP, RINN incorporation IL60 123-1819, USA) and number 2 film (D-speed, Carestream Health, Inc. Rochester, NY 14 608, USA), which was mounted on the anterior bite block (radiographic stent) and connected to the X-ray tube via an adapter ring to evaluate root development and the presence of a periapical lesion.20
Treatment Procedure (Interventions)
Regenerative endodontic treatment has been performed according to the American Association of Endodontics protocol.19
First Appointment
Local anesthesia was administered (2% lidocaine with 1:100,000 adrenaline), (Octocaine 100, Novocol pharmaceutical, Canada), teeth were isolated with a rubber dam (Medium, 6 × 6 inch, CROSSTEX, USA), Teeth cleaning and disinfection were done with 10% providone–iodine BETADINE antiseptic solution (The Nile Co. for Pharmaceuticals and Chemical Industries—A.R.E), and the pulp chamber was accessed by using a round diamond bur (No. 016 long shank, Öko DENT, Germany) and a safe tip fissure carbide bur (Endo-z bur, Dentsply, Maillefer, USA). The working length of the canals was determined radiographically 1 mm shorter than the apical foramen and recorded for reference determined using a size of 40–90 sterile K-file according to the canal width. Mechanical instrumentation of the root canal walls was avoided as only loose or necrotic pulp tissue was removed using suitable endodontic files. Irrigation was done with sodium hypochlorite 2% NaOCl (CHLORA X D 2%, CERKAMED, UL, Poland) (20 mL/canal, 5 minutes). Side-vented ENDO-TOP irrigation needles (25 gauge) (CERKAMED, UL, Poland) were used, with the needle tip positioned about 1 mm from root end, to minimize the cytotoxic effects of sodium hypochlorite on stem cell and vital tissues. Followed by Irrigation with EDTA 17% (20 mL/canal, 5 minutes) (EDTA solution,17% MASTER-DENT 60 mL REF: 12–750 Dentonics Inc. USA), the root canals were dried with suitable size paper points (DiaDent, Korea).
Triple antibiotic paste: Triple antibiotic paste (TAP) consisted of ciprofloxacin tablets—250 mg (SPIMACO El-Dwaia Company, Saudi Arabia); Metronidazole tablets—250 mg (Sanofi Aventis, Cairo, Egypt and minocycline capsules 100 mg (Pfizer, India) were grounded individually by a pestle in separate mortars, producing a fine powder, while the minocycline capsule was evacuated from its powder content into separate mortars. Then by the use of a measuring spoon, equal amounts of ciprofloxacin, metronidazole, and minocycline with a ratio of 1:1:1 by weight were taken. On mixing, the mixed powder was placed inside a mortar for mixing using the pestle with equal amounts of distilled sterile water to form a homogenous paste with a final concentration of 0.1 mg/mL. Then it was delivered into the root canal via a specially designed syringe utilizing the tip of a pediatric canula 2 mm shorter than the working length. All the remaining mix was discarded at the end of the session, as it must be freshly prepared for standardization. Access cavity was sealed by dry sterile cotton and then temporary filling material (Coltosol F, Colten Whaldent, Switzerland) for coronal seal was placed for 21 days.
Second Appointment
Response to initial treatment was assessed. A complete resolution of signs and symptoms (which include pain, swelling, sinus, or fistula) was evaluated. With local anesthesia administration without a vasoconstrictor (3% Mepecaine, Alexandria pharmaceutical, Egypt), rubber dam isolation, and disinfection of operating field via betadine, the temporary restoration was removed using an ultrasonic scaler. Antibiotic dressing was removed by irrigation with 10-mL sterile physiological saline (sodium chloride 0.9%, FIPCO Egypt). Irrigation then was performed with 17% EDTA (20 mL/canal, 5 minutes). Canals were dried with a suitable size sterile paper points; Scaffolds were then created according to the assigned group: blood clot scaffold intervention (control group) and PRP examined group.
Group I Blood Clot Scaffold Intervention (Control Group)
Bleeding was induced by mechanical irritation of periapical tissue and rotational movement of an apically pre-bent file (e.g., long (31 mm) size 40 Hedstrom) (Mani Inc., Japan); the canal was allowed to fill with blood until 2 mm below the CEJ to wait for blood clot formation for 10–15 minutes; bleeding was initiated in all cases first for the following reasons: (a) to ensure that the bleeding was induced, if no bleeding could be initiated, bleeding was tried in the contralateral tooth. (b) In case we could not initiate bleeding in both teeth, blood was withdrawn from the patient's arm and was injected inside the canal. Fortunately, we could initiate bleeding in all cases and no need to withdraw blood from the patient. (c) The time taken about 10–15 minutes for clotting to take place was consumed in preparation of PRP. A collagen matrix (Collacote (Integra Life Sciences Corp., Plainsboro, NJ, USA) was cut to a diameter larger than the coronal part of the root canal and a height of 3–4 mm, place on top of the blood clot, allow the matrix to soak with blood to avoid formation of a hollow space. MTA was placed using an amalgam carrier on top of the collagen matrix in a thin homogeneous layer of about 2 mm underneath the cement–enamel junction.
Group II Platelet-rich Plasma Scaffold (Examined Group)
An estimated 0.5 mL of acid citrate dextrose (ACD) anticoagulant (C3821 Sigma, Sigma-Aldrich, Germany) was injected to a 5 mL sterile plastic syringe (med. EL-Dawlia Ico. Egypt) with a ratio of 9:1. About 4.5 mL of intravenous blood from the antecubital vein was collected in the same sterile plastic syringe and then the syringe was turned upside down ten-times carefully to mix ACD and the collected blood together. Blood from the sterile plastic syringe was transferred to a 5 mL sterile glass tube and then recapped to be centrifuged.
Preparation of Platelet-rich Plasma
PRP was prepared according to the Dohan and Choukroun method.21 Centrifugation machine (i Fuge D06 Bench Top Doctor Centrifuge, Neuation Tech Pvt. Ltd. India) was balanced by adding saline tube opposite to blood-containing tube. The collected blood was centrifuged immediately using a “soft” spin at 2,400 rpm for 10 minutes to separate PRP and platelet-poor plasma (PPP) portions from the red blood cell fraction. The supernatant layer containing PRP and PPP was transferred into another sterile glass tube without the anticoagulant. Again, they were centrifuged at a higher speed (hard spin) at 3,600 rpm for 15 minutes to separate the PRP from the PPP. The upper two-third PPP is removed with a sterile plastic syringe and discarded; then the remaining PRP is mixed with 0.5 mL 10% calcium chloride (EIPICO, Egypt) to activate the platelets and to neutralize the acidity of ACD. Then PRP was soaked on a 2 × 2 mL of sterile collagen sponge and was introduced into the root canal with a sterile tweezer and pushed beyond apical region and was flushed to the level of cemento-enamel junction with a size 30 finger plugger (Fig. 1). An MTA orifice plug extending 3–4 mm in the canal was used to seal the canal orifice covered by a moist cotton pellet and then glass ionomer. The patient was recalled after 3 days to confirm the setting of MTA and then the access cavity is sealed with a layer of Fuji II glass ionomer material (GC America, Alsip, IL) and composite (Z 250, 3M ESPE) to give an effective and durable seal.
Fig. 1.
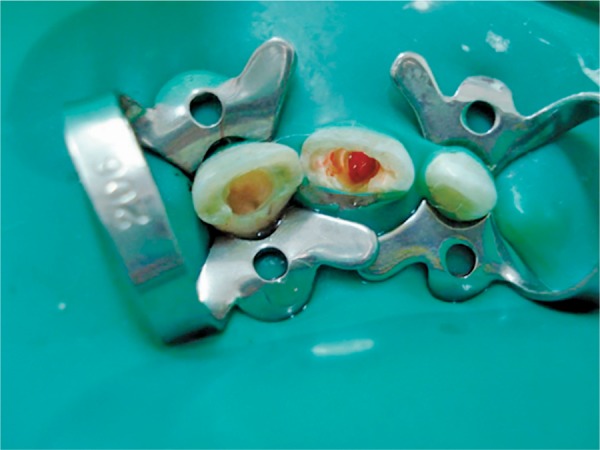
CollaCote soaked with PRP inside the canal
Posttreatment Evaluation
All patients were recalled at 3, 6, 9, and 12 months to evaluate treated teeth. In this study, the primary and secondary outcomes were assessed. The primary outcomes were clinical: pain, mobility, swelling, and sinus/fistula. In addition, radiographic outcome included increased root length and increase in root thickness. On the other hand, secondary outcomes were clinical: discoloration and sensibility test. Radiographic outcome included an increase in bone density measurements and a decrease in the apical diameter (Fig. 2).
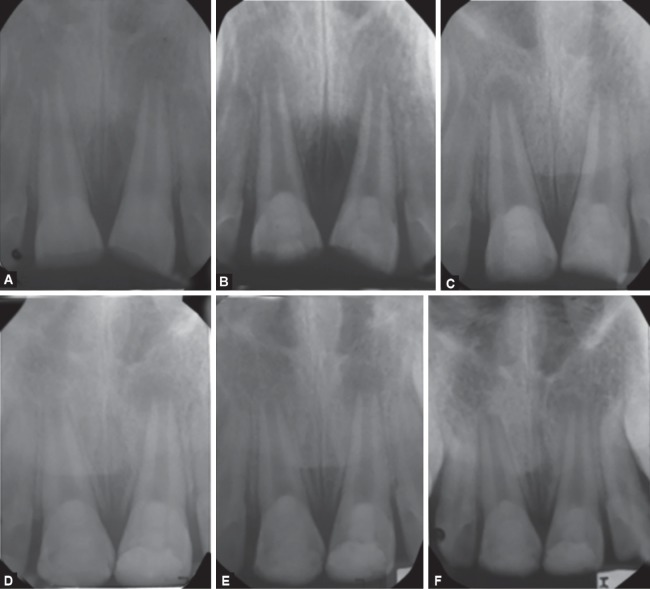
Figs 2A to F.
Group I BC vs group II PRP: immature bilateral, fractured teeth (#11 and 21) with an open apex and large periradicular radiolucency in a 9-year-old boy. 11 was treated with PRP and 21 was treated with blood clot. Periapical radiographs: (A) Preoperative periapical radiolucent lesion with an open apex; (B) After the placement of mineral trioxide aggregate; (C) At the 3-month follow-up, partial regression of periapical radiolucent lesion; (D) At the 6-month follow-up, with marked reduction in periapical lesion with continued development of the root; (E) At the 9-month follow-up, nearly complete healing of periapical lesion with continued development of the root apex; (F) At 1-year follow-up, complete maturation of the root apex
Image Analysis
Quantitative radiographic judgement of the outcome was done using Image J software for easier radiometric measurements and calculation was carried out by the same method described by Nagy et al.22
Statistical Analysis
Statistical analysis was performed using commercially available software program (SPSS statistical version 19). The Wilcoxon signed-rank test can be used as an alternative to the paired Student's t test, t test for matched pairs, or the t test for dependent samples when the population cannot be assumed to be normally distributed, and the size of the sample is less than 30. The Mann–Whitney U test is used to test whether two samples are likely to derive from the same population. The level of significance is set to be p < 0.05.
RESULTS
Patient Flow
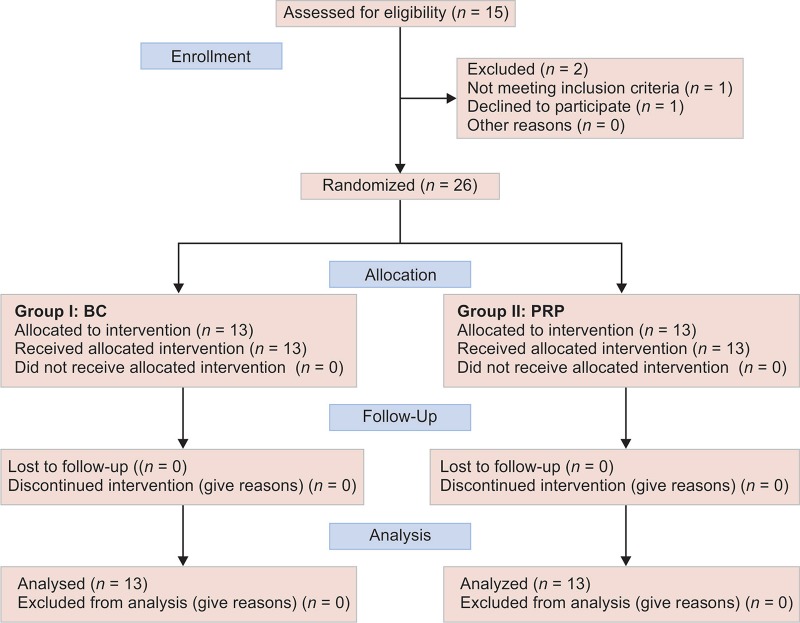
Flowchart 1 shows the flow of the patients through the study.
Flowchart 1.
CONSORT flow diagram presenting the flow of the patients through the study
Demographic Variables
Table 1 describes the demographic data for the participants in each group.
Table 1.
Sample description according to the basic characteristics of the patients
| Variable | BC vs PRP (n = 13) n (%) | |
|---|---|---|
| Age | ||
| Mean ± SD | 9.08 ± 1.038 | |
| Sex | ||
| Male | 7 (53.8%) | |
| Female | 6 (46.2%) | |
| Type of Trauma | Right | Left |
| Enamel-dentin-pulp fracture | 7 (53.8%) | 6 (46.2%) |
| Enamel-dentin | 6 (46.2%) | 7 (53.8%) |
| No loss of tooth structure | 0 (0%) | 0 (0%) |
Primary Clinical Outcomes
No statistically significant difference between the two groups were found in regard to primary clinical outcomes (resolution of pain, swelling, mobility, and sinus/fistula) and all cases showed 100% success.
Primary Radiographic Outcomes
Tables 2 and 3 show the primary radiographic outcomes (root length and thickness).
Table 2.
Increase in root length in millimeters and percentage of the PRP and BC groups in the four evaluation periods
| PRP group (mean ± SD) | BC group (mean ± SD) | p value | |
|---|---|---|---|
| 3 months (mm, %) | 0.225 ± 0.19 (1.52% ± 1.43%) | 0.133 ± 0.217 (0.967% ± 1.75%) | 0.006** |
| 6 months (mm, %) | 0.557 ± 0.23 (3.7% ± 1.43%) | 0.273 ± 0.29 (1.92% ± 2.32%) | 0.0028** |
| 9 months (mm, %) | 0.996 ± 0.35 (6.6% ± 2.4%) | 0.449 ± 0.35 (3.11% ± 2.8%) | 0.001** |
| 12 months (mm, %) | 1.48 ± 0.37 (9.88% ± 2.85%) | 0.68 ± 0.44 (4.68% ± 3.45%) | 0.001** |
*Significant at p value <0
**Highly significant at p value <0.05
Table 3.
Increase in root thickness in millimeters and percentage of PRP and BC in the four evaluation periods
| PRP group (mean ± SD) | BC group (mean ± SD) | p value | |
|---|---|---|---|
| 3 months (mm, %) | 0.153 ± 0.128 (6.03% ± 5.03%) | 0.133 ± 0.27 (5.45% ± 9.48%) | 0.806 |
| 6 months (mm, %) | 0.445 ± 0.41 (18.05% ± 17.45%) | 0.335 ± 0.506 (12.46% ± 17.95%) | 0.019** |
| 9 months (mm, %) | 0.739 ± 0.56 (29.65% ± 23.9%) | 0.49 ± 0.527 (18.44% ± 19.95%) | 0.003** |
| 12 months (mm, %) | 0.97 ± 0.75 (39.27% ± 32.04%) | 0.68 ± 0.678 (25.56% ± 26.5%) | 0.002** |
*Significant at p value <0.1
**Highly significant at p value <0.05
Secondary Clinical Outcomes
All revascularized/revitalized teeth in the present study did not respond to pulp sensibility tests (thermal (cold/heat), and electric pulp tester) at the end of the 12-month study.
BC group showed a higher crown discoloration than PR group, with significant difference between the groups.
Secondary Radiographic Outcomes
Tables 4 and 5 show the secondary radiographic outcomes.
Table 4.
Increase in bone density in grey value and percentage of PRP and BC groups in the four evaluation periods
| PRP group (mean ± SD) | BC group (mean ± SD) | p value | |
|---|---|---|---|
| 3 months (grey value, %) | 24.87 ± 16.63 (27.605% ± 19.89%) | 19.45 ± 12.35 (0.206% ± 1.60%) | 0.027** |
| 6 months (grey value, %) | 34.42 ± 21.03 (42.3% ± 25.46%) | 33.8 ± 17.87 (0.355% ± 0.235%) | 0.027** |
| 9 months (grey value, %) | 52.47 ± 25.39 (57.74% ± 31.36%) | 44.91 ± 21.01 (0.468% ± 0.283%) | 0.027** |
| 12 months (grey value, %) | 65.08 ± 30.043 (71.84% ± 30.043%) | 58.96 ± 19.95 (0.609% ± 0.27%) | 0.027** |
*Significant at p value <0.1
**Highly significant at p value <0.05; SD, standard deviation
Table 5.
Decrease in apical diameter in millimeters and percentage of the PRP and BC groups in the four evaluation periods
| PRP group (mean ± SD) | BC group (mean ± SD) | p value | |
|---|---|---|---|
| 3 months (mm, %) | 0.25 ± 0.167 (9.91%±6.03%) | 0.137 ± 0.063 (6.06% ± 3.7%) | 0.008** |
| 6 months (mm, %) | 0.656 ± 0.43 (27.29%±14.1%) | 0.42 ± 0.27 (18.24% ± 11.11%) | 0.005** |
| 9 months (mm, %) | 2.17 ± 3.86 (51.98%±19.64%) | 1.92 ± 3.9 (40.7% ± 21.43%) | 0.002** |
| 12 months (mm, %) | 2.49 ± 3.93 (64.83% ± 18.5%) | 2.2 ± 3.97 (53.45% ± 19.4%) | 0.003** |
*Significant at p value <0.1
**Highly significant at p value <0.05; SD, standard deviation
DISCUSSION
The split-mouth study formulation is an illustration of a randomization on the spot level where control and intervention groups are randomly categorized to sites of one of the two halves of the mouth. The attractiveness of the split-mouth design is the reduction of much of the inter-subject variability by this means enhancing the power of the study compared to the whole-mouth design. In other words, it aims to remove all elements related to differences between patients, by making within-patient comparisons, rather than between-patient comparisons, thus reducing the sample size.23
Patients were selected with immature necrotic teeth having an apical opening greater than 1 mm. This feature allows the distribution of multipotent mesenchymal stem cells into the root canal space of necrotic immature teeth after a REP.24,25
Bacteria have been shown to penetrate deeper in teeth of younger individuals than older individuals, making bacterial elimination in immature infected teeth a significant challenge.26 The present study mainly relied upon chemical disinfection using 2% sodium hypochlorite and TAP. Sodium hypochlorite was reported to be used in 80% of revascularization/regenerative endodontics published cases.27 We used predetermined concentration of sodium hypochlorite bottles as the market available concentration is labeled below 5% and cannot be diluted by standardized available methods as the original concentration is precisely unknown (<5%). The choice of sodium hypochlorite concentration reveals the need for a weighing scale between sufficient root canal disinfection and survival of the patient's stem cells. It was used in low concentration to circumvent its high concentration harmful effect, as lower concentrations (1–3%) increase the survival of stem cells.28
TAP-containing minocycline have been found to achieve significantly improved results than other pastes as it is capable of diffusing all the way through the dentin thickness and effectively disinfecting deep layers of root canal dentin.26 In addition, a greater increase in dentinal wall thickness was detected with the TAP containing minocycline.29
“All randomized control trials (RCT) assess response variables, or outcomes (end points), for which the groups are compared. Most trials have several outcomes, some of which are of more interest than others. The primary outcome measure is the pre-specified outcome considered to be of greatest importance to relevant stakeholders (such as patients, policy-makers, or clinicians)” as stated by CONSORT statement 2010.30 In this study, the primary and secondary outcomes were assessed. The primary outcomes were clinical: pain, mobility, swelling, and sinus/fistula. In addition, radiographic outcomes included increased root length and increase in root thickness. On the other hand, secondary outcomes were clinical: discoloration and sensibility test. Radiographic outcomes included an increase in bone density measurements and a decrease in apical diameter.
During the follow-up intervals, all of the cases continued their 100% clinical success rate in the two groups with no statistical significant difference between them, which was superior to the success rate of conventional root canal treatment therapy. These data were consistent with the finding presented by Torabinejad and Faras,31 Saoud et al.,32 Alagl et al.33 On the other hand, other studies reported 80–91% success in regenerating endodontics. The difference in success rate might be attributed to different inclusion criteria, different criteria of success, sample size, and different follow-up periods. Regenerative endodontic therapy appears to have a higher clinical success rate in the initial 1–2 years after treatment completion.32
Our study revealed that the mean increase in root length and root dentin thickness in either mm or percentage in PRP group is greater than that of BC for all time points. This difference is statistically significant for all time points with 95% confidence. The findings were in agreement with the results of Shivashankar et al.,34 Jadhav et al.,35 and Turky et al.36 Our findings were in disagreement with Bezgin et al.,37 who concluded that PRP successfully created a scaffold for regenerative endodontic treatment; however, treatment outcomes did not differ significantly between PRP and a conventional blood clot scaffold.
Root growth in length and thickness could be due to the survival of few vital pulp inside the root canal system, which transforms into odontoblasts steered by the intact epithelial root sheath of Hertwig.38 Another possible interpretation that periodontal ligament stem cells, could migrate to the canal via the open apex then differentiate and flourish inside the canal.39 The third possibility relies on the stem cells of apical papilla (SCAPs), where injury with H-file beyond the apical limit of the canal results in the migration of SCAPs into the root canal system. SCAPs may survive infection and retain the capacity for proliferation and differentiation into bone- or dentin-forming cells.40 In addition to all these hypothesis, the use of PRP increased the concentrations of growth factors that can attract stem cells present in the apical tissues and even from periapical lesions.41
All revascularized/revitalized teeth in the study did not respond to pulp sensibility tests during follow-up intervals and at the end of the 12-month study. These findings agreed with Petrino et al.,12 Torabinejad and Fares,42 and Saoud et al.32 Other study reported variable positive responses to pulp sensibility tests.43 Negative results to pulp sensibility test could be due to coronally present MTA layer, which acts as an insulator, or the need of more than 12 months for complete formation of blood vessels and nerve fibers within the root canal.44 Furthermore, thickening of the canal walls is mainly caused by deposition of cementum-like tissue without the tubular structure observed in dentin.45
For teeth discoloration, the percentage of teeth displayed discoloration for BC group is greater and statistically significant than in PRP group for all time points. Scaffold may play a role in discoloration by interacting with MTA during its setting. Blood by itself could lead to discoloration by the accretion of hemoglobin in dentin. Staining of MTA is visible when material sets in interaction with red blood cells (RBCs). It could be hypothesized that iron ions and development of calcium aluminoferrate plays a role in discoloration. Furthermore, porosities that might present in MTA can absorb blood components.46
For bone density measurements, results showed increased density by the time in both groups. However, the mean of the increase of bone density in the PRP group is greater than that of BC group for all time points. This difference is statistically significant. Our findings was in agreement with Alagl et al.,33 and Jadhav et al.35 Results indicate a favorable healing response to the proposed treatment, which was initiated by canal disinfection via the TAP and completed by the application of the scaffold and the MTA plug, where PRP showed an advantage over the BC during the follow-up period. This is attributed to various growth factors, namely PDGF, transforming growth factor β, insulin-like growth factor, and epidermal growth factor present in the PRP. All these factors play a major role in bone regeneration and healing.47
The decrease in the apical diameter for the PRP group is greater than that of the BC group for all time points. This difference is statistically significant. Our findings were in agreement with Jadhav et al.,35 Alagl et al.,33 and Murray.48 Hertwig's epithelial root sheath (HERS) is the most important tissue on the development of the root and the formation of the apical hard tissue barrier. When HERS is damaged, root development is also interrupted; however, apical hard tissue formation can still continue by means of the differentiation of the reservoir cells. If HERS is completely destroyed, apical hard tissue can be formed by cementoblasts and fibroblasts in the apical area.49
CONCLUSION
For necrotic immature teeth, revascularization using PRP is a desirable alternative to BC and shows excellent 12-month prognosis. Though PRP gives better results compared to BC, it requires blood withdrawal from the child, which might be difficult in needle phobic children and also requires expensive materials and equipment.
RECOMMENDATIONS
Regenerative endodontic procedure should be the first choice of treatment in children with immature permanent teeth with necrotic pulp as it displays excellent results.
When deciding to perform regenerative endodontic procedure, PRP should be the first choice as scaffolding material when compared with BC.
LIMITATIONS
Pulp vitality testing that assess pulp blood supply should have been used, which is done by laser doppler flowmetry and a pulse oximeter.
The histology of tissues formed inside root canal could not be assessed owing to ethical reasons.
Footnotes
Source of support: Nil
Conflict of interest: None
REFERENCES
- 1.Cvek M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endo Dent Traumat. 1992;8(2):45–55. doi: 10.1111/j.1600-9657.1992.tb00228.x. DOI: [DOI] [PubMed] [Google Scholar]
- 2.Andreasen J, Farik B, et al. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumat. 2002;18(3):134–137. doi: 10.1034/j.1600-9657.2002.00097.x. DOI: [DOI] [PubMed] [Google Scholar]
- 3.Simon S, Rilliard F, et al. The use of mineral trioxide aggregate in one-visit apexification treatment: a prospective study. Int Endo J. 2007;40(3):186–197. doi: 10.1111/j.1365-2591.2007.01214.x. DOI: [DOI] [PubMed] [Google Scholar]
- 4.Holden D, Schwartz S, et al. Clinical outcomes of artificial root-end barriers with mineral trioxide aggregate in teeth with immature apices. J Endo. 2008;34(7):812–817. doi: 10.1016/j.joen.2008.04.003. DOI: [DOI] [PubMed] [Google Scholar]
- 5.Murray P, Garcia-Godoy F, et al. Regenerative endodontics: a review of current status and a call for action. J Endo. 2007;33(4):377–390. doi: 10.1016/j.joen.2006.09.013. DOI: [DOI] [PubMed] [Google Scholar]
- 6.Verma P, Fouad A. What is the Public Health Need for Regenerative Endodontics? J Endo. 2012;38(3):47. [Google Scholar]
- 7.Shah N, Logani A, et al. Efficacy of Revascularization to Induce Apexification/Apexogensis in Infected, Nonvital, Immature Teeth: A Pilot Clinical Study. J Endod. 2008;34(8):919–925. doi: 10.1016/j.joen.2008.05.001. DOI: [DOI] [PubMed] [Google Scholar]
- 8.Marx RE, Carlson ER. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638–646. doi: 10.1016/S1079-2104(98)90029-4. DOI: [DOI] [PubMed] [Google Scholar]
- 9.Cotti E, Mereu M, et al. Regenerative treatment of an immature, traumatized tooth with apical periodontitis: report of a case. J Endo. 2008;34(5):611–616. doi: 10.1016/j.joen.2008.02.029. DOI: [DOI] [PubMed] [Google Scholar]
- 10.Jung IY, Lee SJ, et al. Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. J Endo. 2008;34(7):876–887. doi: 10.1016/j.joen.2008.03.023. DOI: [DOI] [PubMed] [Google Scholar]
- 11.Chueh L, Ho Y, et al. Regenerative endodontic treatment for necrotic immature permanent teeth. J Endo. 2009;35(2):160–164. doi: 10.1016/j.joen.2008.10.019. DOI: [DOI] [PubMed] [Google Scholar]
- 12.Petrino J, Boda K, et al. Challenges in regenerative endodontics: a case series. J Endo. 2010;36(3):536–541. doi: 10.1016/j.joen.2009.10.006. DOI: [DOI] [PubMed] [Google Scholar]
- 13.Torabinejad M, Faras H. A clinical and histological report of a tooth with an open apex treated with regenerative endodontics using platelet-rich plasma. J Endo. 2012;38(6):864–868. doi: 10.1016/j.joen.2012.03.006. DOI: [DOI] [PubMed] [Google Scholar]
- 14.Soares A, Lins FF, et al. Pulp revascularization after root canal decontamination with calcium hydroxide and 2% chlorhexidine gel. J Endo. 2013;39:417–420. doi: 10.1016/j.joen.2012.10.005. DOI: [DOI] [PubMed] [Google Scholar]
- 15.Pozos-Guillén A, Chavarría-Bolaños D, et al. Split-mouth design in Paediatric Dentistry clinical trials. Eur J Paediatr Dent. 2017;18:1–5. doi: 10.1007/s40368-017-0268-x. DOI: [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Chow S-C. Sample Size Calculation for Comparing Proportions. Wiley Encyclopedia of Clinical Trials; 2007. [Google Scholar]
- 17.Pandis N, Polychronopoulou A, et al. Sample calculation for split-mouth designs. Am J Orthod Dentofacial Orthop. 2011;140(4):142–146. doi: 10.1016/j.ajodo.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Galler KM, Krastl G, et al. European Society of Endodontology position statement: Revitalization procedures. Int Endod J. 2016;49:717–723. doi: 10.1111/iej.12629. DOI: [DOI] [PubMed] [Google Scholar]
- 19.American Association of Endodontics Clinical considerations for a regenerative procedure. www.aae.org 2018. www.aae.org Available at: ,
- 20.Hamanaka EF, Poi WR, et al. A method for the geometric standardization of intraoral radiographs for long-term follow up of replanted teeth: a case report. Dent Traumatol. 2012;29(2):121–126. doi: 10.1111/j.1600-9657.2012.01145.x. DOI: [DOI] [PubMed] [Google Scholar]
- 21.Dohan DM, Choukroun J. PRP, cPRP, PRF, PRG. How to find your way in the jungle of platelet concentrates. Oral Surg Oral Med Oral Pathol Oral Radiol Endo. 2007;103:305–316. doi: 10.1016/j.tripleo.2006.10.009. DOI: [DOI] [Google Scholar]
- 22.Nagy MM, Tawfik HE, et al. Regenerative Potential of Immature Permanent Teeth with Necrotic Pulps after Different Regenerative Protocols. J Endod. 2014;40(2):192–198. doi: 10.1016/j.joen.2013.10.027. DOI: [DOI] [PubMed] [Google Scholar]
- 23.Lesaffre E, Philstrom B, et al. The design and analysis of split-mouth studies: what statisticians and clinicians should know. Stat Med. 2009;28(28):3470–3482. doi: 10.1002/sim.3634. DOI: [DOI] [PubMed] [Google Scholar]
- 24.Lovelace TW, Henry MA, et al. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endod. 2011;37:133–138. doi: 10.1016/j.joen.2010.10.009. DOI: [DOI] [PubMed] [Google Scholar]
- 25.Estefan BS, El Batouty KM, et al. Influence of Age and Apical Diameter on the Success of Endodontic Regeneration Procedures. J Endod. 2016;42(11):1620–1625. doi: 10.1016/j.joen.2016.06.020. DOI: [DOI] [PubMed] [Google Scholar]
- 26.Nosrat A, Kim JR, et al. Tissue Engineering Considerations in Dental Pulp Regeneration. Iran Endod J. 2014;9(1):30–40. [PMC free article] [PubMed] [Google Scholar]
- 27.Kontakiotis EG, Filippatos CG, et al. Regenerative endodontic therapy: a data analysis of clinical protocols. J Endod. 2015;41:146–154. doi: 10.1016/j.joen.2014.08.003. DOI: [DOI] [PubMed] [Google Scholar]
- 28.Martin DE, De Almedia JFA, et al. Concentration-dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J Endod. 2014;40:51–55. doi: 10.1016/j.joen.2013.07.026. DOI: [DOI] [PubMed] [Google Scholar]
- 29.Thibodeau B, Teixeira F, et al. Pulp revascularization of immature dog teeth with apical periodontitis. J Endo. 2007;33(6):680–689. doi: 10.1016/j.joen.2007.03.001. DOI: [DOI] [PubMed] [Google Scholar]
- 30.http://www.consort-statement.org/ [visited 19 October 2018]. http://www.consort-statement.org/
- 31.Torabinejad M, Faras H. A clinical and histological report of a tooth with an open apex treated with regenerative endodontics using platelet-rich plasma. J Endo. 2012;38(6):864–868. doi: 10.1016/j.joen.2012.03.006. DOI: [DOI] [PubMed] [Google Scholar]
- 32.Saoud TMA, Zaazou A, et al. Clinical and radiographic outcomes of traumatized immature permanent necrotic teeth after revascularization/revitalization therapy. J Endod. 2014;40:1946–1952. doi: 10.1016/j.joen.2014.08.023. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alagl A, Bedi S, et al. Use of platelet-rich plasma for regeneration in non-vital immature permanent teeth: Clinical and cone-beam computed tomography evaluation. J Int Med Res. 2017;45(2):583–593. doi: 10.1177/0300060517692935. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shivashankar VY, Johns DA, et al. Comparison of the Effect of PRP, PRF and Induced Bleeding in the Revascularization of Teeth with Necrotic Pulp and Open Apex: A Triple Blind Randomized Clinical Trial. J Clin Diagn Res. 2017;11(6):34–39. doi: 10.7860/JCDR/2017/22352.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jadhav G, Shah N, et al. Revascularization with and without platelet-rich plasma in nonvital, immature, anterior teeth: A pilot clinical study. J Endod. 2012;38(12):1581–1587. doi: 10.1016/j.joen.2012.09.010. DOI: [DOI] [PubMed] [Google Scholar]
- 36.Turky M, Kataia MA, et al. Revascularization Induced Maturogenesis of Human Non-Vital Immature Teeth via Platelets-Rich Plasma (PRP): Radiographic Study. J Dent Oral Health. 2017;3(9):97–101. [Google Scholar]
- 37.Bezgin T, Yilmaz AD, et al. Concentrated platelet-rich plasma used in root canal revascularization: 2 case reports. Int Endod J. 2014;47:41–49. doi: 10.1111/iej.12144. DOI: [DOI] [PubMed] [Google Scholar]
- 38.Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endo. 2004;30(4):196–200. doi: 10.1097/00004770-200404000-00003. DOI: [DOI] [PubMed] [Google Scholar]
- 39.Lieberman J, Trowbridge H. Apical closure of non-vital permanent incisor teeth where no treatment was performed: case report. J Endod. 1983;9(6):257–260. doi: 10.1016/S0099-2399(86)80025-5. DOI: [DOI] [PubMed] [Google Scholar]
- 40.Gronthos S, Mankani M, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci. 2000;97(25):625–630. doi: 10.1073/pnas.240309797. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torabinejad M, Turman M. Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: A case report. J Endod. 2011;37(2):265–268. doi: 10.1016/j.joen.2010.11.004. DOI: [DOI] [PubMed] [Google Scholar]
- 42.Torabinejad M, Faras H. A clinical and histological report of a tooth with an open apex treated with regenerative endodontics using platelet-rich plasma. J Endo. 2012;38(6):864–868. doi: 10.1016/j.joen.2012.03.006. DOI: [DOI] [PubMed] [Google Scholar]
- 43.Cehreli ZC, Isbitiren B, et al. Regenerative endodontictreatment (revascularization) of immature necrotic molars medicated with calcium hydroxide: a case series. J Endod. 2011;37:1327–1330. doi: 10.1016/j.joen.2011.05.033. DOI: [DOI] [PubMed] [Google Scholar]
- 44.Yang J, Yuan J, et al. Pulp Regeneration: Current Approaches and Future Challenges. Front Physiol. 2016;58(7):1–8. doi: 10.3389/fphys.2016.00058. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Thibodeau B, et al. Histologic characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. J Endod. 2010;36:56–63. doi: 10.1016/j.joen.2009.09.039. DOI: [DOI] [PubMed] [Google Scholar]
- 46.Shokouhinejad N, Nekoofar MH, et al. Evaluation and Comparison of Occurrence of Tooth Discoloration after the Application of Various Calcium Silicate-based Cements: An Ex Vivo Study. J Endod. 2016;42(1):140–144. doi: 10.1016/j.joen.2015.08.034. DOI: [DOI] [PubMed] [Google Scholar]
- 47.Carlson E, Roach RB. Platelet-Rich Plasma. Clinical Applications in dentistry. J Am Dent Assoc. 2002;133:1383–1386. doi: 10.14219/jada.archive.2002.0054. [DOI] [PubMed] [Google Scholar]
- 48.Murray PE. Mini review of the clinical efficacy of platelet-rich plasma, platelet-rich fibrin and blood-clot revascularization for the regeneration of immature permanent teeth. World J Stomatol. 2018;6(1):1–5. doi: 10.5321/wjs.v6.i1.1. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He L, Zhong J, et al. Treatment of Necrotic Teeth by Apical Revascularization: Meta-analysis. Sci Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-14412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]