Abstract
Background
Acute pancreatitis (AP) is a common digestive disorder. Its management depends on the severity; therefore, it is essential to stratify AP patients early. D-dimer, a coagulation indicator, appears to be associated with the pathogenesis of AP. The aim of this study was to evaluate D-dimer as an early predictor of the severity of AP.
Material/Methods
This was a single-center retrospective study of 1260 patients diagnosed based on the revised Atlanta classification. Only patients hospitalized within 24 h of onset were included, and 334 patients were enrolled. Blood was collected at admission and 3 times within 48 h of admission. Values at admission and average of the 3 blood samples were evaluated by univariate and multivariate analyses. Furthermore, the area under the receiver-operating characteristic curve (AUC) was used to estimate the validity of the predictor and to define optimal cut-off points for prediction.
Results
We found that 53.3% of the patients had mild AP (MAP), 24.3% had moderately severe AP (MSAP), and 22.4% had severe AP (SAP). D-dimer at admission and the average D-dimer could distinguish MAP patients from MSAP and SAP patients, with cut-off values of 3.355 mg/L and 4.868 mg/L, respectively. No difference in the parameters at admission was observed in multivariate analysis in distinguishing SAP from MSAP, but the average D-dimer level was significantly different with a cut-off value of 7.268 mg/L by comparing Ranson score, APACHE II score, and D-dimer level.
Conclusions
The average value of D-dimer levels could be used as a predictor of severity of AP. In general, patients with an average D-dimer level <4.868 could be diagnosed with MAP, >7.268 would develop into SAP, and between 4.868 and 7.268 would be MSAP.
MeSH Keywords: Clinical Laboratory Techniques, Pancreatitis, Predictive Value of Tests
Background
Acute pancreatitis (AP) is a common gastrointestinal disorder in which there is an acute sterile inflammation of the pancreas [1]. Biliary tract disease, hyperlipidemia, and excessive alcohol consumption are the 3 main etiologies [2]. Most patients have a mild and self-limiting course, but the spectrum of manifestations is broadly variable. Roughly one-fifth of patients are diagnosed with severe AP, with a mortality rate of 30% [3,4].
The management of AP depends on its severity. Therefore, it is vital to stratify AP patients as early as possible. Several biochemical markers and complex scoring systems are in use to estimate the severity of AP [5–7]. However, these previous methods are multifactorial and rather inconvenient for daily use, and some of them have proven to be either impractical or lack sensitivity and specificity for predicting the severe form of AP [8–10].
For all of these aforementioned reasons, an accurate, objective, and easily accessible predictor is urgently needed. One possible pathogenic mechanism of organ dysfunction in AP is release and activation of numerous proinflammatory cytokines leading to hypercoagulation and microvascular thrombosis [11,12]. Therefore, biomarkers of coagulation may be helpful in predicting the severity. D-dimer is a specific indicator of secondary fibrinolysis. Elevated levels of D-dimer found in the present study indicated increased thrombin formation and consequent fibrous deposition with aggravated inflammatory response. Therefore, D-dimer is likely to predict the course of AP, with good sensitivity.
The aim of this study was to assess the utility of D-dimer as an early predictive marker of the severity of AP.
Material and Methods
Patient selection and classification
We evaluated all patients who were diagnosed with AP and who enrolled from March 2012 to March 2017 in our hospital. Sample size was calculated by the formula for sample size of cross-sectional studies. Based on clinical experience, the expected prevalence of MAP is 60% (0.6), α=0.05. After calculation, the sample size n is at least 257.
The regional ethics committee approved the study. Data collected were stored by researchers according to ethical confidentiality standards.
Diagnosis of AP was based on the emergence of at least 2 of the following 3 features: typical signs and symptoms including abdominal tension or specific peritonitis; levels up to 3 times higher than the upper limit of normal values of pancreatic amylase and/or lipase; and imaging showing characteristic findings of AP [13].
Based on the revised Atlanta Classification in 2012, clinical AP severity was classified into 3 groups. Mild AP (MAP) patients were not associated with organ failure (OF) and local or systemic complications. Moderately severe AP (MSAP), which was newly introduced in the Atlanta Classification, was characterized by the presence of transient OF (less than 48 h) or local or systemic complications. Severe AP (SAP) was defined as persistent OF for more than 48 h. The diagnosis of OF was based on the modified Marshall scoring system, and a score of 2 or more was considered to be the presence of OF for the respiratory, cardiovascular, and/or renal systems [14].
Patients who were definitively diagnosed with AP and transferred to our department within 24 h of onset were included in the study. Patients were stratified as MAP, MSAP, or SAP. Patients admitted to the hospital before the revised the Atlanta Classification would be reassessed based on medical records. Patients with any of following features were excluded: 1) a prehospital interval more than 24 h, 2) chronic pancreatitis, 3) recurrent pancreatitis, 4) age less than 18 years old or over 70 years old, 5) cirrhosis, 6) thrombotic diseases, 7) anticoagulant drug use, 8) cancer, 9) recent infection, 10) pregnancy, 11) a history of organ failure, and 12) those who did not undergo treatment for nonmedical reasons.
The demographics of the study population were sex, age, and body mass index (BMI). The cause of AP was determined by history and imaging studies. Complex scoring systems, including the Ranson score and acute Physiology and Chronic Health Examination II (APACHE II) score, were calculated for each patient. Laboratory parameters, including hematocrit (HCT), platelet (PLT), C-reactive protein (CRP), procalcitonin (PCT), and D-dimers (measured through immunoturbidimetry using CS–5100 automatic coagulation analysis system with standard operation procedure, the upper limit of normal value for D-dimer is 0.55 mg/L), were assessed using 3 independent blood samples taken within 2 h, 24 h, and 48 h after admission, respectively. The data at admission and average value of 3 times were compared. All patients accepted standard management, including fasting after admission, parenteral nutrition, and analgesics [15].
Statistical analysis
All analyses were two-tailed and performed using SAS software, version 9.2. Normally distributed data were presented as mean with standard deviation (SD). Comparison between variables was performed using the t test. Non-normally distributed data were presented as median [interquartile range (IQR)]. The Wilcoxon rank-sum test was used for comparison of continuous variables. Categorical variables were expressed as absolute numbers and percent. For association between 2 variables, Pearson’s chi-square test or Fisher’s exact test was applied, as appropriate. The area under the receiver-operating characteristic (ROC) curve (AUC) was used to assess the predictive accuracy of various predictors and to determine the optimum cut-off points with optimal sensitivity and specificity. The AUC was calculated using 95% confidence interval (CI). A p value less than 0.05 was considered statistically significant.
Results
Overall, 1260 AP patients were retrospectively assessed and 926 patients were excluded for various reasons (Figure 1). The baseline characteristics of the 334 patients who were eventually included are shown in Table 1. The etiologies of pancreatitis included biliary tract disease (53.6%), hypertriglyceridemia (31.1%), alcohol abuse (9.0%), and idiopathic causes (6.3%).
Figure 1.
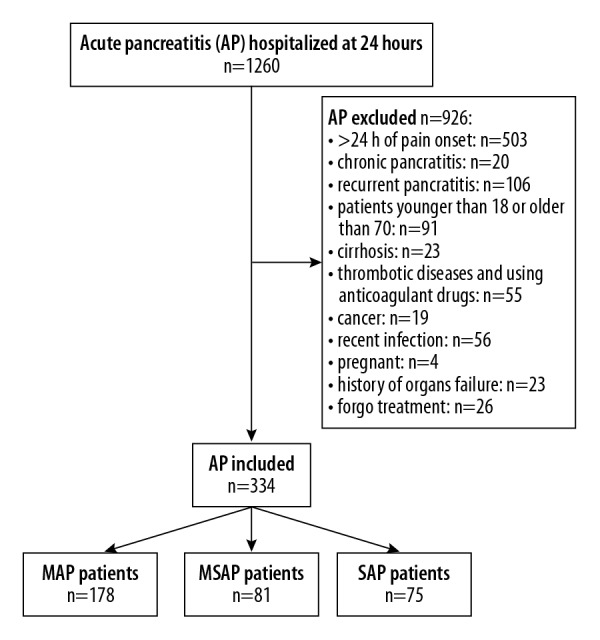
Flowchart of the study.
Table 1.
Patients characteristics.
| Characteristics | Total (n=334) |
|---|---|
| Age (years) | 45.6±13.1 |
| Sex | |
| Male | 220 (65.9%) |
| Female | 114 (34.1%) |
| BMI (kg/m2) | 24.6±5.1 |
| Etiology of AP | |
| Biliary | 179 (53.6%) |
| Hypertriglyceridemia | 104 (31.1%) |
| Alcoholic | 30 (9.0%) |
| Idiopathic | 21 (6.3%) |
| Severity of AP | |
| Mild | 178 (53.3%) |
| Moderately severe | 81 (24.3%) |
| Severe | 75 (22.4%) |
| Time after onset of the symptoms (hours) | 11.6±6.6 |
| Length of hospital stay (days) | 15.7±13.2 |
| Length of stay at the ICU (days) | 2.2±3.1 |
Data presented as mean and standard deviation.
A total of 110 patients experienced OF, of which 75 were persistent. Seventy-seven patients had acute necrotic collection (ANC), and 30 of them progressed to walled-off necrosis (WON). Fifty-one patients had acute peripancreatic fluid collection (APFC), and 11 of them had pancreatic pseudocyst (PPC). Of all the patients, 4 developed systemic complications, all of which were coronary artery diseases. In this study, 178 patients were diagnosed with MAP (53.3%), 81 with MSAP (24.3%), and 75 with SAP (22.4%).
Separation MAP from non-MAP patients
We divided the patients into 2 groups: MAP and non-MAP (MSAP and SAP). We examined several laboratory parameters, clinical parameters, and scoring systems, all of which have a close relationship with AP, in that several of these presented have been confirmed to have predictive value for AP on the basis of prior studies [16–21]. A univariate analysis of the data at admission produced significant differences in BMI, D-dimer, CRP, and PCT levels between the MAP and non-MAP groups (Table 2). Univariate analysis of the average values of 3 independent blood samples revealed that BMI, Ranson score, APACHE II score, D-dimer, PLT, HCT, CRP, and PCT levels showed significant differences between the groups (Table 2).
Table 2.
Predictors in the univariate analysis to distinguish MAP from non-MAP.
| MAP (n=178) | Non-MAP (n=156) | P value | |
|---|---|---|---|
| Sex (Male/Female) | 124/54 | 96/60 | 0.118a |
| Age (years) | 46.3±13.2 | 44.7±13.0 | 0.360b |
| BMI (kg/m2) | 23.8±5.1 | 25.6±4.9 | <0.01b |
| Etiology of AP | 0.385a | ||
| Biliary | 93 (52.3%) | 86 (55.1%) | |
| Hypertriglyceridemia | 55 (30.9%) | 49 (31.4%) | |
| Alcoholic | 15 (8.4%) | 15 (9.6%) | |
| Idiopathic | 15 (8.4%) | 6 (3.9%) | |
| Ranson score | 1 (0, 2) | 3 (1, 4) | <0.0001b |
| APACHE II score | 2 (1, 6) | 6 (2, 10) | <0.0001b |
| At admission | |||
| D-dimer (mg/L) | 1.2 (0.6, 2.3) | 5.0 (3.4, 7.1) | <0.0001b |
| PLT (109/L) | 219.9 (179.1, 277.9) | 207.2 (156.0, 277.2) | 0.107 b |
| HCT (%) | 42.9 (38.6, 47.9) | 44.1 (38.0, 49.5) | 0.378b |
| CRP (mg/L) | 0.8 (0.1, 2.2) | 4.0 (1.5, 9.2) | <0.0001b |
| PCT (ng/ml) | 0.04 (0.01, 0.08) | 0.3 (0.1, 3.1) | <0.0001b |
| Average values | |||
| D-dimer (mg/L) | 3.2 (2.2, 4.0) | 6.8 (5.4, 9.4) | <0.0001b |
| PLT (109/L) | 210.1 (180.3, 257.6) | 167.4 (132.9, 205.7) | <0.0001b |
| HCT (%) | 41.6 (37.9, 45.2) | 38.3 (33.4, 42.5) | <0.0001b |
| CRP (mg/L) | 1.7 (0.4, 6.4) | 8.4 (4.0, 14.1) | <0.0001b |
| PCT (ng/ml) | 0.1 (0.1, 0.2) | 2.1 (0.4, 8.4) | <0.0001b |
Use chi-square tests;
use Wilcoxon tests.
Data presented as median (IQR) except age and BMI (mean and standard deviation).
Multivariable logistic regression models indicated BMI, D-dimer, CRP, and PCT levels were independent risk factors of non-MAP at admission. For the average values, Ranson score, APACHE II score, D-dimer, CRP, and PCT levels were independently associated with non-MAP. In addition, D-dimer at <3.355 mg/L at admission or an average D-dimer level <4.868 mg/L was the optimal diagnostic marker of MAP with an AUC of 0.859 and 0.937, respectively (Figures 2, 3). The sensitivity, specificity, positive predictive value, and negative predictive value of D-dimer for predicting MAP at admission were 86.5%, 75.6%, 73.9%, and 87.5%, respectively, and for the average D-dimer level they were 85.6%, 90.4%, 91.0%, and 84.7%, respectively. The p values, odds ratios with 95% confidence intervals, and area under the ROC curves are provided in Table 3.
Figure 2.
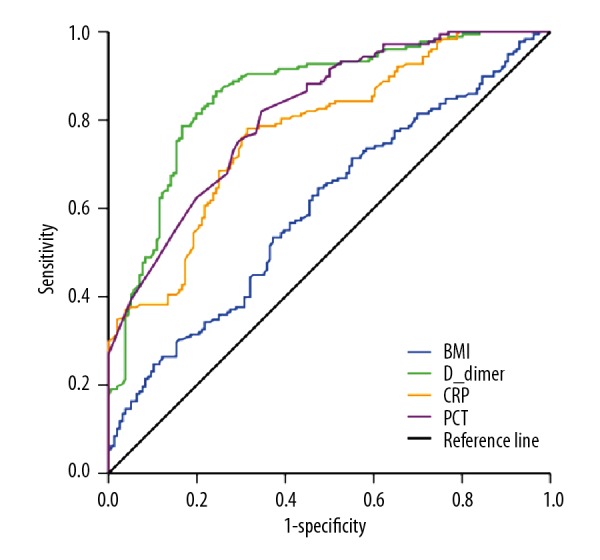
ROC curves for BMI, D-dimer, CRP, and PCT levels at admission in the MAP group and the non-MAP group.
Figure 3.
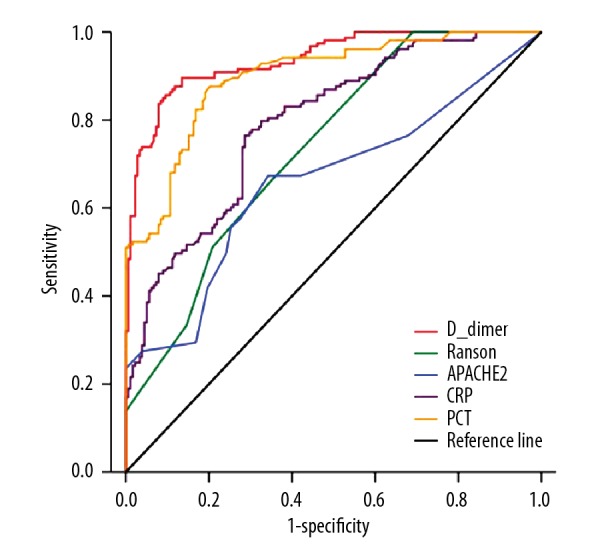
ROC curves for Ranson score, APACHE II score, and average D-dimer, CRP, PCT levels in the MAP group and the non-MAP group.
Table 3.
Predictors in multivariate logistic regression models to distinguish MAP from non-MAP.
| Odds ratio (95% CI) | P value | AUC | Cut-off values | |
|---|---|---|---|---|
| At admission | ||||
| BMI | 1.525 (1.108–2.099) | 0.0097 | 0.601 | 24.95 |
| D-dimer | 1.709 (1.488–1.962) | <0.0001 | 0.859 | 3.355 |
| CRP | 1.318 (1.186–1.464) | <0.0001 | 0.776 | 2.420 |
| PCT | 7.432 (2.620–21.081) | 0.0002 | 0.817 | 0.115 |
| Average values | ||||
| D-dimer | 2.826 (2.212–3.612) | <0.0001 | 0.937 | 4.868 |
| Ranson score | 1.433 (1.164–1.763) | 0.0007 | 0.736 | 1.50 |
| APACHE II score | 1.200 (1.094–1.316) | 0.0001 | 0.659 | 3.50 |
| CRP | 1.124 (1.052–1.201) | 0.0005 | 0.793 | 3.783 |
| PCT | 3.351 (2.076–5.411) | <0.0001 | 0.897 | 0.243 |
Distinguishing SAP patients from MSAP patients
We attempted to distinguish SAP patients from non-MAP patients. Only BMI was predictive of SAP at admission in univariate analyses. However, the average values for BMI, Ranson score, APACHE II score, D-dimer, PLT, HCT, CRP, and PCT levels separated the MSAP from the SAP patients (Table 4). Furthermore, in multivariable logistic regression models, Ranson score, APACHE II score, and D-dimer showed significant differences. By comparing these 3 parameters, we found that the average D-dimer level of >7.268 mg/L was the best predictor of SAP with an AUC of 0.916 (Figure 4). The sensitivity, specificity, positive predictive value, and negative predictive value of average D-dimer level for predicting SAP were 81.3%, 91.0%, 92.2%, and 78.8%, respectively. However, no factor was significantly associated with SAP at admission in multivariate analysis. The p values, odds ratios with 95% confidence intervals, and area under the ROC curves are provided in Table 5.
Table 4.
Predictors in the univariate analysis to distinguish SAP from MSAP.
| MSAP (n=81) | SAP (n=75) | P value | |
|---|---|---|---|
| Sex (Male/Female) | 54/27 | 42/33 | 0.171a |
| Age (years) | 44.7±13.6 | 44.6±12.4 | 0.765b |
| BMI (kg/m2) | 24.4±4.5 | 27.0±5.1 | <0.01b |
| Etiology of AP | |||
| Biliary | 44 (54.3%) | 42 (56.0%) | 0.700a |
| Hypertriglyceridemia | 28 (34.6%) | 21 (28.0%) | <0.0001b |
| Alcoholic | 6 (7.4%) | 9 (12.0%) | <0.0001b |
| Idiopathic | 3 (3.7%) | 3 (4.0%) | |
| Ranson score | 1 (1, 3) | 3 (2, 6) | |
| APACHE II score | 2 (1, 7) | 8 (6, 13) | |
| At admission | |||
| D-dimer (mg/L) | 4.93 (3.51, 6.47) | 5.31 (2.99, 8.75) | 0.3742b |
| PLT (109/L) | 218.90 (165.10, 290.00) | 211.40 (141.80, 249.50) | 0.1363b |
| HCT (%) | 44.56±7.32 | 42.37±9.56 | 0.1161b |
| CRP (mg/L) | 3.30 (1.03, 7.42) | 3.79 (1.92, 9.27) | 0.3657b |
| PCT (ng/ml) | 0.12 (0.05, 2.09) | 0.35 (0.12, 0.89) | 0.0972b |
| Average values | |||
| D-dimer (mg/L) | 5.7 (4.6, 6.5) | 9.3 (7.7, 10.7) | <0.0001b |
| PLT (109/L) | 176.6 (144.3, 215.8) | 156.8 (120.7, 187.0) | 0.006b |
| HCT (%) | 39.1±5.6 | 36.7±7.0 | 0.018b |
| CRP (mg/L) | 7.8 (2.6, 13.0) | 9.3 (4.1, 19.8) | 0.042b |
| PCT (ng/ml) | 0.6 (0.3, 4.1) | 7.0 (0.7, 14.7) | <0.0001b |
Use chi-square tests;
use Wilcoxon tests.
Data presented as median (IQR) except age, BMI, and HCT (mean and standard deviation).
Figure 4.
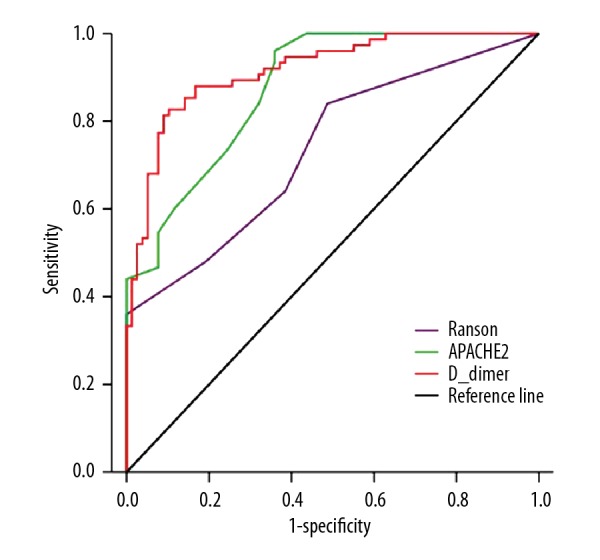
ROC curves for Ranson score, APACHE II score, and average D-dimer level in the MSAP group and the SAP group.
Table 5.
Predictors in multivariate logistic regression models to distinguish SAP from MSAP.
| Odds ratio (95% CI) | P value | AUC | Cut-off values | |
|---|---|---|---|---|
| At admission | ||||
| No factor | ||||
| Average values | ||||
| Ranson score | 2.173 (1.629–2.899) | <0.0001 | 0.736 | 4.50 |
| APACHE II score | 1.841 (1.503–2.255) | <0.0001 | 0.876 | 9.50 |
| D-dimer | 6.514 (3.798–11.175) | <0.0001 | 0.916 | 7.268 |
Outcome comparison between MAP, MSAP and SAP
In our study, 77 patients (23.1%) developed evidence of (peri) pancreatic necrosis. Overall, 301 (90.1%) patients were treated conservatively and 33 (9.9%) underwent interventions. All patients who needed surgical interventions were treated with a step-up approach. Percutaneous catheter drainage was used in 33 patients (100%), and 21 of them (63.6%) underwent further treatment, such as endoscopic retroperitoneal pancreatic necrosectomy. Finally, open necrosectomy was needed in 5 patients (15.2%). The in-hospital mortality rate was 0.9% (3 patients died). Patients with MSAP had a longer length of hospital stay (p<0.0001), a longer length of ICU stay (p<0.0001), and a higher rate of intervention for necrosis (p<0.0001) than those with MAP. Patients with SAP experienced a longer length of hospital stay (p<0.0001), a longer length of ICU stay (p<0.0001), a higher rate of intervention for necrosis (p<0.0001), and a higher mortality rate (p<0.0001) than those with MSAP (Table 6).
Table 6.
Adverse outcomes for MAP, MSAP, and SAP.
| MAP | MSAP | SAP | |
|---|---|---|---|
| N (%) | 178 (53.3) | 81 (24.3) | 75 (22.5) |
| Length of hospital stay (days) | 8.7±3.2 | 18.0±7.6 | 29.9±19.1 |
| Length of stay at the ICU (days) | 0.2±0.5 | 2.3±1.7 | 6.9±2.9 |
| Intervention (%) | 0 (0) | 11 (13.6) | 22 (29.3) |
| Death (%) | 0 (0) | 0 (0) | 3 (4) |
Data presented as mean and standard deviation.
Discussion
AP is a common acute abdominal disease with a wide clinical spectrum ranging from mild to severe. In our study, 178 patients were diagnosed with MAP (53.3%), 81 with MSAP (24.3%), and 75 with SAP (22.4%). The incidence was similar to that reported by Nawaz et al. [16]. However, the proportion of SAP patients in our study was higher than in other studies [22–25]. One possible reason for this is that more MAP patients prefer to receive treatment in local hospitals or smaller hospitals. Therefore, a higher proportion of SAP patients was observed in our study. In addition, the cause of AP is different between studies. For example, hyperlipidemia was the number 2 cause of AP in our study, accounting for 31.1%. Koziel et al., however, reported cholelithiasis and alcohol abuse to be the leading causes in most patients [23].
We had previously found that D-dimer could predict the incidence of infectious complications of AP [26]. In this study, we found that D-dimer was valuable in predicting the severity of the AP, which was reported to prevent the progress of multiple organ failure (MOF) in SAP [27]. The hunt for a predictive indicator for AP has been going on for decades. The computed tomography severity index (CTSI) score, Ranson score, APACHE II score, and other multifactorial prognostic scoring systems are well documented [28,29]. A Ranson score higher than 3 or an APACHE II score higher than 8 were defined as predictors of SAP [12]. CRP and PCT were considered to have predictive value for pancreatic infection in previous reports [30]. Nevertheless, according to the revised Atlanta Classification in 2012, the diagnosis of SAP is based on OF, which means SAP can be confirmed only after 48 h of the occurrence OF [14], but this 2-day delay can be fatal for AP patients. Therefore, an optimal marker is needed to help identify serious cases quickly and accurately.
D-dimer, an easily detectable marker, has previously been assessed in AP. Gomercic and Gupta showed that increased D-dimer level was associated with various complications of AP [31,32]. Yang et al. recently published 2 studies that showed significantly increased D-dimer levels in MSAP patients compared with MAP patients (the sensitivity and specificity were 92.6% and 77.7%, respectively, within 48 h) [33,34]. This conclusion is more noteworthy in hyperlipidemic pancreatitis patients (the sensitivity and specificity were 100% and 83.8%, respectively). Although we drew similar conclusions, our research had significant advantages. First, AP patients with diverse etiologies and SAP patients were included, which means our results are more universally applicable. Second, 3 independent blood samples within 48 h from each patient were assessed, which avoided incidental fluctuations in data collection. In addition, by using more stringent grouping and exclusion criteria, we excluded conditions that might have contributed to increased D-dimer levels. D-dimer levels were significantly different among different studies because they used different units and measurement methods, and “mg/L” was used in our study. Ke et al. previously reported that D-dimer predicted complications of AP with a lower cut-off point than that in our study [35]. Possible reasons for this disparity could be differences in equipment and measurement methods used. In addition, Kong et al. attributed the increase in D-dimer to the increase in triglycerides in AP patients [36]. In this study, however, hyperlipidemia was the second leading cause of AP, accounting for 31.1%, and the higher proportion of hyperlipidemic AP patients contributed to higher D-dimer levels.
Our results were similar to a recently published meta-analysis on sensitivity and specificity of Ranson score and APACHE II score in predicting SAP [37]. Compared with these scoring systems, D-dimer had a greater advantage as a single predictor. D-dimer levels not only have a higher specificity and sensitivity, but it is also easier to obtain. In addition, CRP is mainly synthesized in the liver and increases slowly in the course of AP, which limits the use of CRP as an early predictor. A possible explanation for why we did not observe a significant difference in CRP among the different groups was that 48 h is a short time. PCT is correlated with the presence of bacterial and fungal infection and sepsis [15,38]. Compared with CRP, PCT is a better indicator of bacterial infection [39]. Therefore, PCT is more often used for predicting infection rather than grading disease, which also explains why PCT was not a candidate in the multifactorial analysis.
However, this was a single-center retrospective study, and all patients were from the northeastern region of China. Hence, a similar diet pattern and the retrospective nature could have introduced bias in the study. We excluded 503 patients (39.9%) because they had entered the hospital after 24 h of onset of symptoms, which was a massive loss of sample. However, most of those patients were referrals that had already received treatment and could not be compared with patients who did not receive any treatment. Therefore, more high-quality studies are needed to confirm the present findings.
Conclusions
We confirmed the diagnostic value of D-dimer in predicting the severity of AP. Patients with a D-dimer value of <3.355 mg/mL at admission or an average of 3 values of <4.868 mg/mL within 48 h of admission are more likely to develop MAP. However, an average of >7.268 mg/mL of D-dimer is indicative of SAP. This adds to the small body of literature, suggesting that D-dimer may be a useful early predictive biomarker for AP based on the revised Atlanta classification. This simple, feasible, and reproducible marker could be used in clinical practice to improve the early management of AP.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (grant no. 81770639)
Conflict of interest
None.
References
- 1.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–61. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staubli SM, Oertli D, Nebiker CA. Laboratory markers predicting severity of acute pancreatitis. Crit Rev Clin Lab Sci. 2015;52:273–83. doi: 10.3109/10408363.2015.1051659. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Zak Y, Hernandez-Boussard T, et al. The epidemiology of idiopathic acute pancreatitis, analysis of the nationwide inpatient sample from 1998 to 2007. Pancreas. 2013;42:1–5. doi: 10.1097/MPA.0b013e3182572d3a. [DOI] [PubMed] [Google Scholar]
- 4.Nesvaderani M, Eslick GD, Cox MR. Acute pancreatitis: Update on management. Med J Aust. 2015;202:420–23. doi: 10.5694/mja14.01333. [DOI] [PubMed] [Google Scholar]
- 5.Triester SL, Kowdley KV. Prognostic factors in acute pancreatitis. J Clin Gastroenterol. 2002;34:167–76. doi: 10.1097/00004836-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Windsor AC, Kanwar S, Li AG, et al. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42:431–35. doi: 10.1136/gut.42.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dambrauskas Z, Gulbinas A, Pundzius J, Barauskas G. Value of the different prognostic systems and biological markers for predicting severity and progression of acute pancreatitis. Scand J Gastroenterol. 2010;45:959–70. doi: 10.3109/00365521003770244. [DOI] [PubMed] [Google Scholar]
- 8.Lankisch PG. Natural course of acute pancreatitis: What we know today and what we ought to know for tomorrow. Pancreas. 2009;38:494–98. doi: 10.1097/MPA.0b013e3181a11cb0. [DOI] [PubMed] [Google Scholar]
- 9.Khanna AK, Meher S, Prakash S, et al. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, Apache-II, CTSI scores, IL-6, CRP, and procalcitonin in predicting severity, organ failure, pancreatic necrosis, and mortality in acute pancreatitis. HPB Surg. 2013;2013 doi: 10.1155/2013/367581. 367581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papachristou GI, Muddana V, Yadav D, et al. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105:435–41. doi: 10.1038/ajg.2009.622. quiz 442. [DOI] [PubMed] [Google Scholar]
- 11.Feistritzer C, Wiedermann CJ. Effects of anticoagulant strategies on activation of inflammation and coagulation. Expert Opin Biol Ther. 2007;7:855–70. doi: 10.1517/14712598.7.6.855. [DOI] [PubMed] [Google Scholar]
- 12.Hagiwara S, Iwasaka H, Shingu C, et al. Antithrombin III prevents cerulein-induced acute pancreatitis in rats. Pancreas. 2009;38:746–51. doi: 10.1097/MPA.0b013e3181aba9fa. [DOI] [PubMed] [Google Scholar]
- 13.Bradley EL., 3rd A clinically based classification system for acute pancreatitis. Arch Surg; Summary of the International Symposium on Acute Pancreatitis; Atlanta, GA. September 11–13, 1992; 1993. pp. 586–90. [DOI] [PubMed] [Google Scholar]
- 14.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis – 2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 15.Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1–15. doi: 10.1016/j.pan.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 16.Nawaz H, Mounzer R, Yadav D, et al. Revised Atlanta and determinant-based classification: Application in a prospective cohort of acute pancreatitis patients. Am J Gastroenterol. 2013;108:1911–17. doi: 10.1038/ajg.2013.348. [DOI] [PubMed] [Google Scholar]
- 17.de-Madaria E, Soler-Sala G, Lopez-Font I, et al. Update of the Atlanta Classification of severity of acute pancreatitis: Should a moderate category be included? Pancreatology. 2010;10:613–19. doi: 10.1159/000308795. [DOI] [PubMed] [Google Scholar]
- 18.Acevedo-Piedra NG, Moya-Hoyo N, Rey-Riveiro M, et al. Validation of the determinant-based classification and revision of the Atlanta classification systems for acute pancreatitis. Clin Gastroenterol Hepatol. 2014;12:311–16. doi: 10.1016/j.cgh.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 19.Papachristou GI, Papachristou DJ, Avula H, et al. Obesity increases the severity of acute pancreatitis: Performance of APACHE-O score and correlation with the inflammatory response. Pancreatology. 2006;6:279–85. doi: 10.1159/000092689. [DOI] [PubMed] [Google Scholar]
- 20.Gardner TB, Vege SS, Chari ST, et al. The effect of age on hospital outcomes in severe acute pancreatitis. Pancreatology. 2008;8:265–70. doi: 10.1159/000134274. [DOI] [PubMed] [Google Scholar]
- 21.Koutroumpakis E, Wu BU, Bakker OJ, et al. Admission hematocrit and rise in blood urea nitrogen at 24 h outperform other laboratory markers in predicting persistent organ failure and pancreatic necrosis in acute pancreatitis: A post hoc analysis of three large prospective databases. Am J Gastroenterol. 2015;110:1707–16. doi: 10.1038/ajg.2015.370. [DOI] [PubMed] [Google Scholar]
- 22.Talukdar R, Bhattacharrya A, Rao B, et al. Clinical utility of the revised Atlanta classification of acute pancreatitis in a prospective cohort: Have all loose ends been tied? Pancreatology. 2014;14:257–62. doi: 10.1016/j.pan.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Koziel D, Gluszek S, Matykiewicz J, et al. Comparative analysis of selected scales to assess prognosis in acute pancreatitis. Can J Gastroenterol Hepatol. 2015;29:299–303. doi: 10.1155/2015/392643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Ke L, Tong Z, et al. Association between severity and the determinant-based classification, Atlanta 2012 and Atlanta 1992, in acute pancreatitis: A clinical retrospective study. Medicine (Baltimore) 2015;94:e638. doi: 10.1097/MD.0000000000000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Q, Li M, Chen Y, Hu W. Determinant-based classification and revision of the Atlanta classification, which one should we choose to categorize acute pancreatitis? Pancreatology. 2015;15:331–36. doi: 10.1016/j.pan.2015.05.467. [DOI] [PubMed] [Google Scholar]
- 26.Ji L, Lv JC, Song ZF, et al. Risk factors of infected pancreatic necrosis secondary to severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2016;15:428–33. doi: 10.1016/s1499-3872(15)60043-1. [DOI] [PubMed] [Google Scholar]
- 27.Kibar YI, Albayrak F, Arabul M, et al. Resistin: New serum marker for predicting severity of acute pancreatitis. J Int Med Res. 2016;44:328–37. doi: 10.1177/0300060515605428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brisinda G, Vanella S, Crocco A, et al. Severe acute pancreatitis: Advances and insights in assessment of severity and management. Eur J Gastroenterol Hepatol. 2011;23:541–51. doi: 10.1097/MEG.0b013e328346e21e. [DOI] [PubMed] [Google Scholar]
- 29.Schütte K, Malfertheiner P. Markers for predicting severity and progression of acute pancreatitis. Best Pract Res Clin Gastroenterol. 2008;22:75–90. doi: 10.1016/j.bpg.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Mofidi R, Suttie SA, Patil PV, et al. The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: Systematic review. Surgery. 2009;146:72–81. doi: 10.1016/j.surg.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Gomercic C, Gelsi E, Van Gysel D, et al. Assessment of D-dimers for the early prediction of complications in acute pancreatitis. Pancreas. 2016;45:980–85. doi: 10.1097/MPA.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 32.Gupta S, Shekhawat VP, Kaushik GG. D-dimer, a potential marker for the prediction of severity of acute pancreatitis. Clin Lab. 2015;61:1187–95. doi: 10.7754/clin.lab.2015.150102. [DOI] [PubMed] [Google Scholar]
- 33.Yang N, Hao J, Zhang D. Antithrombin III and D-dimer levels as indicators of disease severity in patients with hyperlipidaemic or biliary acute pancreatitis. J Int Med Res. 2017;45:147–58. doi: 10.1177/0300060516677929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang N, Zhang DL, Hao JY. Coagulopathy and the prognostic potential of D-dimer in hyperlipidemia-induced acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2015;14:633–41. doi: 10.1016/s1499-3872(15)60376-9. [DOI] [PubMed] [Google Scholar]
- 35.Ke L, Ni HB, Tong ZH, et al. D-dimer as a marker of severity in patients with severe acute pancreatitis. J Hepatobiliary Pancreat Sci. 2012;19:259–65. doi: 10.1007/s00534-011-0414-5. [DOI] [PubMed] [Google Scholar]
- 36.Kong H, Ding Z, Zhu XC, et al. D-Dimer change in human acute pancreatitis as determined by serumal triglyceride. Pancreas. 2011;40:1103–6. doi: 10.1097/MPA.0b013e3182204ae3. [DOI] [PubMed] [Google Scholar]
- 37.Karpavicius A, Dambrauskas Z, Gradauskas A, et al. The clinical value of adipokines in predicting the severity and outcome of acute pancreatitis. BMC Gastroenterology. 2016;16:99. doi: 10.1186/s12876-016-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rau BM, Kemppainen EA, Gumbs AA, et al. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): A prospective international multicenter study. Ann Surg. 2007;245:745–54. doi: 10.1097/01.sla.0000252443.22360.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon L, Gauvin F, Amre DK, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin Infect Dis. 2004;39:206–17. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]


