Abstract
Recent innovations have the potential to improve rhythm control therapy in patients with atrial fibrillation (AF). Controlled trials provide new evidence on the effectiveness and safety of rhythm control therapy, particularly in patients with AF and heart failure. This review summarizes evidence supporting the use of rhythm control therapy in patients with AF for different outcomes, discusses implications for indications, and highlights remaining clinical gaps in evidence. Rhythm control therapy improves symptoms and quality of life in patients with symptomatic AF and can be safely delivered in elderly patients with comorbidities (mean age 70 years, 3–7% complications at 1 year). Atrial fibrillation ablation maintains sinus rhythm more effectively than antiarrhythmic drug therapy, but recurrent AF remains common, highlighting the need for better patient selection (precision medicine). Antiarrhythmic drugs remain effective after AF ablation, underpinning the synergistic mechanisms of action of AF ablation and antiarrhythmic drugs. Atrial fibrillation ablation appears to improve left ventricular function in a subset of patients with AF and heart failure. Data on the prognostic effect of rhythm control therapy are heterogeneous without a clear signal for either benefit or harm. Rhythm control therapy has acceptable safety and improves quality of life in patients with symptomatic AF, including in elderly populations with stroke risk factors. There is a clinical need to better stratify patients for rhythm control therapy. Further studies are needed to determine whether rhythm control therapy, and particularly AF ablation, improves left ventricular function and reduces AF-related complications.
Keywords: Atrial fibrillation, Rhythm control therapy, AF ablation, Antiarrhythmic drugs, Heart failure, Stroke, Mortality
Introduction
The prevalence of atrial fibrillation (AF) and its associated mortality and morbidity are expected to double or triple within the next two to three decades, driven by population ageing and increased incidence of AF.1,2 Even on optimal anticoagulation and rate control therapy, patients with AF are at high risk of cardiovascular death, particularly sudden death and death due to heart failure.3,4 Rhythm control therapy using antiarrhythmic drugs, cardioversion, and AF ablation, is clinically used to improve AF-related symptoms.5 Currently, there is no established indication for rhythm control therapy apart from improvement of AF-related symptoms.6–8 The CABANA (Catheter Ablation vs. Anti-arrhythmic Drug Therapy for Atrial Fibrillation) trial recently provided new confirmation on the safety of AF ablation in contemporary AF patients at risk of stroke.9 The smaller CASTLE-AF (Catheter Ablation vs. Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation) suggests that AF ablation could improve outcomes in patients with AF and severe heart failure compared to drug therapy, combining rate control therapy and antiarrhythmic drug therapy.10 Here, we review the available evidence supporting the use of rhythm control therapy in patients with AF, discuss potential implications for indications, and highlight clinical evidence gaps.
Rhythm control therapy improves atrial fibrillation-related symptoms
Restoring and maintaining sinus rhythm indicated to minimize symptoms is a main goal in patients who remain symptomatic despite adequate rate control.11,12 Interestingly, the effects of rhythm control on quality of life are less uniform than their clear effects on maintaining sinus rhythm (Table 1). Both natural variation in patient-reported quality of life, imprecise instruments to assess quality of life, and variable effects of rhythm control therapy on quality of life in individual patients can explain this heterogeneity.13,14 The European Heart Rhythm Association (EHRA) symptom score was introduced in 2007 as a simple clinical tool to quantify AF-related symptoms,15 with subsequent refinement and validation.16 Several disease-specific instruments are available, all with specific strengths and limitations.17 In addition, perceived AF-related symptoms may not always be due to AF, and concomitant cardiovascular diseases and risk factors may affect patient’s health perception in addition to the arrhythmia itself.18,19 Furthermore, patients with paroxysmal AF can be expected to report variable quality of life depending on their rhythm at the time of assessment, on their ability to memorize past symptoms during clusters of AF episodes, and by anxiety related to future episodes of AF.
Table 1.
Effects of rhythm control therapy using antiarrhythmic drugs in controlled clinical trials
| PIAF | CTAF | RACE | AFFIRM | STAF | SAFE-T | AF-CHF | ATHENA | Flec-SL | |
|---|---|---|---|---|---|---|---|---|---|
| Year of publication | 2000 | 2000 | 2002 | 2002 | 2003 | 2005 | 2008 | 2009 | 2012 |
| Number of patients | 252 | 403 | 522 | 4060 | 200 | 665 | 1376 | 4628 | 635 |
| Mean age | 60 | 65 | 68 | 70 | 66 | 67 | 67 | 72 | 64 |
| Sex | 73% male | 56% male | 64% male | 61% male | 64% male | 99% male | 81% male | 53% male | 66% male |
| Inclusion criteria | Symptomatic persistent AF <1 year duration | Symptomatic AF eligible for antiarrhythmic drug therapy | Recurrent persistent AF <1 year duration | >65 years or <65 years with additional risk factor for stroke with AF likely to be recurrent and likely to cause illness or death | Persistent AF either >4 weeks or enlarged LA or heart failure | Persistent AF on anticoagulation | Symptomatic HF (NYHA II–IV), LVEF <36% | Patients with AF, and >70 years with one comorbidity or >75 years | Patients undergoing planned cardioversion |
| Exclusion criteria | NYHA IV, unstable angina | NYHA III–IV, severe CKD, QTc >0.48 | NYHA IV, previous amiodarone, pacemaker | Reversible cause of AF | Permanent AF >2 years, paroxysmal AF | NYHA III–IV, CKD, initially AF >12 months (eliminated later) | AV block, recent decompensation, dialysis | Permanent AF, NYHA IV or unstable HF, bradycardia, AV block | Unsuitable for flecainide |
| AF pattern | Persistent AF | 50% persistent | Persistent AF | 69% AF episode longer than 2 days | Persistent AF | Persistent AF | 2/3 persistent | Not available but 25% were in AF at time of randomization | Persistent AF |
| Duration of AF at baseline (years) | 0.3 (0.3) | <0.5 | 0.9 | 35% first episode of AF | 0.5 (0.2) | 74% < 1 | <1 | Not available | 2.3 |
| Rhythm control intervention | Amiodarone | Amiodarone | Antiarrhythmic drugs | Antiarrhythmic drugs | Antiarrhythmic drugs | Sotalol, amiodarone | Amiodarone | Dronedarone | Flecainide (short and long term) |
| Comparator therapy | Rate control (diltiazem) | Sotalol or propafenone | Rate control | Rate control | Rate control | Placebo | Rate control | Placebo | No antiarrhythmic drug |
| Primary endpoint | Recurrent AF | Recurrent AF | Cardiovascular death, HF, stroke, bleeding, pacemaker, or SAE | Death | MACCE | Recurrent AF | Cardiovascular death | Cardiovascular hospitalization or death | Recurrent AF |
| Method for detecting recurrent AF | 24-h Holter every 3 months | Regular ECG during follow-up | Regular ECG during follow-up | Not specified | Regular ECG upon follow-up | Monthly ECG | Yearly ECG | Yearly ECG | Daily telemetric ECG |
| Sinus rhythm maintenance | 56% at 52 weeks on amiodarone, 10% on diltiazem | 40% at 2 years on sotalol/prop, 60% on amiodarone | 38% in rhythm control group, 10% in rate control during 2.3 years follow-up | 60% in active group, 30% in control group at 5 years | 40% at 12 months, 26% at 24 months in active group | At 12 months: 52% amio, 32% sotalol, 13% placebo | At 48 month visit: 70% (amio) vs. 30% (control), 58% of rhythm control group had AF during follow-up | Median time to first AF recurrence 737 days in dronedarone group and 498 in placebo | 60% (flecainide) vs. 40% (control) at 6 months |
| Outcomes | Improved 6MWT in rhythm control patients | No difference in QoL between groups | No difference in mortality or QoL between groups | No difference in mortality or QoL between groups | No difference in MACCE. Reduced recurrent AF | No difference in mortality or QoL between groups | No difference in mortality or QoL between groups | Lower mortality and less hospitalizations in patients randomized to dronedarone | Improved quality of life in all groups |
All studies found reduced AF recurrences in patients randomized to rhythm control therapy. Several studies reported improved quality of life in patients with successful sinus rhythm maintenance, e.g. in SAFE-T and AF-CHF. AAD antiarrhythmic drug. 6MWT, six minute walking test; QoL, quality of life.
Effectiveness and safety of rhythm control therapy
The success of rhythm control therapy depends on multiple factors including the number, type, and severity of underlying conditions, age, gender, adherence to antiarrhythmic drug therapy, and factors related to the quality of the AF ablation procedure.2,8,20 Furthermore, AF recurrence rates depend on the intensity of electrocardiogram (ECG) monitoring and duration of follow-up.15 Thus, comparing absolute recurrence rates between studies and comparisons to historical controls can be misleading (Table 1).
Effectiveness and safety of antiarrhythmic drug therapy
On average, antiarrhythmic drugs double the proportion of patients who maintain sinus rhythm. Amiodarone is more effective than other antiarrhythmic drugs in maintaining sinus rhythm, and catheter ablation is more effective than antiarrhythmic drugs.8 The long-term complication rates of antiarrhythmic drug therapy are comparable to complications in patients treated with AF ablation.9,21 Although amiodarone has been associated with adverse outcomes in non-randomized analyses of patients at very high risk,22 the safety of antiarrhythmic drug therapy found in recent randomized trials in patients with AF attenuates historical safety concerns,9,21 particularly in patients with heart failure.23 Unlike earlier trials of antiarrhythmic drugs compared to placebo or rate control therapy (Table 1),23–25 antiarrhythmic drug therapy with dronedarone was associated with reduced cardiovascular hospitalizations and cardiovascular deaths compared to placebo.26 The same substance, dronedarone, used as a rate-controlling agent, was associated with higher rates of heart failure, stroke, and cardiovascular death in patients with permanent AF in the PALLAS trial.27 Patients included in PALLAS were not considered suitable for rhythm control therapy, did not receive interventions to restore sinus rhythm (e.g. cardioversion, AF ablation) and had severe heart failure. Hence, they were deprived of any potential to benefit of sinus rhythm. Patients treated with dronedarone in ATHENA, in contrast, received that therapy to restore sinus rhythm. Taken together, these data may suggest that the beneficial effects found in ATHENA could be associated with its rhythm controlling effect, but more data are needed.
Antiarrhythmic drugs are also effective after AF ablation. Two recent randomized studies (AMIO-CAT28 and POWDER-AF29) showed that adding antiarrhythmic drug therapy to AF ablation improves sinus rhythm maintenance for the duration of therapy. This synergistic effect of antiarrhythmic drugs with AF ablation reflects the common (approximately 50% of patients) use of antiarrhythmic drugs 1 year after AF ablation.30 A substudy within AMIO-CAT measuring brain natriuretic peptide suggested that biomarkers may improve identification of patients at risk for recurrent AF,31 pointing potentially towards personalized or stratified selection of patients for specific rhythm control therapies.32
Effectiveness and safety of atrial fibrillation ablation
Initially evaluated in young patients with highly symptomatic AF (mean age around 55 years) who were refractory to antiarrhythmic drug therapy, AF ablation maintains sinus rhythm better than antiarrhythmic drugs.33,34 This was confirmed in CABANA.9 A meta-analysis of randomized trials (6167 patients) found that AF ablation achieves freedom from recurrent AF in approximately half of the patients [53% (46–60), mean (95% confidence interval, CI)], with slightly higher recurrence rates in patients with chronic forms of AF.35 Periprocedural complications occur in ca. 5% of patients (7.8% in EORP AF ablation, 4.8% in CABANA), including tamponade (ca. 1%), stroke, or transient ischaemic attack (ca. 0.5–1% in anticoagulated patients), access site complications (ca. 2–3%), and death (<1%).30,36–38 Reablation is performed in 20–50% of patients undergoing a first AF ablation. During long-term follow-up for up to 10 years, up to 60% of AF ablation patients remain free of clinically relevant recurrences of AF (with around three-fourths in sinus rhythm after 1 year), and approximately half of these patients receive combination therapy with antiarrhythmic drugs.39–41 Whether additional ablation strategies improve these outcomes needs to be investigated.42
Atrial fibrillation ablation compared to antiarrhythmic drug therapy after CABANA
CABANA was designed to test whether AF ablation can reduce mortality compared to antiarrhythmic drugs in patients with AF in need for rhythm control therapy and with stroke risk factors.43 In early 2013, a planned, blind data review identified slow enrolment and lower event rates than anticipated. This resulted in a change in primary endpoint from all-cause mortality to a composite of death, disabling stroke, serious bleeding, or cardiac arrest. In addition, the sample size was reduced. The results have just been reported9: Of the 2204 patients randomized (median age, 68 years; 37% female; 57% persistent AF), 89.3% completed the trial. In patients randomized to AF ablation, 91% underwent the procedure, while AF ablation was performed in 27.5% of the patients randomized to drug therapy, in line with expectations at the start of the trial.43 Safety of rhythm control therapy was good in this elderly patient population (mean age 68 years), with low complication rates in both arms: Patients randomized to AF ablation experienced tamponade (0.8%), haematomas (2.3%), and pseudoaneurysms (1.1%). Patients randomized to antiarrhythmic drug therapy experienced thyroid disorders (1.6%) and proarrhythmia (0.8%). The primary outcome was not different between groups.9 Over a median follow-up of 48.5 months, the primary endpoint occurred in 8.0% of patients randomized to AF ablation, and in 9.2% of patients randomized to antiarrhythmic drug therapy [hazard ratio (HR) 0.86, 95% CI 0.65–1.15; P = 0.30]. Key secondary outcomes were not different between random groups, including all-cause mortality was 5.2% and 6.1% (HR 0.85, 95% CI 0.60–1.21; P = 0.38), death or cardiovascular hospitalization rates were 51.7% and 58.1% for (HR 0.83, 95% CI 0.74–0.93; P = 0.001). Recurrent AF was less common in patients randomized to AF ablation in the subgroup of 1240 patients undergoing systematic ECG monitoring (HR 0.52, 95% CI 0.45–0.60; P < 0.001). Both treatment groups showed improved quality of life, as assessed by the Atrial Fibrillation Effect on Quality of Life (AFEQT) summary score and the Mayo AF-Specific Symptom Inventory (MAFSI). Patients randomized to catheter ablation showed a greater improvement in quality of life (mean difference of 5.3 points).44 This greater effect of AF ablation on quality of life is consistent with the main finding of the Swedish CAPTAF trial.45
Similar to other observational data sets, on-treatment analysis suggested improved outcomes in patients undergoing AF ablation. These findings are additionally supported by a recent study using a large US administrative database of routine patient data, analysing patients who meet the CABANA inclusion criteria.46 Unknown and known confounders, censoring of events—either intentionally by study design or unintentionally because of loss to follow-up—, self-selection of low risk patients to cross over to ablation, and immortal time bias are some of the sources of bias that can explain these findings.47
Rhythm control therapy in patients with atrial fibrillation and heart failure
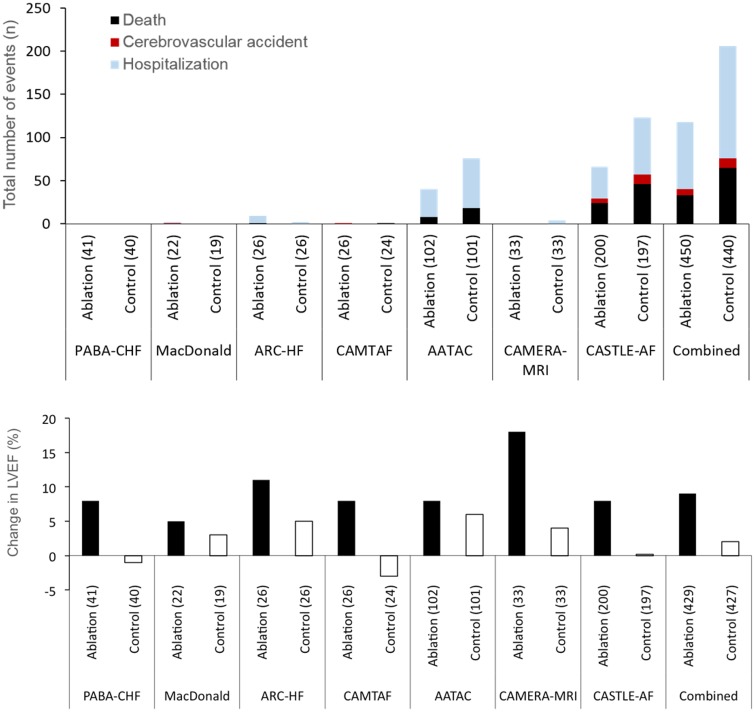
Atrial fibrillation and heart failure (AF+HF) frequently coexist and this is associated with high morbidity and mortality.48 To improve outcomes, restoring and maintaining sinus rhythm has been proposed in patients with AF+HF. Amiodarone is the only antiarrhythmic drug with sufficient safety data in patients with reduced left ventricular ejection fraction.8 Large randomized trials of antiarrhythmic drugs compared to rate control in patients with AF+HF did not find differences in all-cause mortality, cardiovascular mortality, or heart failure hospitalizations.23,49,50 Likewise, patients who maintain sinus rhythm (‘successful rhythm control therapy’) did not have better survival than those with recurrent AF.50 Several small case series and controlled trials found that patients undergoing AF ablation have improved left ventricular function, often using echocardiography to assess left ventricular (LV) function (Table 2): four out of five relatively small studies found improved left ventricular function in patients with AF+HF randomized to AF ablation (Table 2),51–59 largely seen in trials that assessed left ventricular function by echocardiography, which is less reliable in AF than in sinus rhythm.60 There were associated improvements in exercise capacity and brain natriuretic peptide (BNP) levels (Take home figure, bottom panel). Improved exercise capacity and to some extent improved left ventricular function, but not lower BNP, could be partially explained by bias in unblinded trials. These effects have been extrapolated with a certain enthusiasm.61 The largest trial comparing AF ablation with ‘medical therapy’ (mostly rate control, but including antiarrhythmic drugs) in patients with AF+HF is CASTLE-AF (Table 2).10 The quality of rate control therapy may have affected changes in LV function in the control group of the published trials that used rate control as comparator. Thirty-four of the 363 randomized patients were lost to follow-up despite an implanted device allowing home monitoring. In the remaining patients, catheter ablation reduced mortality and HF hospitalizations (28.5% compared with 45%), but had no effect on all-cause hospitalizations and stroke. Details of the drug therapy given to patients randomized to ‘medical therapy’ have not been published. One-third of the patients assigned to medical therapy were on antiarrhythmic drugs at their final follow-up, 22% were in sinus rhythm at 60 months (compared to 63% in the AF ablation arm, Table 2). In line with these findings, the recent update of the AHA/ACC/HRS guidelines for AF included a Class IIb recommendation for AF ablation in patients with heart failure. So far, there is no information about outcomes following catheter ablation for AF in patients with heart failure and a preserved ejection fraction. Despite these limitations, CASTLE-AF and the AATAC trial62 contribute evidence that selected patients with AF+HF benefit from AF ablation (Table 2),51–59 but open questions remain regarding selection of adequate patients and validity of the findings in ‘all-comer’ patients. More research is needed to determine the effect of AF ablation on cardiovascular outcomes in patients with AF+HF.
Table 2.
Randomized studies comparing pharmacological rate or rhythm control, or, in PABA-CHF, AV nodal ablation and biventricular pacing, with catheter ablation in patients with AF and systolic dysfunction with reduced ejection fraction
| PABA-CHF | MacDonald | ARC-AF | CAMTAF | AATAC | CAMERA-MRI | CASTLE-AF | |
|---|---|---|---|---|---|---|---|
| Year of publication | 2008 | 2011 | 2013 | 2014 | 2016 | 2017 | 2018 |
| Number of patients | 81 | 41 | 52 | 50 | 203 | 66 | 363a |
| Age | 61 | 63 | 63 | 58 | 61 | 61 | 64 |
| Sex | >80% male | 78% male | >80% male | 96% male | 74% male | 91% male | 86% male |
| Type of patients | NYHA II–III, LVEF <40% | NYHA II–IV, LVEF <35% | NYHA II–IV, LVEF <35% | NYHA II–IV, LVEF <50% | NYHA II–III, LVEF <40%, dual-chamber ICD or CRT | NYHA II–IV, LVEF <45%b | NYHA II–IV, LVEF <35%, dual-chamber ICD or CRT |
| Exclusion criteria | Post-operative AF, reversible causes of AF or HF, prior AF ablation | Paroxysmal AF, QRS duration >150 ms, myocarditis | Reversible causes of AF and HF | Previous AF ablation, reversible HF cause | Amiodraone therapy, AF <3 months duration, reversible AF | Paroxysmal AF, contraindications to ablation or MRI, ischaemic cardiomyopathy | Prior AF ablation, LA diameter >60 mm |
| Proportion with ischaemic HF aetiology | 70% | 49% | 33% | 26% | 64% | 0% | 46% |
| AF pattern | 52% paroxysmal | 100% chronic | 100% chronic | 100% chronic | 100% chronic | 100% chronic | 33% paroxysmal |
| Duration of AF at baseline | 48 months | 44 months | 51 months | 24 months | 9 months | 22 months | Not known |
| Comparator therapy | Rate control (AV nodal ablation + biventricular ICD) | Pharmacological rate control | Pharmacological rate control | Pharmacological rate control | Rhythm control with amiodarone | Pharmacological rate control | Mixture of rate control and rhythm control |
| Primary endpoint | Composite of LVEF, 6MWT distance, and MLHFQ score | Change in LVEF from randomization to last study visit | Peak VO2 | LVEF at 6 months | Freedom from AF, AFL, or AT of >30 s duration off AAD at follow-up | Change in LVEF from baseline at 6 months on cardiac MRI | Composite of all-cause mortality or worsening of HF requiring unplanned hospitalization |
| Method for AF recurrence assessment | External loop recorder (AF ablation patients only) | 24-h Holter at baseline, 3 and 6 months | 48-h Holter at 6 and 12 months | 48-h Holter at 1, 3, and 6 months (and 12 months in AF ablation patients) | Device interrogation at 3, 6 12, and 24 months | Implanted loop recorder in AF ablation patients | Device interrogation at 3, 6, 12, 24, 36, 48, and 60 months |
| Sinus rhythm maintenance at end of follow-up | 88% | 50% | 88% | 73% | 70% | 75% (56% without antiarrhythmic drugs) | 63% |
| Outcomes | Improved LVEF, 6MWT distance and QoL (MLHFQ) in AF ablation patients | No difference in LV or RV function (measured by cardiac MRI), 6MWT, or BNP between groups | Improved exercise performance, QoL and BNP levels in AF ablation patients | Greater improvement in LVEF, better exercise performance, lower BNP, and improved QoL AF ablation patients | Less unplanned hospitalization, lower mortality, greater improvement of LVEF, 6MWT distance, and QoL (MLHFQ) in AF ablation patients | Greater improvement of LVEF at 6 months in AF ablation patients | Less mortality and HF hospitalizations in AF ablation patients |
Number of randomized patients.
6-Min walk distance and serum brain natriuretic peptide did not support the presence of heart failure in all patients. 6MWT, six minute walking test; AF, atrial fibrillation; BNP, brain natriuretic peptide; CRT, cardiac resynchronization therapy device; ICD, implantable defibrillator; LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire; MRI, magnetic resonance imaging; NYHA class, New York Heart Association functional class; QOL, quality of life; RV, right ventricle.
Take home figure.
AF ablation may affect cardiovascular outcomes (top panel) and appears to improve left ventricular function (bottom panel) in selected patients with Atrial Fibrillation and Heart Failure. Further evidence is needed to underpin these hypothesis-generating findings.
Rhythm control therapy and stroke
The clear association of AF and ischaemic stroke may suggest that maintaining sinus rhythm can help to prevent strokes. There is no signal for reduced strokes in the earlier ‘rate vs. rhythm’ studies (Table 1), including the reasonably large AF-CHF trial.23 There were only three and seven disabling strokes in each arm in CABANA, without differences between groups.9 Interestingly, in a post hoc analysis of the ATHENA trial (Table 1), patients randomized to dronedarone had a lower risk of stroke or transient ischaemic attack (1.2% vs. 1.8%).26 A retrospective, propensity-score matched analysis of a subset of AF patients taken from the Swedish patient registry also suggested that AF ablation may be associated with a lower incidence of ischaemic stroke.63 This is similar to propensity-matched patient comparisons in the largest health maintenance organization in Israel, comparing 969 AF patients undergoing AF ablation to 3772 AF controls.64 These analyses are prone to several biases, including known, unmeasured and unknown confounders, and others.63
Rhythm control therapy and cognitive decline
Atrial fibrillation is associated with cognitive dysfunction and dementia. Anticoagulation appears to reduce dementia in patients with AF in a nationwide cohort analysis.65 While it is unlikely that antiarrhythmic drug therapy causes cerebral complications (stroke, transient ischaemic attack, or cognitive decline), there is a peri-procedural risk of ischaemic stroke (0.3–1%) as well as a risk of magnetic resonance imaging (MRI)-detected clinically silent ischaemic brain lesions in patients undergoing AF ablation.37 This can increase brain damage and subsequently lead to cognitive decline.37 Interestingly, the AXAFA–AFNET 5 study found small MRI-detected brain lesions in ca. 30% of patients undergoing a first AF ablation on continuous anticoagulation, but also detected an improved cognitive function as assessed by Montreal Cognitive Assessment (MoCA) 3 months after AF ablation.38
Rhythm control therapy may reduce AF-related stroke risk by reducing AF burden and subsequent improvement in atrial cardiomyopathy,66 potentially reducing silent embolic lesions, and possibly improving perfusion and metabolism of the brain. A large retrospective observational study found a lower rate of new-onset dementia in 4212 patients undergoing AF ablation compared to 16 848 non-ablated AF patients, while a substudy in the randomized AFFIRM trial did not find a difference in cognitive function between patients randomized to rate or rhythm control therapy, while the AXAFA study found improved cognitive function in 674 patients 3 months after AF ablation compared to baseline.38 The possible cognitive benefits of restoring sinus rhythm in AF patients can be attenuated by atrial cardiomyopathy and by concomitant cardiovascular conditions and other unknown confounders that can cause brain damage, stroke, and cognitive dysfunction in the absence of AF.66,67 Unfortunately, neither CABANA nor CASTLE-AF reported cognitive function outcomes. Ongoing research such as the case–control DIAL-F cohort (NCT01816308) and the randomized EAST-AFNET 4 trial68 will provide further information on the impact of rhythm control therapy including AF ablation on cognitive function.
Rhythm control therapy and atrial cardiomyopathy
The term ‘atrial cardiomyopathy’ summarizes the structural, architectural, contractile, or electrophysiological changes in diseased atria.66 Cardiovascular diseases (e.g. hypertension, heart failure, valvular heart disease, ischaemic heart disease, or diabetes) but also ageing can contribute to an atrial cardiomyopathy. Atrial fibrillation itself accelerates the underlying disease processes, thus contributing to atrial cardiomyopathy.69 Left atrial enlargement, a summative clinical proxy for atrial cardiomyopathy, is partially reversed after AF ablation.7,70,71 Early rhythm control therapy, including AF ablation, has been suggested to slow these processes, thereby simplifying rhythm control therapy and potentially improving long-term outcomes.68 Hence, early rhythm control therapy could slow atrial cardiomyopathy. However, this hypothesis requires confirmation in further studies and trials.
Summary and conclusions
Recent randomized trials and observational data sets including CASTLE-AF and CABANA provide important reassurance on the safety of rhythm control therapy in contemporary patients with AF, including in elderly patients with concomitant cardiovascular diseases. The data confirm the superior effectiveness of AF ablation compared to antiarrhythmic drugs to restore and maintain sinus rhythm, and demonstrate that antiarrhythmic drugs remain effective after AF ablation. Several smaller studies suggest that AF ablation can improve left ventricular function assessed by echocardiography in selected patients with AF and heart failure. Further studies to investigate the impact of rhythm control therapy on LV function in different, clearly defined subsets of patients with AF are warranted. The effects of rhythm control therapy on cardiovascular death, stroke, heart failure, acute coronary syndromes, as well as secondary outcomes such as left atrial, ventricular, and cognitive function require further research, such as the on-going EAST–AFNET 4 trial.68
Acknowledgements
We thank Heidi Oellers at AFNET for expert administrative support in the preparation of this manuscript.
Funding
This work was partially supported by European Union [grant agreement No 633196 (CATCH ME), European Union BigData@Heart (grant agreement EU IMI 116074)], British Heart Foundation (PG/17/30/32961, FS/13/43/30324; and AA/18/2/34218), German Centre for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK, via grants to AFNET and to the DZHK site Hamburg), and Leducq Foundation.
Conflict of interest: C.M. reports personal fees from Abbott, Bayer, Biosense Webster, BMS/Pfizer, Boehringer, Boston Scientific, Daiichi Sankyo. S.W. reports grants and personal fees from Abbott and personal feels from Abbott, Boston Scientific, Boehringer Ingelheim, Bristol Myers Squibb, Bayer Vital, Acutus, and Daiichi Sankyo. K.G.H. reports fees from Bayer, Boehringer, Biotronik, W.L. Gore & Associates BMS/Pfizer, EIP Pharma, Daiichi Sankyo, Edwards Lifesciences, Medtronic, and Sanofi. L.M. reports grants and personal fees from Johnson&johnson, Biosense Webster, Boston Scientific, Medtronic, and Abbott and grants from Biotronik. A.N. reports grants from Boston Scientific, grants and personal fees from Abbott and personal fees from Biosense Webster. Le reports consultant fees, speaking honoraria, and travel expenses from Abbott, Bayer, Biosense Webster, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Medtronic, Pfizer, and Sanofi Aventis. L.E. receives research support from German Research Foundation and German Heart Foundation outside of this work. K.W. reports grants from Biotronik and personal fees from Boston Scientific, Biotronik, and Novartis. J.d.B. reports support for conference attendance from Boston Scientific and Abbott. J.K. reports personal fees from Affera, Abbott, Bayer, Biosense Webster, Biotronik, BMS/Pfizer, Boehringer, Boston Scientific, Daiichi Sankyo, Medtronic, MicroPort. A.B., A.E., I.v.G., A.G., M.G., L.H., L.S., S.T., and H.H. report no disclosures. P.K. receives research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation and has received honoraria from several such companies in the past. P.K. is listed as inventor on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783).
References
- 1. Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC.. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006;27:949–953. [DOI] [PubMed] [Google Scholar]
- 2. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D.. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marijon E, Le Heuzey JY, Connolly S, Yang S, Pogue J, Brueckmann M, Eikelboom JW, Themeles E, Ezekowitz MD, Wallentin L, Yusuf S.. Causes of death and influencing factors in patients with atrial fibrillation: a competing risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation 2013;128:2192–2201. [DOI] [PubMed] [Google Scholar]
- 4. Camm AJ, Amarenco P, Haas S, Hess S, Kirchhof P, Kuhls S, van Eickels M, Turpie AG; XANTUS Investigators. XANTUS: a real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J 2016;37:1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, Kus T, Lambert J, Dubuc M, Gagne P, Nattel S, Thibault B.. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med 2000;342:913–920. [DOI] [PubMed] [Google Scholar]
- 6. Calkins H, Hindricks G, Cappato R, Kim Y-H, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen P-S, Chen S-A, Chung MK, Nielsen JC, Curtis AB, Davies DW, Day JD, d’Avila A, de Groot NMSN, Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao H-M, Verma A, Wilber DJ, Yamane T.. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace 2018;20:157–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kotecha D, Breithardt G, Camm AJ, Lip GYH, Schotten U, Ahlsson A, Arnar D, Atar D, Auricchio A, Bax J, Benussi S, Blomstrom-Lundqvist C, Borggrefe M, Boriani G, Brandes A, Calkins H, Casadei B, Castella M, Chua W, Crijns H, Dobrev D, Fabritz L, Feuring M, Freedman B, Gerth A, Goette A, Guasch E, Haase D, Hatem S, Haeusler KG, Heidbuchel H, Hendriks J, Hunter C, Kaab S, Kespohl S, Landmesser U, Lane DA, Lewalter T, Mont L, Nabauer M, Nielsen JC, Oeff M, Oldgren J, Oto A, Pison L, Potpara T, Ravens U, Richard-Lordereau I, Rienstra M, Savelieva I, Schnabel R, Sinner MF, Sommer P, Themistoclakis S, Van Gelder IC, Vardas PE, Verma A, Wakili R, Weber E, Werring D, Willems S, Ziegler A, Hindricks G, Kirchhof P.. Integrating new approaches to atrial fibrillation management: the 6th AFNET/EHRA Consensus Conference. Europace 2018;20:395–407. [DOI] [PubMed] [Google Scholar]
- 8. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K.. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 9. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, Flaker GC, Pokushalov E, Romanov A, Bunch TJ, Noelker G, Ardashev A, Revishvili A, Wilber DJ, Cappato R, Kuck KH, Hindricks G, Davies DW, Kowey PR, Naccarelli GV, Reiffel JA, Piccini JP, Silverstein AP, Al-Khalidi HR, Lee KL; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bansch D; CASTLE-AF Investigators. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 11. Testa L, Biondi-Zoccai GG, Dello Russo A, Bellocci F, Andreotti F, Crea F.. Rate-control vs. rhythm-control in patients with atrial fibrillation: a meta-analysis. Eur Heart J 2005;26:2000–2006. [DOI] [PubMed] [Google Scholar]
- 12. Lafuente-Lafuente C, Valembois L, Bergmann JF, Belmin J.. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev 2015;3:CD005049. [DOI] [PubMed] [Google Scholar]
- 13. Kirchhof P, Breithardt G, Bax J, Benninger G, Blomstrom-Lundqvist C, Boriani G, Brandes A, Brown H, Brueckmann M, Calkins H, Calvert M, Christoffels V, Crijns H, Dobrev D, Ellinor P, Fabritz L, Fetsch T, Freedman SB, Gerth A, Goette A, Guasch E, Hack G, Haegeli L, Hatem S, Haeusler KG, Heidbuchel H, Heinrich-Nols J, Hidden-Lucet F, Hindricks G, Juul-Moller S, Kaab S, Kappenberger L, Kespohl S, Kotecha D, Lane DA, Leute A, Lewalter T, Meyer R, Mont L, Munzel F, Nabauer M, Nielsen JC, Oeff M, Oldgren J, Oto A, Piccini JP, Pilmeyer A, Potpara T, Ravens U, Reinecke H, Rostock T, Rustige J, Savelieva I, Schnabel R, Schotten U, Schwichtenberg L, Sinner MF, Steinbeck G, Stoll M, Tavazzi L, Themistoclakis S, Tse HF, Van Gelder IC, Vardas PE, Varpula T, Vincent A, Werring D, Willems S, Ziegler A, Lip GY, Camm AJ.. A roadmap to improve the quality of atrial fibrillation management: proceedings from the fifth Atrial Fibrillation Network/European Heart Rhythm Association consensus conference. Europace 2016;18:37–50. [DOI] [PubMed] [Google Scholar]
- 14. Anker SD, Agewall S, Borggrefe M, Calvert M, Jaime Caro J, Cowie MR, Ford I, Paty JA, Riley JP, Swedberg K, Tavazzi L, Wiklund I, Kirchhof P.. The importance of patient-reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J 2014;35:2001–2009. [DOI] [PubMed] [Google Scholar]
- 15. Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener HC, Goette A, Hindricks G, Hohnloser S, Kappenberger L, Kuck KH, Lip GY, Olsson B, Meinertz T, Priori S, Ravens U, Steinbeck G, Svernhage E, Tijssen J, Vincent A, Breithardt G.. Outcome parameters for trials in atrial fibrillation: executive summary: recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NETwork (AFNET) and the European Heart Rhythm Association (EHRA). Eur Heart J 2007;28:2803–2817. [DOI] [PubMed] [Google Scholar]
- 16. Wynn GJ, Todd DM, Webber M, Bonnett L, McShane J, Kirchhof P, Gupta D.. The European Heart Rhythm Association symptom classification for atrial fibrillation: validation and improvement through a simple modification. Europace 2014;16:965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kotecha D, Ahmed A, Calvert M, Lencioni M, Terwee CB, Lane DA.. Patient-reported outcomes for quality of life assessment in atrial fibrillation: a systematic review of measurement properties. PLoS One 2016;11:e0165790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rienstra M, Hobbelt AH, Alings M, Tijssen JGP, Smit MD, Brugemann J, Geelhoed B, Tieleman RG, Hillege HL, Tukkie R, Van Veldhuisen DJ, Crijns H, Van Gelder IC, Investigators R.. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J 2018;39:2987–2996. [DOI] [PubMed] [Google Scholar]
- 19. De With RR, Rienstra CM, Smit MD, Weijs B, Zwartkruis VW, Geelhoed B, Hillege H, Tukkie R, Hemels ME, Tieleman R, Ranchor AV, Van Veldhuisen DJ, Crijns HJGM, van Gelder IC.. Targeted therapy of underlying conditions improves quality of life in patients with persistent atrial fibrillation: results of the RACE 3 study. Europace 2019;21:563–571. [DOI] [PubMed] [Google Scholar]
- 20. Kirchhof P, Breithardt G, Aliot E, Al Khatib S, Apostolakis S, Auricchio A, Bailleul C, Bax J, Benninger G, Blomstrom-Lundqvist C, Boersma L, Boriani G, Brandes A, Brown H, Brueckmann M, Calkins H, Casadei B, Clemens A, Crijns H, Derwand R, Dobrev D, Ezekowitz M, Fetsch T, Gerth A, Gillis A, Gulizia M, Hack G, Haegeli L, Hatem S, Georg Hausler K, Heidbuchel H, Hernandez-Brichis J, Jais P, Kappenberger L, Kautzner J, Kim S, Kuck KH, Lane D, Leute A, Lewalter T, Meyer R, Mont L, Moses G, Mueller M, Munzel F, Nabauer M, Nielsen JC, Oeff M, Oto A, Pieske B, Pisters R, Potpara T, Rasmussen L, Ravens U, Reiffel J, Richard-Lordereau I, Schafer H, Schotten U, Stegink W, Stein K, Steinbeck G, Szumowski L, Tavazzi L, Themistoclakis S, Thomitzek K, Van Gelder IC, von Stritzky B, Vincent A, Werring D, Willems S, Lip GY, Camm AJ.. Personalized management of atrial fibrillation: proceedings from the fourth Atrial Fibrillation competence NETwork/European Heart Rhythm Association consensus conference. Europace 2013;15:1540–1556. [DOI] [PubMed] [Google Scholar]
- 21. Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, Pehrson S, Englund A, Hartikainen J, Mortensen LS, Hansen PS.. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med 2012;367:1587–1595. [DOI] [PubMed] [Google Scholar]
- 22. Adelstein EC, Althouse AD, Davis L, Schwartzman D, Bazaz R, Jain S, Wang N, Saba S.. Amiodarone is associated with adverse outcomes in patients with sustained ventricular arrhythmias upgraded to cardiac resynchronization therapy-defibrillators. J Cardiovasc Electrophysiol 2019;30:348–356. [DOI] [PubMed] [Google Scholar]
- 23. Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, Connolly SJ, Dubuc M, Ducharme A, Guerra PG, Hohnloser SH, Lambert J, Le Heuzey JY, O'Hara G, Pedersen OD, Rouleau JL, Singh BN, Stevenson LW, Stevenson WG, Thibault B, Waldo AL; Atrial Fibrillation and Congestive Heart Failure Investigators. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med 2008;358:2667–2677. [DOI] [PubMed] [Google Scholar]
- 24. Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]
- 25. Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJM, Tijssen JGP, Crijns HJGM.. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002;347:1834–1840. [DOI] [PubMed] [Google Scholar]
- 26. Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, Connolly SJ.. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009;360:668–678. [DOI] [PubMed] [Google Scholar]
- 27. Connolly SJ, Camm AJ, Halperin JL, Joyner C, Alings M, Amerena J, Atar D, Avezum A, Blomstrom P, Borggrefe M, Budaj A, Chen SA, Ching CK, Commerford P, Dans A, Davy JM, Delacretaz E, Di Pasquale G, Diaz R, Dorian P, Flaker G, Golitsyn S, Gonzalez-Hermosillo A, Granger CB, Heidbuchel H, Kautzner J, Kim JS, Lanas F, Lewis BS, Merino JL, Morillo C, Murin J, Narasimhan C, Paolasso E, Parkhomenko A, Peters NS, Sim KH, Stiles MK, Tanomsup S, Toivonen L, Tomcsanyi J, Torp-Pedersen C, Tse HF, Vardas P, Vinereanu D, Xavier D, Zhu J, Zhu JR, Baret-Cormel L, Weinling E, Staiger C, Yusuf S, Chrolavicius S, Afzal R, Hohnloser SH.. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med 2011;365:2268–2276. [DOI] [PubMed] [Google Scholar]
- 28. Darkner S, Chen X, Hansen J, Pehrson S, Johannessen A, Nielsen JB, Svendsen JH.. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J 2014;35:3356–3364. [DOI] [PubMed] [Google Scholar]
- 29. Duytschaever M, Demolder A, Phlips T, Sarkozy A, El Haddad M, Taghji P, Knecht S, Tavernier R, Vandekerckhove Y, De Potter T.. PulmOnary vein isolation With vs. without continued antiarrhythmic Drug trEatment in subjects with Recurrent Atrial Fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J 2018;39:1429–1437. [DOI] [PubMed] [Google Scholar]
- 30. Arbelo E, Brugada J, Blomstrom-Lundqvist C, Laroche C, Kautzner J, Pokushalov E, Raatikainen P, Efremidis M, Hindricks G, Barrera A, Maggioni A, Tavazzi L, Dagres N; on the behalf of the ESC-EHRA Atrial Fibrillation Ablation Long-term Registry Investigators. Contemporary management of patients undergoing atrial fibrillation ablation: in-hospital and 1-year follow-up findings from the ESC-EHRA atrial fibrillation ablation long-term registry. Eur Heart J 2017;38:1303–1316. [DOI] [PubMed] [Google Scholar]
- 31. Darkner S, Goetze JP, Chen X, Henningsen K, Pehrson S, Svendsen JH.. Natriuretic propeptides as markers of atrial fibrillation burden and recurrence (from the AMIO-CAT trial). Am J Cardiol 2017;120:1309–1315. [DOI] [PubMed] [Google Scholar]
- 32. Fabritz L, Guasch E, Antoniades C, Bardinet I, Benninger G, Betts TR, Brand E, Breithardt G, Bucklar-Suchankova G, Camm AJ, Cartlidge D, Casadei B, Chua WW, Crijns HJ, Deeks J, Hatem S, Hidden-Lucet F, Kaab S, Maniadakis N, Martin S, Mont L, Reinecke H, Sinner MF, Schotten U, Southwood T, Stoll M, Vardas P, Wakili R, West A, Ziegler A, Kirchhof P.. Expert consensus document: defining the major health modifiers causing atrial fibrillation: a roadmap to underpin personalized prevention and treatment. Nat Rev Cardiol 2016;13:230–237. [DOI] [PubMed] [Google Scholar]
- 33. Jais P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, Hocini M, Extramiana F, Sacher F, Bordachar P, Klein G, Weerasooriya R, Clementy J, Haissaguerre M.. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008;118:2498–2505. [DOI] [PubMed] [Google Scholar]
- 34. Pappone C, Augello G, Sala S, Gugliotta F, Vicedomini G, Gulletta S, Paglino G, Mazzone P, Sora N, Greiss I, Santagostino A, LiVolsi L, Pappone N, Radinovic A, Manguso F, Santinelli V.. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol 2006;48:2340–2347. [DOI] [PubMed] [Google Scholar]
- 35. Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, Sullivan T, Roberts-Thomson KC, Sanders P.. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc 2013;2:e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Packer D, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, Flaker GC, Pokushalov E, Romanov A, Bunch TJ, Noelker G, Ardashev A, Revishvili A, Wilber DJ, Cappato R, Kuck KH, Hindricks G, Davies DW, Kowey PR, Naccarelli GV, Reiffel JA, Piccini JP, Silverstein AP, Al-Khalidi HR, Lee KL, Investigators C.. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: The CABANA Randomized Clinical Trial. JAMA 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haeusler KG, Kirchhof P, Endres M.. Left atrial catheter ablation and ischemic stroke. Stroke 2012;43:265–270. [DOI] [PubMed] [Google Scholar]
- 38. Kirchhof P, Haeusler KG, Blank B, De Bono J, Callans D, Elvan A, Fetsch T, Van Gelder IC, Gentlesk P, Grimaldi M, Hansen J, Hindricks G, Al-Khalidi HR, Massaro T, Mont L, Nielsen JC, Nolker G, Piccini JP, De Potter T, Scherr D, Schotten U, Themistoclakis S, Todd D, Vijgen J, Di Biase L.. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur Heart J 2018;39:2942–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scherr D, Khairy P, Miyazaki S, Aurillac-Lavignolle V, Pascale P, Wilton SB, Ramoul K, Komatsu Y, Roten L, Jadidi A, Linton N, Pedersen M, Daly M, O’Neill M, Knecht S, Weerasooriya R, Rostock T, Manninger M, Cochet H, Shah AJ, Yeim S, Denis A, Derval N, Hocini M, Sacher F, Haissaguerre M, Jais P.. Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol 2015;8:18–24. [DOI] [PubMed] [Google Scholar]
- 40. Tilz RR, Heeger CH, Wick A, Saguner AM, Metzner A, Rillig A, Wohlmuth P, Reissmann B, Lemes C, Maurer T, Santoro F, Riedl J, Sohns C, Mathew S, Kuck KH, Ouyang F.. Ten-year clinical outcome after circumferential pulmonary vein isolation utilizing the hamburg approach in patients with symptomatic drug-refractory paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol 2018;11:e005250. [DOI] [PubMed] [Google Scholar]
- 41. Dinshaw L, Schaffer B, Akbulak O, Jularic M, Hartmann J, Klatt N, Dickow J, Gunawardene M, Munkler P, Hakmi S, Pecha S, Sultan A, Luker J, Pinnschmidt H, Hoffmann B, Gosau N, Eickholt C, Willems S, Steven D, Meyer C.. Long-term efficacy and safety of radiofrequency catheter ablation of atrial fibrillation in patients with cardiac implantable electronic devices and transvenous leads. J Cardiovasc Electrophysiol 2019;30:679. [DOI] [PubMed] [Google Scholar]
- 42. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque JP, Nardi S, Menardi E, Novak P, Sanders P.. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–1822. [DOI] [PubMed] [Google Scholar]
- 43. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Moretz K, Poole JE, Mascette A, Rosenberg Y, Jeffries N, Al-Khalidi HR, Lee KL, Investigators C.. Catheter ablation versus antiarrhythmic drug therapy for atrial fibrillation (CABANA) trial: study rationale and design. Am Heart J 2018;199:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, Daniels MR, Bahnson TD, Poole JE, Rosenberg Y, Lee KL, Packer DL; CABANA Investigators. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Blomstrom-Lundqvist C, Gizurarson S, Schwieler J, Jensen SM, Bergfeldt L, Kenneback G, Rubulis A, Malmborg H, Raatikainen P, Lonnerholm S, Hoglund N, Mortsell D.. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA 2019;321:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Noseworthy PA, Gersh BJ, Kent DM, Piccini JP, Packer DL, Shah ND, Yao X.. Atrial fibrillation ablation in practice: assessing CABANA generalizability. Eur Heart J 2019;40:1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Packer M, Kowey PR.. Building castles in the sky: catheter ablation in patients with atrial fibrillation and chronic heart failure. Circulation 2018;138:751. [DOI] [PubMed] [Google Scholar]
- 48. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ, Ho JE.. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation 2016;133:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Torp-Pedersen C, Møller M, Bloch-Thomsen PE, Køber L, Sandøe E, Egstrup K, Agner E, Carlsen J, Videbæk J, Marchant B, Camm AJ.. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med 1999;341:857–865. [DOI] [PubMed] [Google Scholar]
- 50. Talajic M, Khairy P, Levesque S, Connolly SJ, Dorian P, Dubuc M, Guerra PG, Hohnloser SH, Lee KL, Macle L, Nattel S, Pedersen OD, Stevenson LW, Thibault B, Waldo AL, Wyse DG, Roy D; AF-CHF Investigators. Maintenance of sinus rhythm and survival in patients with heart failure and atrial fibrillation. J Am Coll Cardiol 2010;55:1796–1802. [DOI] [PubMed] [Google Scholar]
- 51. Marrouche NF, Kheirkhahan M, Brachmann J.. Huff and Puff, this CASTLE is made of bricks. Circulation 2018;138:754–755. [DOI] [PubMed] [Google Scholar]
- 52. Ullah W, Ling LH, Prabhu S, Lee G, Kistler P, Finlay MC, Earley MJ, Sporton S, Bashir Y, Betts TR, Rajappan K, Thomas G, Duncan E, Staniforth A, Mann I, Chow A, Lambiase P, Schilling RJ, Hunter RJ.. Catheter ablation of atrial fibrillation in patients with heart failure: impact of maintaining sinus rhythm on heart failure status and long-term rates of stroke and death. Europace 2016;18:679–686. [DOI] [PubMed] [Google Scholar]
- 53. Anselmino M, Matta M, D’Ascenzo F, Bunch TJ, Schilling RJ, Hunter RJ, Pappone C, Neumann T, Noelker G, Fiala M, Bertaglia E, Frontera A, Duncan E, Nalliah C, Jais P, Weerasooriya R, Kalman JM, Gaita F.. Catheter ablation of atrial fibrillation in patients with left ventricular systolic dysfunction: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol 2014;7:1011–1018. [DOI] [PubMed] [Google Scholar]
- 54. Al Halabi S, Qintar M, Hussein A, Alraies MC, Jones DG, Wong T, MacDonald MR, Petrie MC, Cantillon D, Tarakji KG, Kanj M, Bhargava M, Varma N, Baranowski B, Wilkoff BL, Wazni O, Callahan T, Saliba W, Chung MK.. Catheter ablation for atrial fibrillation in heart failure patients: a meta-analysis of randomized controlled trials. JACC Clin Electrophysiol 2015;1:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Khan MN, Jais P, Cummings J, Di Biase L, Sanders P, Martin DO, Kautzner J, Hao S, Themistoclakis S, Fanelli R, Potenza D, Massaro R, Wazni O, Schweikert R, Saliba W, Wang P, Al-Ahmad A, Beheiry S, Santarelli P, Starling RC, Dello Russo A, Pelargonio G, Brachmann J, Schibgilla V, Bonso A, Casella M, Raviele A, Haissaguerre M, Natale A; PABA-CHF Investigators. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med 2008;359:1778–1785. [DOI] [PubMed] [Google Scholar]
- 56. Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman-Haley SL, McDonagh TA, Underwood SR, Markides V, Wong T.. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol 2013;61:1894–1903. [DOI] [PubMed] [Google Scholar]
- 57. Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V, Goromonzi F, Sawhney V, Duncan E, Page SP, Ullah W, Unsworth B, Mayet J, Dhinoja M, Earley MJ, Sporton S, Schilling RJ.. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol 2014;7:31–38. [DOI] [PubMed] [Google Scholar]
- 58. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, Reddy M, Jais P, Themistoclakis S, Dello Russo A, Casella M, Pelargonio G, Narducci ML, Schweikert R, Neuzil P, Sanchez J, Horton R, Beheiry S, Hongo R, Hao S, Rossillo A, Forleo G, Tondo C, Burkhardt JD, Haissaguerre M, Natale A.. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation 2016;133:1637–1644. [DOI] [PubMed] [Google Scholar]
- 59. Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, Sugumar H, Lockwood SM, Stokes MB, Pathik B, Nalliah CJ, Wong GR, Azzopardi SM, Gutman SJ, Lee G, Layland J, Mariani JA, Ling LH, Kalman JM, Kistler PM.. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI study. J Am Coll Cardiol 2017;70:1949–1961. [DOI] [PubMed] [Google Scholar]
- 60. Kotecha D, Mohamed M, Shantsila E, Popescu BA, Steeds RP.. Is echocardiography valid and reproducible in patients with atrial fibrillation? A systematic review. Europace 2017;19:1427–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen S, Purerfellner H, Meyer C, Acou WJ, Schratter A, Ling Z, Liu S, Yin Y, Martinek M, Kiuchi MG, Schmidt B, Chun KRJ.. Rhythm control for patients with atrial fibrillation complicated with heart failure in the contemporary era of catheter ablation: a stratified pooled analysis of randomized data. Eur Heart J 2019;doi: 10.1093/eurheartj/ehz443. [DOI] [PubMed] [Google Scholar]
- 62. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, Reddy M, Jais P, Themistoclakis S, Dello Russo A, Casella M, Pelargonio G, Narducci ML, Schweikert R, Neuzil P, Sanchez J, Horton R, Beheiry S, Hongo R, Hao S, Rossillo A, Forleo G, Tondo C, Burkhardt JD, Haissaguerre M, Natale A.. Ablation vs. amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation 2016;133:1637–1644. [DOI] [PubMed] [Google Scholar]
- 63. Friberg L, Tabrizi F, Englund A.. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: data from Swedish health registries. Eur Heart J 2016;37:2478–2487. [DOI] [PubMed] [Google Scholar]
- 64. Saliba W, Schliamser JE, Lavi I, Barnett-Griness O, Gronich N, Rennert G.. Catheter ablation of atrial fibrillation is associated with reduced risk of stroke and mortality: a propensity score-matched analysis. Heart Rhythm 2017;14:635–642. [DOI] [PubMed] [Google Scholar]
- 65. Friberg L, Rosenqvist M.. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018;39:453–460. [DOI] [PubMed] [Google Scholar]
- 66. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D'Avila A, Dobrev D, Fenelon G, Gonzalez M, Hatem SN, Helm R, Hindricks G, Ho SY, Hoit B, Jalife J, Kim YH, Lip GY, Ma CS, Marcus GM, Murray K, Nogami A, Sanders P, Uribe W, Van Wagoner DR, Nattel S; Document Reviewers. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace 2016;18:1455–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kamel H, Bartz TM, Elkind MSV, Okin PM, Thacker EL, Patton KK, Stein PK, deFilippi CR, Gottesman RF, Heckbert SR, Kronmal RA, Soliman EZ, Longstreth WT Jr.. Atrial cardiopathy and the risk of ischemic stroke in the CHS (Cardiovascular Health Study). Stroke 2018;49:980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kirchhof P, Breithardt G, Camm AJ, Crijns HJ, Kuck KH, Vardas P, Wegscheider K.. Improving outcomes in patients with atrial fibrillation: rationale and design of the Early treatment of Atrial fibrillation for Stroke prevention Trial. Am Heart J 2013;166:442–448. [DOI] [PubMed] [Google Scholar]
- 69. Schotten U, Verheule S, Kirchhof P, Goette A.. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 2011;91:265–325. [DOI] [PubMed] [Google Scholar]
- 70. Reant P, Lafitte S, JaïS P, Serri K, Weerasooriya R, Hocini M, Pillois X, Clementy J, HaïSsaguerre M, Roudaut R, Reverse remodeling of the left cardiac chambers after catheter ablation after 1 year in a series of patients with isolated atrial fibrillation. Circulation 2005;112:2896–2903. [DOI] [PubMed] [Google Scholar]
- 71. Montserrat S, Sitges M, Calvo N, Silva E, Tamborero D, Vidal B, Berruezo A, Bernado C, Mont L, Brugada J.. Effect of repeated radiofrequency catheter ablation on left atrial function for the treatment of atrial fibrillation. Am J Cardiol 2011;108:1741–1746. [DOI] [PubMed] [Google Scholar]