Abstract
Background
There is a growing interest in nonpharmacological approaches for essential tremor (ET), including tremor cancelation devices. However, the true efficacy of such devices in ET remains unclear.
Methods
A systematic literature review was conducted using standardized criteria regarding efficacy and comfortability. Devices focused on design or experimental testing in which tremor was simulated in a robot were excluded.
Results
Out of 324 articles initially identified, 12 articles were included. Orthoses using biomechanical loading and neuromodulation with electrical stimulation, and external tremor cancelation devices, were the main interventions used to suppress tremor. All devices were designed to control tremor of the upper limbs at different anatomical locations. Overall, an average tremor attenuation of 50–98% was reported (level of evidence III). Interference with voluntary movements and portability was described as the main drawback.
Discussion
In conclusion, this review highlights the growing interest in emerging tremor control devices and the importance of assessing comfort without affecting voluntary movements. However, the level of evidence regarding the efficacy of these tremor control devices remains low. An integrated multidisciplinary combination approach of engineering, robotics, physiology, physiotherapy, and clinical assessment is needed to improve the quality of non-pharmacological interventions for ET.
Keywords: Essential tremor, voluntary movement, devices, neuroprosthesis, orthosis, upper limb
Introduction
Tremor is defined as a rhythmic and involuntary movement of any body part. It is the most common movement disorder that presents in a variety of conditions, including essential tremor (ET), Parkinson’s disease (PD), dystonia, and cerebellar ataxia.1 Tremor is the pathognomonic clinical sign in ET, with an estimated prevalence of approximately 4% of the population over 65 years of age and an incidence rate of 616 per 100,000 annually.1–4 ET classically involves the upper limbs and is generally triggered by arm movement and sustained antigravity postures, thus affecting common daily activities such as writing, using a glass for drinking, or handling cutlery.5 ET is a slowly progressive condition that can also involve the head, voice, and lower limbs.6 More than 65% of the population with upper limb tremor are seriously conditioned when performing their activities of daily living, leading in some cases to social exclusion.2 Furthermore, 34% of the patients with ET present at least mild depression associated with a reduced performance in activities of daily living.5,7 Given that tremor-related disability can be significant, several therapies have been proposed. Initial treatment recommendation includes propranolol, primidone, and topiramate.8,9 However, the symptomatic benefit of these drugs tends to decline over time, likely attributable to disease progression or development of drug tolerance. Second-line agents include gabapentin, nimodipine, and others.8,9 Patients not responding to pharmacological treatment may undergo surgical procedures such as deep brain stimulation, magnetic resonance-guided focused ultrasound thalamotomy, or other ablative techniques like gamma-knife radiosurgery.10
Alternative therapies are then needed for individuals presenting ET who respond poorly to medication, and for those in which surgery is not possible. Therefore, there is a growing interest in nonpharmacological therapies and functional rehabilitation for ET.11 Emerging nonpharmacological therapies for ET include (1) exoskeletons, which are wearable mechatronic systems, in which the physical interface provides a direct transfer of mechanical power and some exchange of information12,13; (2) orthoses, defined as a medical device that acts in parallel to a segment of the body, in order to compensate some dysfunction14,15; and (3) handheld external devices such as spoons.16 However, to date, there is still little evidence on the effectiveness of these devices in tremor suppression. The objective of this paper is to review the main advantages and disadvantages of these emerging therapies from the neurologist’s perspective. A detailed biomechanical description of the mechanism of action of the devices is beyond the scope of this review.
Methods
Search strategy and eligibility criteria
A literature search was performed using PubMed/MEDLINE, Scopus, Physiotherapy Evidence Database (PDRro), and Psychological Database for Brain Impairment Treatment Efficacy (PsycBITE). The search terms used were “essential tremor,” “devices,” “neuroprosthesis,” “orthosis,” and “robot.” Eligible papers included studies on nonpharmacological therapies for ET in either English or Spanish, published between January 1, 2000, and September 1, 2019. Articles on pharmacological or surgical treatments for ET devices focused on design or experimental testing, in which the human motion (both tremor and voluntary movement) was simulated in a robot, and studies including only non-ET tremor patients were excluded. The following outcomes measures were considered in this review: (1) benefit in terms of tremor reduction. On the one hand, clinical benefit was evaluated by different methodologies, including rating scales like the seven-point scale Clinical Global Impression Scale (CGI-S) and tremor rating scales (TRS) including the Fahn–Tolosa–Marin TRS17 and the Essential Tremor Rating Assessment Scale (TETRAS).18 On the other hand, benefit, from a biomechanical point of view, was evaluated by using: (1) the tremor power, measured by power spectral density or PSD, (2) the root mean square (RMS) of the tremor acceleration amplitude, (3) the average tremor acceleration (AA), and (4) the deflection amplitude (AD)19; (2) Comfortability, which was mainly determined by the weight of the device.
The level of evidence for therapeutic studies was based on the recommendations developed by the Centre for Evidence Based Medicine, taken into account the quality of the data (levels from 1, highest quality [systematic review of randomized clinical trials], 2 [cohort studies], 3 [case-control studies], 4 [case series], to 5, lowest quality (expert opinions, physiology bench research, or first principles]).20
Results
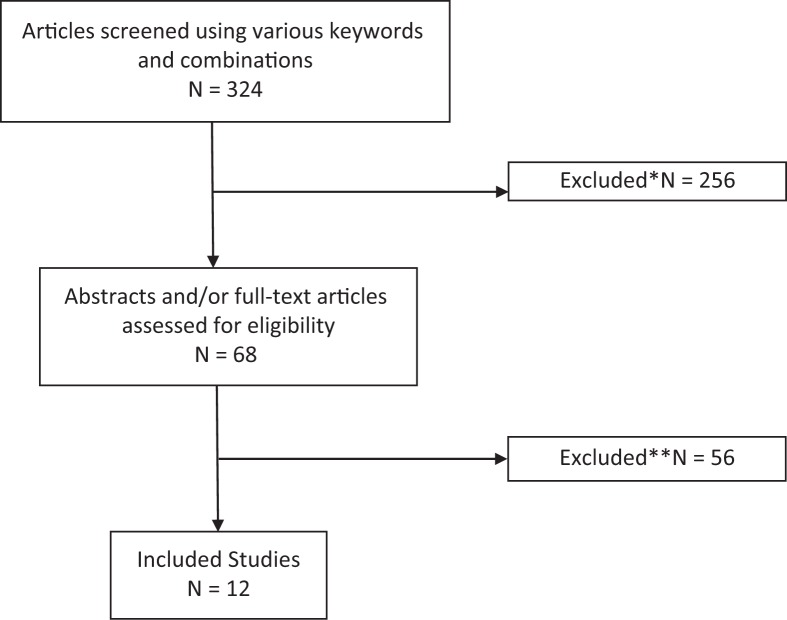
Following eligibility criteria, by consensus of the authors, 12 articles were selected and reviewed (Figure 1 and Table 1). Devices were classified as orthoses using neuromodulation with electrical stimulation of pairs of antagonist muscles opposing tremor oscillations,14,21–26 biomechanical loading with suppressive technologies like viscous and magnetic fluids, magnetic particle brakes, pneumatic actuators and motors, exoskeleton robots with tremor suppression control,27–32 and external tremor cancelation devices.16 All devices were primarily designed to control tremor of the upper limbs. Five studies analyzed the devices that controlled the entire upper limb (including elbow/forearm and wrist), seven studies tested efficacy in one single joint, four studies addressed just the elbow, and three studies the wrist (Table 1). In one study, an external tremor control device (a spoon) was tested.16 Below, a brief description of each device functionality is provided.
Figure 1.
PRISMA Flow Diagram for Identification of Relevant Studies. Reasons for exclusion: *non-tremor control device studies; **abstracts, non-matched inclusion criteria studies, language (non-English or Spanish).
Table 1.
Main Results of Search for Tremor Suppression Devices
| Author | Sample (n) | Mechanism | Upper arm location | UsabilityTotal weight (Kg) | Evidence level |
|---|---|---|---|---|---|
| Manto et al.27 | ET (6) | Orthosis biomechanical loading |
Elbow and wrist | 0.850 | III |
| Loureiro et al.28 | Tremor (33)* | Orthosis biomechanical loading |
Forearm, wrist | <0.200 | III |
| Rocon et al.11,12 | ET (6), others (4) (PD, Multiple sclerosis, post-anoxic tremor) | Exoskeleton biomechanical loading and notch filtering |
Elbow and wrist | 0.850 | III |
| Gallego et al.13 | Tremor (17) including PD, ET, cerebellar disorders | Soft-wearable robot (sleeve) biomechanical loading + functional electrical stimulation |
Elbow and wrist | ? | III |
| Seki et al.21,22 | ET (1) | Myoelectric-controlled exoskeletal robot | Elbow | ? | III |
| Popovic´ et al.26 | PD (4) ET (3) |
Orthosis biomechanical loading Functional electric stimulation |
Wrist | ? | III |
| Ando et al.23 | ET (1) | Myoelectric-controlled exoskeletal robot | Elbow | 0.330 | III |
| Gallego et al.24 | ET (4) PD (2) |
Orthosis (textile) Functional electrical stimulation | Elbow and wrist | ? | III |
| Belda et al.30 | ET (12) | Orthosis biomechanical loading |
Wrist | ? | III |
| Pathak et al.16 | ET (15) | Spoon | External (hand) | 0.100 | III |
| Gallego et al.25 | ET (1) | Orthosis functional electrical stimulation |
Elbow | ? | III |
| Hermstadt et al. 31,32 | ET (9) | Orthosis biomechanical loading |
Elbow | 1.7 | III |
Abbreviations: ET, Essential Tremor; PD, Parkinson’s Disease.
Tremor was not clinically classified.
Manto et al.27 conducted a study in 12 subjects with tremor, in which they intended to develop the prototype of a wearable active device, by characterizing the contribution of each joint to the observed tremor of the upper limb, and analyzing the loads/forces needed to counteract the oscillations. In this article, parameters and constraints for constructing workable and usable active orthoses were presented, although no specific data in terms of efficacy or side effects were reported.
Loureiro et al.28 developed an orthosis that can dynamically suppress pathological tremor by applying controlled viscous damping to the affected limb. This orthosis has an actuator based on magnetorheological fluids that deliver the so-called damping action, meaning the dissipation of the energy stored in an oscillatory movement, which reduces the occurrence and the amplitude of tremor. Kinematic sensors analyze the mechanical characteristics of tremor and discriminate between desired and undesired components of motion. Biomechanical data from 33 patients with tremor allowed for the implementation of a prototype that was applied to one ET patient. This device, in which the damping coefficient was 4 N·s/mm, significantly reduced tremor at the wrist and showed no side effects.
Rocon et al.12 tested in six subjects a robotic exoskeleton called wearable orthosis for tremor assessment and suppression (WOTAS). The device was based on two mechanisms: (1) tremor reduction through impedance control (defined by the relation between force and position), by modifying the stiffness, damping, and mass properties of the upper limb; and (2) notch filtering, a system that attenuates only those frequencies coinciding with tremor. The exoskeleton has gyro sensors in each joint that provide input signals for control. The efficacy of the device was assessed in 10 patients with tremor disorders, by using both a passive control mode, analyzing the change in the biomechanical properties of the upper limb (viscosity and inertia); and in an active control mode, by applying opposite forces to the tremor movement. A better tremor control was obtained with the active control mode (81.2% mean power reduction) compared to the passive suppression mode (70% mean power reduction). In a second study, as performed in six subjects with ET,13 the authors observed a reduction of tremor up to 98%. However, users considered the exoskeleton a bulky and heavy apparatus that hindered adequate social life.
Gallego et al.14,24,25 tested the TREMOR neuro-robot, a type of active wearable outfit, which incorporated an array of electrodes sewn to the garment, both for electrical stimulation and recording. It included a brain–neural computer interface that monitored the whole neuromusculoskeletal system. This device detects both voluntary movements and tremor, and is able to electrically stimulate pairs of antagonist muscles exactly opposing tremor oscillations. Preliminary results indicated that efficacy varied considerably between patients with different types of tremor. In a PD patient, modification of joint impedance was effective in the suppression of tremor (reaching 60% of the peak-to-peak value, in tremor amplitude and frequency of the PSD). In another ET patient, the average tremor reduction was 16.77 ± 8.33% of the PSD. They also showed that tremor tended to migrate to proximal joints when suppressed more distally, thus supporting the need of independent joint controllers.
Seki et al.21,22 conducted a study aimed to develop a filter, which used a neural network learning algorithm that reduces tremor-related electromyographic noise, to better characterize tremor oscillations. They developed a prototype with a surface myoelectric sensor that collected input signals, a signal processor that subtracted the voluntary movement, and a tremor recognition system that provided an output signal. The actuator was a harmonic drive system that suppressed the tremor. The results demonstrated that the proposed filter optimized motion recognition, especially on flexed postures. Using a similar prototype, Ando et al.23 developed an algorithm able to accurately recognize voluntary movements of the elbow joint in patients with ET (89.5% average recognition rate). In one ET patient, a 50–80% reduction of action tremor while eating was observed, without significant side effects.
Popovic et al.26 developed a programmable multichannel stimulator that supported asynchronous activation of several electrodes, and used inertial sensors attached to several joints. The software provided adaptive sensor-driven control for the out-of-phase stimulation. This system was used in seven patients (PD and ET) to improve wrist joint tremor. In all but one patient, the adaptive out-of-phase stimulation resulted in a significant decrease in the amplitude of tremor (67 ± 13%).
Belda et al.30 conducted a study over 12 ET patients using an orthosis prototype, with a rotary damper mechanism, which combined dry friction and viscous damping. Efficacy was evaluated with the TRS, spirals, and actions tasks. They observed a 33% reduction in tremor severity with no side effects.
Pathak et al.16 analyzed a handheld device that carried an active tremor cancelation device aimed to improve the use of a regular spoon. Three specific tasks were tested in 15 ET subjects with this device turned “on” and “off.” Tremor severity was evaluated with the TRS, subjective improvement with the CGI-S, and tremor amplitude with device-embedded accelerometers. TRS scores for all three tasks significantly improved when the device was on versus off (holding, p = 0.01; eating, p = 0.001; and transferring, p = 0.001). CGI-S improved when eating and transferring but not for the holding task, and accelerometer measurements demonstrated a 71–76% reduction in tremor when the device was on. However, this tremor cancelation system could not be used in two subjects with severe tremor, who had their DBS turned off, suggesting that this device is most suitable for mild-moderate cases. Side effects were not observed.
Hernstadt et al.31,32 conducted a study to evaluate the feasibility of a voluntary-driven, speed-controlled tremor suppression robotic orthosis for elbow tremor. They developed a prototype using a brushless rotatory electrical motor, connected to a commercial spur gearbox in combination with a custom gear reduction, embedded with a torque sensor and an encoder. Nine participants (ET and PD) performed computerized pursuit tracking tasks following a sinusoid and a random target, both with and without the tremor suppression orthosis. Efficacy was determined by the relative power change calculated with the PSD. Tremor severity was assessed with the performance section of TETRAS. The suppressive orthosis resulted in a 94% mean power reduction of tremor (p < 0.001). No significant impact on voluntary movements was observed. Nevertheless, the size and weight of the device were considered a troublesome issue for the participants. They did not observe, however, migration of tremor to nearby joints.
Discussion
This literature review shows that the level of evidence regarding the efficacy of the several types of tremor suppression devices remains low. Most of them are prototypes, tested in small and heterogeneous groups of patients harboring different tremor disorders, providing little information on comfort and impact of its use on daily life activities and quality of life. To develop and validate efficient tremor suppression devices, we need a better understanding of (1) the anatomical features of tremor, (2) how tremor originates, (3) what muscles are involved, and (4) how does it propagate to the hand, where it results most disabling.
The best anatomical location to place a tremor controller device of the upper limb still remains controversial. Likewise, it is not fully established what type of movement control, and muscle stimulation in each joint, needs to be addressed for tremor improvement33: (1) elbow: flexion/extension, (2) forearm: pronation/supination, (3) wrist: flexion/extension, or (4) wrist: abduction/adduction. Previous studies15 indicate that the first three pairs of motions do have a significant impact on disability. Moreover, studies focused on the elbow joint31 have shown that elbow motion is the key to successfully perform most activities of daily living.33
In terms of daily living activities and quality of life impairment, there is also still controversy whether disability of ET patients is more associated with the frequency of tremor,34 or with its amplitude,35,36 Of note, another unsolved issue is tremor migration. Some authors have observed that tremor tends to migrate toward proximal joints when it is suppressed more distally; for example, if tremor is suppressed at the wrist level, it may appear or increase at the elbow or shoulder. This phenomenon has been reported in both PD and ET patients,25,37,38 although a specific physiologic explanation still lacks.
To develop tremor cancelation device prototypes, a few basic characteristics have been suggested.19,25,32 The design of the tremor suppression part of an upper limb orthosis, the actuator, needs to include a sensing mechanism able to provide position, velocity, and acceleration information to the control and feedback system with minimum delay. These sensors need to extract information following a hierarchical integration scheme: first, to detect user’s intention to perform a certain voluntary movement; second, to evaluate tremor characteristics with an estimation of its amplitude, frequency, and phase; and third, to determine how tremor affects each joint and which muscles contribute to joint tremor.14,23 The actuator, however, should ideally be light, small, easily attachable to the orthosis, noiseless; should respond fast; and consume battery as little as possible. On the other hand, it is also very important to acknowledge patient’s comfort regarding the external appearance of the device such as size and weight. Thus, indicators of voluntary movement restriction, like the degree of freedom (DOF) coefficient, and the weight per DOF, have been developed as recommended measurement tools of usability/convenience.19
To effectively compare tremor suppression devices, some methodological aspects need to be considered. First, elaboration of a standard common protocol, describing the tasks to be performed, is needed for adequate comparison. Second, tremor suppression efficacy needs objective assessments of tremor with specific validated biomechanical outcomes. Third, clinicians and engineers should use validated clinical rating scales/objective measurements for ET, reflecting tremor severity, impact on daily living activities, and quality of life. Fourth, researchers should also evaluate combined outcome measures, like cost-effectiveness variables,39 and the so-called minimum significant difference,40 defined as the smallest clinically significant change of a specific variable, that the patient deems important.
Finally, validation of tremor cancelation devices should adopt the same scientific methodology used for clinical trials in order to provide an adequate level of evidence and extrapolation of data. Well-designed longitudinal studies with a priori sample size calculation, adequate randomization and appropriate ethical compliance are therefore needed. In this regard, given that adequate blinding within trials can be challenging, the use of objective tremor motion measurements used in these devices may overcome this difficulty.
This review has some limitations. It is widely recognized that Japanesse researchers are considered pioneers in robotics; however, translation into English or Spanish of some papers written in Japanesse was not available for analysis. Additionally, we did not include some tremor cancelation devices, which were directly advertised at different websites, which did not provide any scientific literature that could be reviewed.
In conclusion, this review highlights the growing interest in emerging tremor control devices and the importance of assessing comfort without affecting voluntary movements. However, the level of evidence regarding the efficacy of these tremor control devices remains low. An integrated multidisciplinary combination approach of engineering, robotics, physiology, physiotherapy, and clinical assessment is needed to improve the quality of nonpharmacological interventions for ET.
Acknowledgments
The authors would like to thank Fundación Burgos por la Investigación de la Salud for its support.
Footnotes
Citation: Castrillo-Fraile V, Peña EC, Gabriel y Galán JMT, Delgado-López PD, Collazo C & Cubo E. Tremor Control Devices for Essential Tremor: A Systematic Literature Review. Tremor Other Hyperkinet Mov. 2019; 9. doi: 10.7916/tohm.v0.688
Editor: Ruth Helen Walker, Mount Sinai School of Medicine, USA
Funding: Financial support for this study was provided by Fundación Burgos por la Investigación de la Salud.
Financial Disclosures: None.
Conflicts of Interest: The authors report no conflicts of interest.
Ethics Statement: Not applicable for this category of article.
References
- 1.Bermejo-Pareja F, Benito-Leon J, Vega Q S, Díaz-Guzmán J, Rivera-Navarro J, Molina JA, et al. [The NEDICES cohort of the elderly. Methodology and main neurological findings]. Rev Neurol 2008;46:416-23. doi: 10.33588/rn.4607.2008134 [DOI] [PubMed] [Google Scholar]
- 2.Benito-Leon J, Bermejo-Pareja F, Louis ED, Neurological Disorders in Central Spain Study G. Incidence of essential tremor in three elderly populations of central Spain. Neurology 2005;64:1721-5. doi: 10.1212/01.WNL.0000161852.70374.01 [DOI] [PubMed] [Google Scholar]
- 3.Cacho J, Benito-Leon J, Louis ED, Group NS. Methods and design of the baseline survey of the neurological disorders in Salamanca (NEDISA) cohort: a population-based study in Central-Western Spain. Neuroepidemiology 2011;36:62-8. doi: 10.1159/000323269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord 2010;25:534-41. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz D, Schwieger D, Moises H, Deuschl G. Quality of life and personality in essential tremor patients. Mov Disord 2006;21:1114-8. doi: 10.1002/mds.20884 [DOI] [PubMed] [Google Scholar]
- 6.Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, et al. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 2018;33:75–87. doi: 10.1002/mds.27121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller KM, Okun MS, Fernandez HF, Jacobson CEt, Rodriguez RL, Bowers D. Depression symptoms in movement disorders: comparing Parkinson's disease, dystonia, and essential tremor. Mov Disord 2007;22:666–72. doi: 10.1002/mds.21376 [DOI] [PubMed] [Google Scholar]
- 8.Rajput AH, Rajput A. Medical treatment of essential tremor. J Cent Nerv Syst Dis 2014;6:29––39.. doi: 10.4137/JCNSD.S13570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira JJ, Mestre TA, Lyons KE, Benito-León J, Tan EK, Abbruzzese G, et al. MDS evidence-based review of treatments for essential tremor. Mov Disord 2019;34:950-8. doi: 10.1002/mds.27700 [DOI] [PubMed] [Google Scholar]
- 10.Fasano A, Lozano AM, Cubo E. New neurosurgical approaches for tremor and Parkinson's disease. Curr Opin Neurol 2017;30:435-46. doi: 10.1097/WCO.0000000000000465 [DOI] [PubMed] [Google Scholar]
- 11.Dosen S, Muceli S, Dideriksen JL, Romero JP, Rocon E, Pons J, et al. Online tremor suppression using electromyography and low-level electrical stimulation. IEEE Trans Neural Syst Rehabil Eng 2015;23:385–95. doi: 10.1109/TNSRE.2014.2328296 [DOI] [PubMed] [Google Scholar]
- 12.Rocon E, Belda-Lois JM, Ruiz AF, Manto M, Moreno JC, Pons JL. Design and validation of a rehabilitation robotic exoskeleton for tremor assessment and suppression. IEEE Trans Neural Syst Rehabil Eng 2007;15:367–78. doi: 10.1109/TNSRE.2007.903917 [DOI] [PubMed] [Google Scholar]
- 13.Rocon E, Gallego JA, Belda-Lois JM, Benito-Leon J, Luis Pons J. Biomechanical loading as an alternative treatment for tremor: a review of two approaches. Tremor Other Hyperkinet Mov (N Y) 2012;2:02–77-495-1. doi: 10.7916/D82Z147G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallego JA, Rocon E, Belda-Lois JM, Pons JL. Closed-loop modulation of a notch-filter stimulation strategy for tremor management with a neuroprosthesis. XIII Mediterranean Conference on Medical and Biological Engineering and Computing 2013 IFMBE Proceedings. 2014;41:1747–1750. doi: 10.1007/978-3-319-00846-2_431 [DOI] [Google Scholar]
- 15.Belda JM, Prieto L, Bermejo I, Fernández P, Fernández L, Castillo A, et al. Ortesis para reducir el temblor esencial. Revista de biomecánica 2013:35–38. [Google Scholar]
- 16.Pathak A, Redmond JA, Allen M, Chou KL. A noninvasive handheld assistive device to accommodate essential tremor: a pilot study. Mov Disord 2014;29:838-42. doi: 10.1002/mds.25796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahn S, Tolosa E, Marin C. Clinical Rating Scale for Tremor. In:, editors. Parkinson’s Disease and Movement Disorders. 2nd ed. Williams & Wilkins; Baltimore, MD: 1993. pp. 271–280. doi: 10.1002/mds.25648 [DOI] [Google Scholar]
- 18.Elble R, Comella C, Fahn S, Hallett M, Jankovic J, Juncos JL, et al. Reliability of a new scale for essential tremor. Mov Disord 2012;27:1567–9. doi: 10.1002/mds.25162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fromme NP, Camenzind M, Riener R, Rossi RM. Need for mechanically and ergonomically enhanced tremor-suppression orthoses for the upper limb: a systematic review. J Neuroeng Rehabil 2019;16:93. doi: 10.1186/s12984-019-0543-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centre for Evidence Based Medicine [Accessed July 1, 2019]; Available at http://www.cebm.net.
- 21.Seki M, Matsumoto Y, Ando T, Kobayashi Y, Fujie MG, Iijima H, et al. Development of robotic upper limb orthosis with tremor suppressiblity and elbow joint movability. Systems, Man, and Cybernetics (SMC; ), 2011 IEEE International Conference. 2011; 729 –735. doi: 10.1109 [Google Scholar]
- 22.Seki M, Matsumoto Y, Ando T, et al. The weight load inconsistency effect on voluntary movement recognition of essential tremor patient. Robotics and Biomimetics (ROBIO), 2011 IEEE International Conference; 2011, p. 901–7. [Google Scholar]
- 23.Ando T, Watanabe M, Nishimoto K, Matsumoto Y, Seki M, Fujie MG. Myoelectric-controlled exoskeletal elbow robot to suppress essential tremor: Extraction of elbow flexion movement using STFTs and TDNN. Journal of Robotics and Mechatronics. 2012;24 1:141–149. doi: 10.20965 [Google Scholar]
- 24.Gallego JA, Rocon E. “A soft wearable robot for tremor assessment and suppression,” 2011 IEEE International Conference on Robotics and Automation, Shanghai, pp. 2249–2254. doi: 10.1109/icra.2011.5979639 [DOI] [Google Scholar]
- 25.Gallego JA, Rocon E, Belda-Lois JM, Pons JL. A neuroprosthesis for tremor management through the control of muscle co-contraction. J Neuroeng Rehabil 2013;10:36. doi: 10.1186/1743-0003-10-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popovic Maneski L, Jorgovanovic N, Ilic V, Došen S, Keller T, Popović MB, et al. Electrical stimulation for the suppression of pathological tremor. Med Biol Eng Comput 2011;49:1187–93. doi: 10.1007/s11517-011-0803-6 [DOI] [PubMed] [Google Scholar]
- 27.Manto M, Topping M, Soede M, Sánchez-Lacuesta J, Harwin W, Williams J, et al. Dynamically responsive intervention for tremor suppression. IEEE Eng Med Biol Mag 2003;22:120–32. doi: 10.1109/memb.2003.1213635 [DOI] [PubMed] [Google Scholar]
- 28.Loureiro R, Belda-louis JM, Lima ER, Pons JL, Sanchez-Lacuesta JJ. & Harwin WS. Upper Limb tremor supuppession in ADL via an Orthosis Incorporating a Controllable Double Viscous Bean Actuator. Proceedings of the 2005 IEEE 9th International Conference on Rehabilitation Robotics. 2005; 119–122. doi: 10.1109 [Google Scholar]
- 29.Rocon E, Manto M, Pons J, Camut S, Belda JM. Mechanical suppression of essential tremor. Cerebellum 2007;6:73––8.. doi: 10.1080/14734220601103037 [DOI] [PubMed] [Google Scholar]
- 30.Belda JM, Rocon E, Sánchez-Lacuesta JJ, Ruiz AF, Pons JL. “Functional assessment of tremor in the upper limb,” in Euro Conf Advancement Assistive Techn in Europe, 2005. [Google Scholar]
- 31.Herrnstadt G, Menon C. Voluntary-Driven Elbow Orthosis with Speed-Controlled Tremor Suppression. Front Bioeng Biotechnol 2016;4:29. doi: 10.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrnstadt G, McKeown MJ, Menon C. Controlling a motorized orthosis to follow elbow volitional movement: tests with individuals with pathological tremor. J Neuroeng Rehabil 2019;16:23. doi: 10.1186/s12984-019-0484-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aizawa J, Masuda T, Koyama T, Nakamaru K, Isozaki K, Okawa A. et al. Three-dimensional motion of the upper extremity joints during various activities of daily living. J Biomech 2010;43:2915–22. doi: 10.1016/j.jbiomech.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 34.Elble RJ, Higgins C, Hughes L. Longitudinal study of essential tremor. Neurology 1992;42:441–3. [DOI] [PubMed] [Google Scholar]
- 35.Calzetti S, Baratti M, Gresty M, Findley L. Frequency/amplitude characteristics of postural tremor of the hands in a population of patients with bilateral essential tremor: implications for the classification and mechanism of essential tremor. J Neurol Neurosurg Psychiatry 1987;50:561–7. doi: 10.1136/jnnp.50.5.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elble RJ, Pullman SL, Matsumoto JY, Raethjen J, Deuschl G, Tintner R, et al. Tremor amplitude is logarithmically related to 4- and 5-point tremor rating scales. Brain 2006;129:2660-6. doi: 10.1093/cerebro/punzon190 [DOI] [PubMed] [Google Scholar]
- 37.Aisen ML, Arnold A, Baiges I, Maxwell S, Rosen M. The effect of mechanical damping loads on disabling action tremor. Neurology 1993;43:1346–50. doi: 10.1212/wnl.43.7.1346 [DOI] [PubMed] [Google Scholar]
- 38.Hwang IS, Lin CC, Wu PS. Tremor modulation in patients with Parkinson's disease compared to healthy counterparts during loaded postural holding. J Electromyogr Kinesiol 2009;19:e520-8. doi: 10.1016/j.jelekin.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 39.Saha S, Hoerger TJ, Pignone MP, Teutsch SM, Helfand M, Mandelblatt JS, et al. The art and science of incorporating cost effectiveness into evidence-based recommendations for clinical preventive services. Am J Prev Med 2001;20:36–43. doi: 10.1016/s0749-3797 [DOI] [PubMed] [Google Scholar]
- 40.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407––15.. doi: 10.1016/0197-2456 [DOI] [PubMed] [Google Scholar]