Abstract
Background
Organic acidemias, especially propionic acidemia (PA) and methylmalonic acidemia (MMA), may manifest clinically within the first few hours to days of life. The classic presentation in the newborn period includes metabolic acidosis, hyperlactatemia, and hyperammonemia that is precipitated by unrestricted protein intake. Implementation of newborn screening to diagnose and initiate early treatment has facilitated a reduction in neonatal mortality and improved survival. Despite early diagnosis and appropriate management, these individuals are prone to have recurrent episodes of metabolic acidosis and hyperammonemia resulting in frequent hospitalizations. Liver transplantation (LT) has been proposed as a treatment modality to reduce metabolic decompensations which are not controlled by medical management. Published reports on the outcome of LT show heterogeneous results regarding clinical and biochemical features in the post transplantation period. As a result, we evaluated the outcomes of LT in our institution and compared it to the previously published data.
Study design/Methods
We performed a retrospective chart review of nine individuals with PA or MMA who underwent LT and two individuals with MMA who underwent LT and kidney transplantation (KT). Data including number of hospitalizations, laboratory measures, cardiac and neurological outcomes, and growth parameters were collected.
Results
The median age of transplantation for subjects with MMA was 7.2 years with a median follow up of 4.3 years. The median age of transplantation for subjects with PA was 1.9 years with a median follow up of 5.4 years. The survival rate at 1 year and 5 years post-LT was 100%. Most of our subjects did not have any episodes of hyperammonemia or pancreatitis post-LT. There was significant reduction in plasma glycine post-LT. One subject developed mild elevation in ammonia post-LT on an unrestricted protein diet, suggesting that protein restriction may be indicated even after LT.
Conclusion
In a large single center study of LT in MMA and PA, we show that LT may reduce the incidence of metabolic decompensation. Moreover, our data suggest that LT may be associated with reduced number of hospitalizations and improved linear growth in individuals with PA and MMA.
Keywords: Organic acidemia, Propionic acidemia, Methylmalonic acidemia, Liver transplantation
1. Introduction
Organic acidemias (OA) are inborn errors of metabolism that result from defective catabolism of specific amino acids and are characterized by abnormal accumulation and excretion of organic acids. Propionic acidemia (PA) (OMIM# 606054) and methylmalonic acidemia (MMA) (OMIM #251000) are such classical organic acidemias. Both PA and MMA result from defective catabolism of valine, isoleucine, methionine, threonine and odd chain fatty acids. Deficiency of propionyl-CoA carboxylase (PCC; EC 6.4.1.3), a dodecamer comprised of 6 subunits of propionyl CoA carboxylase A (PCCA) and 6 subunits of propionyl CoA carboxylase B (PCCB) causes PA whereas, deficiency of methylmalonyl Co-A mutase (MCM; EC 5. 4.99.2) causes one form of MMA. The incidence of MMA is estimated to be approximately 1: 90,000, [1] and the incidence of PA is estimated to be approximately 1:105,000-1:130,000 in the U.S. [2]
Both MMA and PA can present with emesis, lethargy, poor feeding, metabolic acidosis and hyperammonemia in the neonatal period. Acute metabolic decompensation in PA and MMA can be treated through reversal of catabolism using intravenous glucose, insulin infusion, lipids, and carnitine, in addition to dietary protein restriction [3][4]. Oral N-carbamoyl glutamate/carglumic acid (a prodrug of N-acetylglutamate) has been used to treat hyperammonemia during acute metabolic decompensations [5][6].
Chronic complications of PA include developmental delay, intellectual disability, impaired growth, pancreatitis, bone marrow suppression, optic nerve atrophy, sensorineural hearing loss and cardiomyopathy [7][8][9][10]. MMA has similar chronic complications; however, renal failure is more common and cardiac manifestations are less common in MMA compared to PA [11][12][13]. Chronic management of MMA and PA consists of dietary protein restriction in combination with medical foods to restrict dietary intake of propiogenic amino acids (isoleucine, methionine, threonine, valine), L-carnitine supplementation is used to promote excretion of accumulating organic acids and oral antibiotics may be given to reduce propionate production by the intestinal microbiome [3][14]. Routine clinical surveillance of individuals with MMA and PA is recommended in order to detect and minimize the long-term cardiac, renal and ophthalmologic complications [14][3][15].
Liver transplantation (LT) has been proposed as a treatment modality to increase the enzyme activity in the liver in individuals with PA and MMA who have frequent metabolic decompensations despite appropriate medical treatment [16]. Clinical studies have demonstrated that LT reduces the risk of metabolic decompensation and chronic complications that are associated with MMA and PA. The overall survival rate for individuals with MMA and PA at 1 year post LT improved from 72.2% [17] in earlier reports to 100% over the last 10-15 years [18][19]. Simultaneous liver and kidney transplantation (LT/KT) has been recommended in individuals with MMA who have associated chronic kidney disease [20]. LT in both MMA and PA has been associated with improvement in metabolic stability with regards to hyperammonemia, pancreatitis and metabolic acidosis with a concomitant decrease in hospitalizations. Some reports suggest no significant changes in biochemical parameters such as plasma propionylcarnitine (C3), glycine and urinary methylcitrate. [21][22][23][24][17] (Supplemental table 1 & 2). In contrast, there are reports that document significant reduction in C3 [18][25] , glycine and plasma methylmalonic acid levels after LT [19].
Despite the reported improved clinical outcomes of individuals with MMA and PA after LT, studies suggest that LT is not curative for these disorders. Case reports show evidence of metabolic strokes and conflicting evidence of neurological improvement and degeneration in individuals with organic acidemias after LT [26][27] [28] [29] (Supplemental table 1 & 2). Given this conflicting evidence, more research is needed to determine the long-term clinical outcomes of LT for the treatment of MMA and PA. The primary objective of this study was to investigate if LT improved chronic complications, such as biochemical, clinical and growth outcomes, in a large, single-center cohort of individuals with PA and MMA.
2. Methods
2.1. Study Design and Protocol
We performed a retrospective chart review of patients with PA and MMA who had LT or simultaneous LT/KT at Texas Children’s Hospital (Houston, Texas). Inclusion criteria included the following: 1) confirmed molecular or biochemical diagnosis of PA or MMA; 2) LT performed between January 1999 to December 2017, and 3) routine follow up visits in the Transplant Clinic at Texas Children’s Hospital. We included all available data from each subject’s clinical charts through March 31, 2019. Exclusion criteria included individuals with MMA or PA who did not have medical records available for review. Charts of eleven individuals (MMA, n=3; PA, n=8) who had LT or LT/KT between January 1999 and December 2017 and who met the inclusion criteria were evaluated. The study was approved by the Institutional Review Board of Baylor College of Medicine.
2.2. Clinical Measurements
Clinical data for the 11 subjects with MMA or PA enrolled in this study were collected. The following data were collected pre- and post-LT: 1) number and severity of hospitalizations; 2) laboratory measures in plasma; 3) long-term clinical outcome data, including cardiac manifestations, episodes of pancreatitis, and neurological outcomes; 4) dietary protein intake; and 5) growth parameters including weight and height Z scores prior to LT, at 1 year post-LT, at 2 years post-LT and at the most recent follow-up visit. Every hospitalization reported in the Texas Children’s Hospital medical record during the six months prior to transplantation and the twelve months after transplantation was included in the analysis regardless of the medical reason for the hospitalization. The hospitalization during which the LT or LT/KT was planned or performed was not included in the statistical analyses. Our analysis of hospitalizations was restricted to six months before and twelve months after transplantation because all hospitalizations should be recorded in the Texas Children’s Hospital medical record in this time frame. No information about hospitalizations was available for one subject.
For the analysis of hyperammonemia episodes and pancreatitis episodes, only those episodes recorded in the Texas Children’s Hospital medical record were used, and thus, our data may underestimate the true number of episodes. In this study, a hyperammonemia episode is defined by venous ammonia ≥100 μmol/L. Serum lipase level (≥ three times the upper limit of normal or 360 U/L) was used to define pancreatitis in our study. Growth parameters collected during routine outpatient follow-up visits at the Transplant Clinic were included in this study. If growth parameters were not available from a pre-LT outpatient visit in the Transplant Clinic, growth parameters from day of LT were used. All biochemical laboratory measures reported in each subject’s Texas Children’s Hospital medical records during the pre- and post-LT periods were included in this study.
2.3. Statistical Analysis
All statistical analyses were performed in SAS version 9.4 (SAS Institute, Inc.). Growth parameters and hospitalizations were analyzed using a two-way repeated measures ANOVA using PROC MIXED with main effects for disease (MMA or PA), time (height and weight Z scores: pre-LT, 1 year post-LT, 2 years post-LT; hospitalizations: pre-LT, 0-6 months post-LT and 6-12 months post-LT) and the disease × time interaction. The model included random effects for subject (disease). When data were skewed, a log transformation was performed. To compare height and weight Z scores versus the most recent growth measure obtained, paired t-tests were performed. Because the repeated measures ANOVA and paired t-tests require balanced data to assess the parameters of interest across specified time points, subjects missing one or more observations were excluded from the final statistical analyses (hospitalizations, 1/11 subjects excluded; height and weight Z scores, 3/11 subjects excluded).
Statistical analyses to assess plasma concentrations of C3, MMA and glycine were performed using PROC MIXED (SAS Institute, Inc.). Values for each subject were averaged for pre-LT and post-LT separately. Two-way ANOVA using a completely randomized design with crossed data was performed to test for the main effects for disease (MMA or PA), treatment (pre-LT or post-LT) and the disease × treatment interaction. The model included random effects for subject. If the data were skewed, a log transformation was performed. Subjects that had no reported lab values pre-LT and/or post-LT were excluded from the final statistical analyses for plasma C3 (3/11 subjects) and glycine (1/11 subjects). The threshold for statistical significance was p<0.05.
3. Results
3.1. Subject Characteristics
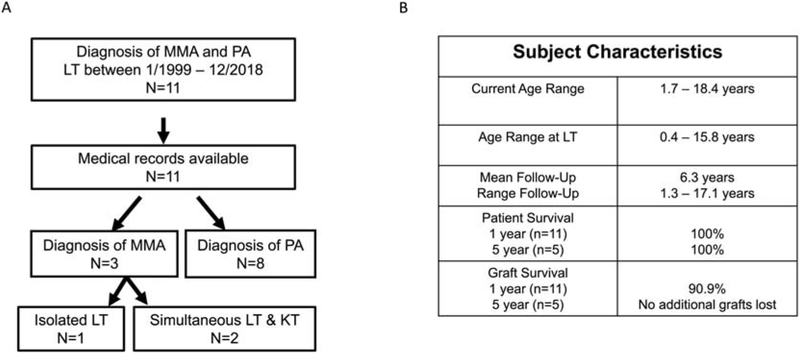
Of the eleven subjects, three had a diagnosis of MMA and eight had a diagnosis of PA. The age of presentation for the individuals with MMA was 2-3 days of age. The age of initial presentation of P3 was unavailable since the subject had pre-LT care at an outside hospital, and the specific age at presentation was not documented in the transfer summary. The three subjects with MMA had the diagnosis confirmed with molecular testing (Table 1). Two of three subjects with MMA had simultaneous LT/KT whereas one subject had isolated LT. The median age of LT for subjects with MMA was 7.2 years (range: 3.5 – 15.8 years) with a median follow up of 4.3 years (range: 2.6 – 7.3 years) (Figure 1). The median age of initial presentation for subjects with PA was 3 days (range: 1 – 30 days, table 2). Five subjects with PA had their diagnosis confirmed by molecular testing. One PA diagnosis (P9) was confirmed by enzyme testing in skin fibroblasts while two PA diagnoses (P10 and P11) were confirmed by biochemical analyte testing. The median age of LT for subjects with PA was 1.9 years (range: 0.4– 9.4 years) with a median follow up of 5.4 years (range: 1.3 – 17.1 years) (Table 1, Figure 1).
Table 1:
Study Subjects
| Patient ID |
Genetic Testing | Current age |
Age at LT/KT |
Indication for LT |
Transplant | Complications | Explant pathology | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatic | Renal | Steatosis | Mega mitochondria |
Fibrosis | Neutrophil/ leukocyte infiltrate |
||||||
| P1 (MMA) | c.878A>C (homozygous) in MUT | 14 yr 5 m | 7 yr 2 m | Renal failure, recurrent pancreatitis | Liver, kidney | Portal vein tear requiring repair; GI bleed likely secondary to duodenal varices requiring blood transfusion | Ureteral stricture requiring stent placement × 2 and nephrostomy tube placement × 2, de novo immune complex crescentic glomerulonephritis requiring pulse steroids, massive ascites of unclear etiology with severe HTN | + (patchy, macrovesicular, mild) | - | - | + (patchy sinusoidal and central vein neutrophilic aggregates) |
| P2 (MMA) | c.454C>T, c.1792_1808+6del23 in MUT | 7 yr 10 m | 3 yr 6 m | Recurrent pancreatitis and poor metabolic control | Liver | Biliary obstruction/occlusion requiring drain placement and multiple bilateraly stent placement; episode of pseudomonas cholangitis | N/A | + (patchy, macrovesicular) | + (occasional) | - | + (scattered lobular neutrophilic inflammation) |
| P3 (MMA) | c.322C>T, c.280G>A in MUT | 18 yr 5 m | 15 yr 10 m | Renal failure and poor metabolic control | Liver, kidney | None | Delayed renal graft function requiring CRRT and then intermittent dialysis with concern for poor perfusion requiring exploratory laparotomy and renal biopsy showing focal areas of ischemic necrosis | - | + (occasional) | - | - |
| P4 (PA) | c.1891G>C, c.2119-1G>C in PCCA | 1 yr 8 m | 5 m | Recurrent hyper - ammonemia, poor metabolic control | Liver | None | N/A | - | - | - | + (focal, mild) |
| P5 (PA) | c.1218_1231delins12 (homozygous) in PCCB | 6 yr 5 m | 1 yr 9 m | Poor metabolic control | Liver | Tacrolimus-induced hepatic toxicity, multiple episodes of acute cellular rejection requiring steroid pulse, Autoimmune hepatitis requiring steroid pulse | N/A | + (patchy, periportal macrosteatosis) | - | - | + (single focus of chronic lobular portal inflammation) |
| P6 (PA) | c.386_387delTTinsA AC, c.836C>T in PCCB | 12 yr 11 m | 6 yr 9 m | Recurrent pancreatitis, poor metabolic control | Liver | Acute cellular rejection requiring steroid pulse, Alloimmune hepatitis requiring multiple steroid pulses, Early chronic rejection requiring initiation of sirolimus | N/A | + (macrovesicular) | - | + (mild, portal fibrosis) | + (mild chronic portal inflammation) |
| P7 (PA) | c.425G>A (homozygous) in PCCA | 3 yr 3 m | 1 yr 6 m | Poor metabolic control | Liver | None | N/A | - | - | - | - |
| P8 (PA) | c.1593_1595delATT, c.2041-2A>G in PCCA | 6 yr 8 m | 2 yr 1 m | Recurrent hyper ammonemia, poor metabolic control | Liver | None | N/A | + (focal microvesicular and macrovesicular steatosis) | - | - | + (focal minimal periportal chronic inflammation) |
| P9 (PA) | N/A1 | 18 yr 3 m | 1 yr 2m | Poor metabolic control | Liver | None | N/A | - | - | + (some triads have increased fibrous tissue surrounding the branch of the hepatic artery) | + (muscular wall is slightly thickened and there is chronic inflammation in the lamina propria. Portal tracts have a very mild chronic inflammatory infiltrate) |
| P10 (PA) | N/A2 | 16 yr 3 m | 2 yr 5 m | Poor metabolic control | Liver* | HAT with bile duct necrosis, recurrent bile leak with superinfected Enterobacter peritonitis, IVC stenosis requiring balloon procedure ×2 | N/A | + | - | - | + |
| P11 (PA) | N/A2 | 15 yr 9 m | 9 yr 5 m | Poor metabolic control | Liver | Portal vein stenosis at anastomosis, stable with no intervention | N/A | + (micrvesicular and macrovesicular) | - | + (portal fibrosis with incomplete portal to portal bridging) | + (mild chronic portal inflammation) |
MUT variants are provided using NM_000255.3. PCCA variants are provided using NM_000282.4. PCCB variants are provided using NM_000532.5.
Required re-transplantation due to hepatic artery stenosis
Diagnosed by enzyme testing
Diagnosed by biochemical testing
CRRT: continuous renal replacement therapy; HAT: hepatic artery stenosis; HTN: hypertension; IVC: inferior vena cava; KT: kidney transplantation; m: month; LT: liver transplantation; MMA: methylmalonic acidemia; N/A: not available for review; PA: propionic acidemia; yr: year
Figure 1:
Study subjects. A: Details about the study cohort are provided. B: Clinical characteristics of the subjects in the study are provided. MMA: methylmalonic acidemia; LT: liver transplantation, KT: kidney transplantation; PA, propionic acidemia.
Table 2:
Clinical, biochemical and diet parameters in subjects with MMA and PA pre-LT.
| P1 (MMA) | P2 (MMA) | P3 (MMA) | P4 (PA) | P5 (PA) | P6 (PA) | P7 (PA) | P8 (PA) | P9 (PA) | P10 (PA) | P11 (PA) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at initial presentation | 2 days | 3 days | N/A | 3 days | 1 day | 1 month | 3 days | 2 days | 2 days | 7 days | 7 days |
| Mean MMA (n; SD) (Normal: 0-0.5 nmol/L) | 1,776 (5; 1,243) | 305 (18; 345) | 280 (16; 402) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Mean C3 (n; SD) (Normal: 0-870 nmol/L) | N/A | 68,309 (3; 16,613) | 21,229 (6; 16,342) | N/A | 35,727 (1; N/A) | 12,156 (5; 3,972) | 60,498 (4; 16,683) | 33,415 (1; N/A) | N/A | 41,855 (5; 12,603) | 91,337 (1; N/A) |
| Mean Glycine (n; SD) (Normal: 92-346 μmol/L) | 303 (6; 126) | 400 (27; 137) | 692 (32; 295) | 386 (1; N/A) | 800 (13; 266) | 500 (23; 194) | 803 (6; 324) | N/A | 788 (2; 100) | 901 (15; 277) | 454 (1; N/A) |
| EKG | T wave inversion in lateral leads, prolonged QTc | Normal | T wave inversion, borderline prolonged QTc | Normal | Non-specific T wave abnormality | Borderline QTc prolongation, nonspecific T wave abnormality, history of atrial and ventricular escape beats | Normal | Normal | N/A | Normal | N/A |
| Echocardiogram | Trivial TR and MR, moderate concentric LVH | Hyperdynamic systolic function, reduced LV end diastolic dimension | Mild LVH | Mild dilation of pulmonary valve and main pulmonary artery, fenestrated secundum ASD | Small PFO | Normal | Normal | Normal | Small PFO | Normal | Normal |
| Brain MRI | Cerebral volume loss, focal gliosis or hypomyelination of left frontal and right parietal subcortical white matter (4 m pre-LT) | N/A | N/A | N/A | N/A | Delayed myelination, diffusion restriction in globus pallidus, anteroinferior thalami and upper midbrain;T2 hyperintensity of left frontal lobe, and prominent CSF spaces (7 yrs pre-LT) | Brain parenchymal insult in subcortical cerebral white matter and globi pallidi,. Patchy restricted diffusion in cerebral cortex. Abnormal T2 hyperintense signal in the striatum, cerebral peduncles, periventricular white matter, and the central brainstem. Increased cerebral volume loss, now moderate in severity. | Metabolic stroke of the basal ganglia | Bilateral basal ganglial and splenium signal abnormality. The white matter appears generally hyperintense on T2 weighted imaging. The extra-axial spaces are generous | N/A | Diffusion abnormality in bilateral thalami and basal ganglia |
| Protein intake, g/kg/day (% of DRI) | 1.1 (115%) | 1.1 (100%) | 2.0 (168%) | 2.3 (150%) | 1.2 (112%) | 1.0 (105%) | 0.8 (73%) | 1.2 (112%) | 1.9 (127%) | 1.2 (109%) | 1.0 (105%) |
| Dietary protein source, percentage of intake [Intact Protein vs Medical Food (MF)] | 75% Intact 25% MF | 70% Intact 30% MF | 30% Intact 70% MF | 36% Intact 64% MF | 60% Intact 40% MF | 60% Intact 40% MF | 70% Intact 30% MF | 70% Intact 30% MF | 65% Intact 35% MF | 50% Intact 50% MF | 68% Intact 32% MF |
ASD: atrial septal defect; C3: propionyl carnitine; CSF: cerebrospinal fluid; DRI: Dietary Reference Intake; LT: liver transplantation; LV: left ventricle; LVH: left ventricular hypertrophy; MF: medical food; MMA: methylmalonic acidemia or methylmalonic acid; MR: mitral regurgitation; N/A: not available for review; PA: propionic acidemia; PFO: patent foramen ovale; TR: tricuspid regurgitation, yrs: years.
3.2. Survival Rate and LT Course
All subjects had deceased donor orthotopic LT or simultaneous deceased donor orthotopic LT/KT. In our cohort, the survival rate at 1 year and 5 years post-LT was 100%. The follow up time ranges from 1.3 to 17.1 years post-LT. Graft survival rate in our cohort was 90.9% at 1 year post-LT as one individual required re-transplantation within 6 months (P10) due to hepatic artery thrombosis. No additional grafts were lost during the follow up period (Table 1). In addition to the hepatic artery thrombosis in P10, other hepatic complications included portal vein and inferior vena cava stenosis requiring dilatation 9 years after re-transplantation in the same subject, portal vein tear requiring repair in P1, and biliary stricture post-LT requiring multiple stent placements in P2. Two individuals had moderate to severe acute rejection of the transplanted liver which was managed medically. One subject with MMA (P1) had renal complications, including ureteral stricture, de novo immune complex crescentic glomerulonephritis, and ascites treated with regular pulse steroid post-transplantation. Another subject with MMA (P3) had delayed renal graft function and required dialysis for 1.5 months before making recovery. Post-LT complications are summarized in Table 1. Histopathological signs of chronic liver disease, such as macrovesicular and microvesicular steatosis, megamitochondria, fibrosis and inflammation (neutrophilic infiltrates) were noted in the explanted livers from several subjects (Table 1).
3.3. Hospitalizations and Clinical Features
3.3.1. Hospitalizations
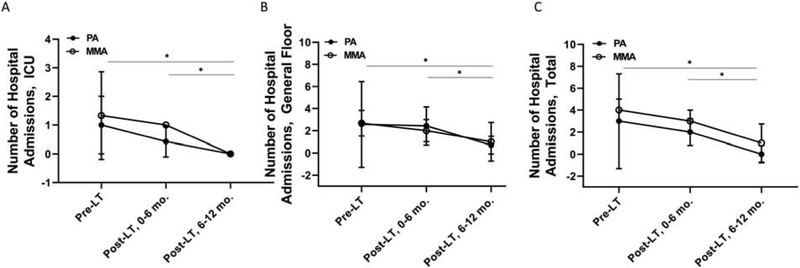
We assessed the total number of hospitalizations and the number of hospitalizations to the general floor and intensive care unit (ICU) in the 6 months pre-LT, 0-6 months post-LT, and 6-12 months post-LT (Figure 2). We only evaluated the number of hospitalizations in the six months prior to LT because we did not have complete records for all hospitalizations at other centers prior to the 6 months pre-LT. The hospitalization during which the LT or LT/KT was planned or performed was not included in the statistical analyses. No statistically significant differences in the number of hospitalizations to the general floor, ICU or in total were detected pre-LT versus 0-6 months post-LT (Figure 2). However, there were significant reductions in the number of hospitalizations to the general floor, ICU and in total when we compared the six-month period pre-LT versus 6-12 months post-LT. Moreover, similar results were obtained when we compared hospitalizations 0-6 months post-LT versus 6-12 months post-LT.
Figure 2:
Reductions in the number of hospitalizations in subjects with MMA and PA post-LT. Samples sizes included 3 subjects with MMA and 7 subjects with PA. Values are means ± SD. Statistical analysis included repeated measures ANOVA with main effects for disease (MMA or PA), time (pre-LT, 0-6 months post-LT, 6-12 months post-LT) and disease × time interaction. A: Subjects with MMA and PA had significantly fewer hospital admissions to the ICU pre-LT or 0-6 months post-LT versus 6-12 months post-LT (disease, p=0.29; time, p=0.02; disease × time, p=0.60). B. Subjects with MMA and PA had significantly fewer hospital admissions to the general floor pre-LT or 0-6 months post-LT versus 6-12 months post-LT (disease, p=0.67; time, p=0.04; disease × time, p=0.75). C. Subjects with MMA and PA had significantly fewer total hospital admissions pre-LT or 0-6 months post-LT versus 6-12 months post-LT (disease, p=0.52; time, p=0.003; disease × time, p=0.81). ICU: intensive care unit; LT: liver transplantation; MMA: methylmalonic acidemia; mo.: months; PA, propionic acidemia.
3.3.2. Hyperammonemia and Pancreatitis
Review of available records revealed that one subject with MMA (P2) had four episodes of hyperammonemia > 100 μmol/L prior to LT. None of the subjects with MMA had any known recurrence of hyperammonemia (> 100 μmol/L) in the post-LT period. P1 had one episode of pancreatitis at our center, and P2 had eleven episodes of pancreatitis recorded in the medical record before LT. After LT, P1 had two episodes of pancreatitis, which were attributed to a diagnosis of hypertriglyceridemia in the post LT period with exome analysis failing to identify a genetic etiology. No episodes of pancreatitis were noted in the other two subjects with MMA after LT (Table 2 & 3).
Table 3:
Clinical, biochemical and diet parameters post-LT
| P1 (MMA) | P2 (MMA) | P3 (MMA) | P4 (PA) | P5 (PA) | P6 (PA) | P7 (PA) | P8 (PA) | P9 (PA) | P10 (PA) | P11 (PA) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean MMA (n; SD) (Normal: 0-0.5 nmol/L) | 184 (24; 100) | 41 (4; 23) | 174 (3; 74) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Mean C3 (n; SD) (Normal: 0-870 nmol/L) | 58,203 (3; 16,582) | 66,729 (1; N/A) | 128,115 (2; 21,481) | 26,812 (2; 22,402) | 32,805 (7; 9,918) | 57,617 (10; 25,349) | 37,580 (2; 9,962) | 54,680 (1; N/A) | 19,298 (14; 16,473) | 32,581 (8; 23,331) | 45,862 (1; N/A) |
| Mean Glycine (n; SD) (Normal: 92-346 μmol/L) | 381 (7; 81) | 410 (7; 55) | 352 (2; 135) | 304 (5; 53) | 390 (8; 78) | 413 (5; 147) | 346 (2; 2) | N/A | 325 (8; 162) | 420 (19; 129) | 196 (1; N/A) |
| EKG | Non-specific ST and T wave abnormality | Normal | Borderline prolonged QTc | Normal | Normal | R axis deviation, nonspecific T wave abnormality | N/A | N/A | N/A | Normal | T-wave inversion |
| Echocardio gram | Trivial TR, MR, AR, and PI. Mild to moderate concentric LVH | Normal | Normal | N/A | Normal | Normal | Normal | N/A | Normal | Normal | N/A |
| Brain MRI (Imaging time line) | Diffuse parenchymal volume loss, focal gliosis in subcortical white matter from prior insult, stable corpus callosum changes (6 yrs post-LT) | Small bilateral and symmetric areas of abnormal signal in anterior globus pallidus, mildly prominent ventricles and sulci consistent with mild parenchymal volume loss (4 yrs post-LT) | Symmetric abnormal signal and volume loss of bilateral globi pallidi with focal white matter signal abnormality, also with signal abnormality of mid-pons and parenchymal volume loss (1m post-LT) | N/A | N/A | Mild diffusion restriction in anterior and medial putamina bilaterally, mild increased FLAIR signal in the frontal and parietal white matter bilaterally (3 yrs post-LT) | N/A | N/A | Stable symmetric increased t2 weighted signal in the anterior putamina bilaterally (2 yrs post-LT) | Persistent evidence for gliotic change affecting the bilateral lentiform nuclei, bilateral thalami and subcortical white matter of both prefrontal lobes. T2 hyperintensity returned from the bilateral posterior centrum semiovale (12 yrs post-LT) | N/A |
| Protein intake, g/kg/day (% of DRI) | 1.0 (105%) | 1.0 (105%) | Low protein diet | 1.4 (127%) | 1.0 (100%) | 0.7 (79%) | 0.9 (78%) | 2.8 (294%) | Un-restricted | Un-restricted | Un-restricted |
| Dietary Protein Source, Percentage of Intake [Intact Protein vs Medical Food (MF)] | 100% Intact | 100% Intact | 100% Intact | 81% Intact 19% MF | 100% Intact | 100% Intact | 100% Intact | 100% Intact | 100% Intact | 100% Intact | 100% Intact |
a Developed de novo immune complex crescentic glomerulonephritis requiring regular pulse steroid treatment, Had complication post LT, *a Had biliary stenosis post LT requiring ERCP and multiple stent placements, *b Required re-transplant due to hepatic artery thrombosis and IVC dilatation for IVC/Portal vein stenosis, **Diagnosed with moderate rejection acute post LT requiring periodic liver biopsy. AR: aortic regurgitation; DRI, Dietary Reference Intake; LT: liver transplantation; LVH: left ventricular hypertrophy; MF, medical food; MMA: methylmalonic acidemia or methylmalonic acid; MF: medical food; MR: mitral regurgitation; N/A: not available for review; PA: propionic acidemia; PI: pulmonary insufficiency; yrs: years
Prior to LT, five subjects with PA had multiple hyperammonemic episodes (1-9 episodes per subject with ammonia > 100 μmol/L) that required hospitalization. There were no known episodes of hyperammonemia (> 100 μmol/L) in the PA subjects after transplantation. However, one subject (P10), who was consuming an unrestricted protein diet, had one episode of elevated ammonia with a peak ammonia of 94 μmol/L 13 years after LT. He had no infections, no evidence of graft dysfunction, and no evidence of a portosystemic shunt contributing to the episode. The increased protein intake was suspected to be the cause for this episode, and thus, a low protein diet was instituted (Table 2 & 3). No subjects with PA had documented episodes of pancreatitis with lipase ≥ 480 U/L in the pre-LT period or post-LT period.
3.3.3. Cardiac Manifestations
To evaluate the prevalence of cardiac manifestations in our subjects with MMA and PA, we compared the available echocardiograms and electrocardiograms (EKG) data obtained pre- and post-LT, if available (Table 2 & 3). Two of the three subjects with MMA (P1 and P3) had concentric left ventricular hypertrophy (LVH) detected by echocardiogram before LT that resolved by one-year post-LT only in P3. Both P1 and P3 had hypertension due to her underlying chronic renal disease pre-LT. However, P1 and P3 had persistence of hypertension at the time of the post-LT/KT echocardiogram. One of three subjects with MMA (P2) demonstrated decreased left ventricular diastolic volume, which normalized within one year post-LT. The same subjects with MMA who had LVH (P1 and P3) had prolonged QTc interval and T wave inversions suggestive of increased risk of arrhythmias, which normalized post-LT/KT based on the EKG. Interestingly, none of the subjects with PA had evidence of cardiomyopathy or prolonged QTc interval prior to LT. One subject with PA (P11) had an EKG showing T wave inversion, which normalized post-LT. No abnormal findings on EKG or echocardiogram post-LT were identified in any of our subjects who had a normal pre-LT EKG or echocardiogram.
3.3.4. Neurological Manifestations:
One subject with MMA (P3) had one episode of metabolic stroke pre-LT, which resulted in loss of developmental milestones. P3 had regression of motor skills following an episode of altered mental status at the age of 5 years during which, bilateral symmetric hypodensities in the globus pallidi with a nonspecific hypodense ischemic insult in the right frontal corona radiata white matter were reported on computed tomography scan of the brain. None of the subjects with MMA had any known metabolic strokes post-LT. Compared to the brain MRI done 3 months prior to LT, P1 had new areas of focal gliosis and hypomyelination on brain MRI performed 6 years after LT. T2 hyperintensity and volume loss in bilateral globus pallidi were found in P2 and P3 on imaging done 4 years and 1 month post-LT respectively; however, MRI data for these subjects prior to LT was not available for comparison.
Two subjects with PA (P7 and P8) had one episode of metabolic stroke reported in the medical record prior to LT. P7 and P8 had their clinical care at an outside hospital at the time of the stroke and hence imaging was not available for review. However, the medical records state that there was regression of developmental skills and hypotonia associated with the stroke. None of the subjects with PA had any known episodes of stroke post-LT. Two of eight subjects with PA (P6 and P9) had MRI scans of the brain both pre and post-LT. Compared to the acute changes such as diffusion restriction in globus pallidus and thalamus seen on imaging 7 years pre-LT, P6 showed areas of T2 hyperintensity in bilateral parietal and frontal lobes, putamen and cerebellar volume loss on MRI done 2 years post-LT. P9 had a completely normal imaging 2 years pre-LT but follow up imaging 2 years post-LT showed T2 hyper intensities in putamen.
3.3.5. Development
Formal developmental assessment data was not available for all subjects. However, information about developmental milestones for each subject was collected from clinic notes. Despite the existing developmental delays, all of our subjects continued to attain new milestones post-LT. Developmental regression or stagnation was not detected in the post-LT period. Moreover, P4 who had LT at the age of 5 months continues to meet developmental milestones appropriate for her age without any concerns for developmental delay. P6 and P10 were diagnosed with autism spectrum disorder and attention deficit hyperactivity disorder post-LT in their teenage years through neuropsychology evaluation.
3.4. Dietary Intake and Growth
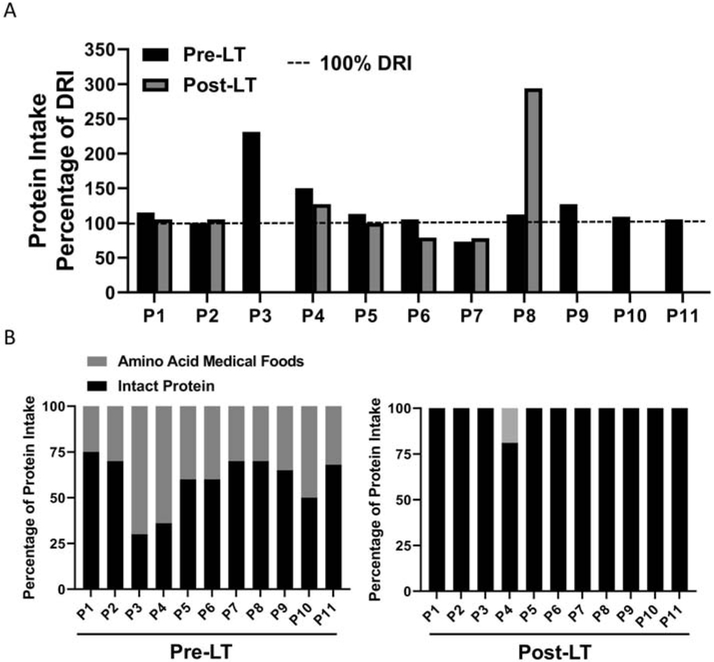
To assess for differences in dietary management as related to clinical practice in the subjects with MMA and PA pre- and post-LT, we assessed total dietary protein intake as a percentage of the Dietary Reference Intake (DRI) and percentage of prescribed dietary protein intake from intact protein versus amino acid medical foods (Tables 2-3, Figure 3). Dietary protein intake as a percentage of the DRI was 100% in 10 of 11 subjects pre-LT. Five of 7 subjects obtained 100% of the DRI for dietary protein post-LT. Four subjects (P3, P9, P10, P11) reported that they followed an unrestricted diet post-LT; therefore, information regarding dietary protein intake as a percentage of the DRI was not obtained. Medical records indicated that the transition to an unrestricted diet for these four subjects was against medical advice, but it is possible that these subjects were self-restricting their dietary protein intake. As described previously, dietary protein restriction was initiated for P10 after the occurrence of an elevated ammonia level on an unrestricted diet. All subjects with MMA and PA were prescribed a percentage of protein intake from intact protein and amino acid medical foods pre-LT; however, 10 of 11 subjects were prescribed 100% of dietary protein intake from intact protein post-LT.
Figure 3:
Dietary protein intake patterns in subjects with MMA and PA pre- and post-LT. A: Ten of 11 subjects consumed 100% or more of the DRI for dietary protein pre-LT, whereas 5 of 7 subjects consumed 100% or more of the DRI for dietary protein post-LT. Protein intake data as a percentage of the DRI were missing for 4 of 11 subjects during the post-LT period because these subjects were following an unrestricted diet (n=1) or did not document their dietary protein intake (n=3). B: Subjects with MMA and PA ingested a combination of dietary protein from intact sources and amino acid medical foods pre-LT. Ten of 11 subjects transitioned to consume all dietary protein from intact sources post-LT. P3 underwent hemodialysis during the pre-LT period, which may have impacted dietary protein intake patterns. DRI: Dietary Reference Intake; LT: liver transplantation; MMA: methylmalonic acidemia; PA, propionic acidemia.
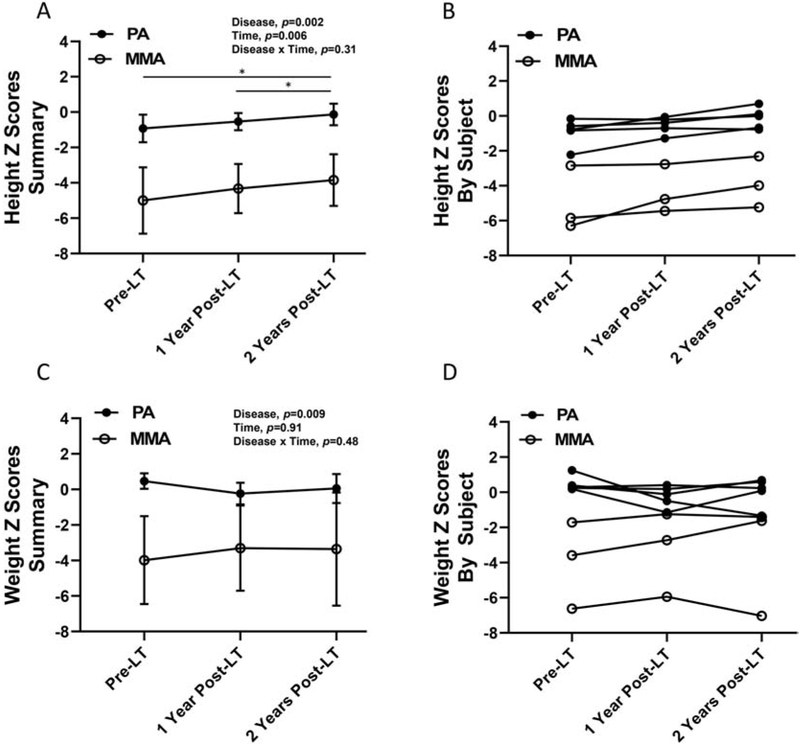
Growth parameters, height and weight Z scores, were collected pre-LT, 1 year post-LT, and 2 years post-LT (Figure 4). Importantly, subjects with PA and MMA had significant increases in height Z scores pre-LT versus 2 years post-LT and 1 year post-LT versus 2 years post-LT. Regardless of the time point, subjects with PA had significantly higher height and weight Z scores than subjects with MMA. No significant differences were noted in weight Z scores in subjects with MMA and PA pre- versus post-LT. In addition, height and weight Z scores from the most recent clinical visit (if beyond 2 years post-LT) were compared with pre-LT values (Supplemental Figure 1). Consistent with the previous observations, subjects with MMA and PA demonstrated a trend suggesting an increase in height Z scores (height Z scores, means±SD; pre-LT, −2.0±2.1; post-LT, −1.1±1.5; p=0.06), whereas no significant differences were observed in weight Z scores.
Figure 4:
Height and weight Z scores pre- and post-LT. Samples sizes included 3 subjects with MMA and 5 subjects with PA. Values are means ± SD. Statistical analysis included repeated measures ANOVA with main effects for disease (MMA or PA), time (pre-LT, 1 year post-LT, 2 years post-LT) and disease × time interaction. A. Subjects with PA had significant higher height Z scores compared to subjects with PA (disease, p=0.002). Subjects with MMA and PA had significantly greater height Z scores pre-LT or 1 year post-LT versus 2 years post-LT (time, p=0.006). There was not a significant interaction (disease × time, p=0.31). B. Height Z scores pre-LT, 1 year post-LT and 2 year post-LT are shown by subject. C. Subjects with PA had significant higher weight Z scores compared to subjects with PA (disease, p=0.0099). Weight Z scores were similar pre-LT, 1 year post-LT and 2 years post-LT in subjects with MMA and PA (time, p=0.91). There was not a significant interaction (disease × time, p=0.48). B. Height Z scores pre-LT, 1 year post-LT and 2 years post-LT are shown by subject. LT: liver transplantation; MMA: methylmalonic acidemia; PA, propionic acidemia.
3.5. Biochemical Measurements in Plasma
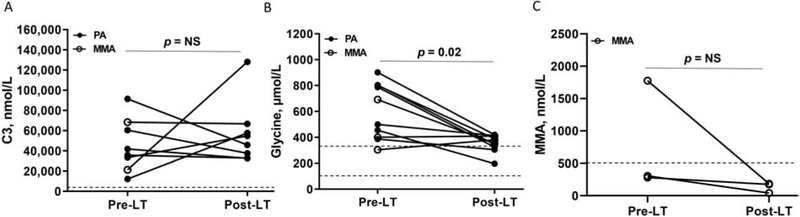
To evaluate whether there were changes in biochemical parameters after LT, we compared plasma C3, glycine and methylmalonic acid levels pre- and post-LT. When compared to pre-LT values, no significant difference in C3 values was detected post-transplantation. However, plasma glycine concentrations significantly decreased after LT or LT/KT in subjects with MMA and PA. Plasma methylmalonic acid levels were not significantly different after transplantation in subjects with MMA (Supplemental Table 1 & 2, Figure 5). However, some of the pre-LT and post-LT laboratory measures for one subject (P3) may be confounded by hemodialysis before and after the LT.
Figure 5:
Plasma laboratory measures obtained pre- and post-LT in subjects with MMA and PA. Statistical analysis for Fig. 4A-B included a two-way ANOVA utilizing a completely randomized design with crossed data to assess main effects for disease (MMA or PA), treatment (pre-LT vs post-LT) and the disease × treatment interaction. Statistical analysis for Fig. 4C included a one-way ANOVA utilizing completely randomized design with crossed data to assess main effects for treatment (pre-LT vs post-LT). Both models included random effects for the subject. Results show the pre-LT laboratory measure and corresponding post-LT laboratory measure for each subject with PA (black circle) or MMA (white circle). A. Plasma C3 concentrations were similar in subjects with MMA and PA pre- and post-LT (disease, p=0.34; treatment, p=0.16; disease × treatment, p=0.19, n=2 MMA/6 PA). B. Plasma glycine concentrations significantly reduced post-LT in subjects with MMA and PA (disease, p=0.44; treatment, p=0.02; disease × treatment, p=0.053; n=3 MMA/7 PA). C. Plasma MMA concentrations were similar in subjects with MMA pre- and post-LT (treatment, p=0.36; n=3). Dotted lines indicate the reference ranges for the plasma laboratory measures, which included the following: C3, 0-870 nmol/L; glycine, 90-346 μmol/L; MMA, 0-500 nmol/L. C3: propionyl carnitine; LT: liver transplantation; MMA: methylmalonic acidemia or methylmalonic acid; NS, non-significant; PA: propionic acidemia.
3.6. Medication Use
All subjects were prescribed L-carnitine before and after LT. One subject with PA (P4) received daily carglumic acid before LT, and this medication was discontinued after LT. Three subjects with PA used nitrogen scavenging agents (sodium benzoate, n=2; sodium phenylbutyrate, n=1) before LT, which were discontinued after LT. One subject with MMA (P2) and 4 subjects with PA (P5, P9, P10 and P11) used metronidazole before LT, and this medication was discontinued after LT. One subject with MMA (P1) continued to use fenofibrate for treatment of hypertriglyceridemia of unknown etiology. For immunosuppression after LT, all of the eleven subjects were on tacrolimus or sirolimus. Four subjects (1 of 3 subjects with MMA, 3 of 8 subjects with PA) used mycophenolate, in addition to tacrolimus, for immunosuppression after LT. Secondary complications due to the medications post-LT included steroid-induced hyperglycemia requiring insulin therapy in one subject with MMA (P1) and one subject with PA (P5). P1 (subject with MMA, a liver-kidney recipient) continued insulin therapy in combination with steroid therapy due to complications from glomerulonephritis. In contrast, hyperglycemia was transient in P5 (subject with PA) and thus, did not require insulin therapy. P1 is on labetalol, lisinopril and clonidine patch for the hypertension related to the post-transplant complication glomerulonephritis and ascites. Similarly, P3 is on amlodipine for the hypertension related to delayed renal graft function post-LT.
4. Discussion
Despite early diagnosis and medical management, individuals with PA and MMA are prone to recurrent hospitalizations for metabolic acidosis and hyperammonemia. LT has been proposed as a treatment modality to reduce metabolic decompensations associated with PA and MMA [17][30]. In this large, single center study of LT and LT/KT in MMA and PA, we investigated whether LT led to improved outcomes, such as reduction in hospitalizations and chronic complications, improved biochemical parameters, and improved growth outcomes.
According to the Studies in Pediatric Liver Transplantation registry , 14.9% of pediatric LT occurs in individuals with metabolic disorders [31]. Data from the United Network for Organ Sharing (UNOS) show that only 4% of individuals with OA received living donor LT [32]. Likewise, in our cohort, all subjects had cadaveric LT. However, there are multiple reports of heterozygous living related donor LT in PA and MMA [33][34][24].
Historically, the survival rate after LT in PA and MMA was between 50-75% [35][36] [37]. The one year and five year patient survival rate in our cohort was 100%, which is consistent with the improved outcomes from the more recent publications [18][23][19][25]. The 1 year graft survival rate was 90.9% in our series due to hepatic artery thrombosis in one subject requiring re-transplantation. Hepatic artery thrombosis has been documented as a frequent complication in pediatric LT recipients [38]. Following LT in PA and MMA, several studies have suggested that hepatic artery thrombosis may be common, although a recent study has reported no difference in the incidence of this complication in individuals with PA versus individuals without PA ([30][25][39]. We also report biliary stricture in one subject who required multiple stent placements.
As previously reported (Supplemental Tables 1 & 2), improved metabolic control was noted in our cohort with the absence of hyperammonemia post-LT compared to the pre-LT period except for the single episode of mild elevation in ammonia in one subject consuming an unrestricted diet. Similarly, there was a cessation of the occurrence of pancreatitis in most of our subjects. However, isolated case reports describe the presence of metabolic decompensation even after LT indicating the need for careful monitoring during periods of stress [40][33][29][27][41][23]. Metabolic stroke is a well known complication of PA and MMA [42]. In comparison to ischemic or hemorragic stroke, metabolic stroke is not associated with any large vessel occlusion or rupture. Underlying mitochondrial dysfunction, neuronal swelling due to toxic byproducts, and susceptibility of basal ganglia to decreased perfusion have all been suspected to contribute to metabolic strokes in this population [43]. Although the subjects in our cohort had no episodes of metabolic stroke during the post-LT period, previous reports have described metabolic stroke in the post-LT period in more individuals with MMA than PA [26][44][21].
Metabolic decompensations led to frequent hospitalizations for several of our subjects pre-LT. In the immediate post-LT period, there was no significant reduction in number of hospitalizations. However, there was a significant reduction in the number of hospitalizations in months 6 - 12 post-LT. Further analysis of the data beyond one year post-LT will determine whether this trend persists, and if so, this finding would support the findings of Li et al suggesting that LT is cost-effective [45].
Cardiomyopathy and prolonged QTc interval are two cardiac complications observed in PA [14]. The incidence of cardiomyopathy in PA has been estimated to be approximately 9-23% [3]. Reversal of cardiomyopathy in individuals with PA after LT has been previously reported [35][8][30][46][18] (Supplemental table 1). Similarly prolonged QTc interval with normalization post-LT has been previously reported in individuals with PA [47][48][9]. Although cardiomyopathy is rare in patients with MMA, it has been previously reported. [49][50] [46]. Interestingly, in two of three (66%) subjects with MMA, left ventricular hypertrophy and prolonged QTc were noted pre-LT/KT but these resolved post-LT/KT in one subject. It is difficult to assess the extent in which underlying hypertension from chronic kidney disease contributed to the LVH and prolonged QTc especially given the fact that the high blood pressure persisted even at the time of post-LT echocardiogram (Table 1 & 2).
Neuroimaging findings in PA and MMA are non-specific for the disorder and could be classified into acute and chronic changes. During acute decompensation, hallmarks of decreased perfusion such as diffusion restriction and hyperintensity on T2 and FLAIR sequences are usually seen in the basal ganglia [51] [52]. In the chronic phase, delayed myelination, cortical and white matter atrophy and gliosis characterized by hyperintensity on T2 and FLAIR sequences are seen [51][52]. Nagao et al reported improvement in brain volume and myelination after LT in a child with PA [24]. In our cohort, subjects had persistence of signal abnormalities suggestive of gliosis and volume loss in the post-LT brain imaging. New or acute findings were not seen in these subjects post-LT again suggesting absence of metabolic decompensations or strokes in the post-LT period. Behavioral abnormalities including autism spectrum disorder have been previously reported in individuals with PA [53][54]. Interestingly, despite having LT at 2.5 years of age, P10 was diagnosed with autism spectrum disorder at 10 years of age. P4, the youngest subject in our cohort to receive LT, has continued to meet all developmental milestones at the expected age despite four hyperammonemic episodes within the first 5 months of life. This finding may suggest that earlier LT may lead to improved outcome but larger studies with longer-term follow-up are needed.
Diet management post-LT varies from dietary protein restriction [19] to increased protein intake with avoidance of high protein foods [19][18][40], normalization of protein intake [55] , and even unrestricted diets with the caveat that subjects may self-select low protein foods [21] (Supplemental table 1 & 2). Diet liberalization in some cases was based on monitoring of propionate metabolite levels [25]. Metabolic decompensations post-LT are rare even with liberalization of diet in previous studies (Supplemental Tables 1 & 2) with one episode of metabolic decompensation reported in one subject 3 years post-LT [40]. Significant improvement in height has been documented when LT was done at a younger age (Supplemental table 1 & 2) [40][23]. However Yorifugi et al. reported no improvements in growth in the first 2 years post-LT due to steroid therapy [40]. In comparison, our subjects had improvements in growth 2 years post-LT with a similar trend at most recent follow up post-LT.
Limitations of our study include the retrospective nature, small sample size and variable length of follow-up post-LT. In addition, since some of our subjects were followed at outside institutions prior to the pre-transplantation evaluation, records for some subjects were incomplete in the pre-LT period. Because of the incomplete medical records, our analysis of hospitalizations was limited to the six months before and twelve months after LT. Of note, lengthy hospitalizations may be a confounder for the analysis of number of hospitalizations especially in the six months immediately following LT. Because of relocation, records for one subject were limited beyond one year post-LT.
In conclusion, in a large, single center study of PA and MMA post-LT, we report 100% patient survival rate in our subjects with PA and MMA who underwent LT or LT/KT. Our study shows that LT or LT/KT was associated with a reduction in the incidence of hyperammonemia and pancreatitis in most subjects, improved linear growth parameters, and reduction in the number of hospitalizations. Even with significant reduction in plasma glycine levels, absence of significant reduction in other biochemical parameters including C3 denote that transplantation does not result in metabolic cure in individuals with PA and MMA. This is not surprising in that other tissues such as skeletal muscle continue to provide a source for circulating propionic acid.
Supplementary Material
Supplemental Figure 1: Height and weight Z scores in subjects with MMA and PA pre-LT and from a recent clinical visit post-LT. Sample sizes included 2 subjects with MMA and 5 subjects with PA. Statistical analysis included paired t-tests. A. Subjects with MMA and PA demonstrated a non-significant increase in height Z scores at a recent clinical visit post-LT versus pre-LT (p=0.06). B. Weight Z scores in subjects with MMA and PA were similar pre-LT and at a recent clinical visit post-LT (p=0.65). LT: liver transplantation; MMA: methylmalonic acidemia or methylmalonic acid; NS, non-significant; PA: propionic acidemia.
Acknowledgments
Funding: NRP is supported by Sanofi Genzyme ACMGF Next Generation Training Award. LCB is supported by NIH K08DK106453 and holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund. BMS is supported by NIH T32DK007664-28 (BS) and the US Public Health Service grant P30DK56338, which funds the Texas Medical Center Digestive Disease Center. BJS is supported by the Takeda Next Generation Medical Biochemical Subspecialty Fellowship.
Abbreviations
- C0
Free carnitine
- C2
Acetylcarnitine
- C3
Propionylcarnitine
- DRI
Dietary reference intake
- EKG
Electrocardiogram
- ICU
Intensive care unit
- KT
Kidney transplantation
- LT
Liver transplantation
- LVH
Left ventricular hypertrophy
- MMA
Methylmalonic acidemia
- OA
Organic acidemia
- PA
Propionic acidemia
Footnotes
Ethical Approval: This study was approved by the Institutional Review Board at Baylor College of Medicine under Protocol number: H-39130
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Chace DH, DiPerna JC, Kalas TA, Johnson RW, Naylor EW, Rapid diagnosis of methylmalonic and propionic acidemias: Quantitative tandem mass spectrometric analysis of propionylcarnitine in filter-paper blood specimens obtained from newborns, Clin. Chem 47 (2001) 2040–2044. [PubMed] [Google Scholar]
- [2].Couce ML, Castiñeiras DE, Bóveda MD, Baña A, Cocho JA, Iglesias AJ, Colón C, Alonso-Fernández JR, Fraga JM, Evaluation and long-term follow-up of infants with inborn errors of metabolism identified in an expanded screening programme, Mol. Genet. Metab 104 (2011) 470–475. 10.1016/j.ymgme.2011.09.021. [DOI] [PubMed] [Google Scholar]
- [3].Baumgartner MR, Hörster F, Dionisi-Vici C, Haliloglu G, Karall D, Chapman KA, Huemer M, Hochuli M, Assoun M, Ballhausen D, Burlina A, Fowler B, Grünert SC, Grünewald S, Honzik T, Merinero B, Pérez-Cerdá C, Scholl-Bürgi S, Skovby F, Wijburg F, MacDonald A, Martinelli D, Sass JO, Valayannopoulos V, Chakrapani A, Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia, Orphanet J. Rare Dis 9 (2014). 10.1186/s13023-014-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chapman KA, Gropman A, MacLeod E, Stagni K, Summar ML, Ueda K, Mew NA, Franks J, Island E, Matern D, Pena L, Smith B, Sutton VR, Urv T, Venditti C, Chakrapani A, Acute management of propionic acidemia, in: Mol. Genet. Metab, 2012: pp. 16–25. 10.1016/j.ymgme.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schwahn BC, Pieterse L, Bisset WM, Galloway PG, Robinson PH, Biochemical efficacy of N-carbamylglutamate in neonatal severe hyperammonaemia due to propionic acidaemia, Eur. J. Pediatr 169 (2010) 133–134. 10.1007/s00431-009-1036-7. [DOI] [PubMed] [Google Scholar]
- [6].Filippi L, Gozzini E, Fiorini P, Malvagia S, La Marca G, Donati MA, N-carbamylglutamate in emergency management of hyperammonemia in neonatal acute onset propionic and methylmalonic aciduria, Neonatology. 97 (2010) 286–290. 10.1159/000255168. [DOI] [PubMed] [Google Scholar]
- [7].Grünert SC, Müllerleile S, De Silva L, Barth M, Walter M, Walter K, Meissner T, Lindner M, Ensenauer R, Santer R, Bodamer OA, Baumgartner MR, Brunner-Krainz M, Karall D, Haase C, Knerr I, Marquardt T, Hennermann JB, Steinfeld R, Beblo S, Koch HG, Konstantopoulou V, Scholl-Bürgi S, Van Teeffelen-Heithoff A, Suormala T, Sperl W, Kraus JP, Superti-Furga A, Schwab KO, Sass JO, Propionic acidemia: Neonatal versus selective metabolic screening, J. Inherit. Metab. Dis 35 (2012) 41–49. 10.1007/s10545-011-9419-0. [DOI] [PubMed] [Google Scholar]
- [8].Romano S, Valayannopoulos V, Touati G, Jais JP, Rabier D, de Keyzer Y, Bonnet D, de Lonlay P, Cardiomyopathies in Propionic Aciduria are Reversible After Liver Transplantation, J. Pediatr 156 (2010) 128–134. 10.1016/j.jpeds.2009.07.002. [DOI] [PubMed] [Google Scholar]
- [9].Kölker S, Valayannopoulos V, Burlina AB, Sykut-Cegielska J, Wijburg FA, Teles EL, Zeman J, Dionisi-Vici C, Barić I, Karall D, Arnoux JB, Avram P, Baumgartner MR, Blasco-Alonso J, Boy SPN, Rasmussen MB, Burgard P, Chabrol B, Chakrapani A, Chapman K, Cortès i Saladelafont E, Couce ML, de Meirleir L, Dobbelaere D, Furlan F, Gleich F, González MJ, Gradowska W, Grünewald S, Honzik T, Hörster F, Ioannou H, Jalan A, Häberle J, Haege G, Langereis E, de Lonlay P, Martinelli D, Matsumoto S, Mühlhausen C, Murphy E, de Baulny HO, Ortez C, PedrColón CC, Pintos-Morell G, Pena-Quintana L, Ramadža DP, Rodrigues E, Scholl-Bürgi S, Sokal E, Summar ML, Thompson N, Vara R, Pinera IV, Walter JH, Williams M, Lund AM, Garcia Cazorla A, The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 2: the evolving clinical phenotype, J. Inherit. Metab. Dis 38 (2015) 1059–1074. 10.1007/s10545-015-9840-x. [DOI] [PubMed] [Google Scholar]
- [10].Pena L, Burton BK, Survey of health status and complications among propionic acidemia patients, Am. J. Med. Genet. Part A 158 A (2012) 1641–1646. 10.1002/ajmg.a.35387. [DOI] [PubMed] [Google Scholar]
- [11].Kruszka PS, Manoli I, Sloan JL, Kopp JB, Venditti CP, Renal growth in isolated methylmalonic acidemia, Genet. Med 15 (2013) 990–996. 10.1038/gim.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hörster F, Baumgartner MR, Viardot C, Suormala T, Burgard P, Fowler B, Hoffmann GF, Garbade SF, Kölker S, Baumgartner ER, Long-term outcome in methylmalonic acidurias is influenced by the underlying defect (mut0, mut-, cblA, cblB), Pediatr. Res 62 (2007) 225–230. 10.1203/PDR.0b013e3180a0325f. [DOI] [PubMed] [Google Scholar]
- [13].Walter JH, Michalski A, Wilson WM, Leonard JV, Barratt TM, Dillon MJ, Chronic renal failure in methylmalonic acidaemia, Eur. J. Pediatr 148 (1989) 344–348. 10.1007/BF00444131. [DOI] [PubMed] [Google Scholar]
- [14].Sutton VR, Chapman KA, Gropman AL, MacLeod E, Stagni K, Summar ML, Ueda K, Mew NA, Franks J, Island E, Matern D, Peña L, Smith B, Urv T, Venditti C, Chakarapani A, Chronic management and health supervision of individuals with propionic acidemia, in: Mol. Genet. Metab, 2012: pp. 26–33. 10.1016/j.ymgme.2011.08.034. [DOI] [PubMed] [Google Scholar]
- [15].Fraser JL, Venditti CP, Methylmalonic and propionic acidemias: Clinical management update, Curr. Opin. Pediatr (2016). 10.1097/MOP.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Deodato F, Boenzi S, Santorelli FM, Dionisi-Vici C, Methylmalonic propionic aciduria, Am. J. Med. Genet. - Semin. Med. Genet (2006). 10.1002/ajmg.c.30090. [DOI] [PubMed] [Google Scholar]
- [17].Barshes NR, Vanatta JM, Patel AJ, Carter BA, O’Mahony CA, Karpen SJ, Goss JA, Evaluation and management of patients with propionic acidemia undergoing liver transplantation: A comprehensive review, Pediatr. Transplant 10 (2006) 773–781. 10.1111/j.1399-3046.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- [18].Silva HM, Nassogne MC, Smets F, Stéphenne X, Scheers I, Veyckemans F, Pirotte T, Bourdeaux C, Magnée CDE, Reding R, Sokal E, Liver Transplantation for Propionic Acidemia, J. Pediatr. Gastroenterol. Nutr 64 (2017) e73–e76. 10.1097/MPG.0000000000000626. [DOI] [PubMed] [Google Scholar]
- [19].Critelli K, McKiernan P, Vockley J, Mazariegos G, Squires RH, SLTys K, Squires JE, Liver Transplantation for Propionic Acidemia and Methylmalonic Acidemia: Perioperative Management and Clinical Outcomes, Liver Transplant. (2018). 10.1002/lt.25304. [DOI] [PubMed] [Google Scholar]
- [20].Niemi AK, Kim IK, Krueger CE, Cowan TM, Baugh N, Farrell R, Bonham CA, Concepcion W, Esquivel CO, Enns GM, Treatment of methylmalonic acidemia by liver or combined liver-kidney transplantation, J. Pediatr 166 (2015) 1455–1461.e1. 10.1016/j.jpeds.2015.01.051. [DOI] [PubMed] [Google Scholar]
- [21].Vara R, Turner C, Mundy H, Heaton ND, Rela M, Mieli-Vergani G, Champion M, Hadzic N, Liver transplantation for propionic acidemia in children, Liver Transplant. 17 (2011) 661–667. 10.1002/lt.22279. [DOI] [PubMed] [Google Scholar]
- [22].Li M, Dick A, Montenovo M, Horslen S, Hansen R, Cost-effectiveness of liver transplantation in methylmalonic and propionic acidemias, Liver Transplant. 21 (2015) 1208–1218. 10.1002/lt.24173. [DOI] [PubMed] [Google Scholar]
- [23].Sakamoto R, Nakamura K, Kido J, Matsumoto S, Mitsubuchi H, Inomata Y, Endo F, Improvement in the prognosis and development of patients with methylmalonic acidemia after living donor liver transplant, Pediatr. Transplant 20 (2016) 1081–1086. 10.1111/petr.12804. [DOI] [PubMed] [Google Scholar]
- [24].Nagao M, Tanaka T, Morii M, Wakai S, Horikawa R, Kasahara M, Improved neurologic prognosis for a patient with propionic acidemia who received early living donor liver transplantation, Mol. Genet. Metab 108 (2013) 25–29. 10.1016/j.ymgme.2012.10.022. [DOI] [PubMed] [Google Scholar]
- [25].Quintero J, Molera C, Juamperez J, Redecillas S, Meavilla S, Nunez R, Garcia C, Del Toro M, Garcia A, Ortega J, Segarra O, Martin de Carpi J, Bilbao I, Charco R, The role of liver transplantation in propionic acidemia., Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc (2018). 10.1002/lt.25344. [DOI] [PubMed] [Google Scholar]
- [26].Chakrapani A, Sivakumar P, McKiernan PJ, Leonard JV, Metabolic stroke in methylmalonic acidemia five years after liver transplantation, J. Pediatr 140 (2002) 261–263. 10.1067/mpd.2002.121698. [DOI] [PubMed] [Google Scholar]
- [27].Mc Guire PJ, Lim-Melia E, Diaz GA, Raymond K, Larkin A, Wasserstein MP, Sansaricq C, Combined liver-kidney transplant for the management of methylmalonic aciduria: A case report and review of the literature, Mol. Genet. Metab 93 (2008) 22–29. 10.1016/j.ymgme.2007.08.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nyhan WL, Gargus JJ, Boyle K, Selby R, Koch R, Progressive neurologic disability in methylmalonic acidemia despite transplantation of the liver, Eur. J. Pediatr 161 (2002) 377–379. 10.1007/s00431-002-0970-4. [DOI] [PubMed] [Google Scholar]
- [29].Kasahara M, Horikawa R, Tagawa M, Uemoto S, Yokoyama S, Shibata Y, Kawano T, Kuroda T, Honna T, Tanaka K, Saeki M, Current role of liver transplantation for methylmalonic acidemia: A review of the literature, Pediatr. Transplant 10 (2006) 943–947. 10.1111/j.1399-3046.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- [30].Charbit-Henrion F, Lacaille F, McKiernan P, Girard M, De Lonlay P, Valayannopoulos V, Ottolenghi C, Chakrapani A, Preece M, Sharif K, Chardot C, Hubert P, Dupic L, Early and late complications after liver transplantation for propionic acidemia in children: A two centers study, Am. J. Transplant 15 (2015) 786–791. 10.1111/ajt.13027. [DOI] [PubMed] [Google Scholar]
- [31].Oishi K, Arnon R, Wasserstein MP, Diaz GA, Liver transplantation for pediatric inherited metabolic disorders: Considerations for indications, complications, and perioperative management, Pediatr. Transplant (2016). 10.1111/petr.12741. [DOI] [PMC free article] [PubMed]
- [32].Perito ER, Rhee S, Roberts JP, Rosenthal P, Pediatric liver transplantation for urea cycle disorders and organic acidemias: United network for organ sharing data for 2002-2012, Liver Transplant. 20 (2014) 89–99. 10.1002/lt.23765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Morioka D, Kasahara M, Takada Y, Corrales JPG, Yoshizawa A, Sakamoto S, Taira K, Yoshitoshi EY, Egawa H, Shimada H, Tanaka K, Living donor liver transplantation for pediatric patients with inheritable metabolic disorders., Am. J. Transplant 5 (2005) 2754–2763. 10.1111/j.1600-6143.2005.01084.x. [DOI] [PubMed] [Google Scholar]
- [34].Morioka D, Kasahara M, Horikawa R, Yokoyama S, Fukuda a, Nakagawa a, Efficacy of living donor liver transplantation for patients with methylmalonic acidemia., Am. J. Transplant 7 (2007) 2782–2787. 10.1111/j.1600-6143.2007.01986.x. [DOI] [PubMed] [Google Scholar]
- [35].Saudubray JM, Touati G, Delonlay P, Jouvet P, Schlenzig J, Narcy C, Laurent J, Rabier D, Kamoun P, Jan D, Revillon Y, Liver transplantation in propionic acidaemia., Eur. J. Pediatr 158 Suppl (1999) S65–9. 10.1007/PL00014325. [DOI] [PubMed] [Google Scholar]
- [36].V Leonard J, Walter JH, McKiernan PJ, The management of organic acidaemias: The role of transplantation, J. Inherit. Metab. Dis 24 (2001) 309–311. 10.1023/A:1010395724012. [DOI] [PubMed] [Google Scholar]
- [37].van’t Hoff W, McKiernan PJ, Surtees RA, V Leonard J, Liver transplantation for methylmalonic acidaemia., Eur. J. Pediatr 158 Suppl (1999) S70–4. 10.1007/PL00014326. [DOI] [PubMed] [Google Scholar]
- [38].Hamby BA, Ramirez DE, Loss GE, Bazan HA, Smith TA, Bluth E, Sternbergh WC, Endovascular treatment of hepatic artery stenosis after liver transplantation, J. Vasc. Surg (2013). 10.1016/j.jvs.2012.10.086. [DOI] [PubMed] [Google Scholar]
- [39].Alexopoulos SP, Matsuoka L, Hafberg E, Morgan T, Thurm C, Hall M, Godown J, Liver Transplantation for Propionic Acidemia, J. Pediatr. Gastroenterol. Nutr (2019) 1 10.1097/MPG.0000000000002534. [DOI] [PubMed] [Google Scholar]
- [40].Yorifuji T, Kawai M, Mamada M, Kurokawa K, Egawa H, Shigematsu Y, Kohno Y, Tanaka K, Nakahata T, Living-donor liver transplantation for propionic acidaemia, J. Inherit. Metab. Dis 27 (2004) 205–210. 10.1023/B:BOLI.0000028778.54210.13. [DOI] [PubMed] [Google Scholar]
- [41].Baba C, Kasahara M, Kogure Y, Kasuya S, Ito S, Tamura T, Fukuda A, Horikawa R, Suzuki Y, Perioperative management of living-donor liver transplantation for methylmalonic acidemia, Paediatr. Anaesth (2016). 10.1111/pan.12930. [DOI] [PubMed] [Google Scholar]
- [42].Testai FD, Gorelick PB, Inherited Metabolic Disorders and Stroke Part 2, Arch. Neurol (2010). 10.1001/archneurol.2009.333. [DOI] [PubMed] [Google Scholar]
- [43].Zinnanti WJ, Lazovic J, Housman C, Antonetti DA, Koeller DM, Connor JR, Steinman L, Mechanism of metabolic stroke and spontaneous cerebral hemorrhage in glutaric aciduria type I, Acta Neuropathol. Commun (2014). 10.1186/2051-5960-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kaplan P, Ficicioglu C, Mazur AT, Palmieri MJ, Berry GT, Liver transplantation is not curative for methylmalonic acidopathy caused by methylmalonyl-CoA mutase deficiency, Mol. Genet. Metab 88 (2006) 322–326. 10.1016/j.ymgme.2006.04.003. [DOI] [PubMed] [Google Scholar]
- [45].Li M, Dick A, Montenovo M, Horslen S, Hansen R, Cost-effectiveness of liver transplantation in methylmalonic and propionic acidemias, Liver Transplant. (2015). 10.1002/lt.24173. [DOI] [PubMed] [Google Scholar]
- [46].Arrizza C, De Gottardi A, Foglia E, Baumgartner M, Gautschi M, Nuoffer JM, Reversal of cardiomyopathy in propionic acidemia after liver transplantation: A 10-year follow-up, Transpl. Int 28 (2015) 1447–1450. 10.1111/tri.12677. [DOI] [PubMed] [Google Scholar]
- [47].Kakavand B, Schroeder VA, Di Sessa TG, Coincidence of long QT syndrome and propionic acidemia, Pediatr Cardiol 27 (2006) 160–161. 10.1007/s00246-005-1129-7. [DOI] [PubMed] [Google Scholar]
- [48].Jameson E, Walter J, Cardiac arrest secondary to long QTC in a child with propionic acidemia, Pediatr. Cardiol 29 (2008) 969–970. 10.1007/s00246-007-9160-5. [DOI] [PubMed] [Google Scholar]
- [49].Prada CE, Al Jasmi F, Kirk EP, Hopp M, Jones O, Leslie ND, Burrow TA, Cardiac disease in methylmalonic acidemia., J. Pediatr 159 (2011) 862–4. 10.1016/j.jpeds.2011.06.005. [DOI] [PubMed] [Google Scholar]
- [50].Horster F, Garbade SF, Zwickler T, Aydin HI, Bodamer OA, Burlina AB, Das AM, De Klerk JBC, Dionisi-Vici C, Geb S, Gokcay G, Guffon N, Maier EM, Morava E, Walter JH, Schwahn B, Wijburg FA, Lindner M, Grunewald S, Baumgartner MR, Kolker S, Prediction of outcome in isolated methylmalonic acidurias: combined use of clinical and biochemical parameters., J. Inherit. Metab. Dis 32 (2009) 630 10.1007/s10545-009-1189-6. [DOI] [PubMed] [Google Scholar]
- [51].Karimzadeh P, Jafari N, Ahmad Abadi F, Jabbedari S, Taghdiri MM, Alaee MR, Ghofrani M, Tonekaboni SH, Nejad Biglari H, Propionic acidemia: Diagnosis and neuroimaging findings of this neurometabolic disorder, Iran. J. Child Neurol (2014). [PMC free article] [PubMed] [Google Scholar]
- [52].Reddy N, Calloni SF, Vernon HJ, BLTshauser E, Huisman TAGM, Soares BP, Neuroimaging Findings of Organic Acidemias and Aminoacidopathies, RadioGraphics. (2018). 10.1148/rg.2018170042. [DOI] [PubMed] [Google Scholar]
- [53].Witters P, Debbold E, Crivelly K, Vande Kerckhove K, Corthouts K, Debbold B, Andersson H, Vannieuwenborg L, Geuens S, Baumgartner M, Kozicz T, Settles L, Morava E, Autism in patients with propionic acidemia, Mol. Genet. Metab (2016). 10.1016/j.ymgme.2016.10.009. [DOI] [PubMed] [Google Scholar]
- [54].de la Bâtie CD, Barbier V, Roda C, Brassier A, Arnoux JB, Valayannopoulos V, Guemann AS, Pontoizeau C, Gobin S, Habarou F, Lacaille F, Bonnefont JP, Canouï P, Ottolenghi C, De Lonlay P, Ouss L, Autism spectrum disorders in propionic academia patients, J. Inherit. Metab. Dis (2018). 10.1007/s10545-017-0070-2. [DOI] [PubMed] [Google Scholar]
- [55].Spada M, Calvo PL, Brunati A, Peruzzi L, Dell’Olio D, Romagnoli R, Porta F, Early Liver Transplantation for Neonatal-Onset Methylmalonic Acidemia., Pediatrics. 136 (2015) e252–6. 10.1542/peds.2015-0175. [DOI] [PubMed] [Google Scholar]
- [56].Kayler LK, Merion RM, Lee S, Sung RS, Punch JD, Rudich SM, … Magee JC (2002). Long-term survival after liver transplantation in children with metabolic disorders. Pediatr Transplant, 6(4), 295–300. [DOI] [PubMed] [Google Scholar]
- [57].Rela M, Battula N, Madanur M, Mieli-Vergani G, Dhawan A, Champion M, … Heaton N (2007). Auxiliary liver transplantation for propionic acidemia: A 10-year follow-up. American Journal of Transplantation, 7(9), 2200–2203. 10.1111/j.1600-6143.2007.01899.x [DOI] [PubMed] [Google Scholar]
- [58].Sato S, Kasahara M, Fukuda A, Mizuguchi K, Nakagawa S, Muguruma T, … Horikawa R (2009). Liver transplantation in a patient with propionic acidemia requiring extra corporeal membrane oxygenation during severe metabolic decompensation. Pediatric Transplantation, 13(6), 790–793. 10.1111/j.1399-3046.2008.01029.x [DOI] [PubMed] [Google Scholar]
- [59].Davison JE, Davies NP, Wilson M, Sun Y, Chakrapani A, McKiernan PJ, … Peet AC (2011). MR spectroscopy-based brain metabolite profiling in propionic acidaemia: Metabolic changes in the basal ganglia during acute decompensation and effect of liver transplantation. Orphanet Journal of Rare Diseases, 6(1). 10.1186/1750-1172-6-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kasahara M, Sakamoto S, Kanazawa H, Karaki C, Kakiuchi T, Shigeta T, … Horikawa R (2012). Living-donor liver transplantation for propionic acidemia. Pediatric Transplantation, Vol. 16, pp. 230–234. 10.1111/j.1399-3046.2011.01607.x [DOI] [PubMed] [Google Scholar]
- [61].Ryu J, Shin YH, Ko JS, Gwak MS, & Kim GS (2013). Intractable metabolic acidosis in a child with propionic acidemia undergoing liver transplantation -a case report-. Korean Journal of Anesthesiology, 65(3), 257–261. 10.4097/kjae.2013.65.3.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Nagarajan S, Enns GM, Millan MT, Winter S, & Sarwal MM (2005). Management of methylmalonic acidaemia by combined liver - Kidney transplantation. Journal of Inherited Metabolic Disease, 28(4), 517–524. 10.1007/s10545-005-0517-8 [DOI] [PubMed] [Google Scholar]
- [63].Chen PW, Hwu WL, Ho MC, Lee NC, Chien YH, Ni YH, & Lee PH (2010). Stabilization of blood methylmalonic acid level in methylmalonic acidemia after liver transplantation. Pediatric Transplantation, 14(3), 337–341. 10.1111/j.1399-3046.2009.01227.x [DOI] [PubMed] [Google Scholar]
- [64].Kamei K, Ito S, Shigeta T, Sakamoto S, Fukuda A, Horikawa R, … Kasahara M (2011). Preoperative Dialysis for Liver Transplantation in Methylmalonic Acidemia. Therapeutic Apheresis and Dialysis. 10.1111/j.1744-9987.2011.00974.x [DOI] [PubMed] [Google Scholar]
- [65].Vernon HJ, Sperati CJ, King JD, Poretti A, Miller NR, Sloan JL, … Valle D (2014). A detailed analysis of methylmalonic acid kinetics during hemodialysis and after combined liver/kidney transplantation in a patient with mut 0 methylmalonic acidemia. Journal of Inherited Metabolic Disease, 37(6), 899–907. 10.1007/s10545-014-9730-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wilnai Y, Enns GM, Niemi AK, Higgins J, & Vogel H (2014). Abnormal hepatocellular mitochondria in methylmalonic acidemia. Ultrastructural Pathology, 38(5), 309–314. 10.3109/01913123.2014.921657 [DOI] [PubMed] [Google Scholar]
- [67].Splinter K, Niemi A-K, Cox R, Platt J, Shah M, Enns GM, … Bernstein JA (2015). Impaired Health-Related Quality of Life in Children and Families Affected by Methylmalonic Acidemia. Journal of Genetic Counseling, 936–944. 10.1007/s10897-015-9921-x [DOI] [PubMed] [Google Scholar]
- [68].Chu TH, Chien YH, Lin HY, Liao HC, Ho HJ, Lai CJ, … Niu DM (2019). Methylmalonic acidemia/propionic acidemia - The biochemical presentation and comparing the outcome between liver transplantation versus non-liver transplantation groups. Orphanet Journal of Rare Diseases, 14(1). 10.1186/s13023-019-1045-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Height and weight Z scores in subjects with MMA and PA pre-LT and from a recent clinical visit post-LT. Sample sizes included 2 subjects with MMA and 5 subjects with PA. Statistical analysis included paired t-tests. A. Subjects with MMA and PA demonstrated a non-significant increase in height Z scores at a recent clinical visit post-LT versus pre-LT (p=0.06). B. Weight Z scores in subjects with MMA and PA were similar pre-LT and at a recent clinical visit post-LT (p=0.65). LT: liver transplantation; MMA: methylmalonic acidemia or methylmalonic acid; NS, non-significant; PA: propionic acidemia.