Abstract
Background
This study aimed to investigate the expression of long noncoding RNA (lncRNA) loc285194 in cervical squamous cell carcinoma (CSCC) biopsies that were positive and negative for human papillomavirus (HPV) and in human CSCC cell lines SiHa and C33A and to investigate the overexpression of lncRNA loc285194.
Material/Methods
Cervical biopsy tissue and plasma samples from 66 patients with histologically confirmed CSCC, that were HPV16-positive (N=22), HPV18-positive (N=27), and HPV-negative (N=17), and healthy controls (N=20) and human CSCC cell lines SiHa (HPV16-positive) and C33A (HPV-negative) were studied. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was used to measure the expression of lncRNA loc285194 in cervical biopsies and plasma. Enzyme-linked immunosorbent assay (ELISA) and Western blot were used to measure levels of transforming growth factor-β1 (TGF-β1). A lncRNA loc285194 expression vector was constructed and transfected into SiHa and C33A cells that underwent a transwell assay for cell migration.
Results
Expression of lncRNA loc285194 was downregulated in HPV-positive and HPV-negative tissue samples and plasma from patients with CSCC and distinguished between patients and healthy controls. Plasma levels of loc285194 and TGF-β1 were significantly correlated with the presence of CSCC. In SiHa and C33A cells, TGF-β1 expression was downregulated, and cell migration was inhibited following lncRNA loc285194 overexpression. Although lncRNA loc285194 expression was not affected by TGF-β1 treatment, its effects on cell migration were reduced by TGF-β1.
Conclusions
The expression of lncRNA loc285194 inhibited the migration of CSCC cells in vitro through the inactivation of TGF-β1.
MeSH Keywords: Cerclage, Cervical; Human papillomavirus 16; RNA, Long Noncoding
Background
Metastasis from malignant tumors is the main cause of patient morbidity and mortality [1]. Therefore, the prevention and treatment of late-stage malignancy remain a therapeutic challenge [2]. Worldwide, cervical cancer is a common gynecological cancer [3], with cervical squamous cell carcinoma (CSCC) being the main subtype [4]. Infections with human papillomavirus (HPV) is now recognized as the major risk factor for CSCC [5]. Screening programs to detect cytological changes of cervical dysplasia and CSCC and HPV infection have reduced the incidence of invasive CSCC, but because the pathogenesis of CSCC remains unclear, clinical management remains controversial [6].
Transforming growth factor-β1 (TGF-β1) signaling has pivotal roles in tumor growth and metastasis in several types of human malignancy, including cervical cancer [8]. Therefore, inhibition of TGF-β signaling may be a promising target for the treatment of human cancer [9]. Previous studies have shown crosstalk between TGF-β signaling and long noncoding RNA (lncRNA) in the pathogenesis of some human diseases [10,11], and lncRNA loc285194, also known as LSAMP antisense RNA 3, has been shown to have a role as a tumor suppressor [12]. However, the role lncRNA loc285194 in CSCC and its interaction with TGF-β signaling are unknown.
Therefore, this study aimed to investigate the expression of lncRNA loc285194 in CSCC biopsy tissue, positive and negative for HPV, and in the human CSCC cell lines SiHa (HPV16-positive) and C33A (HPV-negative) and to investigate overexpression of lncRNA loc285194.
Material and Methods
Patients and specimens
This study included 66 patients with a mean age of 46.4±5.6 years (range, 28–66 years) who were diagnosed with cervical squamous cell carcinoma (CSCC) and treated in Gansu Provincial Maternity and Child Care Hospital from March 2015 to January 2018. The study inclusion criteria included a histologically confirmed diagnosis of CSCC on biopsy and newly diagnosed cases. The exclusion criteria included a diagnosis of other malignancies and other cervical disease, and patients who had received treatment, and patients who refused to participate in the study. Of the 66 cases of CSCC, 22 cases were HPV16-positive, 27 cases were HPV18-positive, and 17 cases were HPV-negative. No significant differences in age, gender, clinical stages, and other clinical factors were found between the three patient subgroups. During the same period, from 102 women who underwent cervical biopsy, 20 women with normal cervical biopsies were matched for the age distribution of the patient group and were included as the control group. Plasma and cervical biopsies of all study participants were obtained from the samples stored at Gansu Provincial Maternity and Child Care Hospital. All specimens were stored in liquid nitrogen before use. This study was approved by the Ethics Committee of Gansu Provincial Maternity and Child Care Hospital. All study participants signed informed consent.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
TRIzol reagent (Invitrogen, Carlsbad, CA, USA), SuperScript III reverse transcriptase (Thermo Fisher Scientific, Waltham, MA, USA), and SYBR Green Master Mix (Bio-Rad, Hercules, CA, USA) were used for RNA extraction, reverse transcription, and PCR mixture preparation, respectively. The primer sequences were:
lncRNA loc285194, forward: 5′-TGTGCCTGTTTGACCTCT-3′;
lncRNA loc285194, reverse; 5′-AGGAAGGATAAAAGACCGAC-3;
β-actin, forward: 5′-GACCTCTATGCCAACACAGT-3′;
β-actin, reverse: 5′-AGTACTTGCGCTCAGGAGGA-3′.
Thermal cycling conditions for the PCR reactions were: 1 min at 95°C, followed by 15s at 95°C, and 35s at 55°C, for a total of 40 cycles. Expression of lncRNA loc285194 was normalized to β-actin using the 2–ΔΔCT method.
Enzyme-linked immunosorbent assay (ELISA)
An ELISA kit (Sigma-Aldrich, St. Louis MO, USA) was used to detect plasma levels of transforming growth factor-β1 (TGF-β1), which were expressed as ng/ml.
Cell lines and cell culture
SiHa (HPV-positive) and C33A (HPV-negative) human CSCC cell lines (ATCC, Manassas, VA, USA) were used. A mixture of 90% Eagle’s minimum essential medium (MEM) and 10% fetal bovine serum (FBS) were used to culture the cells at a temperature of 37°C and 5% CO2. Full-length lncRNA loc285194 underwent splicing with EcoRI restriction enzyme and PCR amplification. The lncRNA loc285194 expression vector was constructed, and cDNA in the EcoRI-EcoRI fragment was then inserted into a pIRSE2 vector (Clontech, Palo Alto, CA, USA). Cells were harvested when they reached 80–90% confluence, and 10 nM vectors, empty vector and negative control (NC) were transfected into 4×105 cells using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA). Control (C) cells were non-transfected cells. The subsequent experiment was performed only in cases of overexpression rate reached 200%, when compared with the control (C) and negative control (NC) cells.
Transwell assay
Cell suspensions were prepared by mixing 4×104 cells with 1 ml of a mixture of 99% Eagle’s MEM and 1% FBS. The upper transwell chamber was filled with cell suspension, and the lower chamber was filled with a mixture of 80% Eagle’s MEM and 20% FBS. Cell migration was observed for 12 h. Then, 0.5% crystal violet (Sigma-Aldrich, St. Louis MO, USA) staining was performed at room temperature for 30 minutes.
Western blot
Radioimmunoprecipitation assay (RIPA) buffer and a bicinchoninic acid (BCA) assay kit (Thermo Fisher Scientific, Waltham, MA, USA) were used for protein extraction and measurement, respectively. Protein samples were maintained in boiling water for 10 min to denature the protein, followed by electrophoresis using a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel with 20 μg protein in each well. Proteins were transferred to polyvinylidene fluoride (PVDF) membranes, which were then blocked by incubation in 5% dried skimmed milk powder for 1h. Incubation was performed with a primary rabbit polyclonal antibody to TGF-β1 (1: 1200) (ab92486) (Abcam, Cambridge, MA, USA) and a rabbit monoclonal antibody to GAPDH (1: 1200) (ab37168) (Abcam, Cambridge, MA, USA) overnight at 4°C. The second incubation was performed with goat anti-rabbit IgG conjugated with horseradish peroxidase (HRP) (1: 1000) (MBS435036) (MyBioSource, San Diego, CA, USA) at 25°C for 2 h. The signal was developed using enhanced chemiluminescence (ECL) (Sigma-Aldrich, St. Louis MO, USA). The results were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The experiments were performed in triplicate, and the mean values were calculated. Differences were analyzed using a t-test to compare two groups or one-way analysis of variance (ANOVA) and Tukey’s test for multiple groups. Correlation between plasma levels of lncRNA loc285194 and TGF-β1 were analyzed using Pearson’s correlation coefficient. Receiver operating curve (ROC) analysis was performed to determine the diagnostic performance of lncRNA loc285194 expression in patients with CSCC compared with controls. P<0.05 represented a statistically significant difference.
Results
Long noncoding RNA (lncRNA) loc285194 was downregulated in patients with cervical squamous cell carcinoma (CSCC)
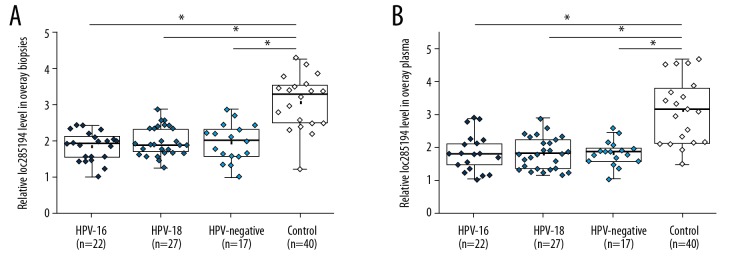
Expression of lncRNA loc285194 in biopsies and plasma of 66 patients with CSCC, including 22 patients with HPV16-positive CSCC, 27 patients with HPV18-positive CSCC, and 17 patients with HPV-negative CSCC, and 20 healthy controls was detected by quantitative reverse transcription-polymerase chain reaction (qRT-PCR). As shown in Figure 1, compared with the control group, expression levels of lncRNA loc285194 in biopsies (Figure 1A) and plasma (Figure 1B) were significantly lower in the three groups of patients with CSCC (p<0.05). Expression levels of lncRNA loc285194 were not significantly different between the three groups of patients with CSCC, indicating that lncRNA loc285194 expression was associated with CSCC.
Figure 1.
Long noncoding RNA (lncRNA) loc285194 was significantly downregulated in patients with cervical squamous cell carcinoma (CSCC) compared with healthy controls. The expression of lncRNA loc285194 in biopsies (A) and plasma (B) were detected using quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and compared between the patient and the control groups using the unpaired t-test (* P<0.05).
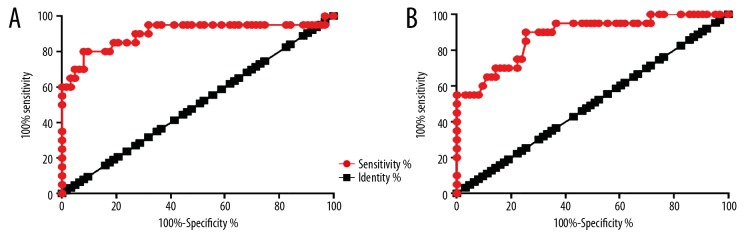
Receiver operating curve (ROC) analysis was performed to determine the diagnostic performance of lncRNA loc285194 expression in patients with CSCC compared with controls. For lncRNA loc285194 expression in cervical biopsies, the area under the curve (AUC) was 0.9012, with a standard error of 0.05070 and 95% confidence interval (95% CI) of 0.8018–1.001 (p<0.0001) (Figure 2A). For loc285194 expression in plasma, the AUC was 0.8813, with standard error of 0.04427 and 95% CI, 0.7946–0.9681 (p<0.0001) (Figure 2B). There was an association between the expression of lncRNA loc285194 and TGF-β1 in all three groups of patients with CSCC.
Figure 2.
Long noncoding RNA (lncRNA) loc285194 as a diagnostic marker of cervical squamous cell carcinoma (CSCC). Receiver operating curve (ROC) analysis was performed to determine the diagnostic performance of lncRNA loc285194 expression in patients with CSCC compared with controls for cervical biopsies (A) and plasma (B).
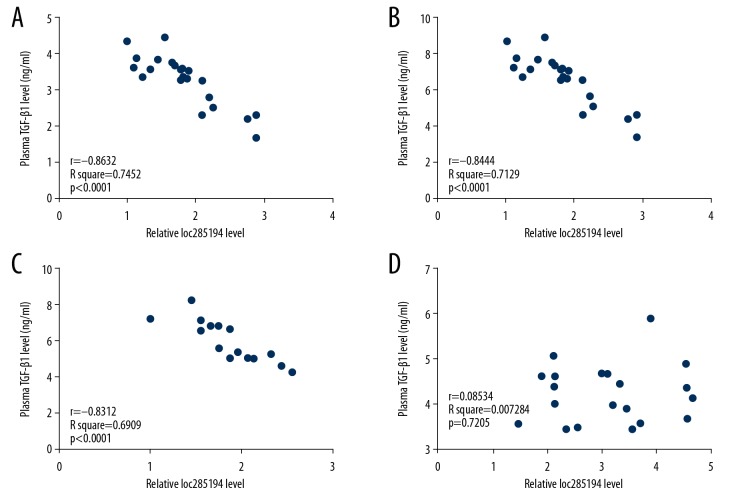
Correlations between plasma lncRNA loc285194 and TGF-β1 were analyzed using Pearson’s correlation coefficient. As shown in Figure 3, plasma lncRNA loc285194 and TGF-β1 were correlated in 22 HPV16-positive patients with CSCC (Figure 3A), 27 HPV18-positive patients with CSCC (Figure 3B) and 17 HPV-negative patients (Figure 3C) but not in 20 healthy controls (Figure 3D). Also, expression of lncRNA loc285194 inhibited TGF-β1.
Figure 3.
Plasma levels of long noncoding RNA (lncRNA) loc285194 and transforming growth factor-β1 (TGF-β1) were significantly correlated with the presence of cervical squamous cell carcinoma (CSCC) in the three study groups, but not in healthy controls. The correlations between lncRNA loc285194 and TGF-β1 in patients with HPV16-positive CSCC (N=22) (A), in patients with HPV18-positive CSCC (N=27) (B), in patients with HPV-negative CSCC (C), and 20 healthy controls (D), were analyzed using Pearson’s correlation coefficient.
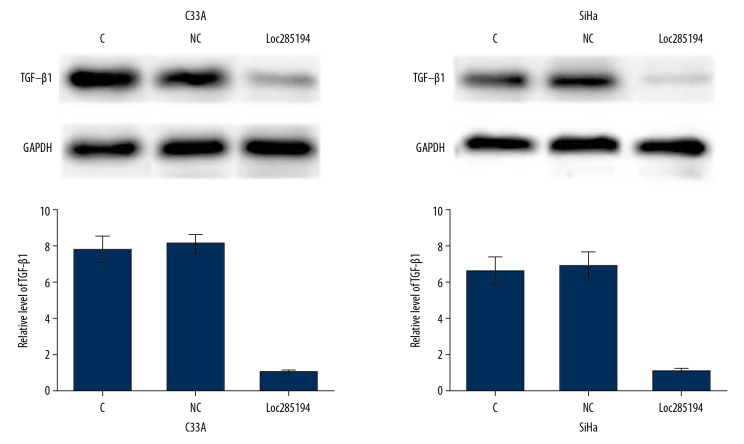
SiHa (HPV-positive) and C33A (HPV-negative) cells were transfected with lncRNA loc285194 expressed vector. As shown in Figure 4, compared with the control groups, lncRNA loc285194 overexpression significantly downregulated TGF-β1 (p<0.05). However, treatment with exogenous TGF-β1 (Sigma-Aldrich, St. Louis MO, USA) at doses of 1, 10, 20, and 40 ng/ml showed no significant effects on lncRNA loc285194 expression (data not shown).
Figure 4.
Long noncoding RNA (lncRNA) loc285194 overexpression promoted transforming growth factor-β1 (TGF-β1) expression in SiHa (HPV16-positive) and C33A (HPV-negative) human cervical squamous cell carcinoma (CSCC) cells. Western blot was performed to analyze the expression of TGF-β1 in CSCC cells transfected with the lncRNA loc285194 expression vector, and data were compared between groups by one-way analysis of variance (ANOVA) and Tukey’s test. (* P<0.05).
lncRNA loc285194 inhibited cell migration SiHa and C33A cells
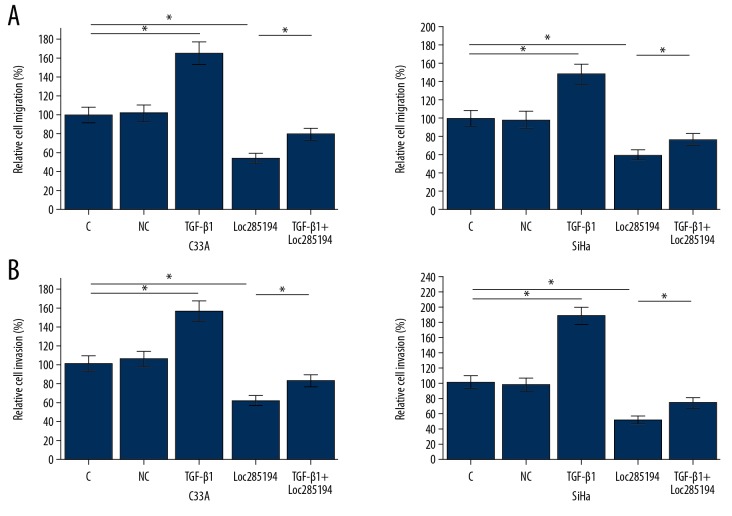
As shown in Figure 5, compared with the control groups, lncRNA loc285194 overexpression significantly inhibited SiHa and C33A cell migration, and treatment with exogenous TGF-β1 at a dose of 10 ng/ml, promoted cell migration (Figure 5A, 5B) (p<0.05). Treatment with exogenous TGF-β1 at a dose of 10 ng/ml reduced the effects of lncRNA loc285194 overexpression on cell migration of SiHa and C33A cells (Figure 5A, 5B) (p<0.05).
Figure 5.
Long noncoding RNA (lncRNA) loc285194 overexpression inhibited migration of SiHa (HPV16-positive) and C33A (HPV-negative) human cervical squamous cell carcinoma (CSCC) cells. The transwell migration assay was performed to analyze the effects of lncRNA loc285194 overexpression and exogenous TGF-β1 on cell migration (A, B) of cells of SiHa and C33A cells (* P<0.05).
Discussion
The aim of this study was to investigate the expression of long noncoding RNA (lncRNA) loc285194 in cervical biopsies from women diagnosed with cervical squamous cell carcinoma (CSCC) who were positive and negative for human papillomavirus (HPV), and in human CSCC cell lines, SiHa (HPV16-positive) and C33A (HPV-negative) and to investigate the overexpression of lncRNA loc285194. The hypothesis that drove this study was that lncRNA loc285194 might play a role as a tumor suppressor in CSCC. The downregulation of TGF-β1 expression at least partially mediated the action of lncRNA loc285194 in CSCC. The expression patterns and functionality of lncRNA loc285194 have been previously investigated in several types of human cancer. Ding et al. reported that lncRNA loc285194 was downregulated in pancreatic ductal adenocarcinoma and predicted poor prognosis [13]. In another study, the downregulation of lncRNA loc285194 was also observed in patients with non-small cell lung cancer (NSCLC) [14]. Human papillomavirus (HPV) infection is the main cause of CSCC [5]. The present study included patients who were positive for HPV16 and HPV18, which are the two most common HPV subtypes detected in patients with CSCC. The data in this study showed that lncRNA loc285194 might have a role in the pathogenesis of CSCC, but not through HPV-dependent pathways because both HPV-positive and HPV-negative CSCC showed downregulated lncRNA loc285194.
Early diagnosis of CSCC is critical for treatment, and patients with metastatic CSCC can have a poor clinical outcome. Therefore, timely treatment at an early stage is important. The detection of downregulated lncRNA loc285194 is a potentially promising diagnostic biomarker for colorectal cancer [15]. In the present study, the differential expression of lncRNA loc285194 expression in both plasma and cervical biopsies distinguished between patients with CSCC and controls, but the sensitivity and specificity of lncRNA loc285194 as a diagnostic marker remains to be evaluated by controlled and large scale clinical studies. Compared with cervical biopsies, plasma is more easily available for diagnostic testing and could be used in future large-scale studies. However, because lncRNA loc285194 is downregulated in multiple cancers, for future studies to evaluate the role of lncRNA loc285194 in CSCC, detection of lncRNA loc285194 should be combined with other diagnostic markers to improve diagnostic specificity.
Transforming growth factor-β1 (TGF-β1) signaling has opposing roles in different stages of several types of malignancy [16]. Expression of TGF-β suppresses tumor growth at the early stages of tumor development but promotes tumor metastasis in more advanced stages of malignancy [17,18]. lncRNA loc285194 is a tumor-suppressor lncRNA regulated by p53, which interacts with TGF-β signaling to exert its role in the pathogenesis of malignancy [19]. Therefore, lncRNA loc285194 may also show crosstalk with TGF-β signaling. In the present study, plasma levels of TGF-β1 were significantly increased in patients with CSCC, which is consistent with the finding that most patients with CSCC in this study had advanced-stage malignant disease. The results also showed that treatment with exogenous TGF-β1 promoted cell migration of SiHa and C33A CSCC cells, which supported the role of TGF-β signaling on promoting metastasis in CSCC. The study findings also showed that lncRNA loc285194 was likely to be an upstream inhibitor of TGF-β1 as lncRNA loc285194 overexpression resulted in increased expression levels of TGF-β1 and treatment with exogenous TGF-β1 showed no significant effects on lncRNA loc285194 expression. Also, the effects of lncRNA loc285194 overexpression on CSCC cell behaviors were reduced by treatment with exogenous TGF-β1. However, the impact of lncRNA loc285194 on TGF-β1 might have been indirect, due to the lack of a significant correlation between lncRNA loc285194 and TGF-β1 in healthy controls. CSCC-specific mediators may exist between lncRNA loc285194 and TGF-β1, as it has previously been shown that specific lncRNAs may regulate the methylation of protein-coding genes and affect gene expression [20]. The role of lncRNA loc285194 on methylation of the TGF-β1 gene requires future study.
Conclusions
This study aimed to investigate the expression of long noncoding RNA (lncRNA) loc285194 in biopsies of cervical squamous cell carcinoma (CSCC) that were positive and negative for human papillomavirus (HPV) and in human CSCC cell lines SiHa and C33A and to investigate the overexpression of lncRNA loc285194. The findings showed that lncRNA loc285194 was downregulated in both HPV-positive and HPV-negative CSCC. The expression of lncRNA loc285194 also inhibited the migration of CSCC cells in vitro through the inactivation of TGF-β1, which indicated that lncRNA loc285194 might inhibit the metastasis in CSCC by downregulating TGF-β1.
Footnotes
Source of support: This study was funded by the Gansu Provincial Department of Science and Technology International Exchanges and Cooperation (No. 1504WKCA060)
Conflict of interest
None.
References
- 1.Valastyan S, Weinberg RA. Tumor metastasis: Molecular insights and evolving paradigms. Cell. 2011;147(2):275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steeg PS. Tumor metastasis: Mechanistic insights and clinical challenges. Nat Med. 2006;12(8):895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Yang PM, Chou CJ, Tseng SH, et al. Bioinformatics and in vitro experimental analyses identify the selective therapeutic potential of interferon gamma and apigenin against cervical squamous cell carcinoma and adenocarcinoma. Oncotarget. 2017;8(28):46145–62. doi: 10.18632/oncotarget.17574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crosbie EJ, Einstein MH, Franceschi S, et al. Human papillomavirus and cervical cancer. Lancet. 2013;382(9895):889–99. doi: 10.1016/S0140-6736(13)60022-7. [DOI] [PubMed] [Google Scholar]
- 6.Hildesheim A, Gonzalez P, Kreimer AR, et al. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. Am J Obstet Gynecol. 2016;215(2):212e1–e15. doi: 10.1016/j.ajog.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drabsch Y, Ten Dijke P. TGF-β signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev. 2012;31(3–4):553–68. doi: 10.1007/s10555-012-9375-7. [DOI] [PubMed] [Google Scholar]
- 8.Noordhuis MG, Fehrmann RSN, Wisman GBA, et al. Involvement of the TGF-β and β-catenin pathways in pelvic lymph node metastasis in early-stage cervical cancer. Clin Cancer Res. 2011;17(6):1317–30. doi: 10.1158/1078-0432.CCR-10-2320. [DOI] [PubMed] [Google Scholar]
- 9.Connolly EC, Freimuth J, Akhurst RJ. Complexities of TGF-β targeted cancer therapy. Int J Biol Sci. 2012;8(7):964–78. doi: 10.7150/ijbs.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan Y, Shen B, Tan M, et al. TGF-β–induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin Cancer Res. 2014;20(6):1531–41. doi: 10.1158/1078-0432.CCR-13-1455. [DOI] [PubMed] [Google Scholar]
- 11.Zhao JJ, Hao S, Wang LL, et al. Long non-coding RNA ANRIL promotes the invasion and metastasis of thyroid cancer cells through TGF-β/Smad signaling pathway. Oncotarget. 2016;7(36):57903–18. doi: 10.18632/oncotarget.11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Q, Huang J, Zhou N, et al. LncRNA loc285194 is a p53-regulated tumor suppressor. Nucleic Acids Res. 2013;41(9):4976–87. doi: 10.1093/nar/gkt182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding YC, Yu W, Ma C, et al. Expression of long non-coding RNA LOC285194 and its prognostic significance in human pancreatic ductal adenocarcinoma. Int J Clin Exp Pathol. 2014;7(11):8065–70. [PMC free article] [PubMed] [Google Scholar]
- 14.Shi X, Chen Y, Chen J, et al. Antisense long noncoding RNA LOC285194 is downregulated in NSCLC and associated with poor prognosis. Chest. 2016;150(1):261. doi: 10.1016/j.chest.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Yu J, Han Y, et al. Long non-coding RNAs LOC285194, RP11-462C24. 1 and Nbla12061 in serum provide a new approach for distinguishing patients with colorectal cancer from healthy controls. Oncotarget. 2016;7(43):70769–78. doi: 10.18632/oncotarget.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akhurst RJ, Derynck R. TGF-β signaling in cancer – a double-edged sword. Trends Cell Biol. 2001;11(11):S44–51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 17.Seoane J, Gomis RR. TGF-β family signaling in tumor suppression and cancer progression. Cold Spring Harb Perspect Biol. 2017;9(12) doi: 10.1101/cshperspect.a022277. pii: a022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29(2):117–29. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 19.Ali A, Wang Z, Fu J, et al. Differential regulation of the REGγ – proteasome pathway by p53/TGF-β signalling and mutant p53 in cancer cells. Nat Commun. 2013;4:2667. doi: 10.1038/ncomms3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang F, Deng X, Ma W, et al. The lncRNA Firre anchors the inactive X chromosome to the nucleolus by binding CTCF and maintains H3K27me3 methylation. Genome Biol. 2015;16(1):52. doi: 10.1186/s13059-015-0618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]