Abstract
Biological electron transfer is challenging to directly regulate using environmental conditions. To enable dynamic, protein-level control over energy flow in metabolic systems for synthetic biology and bioelectronics, we created ferredoxin logic gates that utilize transcriptional and post-translational inputs to control energy flow through a synthetic electron transfer pathway that is required for bacterial growth. These logic gates were created by subjecting a thermostable, plant-type ferredoxin to backbone fission and fusing the resulting fragments to a pair of proteins that self-associate, a pair of proteins whose association is stabilized by a small molecule, and to the termini of a ligand-binding domain. We show that the latter domain insertion design strategy yields an allosteric ferredoxin switch that acquires an oxygen-tolerant 2Fe-2S cluster and can use different chemicals, including a therapeutic drug and an environmental pollutant, to control the production of a reduced metabolite in Escherichia coli and cell lysates.
INTRODUCTION
Achieving direct, dynamic control over biological electron transfer (ET) through synthetic biology is critical for creating living sensors and bioelectronics1–4, controlling microbial electro-synthesis and -fermentation5, and building efficient metabolic pathways for chemical synthesis6,7. At the hub of mediating the transfer of low potential electrons is the ferredoxin (Fd) family, ancient Fe-S proteins that are deeply rooted in the tree of life8,9. These electron carriers are important for ancient metabolic processes, such as hydrogen-dependent CO2 fixation by autotrophs (acetogens and methanogens) and light-dependent CO2 fixation by photoautotrophs10. Additionally, Fds are widespread across all three domains of life. Some organisms contain over a dozen Fd paralogs, and Fds have been found to support ET to over eighty classes of oxidoreductases11, suggesting that these electron carriers helped spur the evolution of diverse bioenergetics strategies12. Recently, Fds have been used to construct synthetic ET chains. These pathways have been developed to evolve Fd-dependent enzymes with improved catalytic functions13 and to understand Fd-partner specificity14. These efforts have demonstrated the potential for manipulating protein electron carriers for synthetic biology. However, we still lack Fds whose ET can be switched “on” and “off” in response to specific environmental conditions.
Some organisms use Fds to dynamically control ET through post-translational modifications. Upon Fe-S cluster oxidation, nitrogenase-protecting Fds alter their conformation and binding affinity to nitrogenase to protect from oxidative damage15,16. Phosphorylated and calcium-bound forms of Fds were recently discovered in cyanobacteria17 and rhizobia18, respectively. These discoveries suggest that Fd ET may also be dynamically regulated through signaling cascades, although the exact mechanisms by which these modifications affect Fd ET remain unclear. Additionally, protein design efforts have investigated whether Fd ET can be deliberately controlled. Fds have been rationally mutated using structural information19 and fused to acceptor proteins to tune the relative proportions of electrons transferred to different metabolic pathways20–22. While these efforts have shown how protein evolution can redirect energy flow, they have yet to yield insight into the ways that Fds acquire new allosteric functions.
Previous studies have shown that proteins can be fragmented into polypeptides whose stable association and cooperative activities are dependent upon ligand-binding events23. This has been achieved by fusing engineered fragments to pairs of proteins whose association is stabilized by ligand binding24,25, and by fusing protein fragments to the termini of ligand binding domains25,26. To investigate if a similar design approach can be used to create protein electron carriers that are regulated by environmental conditions, we rationally designed four split Fds (sFds) and evaluated their ability to support ET in cells. All four sFds supported ET in cells when they were fused to peptides that stabilize fragment association, and three of the sFds could be used as transcriptional AND gates in which a pair of conditional promoters regulate Fd-mediated ET. One of the sFds could also be used to create a three-hybrid system that only supports cellular ET transfer when both fragments are expressed and cells are exposed to the small-molecule rapamycin. Additionally, when the fragments of this sFd were fused to the termini of the estrogen receptor (ER) ligand-binding domain (LBD), the resulting polypeptide presented ET that could be enhanced post-translationally by different estrogen-receptor modulators.
RESULTS
Monitoring protein electron transfer using a selection.
To rapidly assay ET mediated by engineered Fds, we utilized Escherichia coli EW1113, a strain that cannot grow in minimal medium containing sulfate as a sulfur source unless it expresses a synthetic ET chain consisting of a Fd-NADP+ reductase (FNR) electron donor, a Fd electron carrier, and a Fd-dependent sulfite reductase (SIR) electron acceptor (Fig. 1a). Endogenous oxidoreductases were systematically removed from the genome of this strain to insulate Fd-dependent redox pathways from native redox reactions13. To evaluate Fd ET in cells, we constitutively expressed Zea mays FNR and SIR and used a TetR-repressible promoter to control the expression of plant-type 2Fe-2S Fds. With this expression strategy, growth depended upon the level of anhydrotetracyline (aTc) (Fig. 1b), and thus, the amount of Fd available to mediate ET.
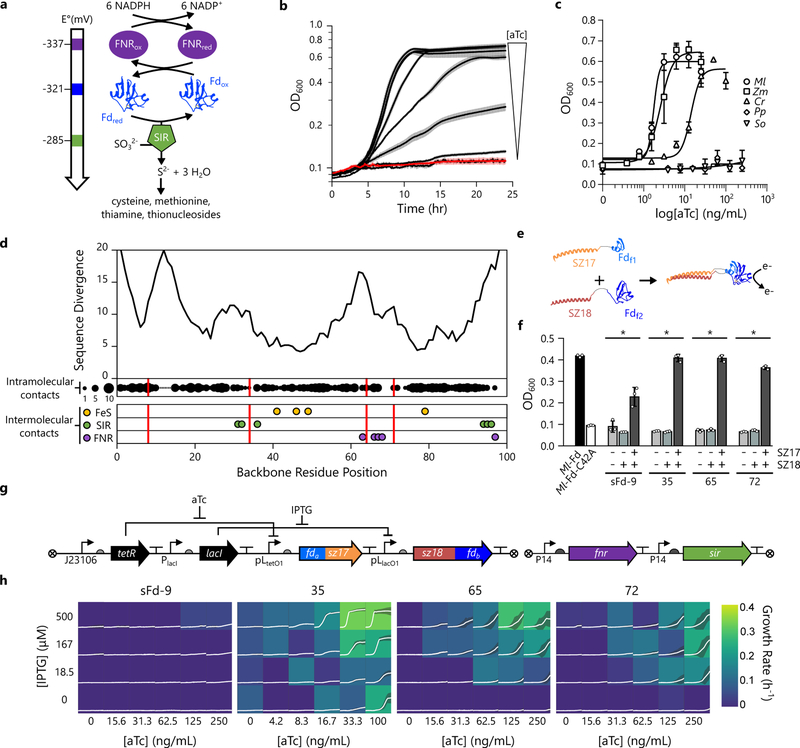
Figure 1. Designing split Fds that transfer electrons within cells.
(a) A synthetic ET pathway that couples NADPH oxidation by FNR to sulfide production by SIR using Fd-mediated ET. (b) The effect of varying concentrations of aTc (0.4, 0.8, 1.6, 3.1, 6.3, 12.5, and 25 ng/mL) on the growth of cells expressing Ml-Fd from an aTc-inducible PLtetO1 promoter and FNR and SIR from constitutive promoters. The red line represents the mean in the absence of aTc, black lines represents the mean in the presence of aTc, and the error bars represent the s.d. (n=3 biologically independent samples). (c) Growth complementation observed after 24 hours with Mastigocladus laminosus (Ml; k1/2 = 1.8 ng/mL aTc), Zea mays root (Zm; k1/2 = 2.5 ng/mL aTc), Chlamydomonas reinhardtii (Cr; k1/2 = 14.2 ng/mL aTc), Spinacia oleracea (So; k1/2 > 250 ng/mL), and Prochlorococcus phage (Pp; k1/2 > 250 ng/mL) Fds. Symbols and error bars represent the mean and s.d., respectively (n=3 biologically independent samples). (d) The number of different amino acids observed at each residue position within a Fd multiple sequence alignment mapped onto Ml-Fd primary structure (top). The backbone fission sites used to generate sFds (red lines) are compared with the number of intramolecular residue-residue contacts (n = 0 to 10 displayed as proportionally-sized black circles) within the structure of Ml-Fd (PDB = 1RFK), as well as intermolecular binding residues, including the cysteines that coordinate the 2Fe-2S cluster (yellow) and the residues that contact SIR29 (green) and FNR30 (purple). (e) Each pair of sFd fragments, Fdf1 and Fdf2 (blue) was expressed as a fusion to SYNZIP-17 (SZ17; orange) and SYNZIP-18 (SZ18; red), respectively. (f) Complementation of E. coli EW11 growth by Ml-Fd and a C42A mutant of Ml-Fd that lacks a cysteine required for coordinating iron is compared with the complementation observed in cells expressing Fdf1 and Fdf2 alone, Fdf1 and the SZ18-Fdf2 fusion, and Fdf1 and Fdf2 fused to SZ17 and SZ18, respectively. Bars and error bars represent the average and s.d., respectively (n=3 biologically independent samples). * represents significant growth relative to the C42A mutant (p-values [sFd-9: 2×10−3; sFd-35: 8×10−4; sFd-65: 6×10−4; sFd-72: 1×10−4] using a two-tailed, independent t-test). (g) Genetic circuit used to regulate Fd fragment expression. The Fdf1-S17 fusion is expressed using an aTc-inducible promoter, the S18-Fdf2 fusion is expressed using an IPTG-inducible promoter, and the partner proteins are constitutively expressed. (h) Effects of sFd fragment concentration, controlled by aTc and IPTG, on E. coli EW11 complementation. Each box shows cell density from cultures with the growth rate (h−1) indicated by the color. The white lines and shaded region within each box represent the cell density mean and s.d., respectively (n=3 biologically independent samples).
The effect of aTc on complementation by five 2Fe-2S Fds was analyzed, including plant, cyanobacterial, algal, and phage family members (Fig. 1c). Two of the Fds were unable to complement growth, including Spinacia oleracea and Prochlorococcus phage P-SSM2 Fds. The other three Fds presented half-maximal concentration at different aTc concentrations. Mastigocladus laminosus Fd (Ml-Fd) yielded half-maximal complementation at the lowest aTc concentration. This thermostable Fd, which displays optimal ET at 65 °C27, was targeted for all subsequent design efforts to minimize the destabilizing effects of mutations, since previous studies have shown that tolerance to fission correlates with thermostability28.
Design of split protein electron carriers.
To identify backbone fission locations that are non-disruptive to Ml-Fd structure, we generated a multiple sequence alignment of 2Fe-2S Fds (Supplementary Figure 1) and calculated the number of different amino acids at each native Ml-Fd position (Fig. 1d). We targeted four sites with moderate sequence divergence for backbone fission, including the peptide bonds following residues 9, 35, 65, and 72. We chose these sites because they vary in their distances from the 2Fe-2S cluster, and they differ in the number of cofactor and partner-binding residues encoded distributed across the pair of fragments.
In the Ml-Fd structure27, the backbone cleavage sites occur 9.5 to 18.5 Å from the 2Fe-2S cluster (Supplementary Figure 2). For two of the split Fd (sFd) proteins, sFd-9 and sFd-35, all of the cysteines that coordinate iron reside on a single polypeptide (Fig. 1d). The other split proteins, sFd-65 and sFd-72, have their iron-coordinating cysteines dispersed across the fragments, potentially requiring 2Fe-2S cluster maturation following fragment complementation. Mapping the backbone fission sites onto the Fd-SIR complex structure29 reveals that the SIR contacting residues in these sFds are divided among the different polypeptide fragments in sFd-35, sFd-65, and sFd-72 (Supplementary Figure 3). In contrast, the FNR contacting residues in the Fd-FNR complex30 are only divided among the different fragments in sFd-65 and sFd-72.
Split ferredoxins support electron transfer in cells.
To assist with sFd ET, each pair of Fd fragments was initially expressed as fusions to SYNZIP-17 and SYNZIP-18 (Fig. 1e), synthetic peptides that stably associate to form a coiled coil31. When the sFds were expressed in E. coli EW11, complementation was observed with all four variants (Fig. 1f), albeit to varying extents. Removal of one or both SYNZIP peptides abolished growth in all cases, suggesting that these split proteins uniformly require assistance with fragment association for efficient ET. TetR- and LacI-repressible promoters were also used to control the expression of each Fd-fragment fusion (Fig. 1g), and complementation was analyzed over a range of aTc and isopropyl β-D-1-thiogalactopyranoside (IPTG) concentrations (Fig. 1h). sFd-35, sFd-65, and sFd-72 all functioned as two-input AND gates to control the production of a reduced sulfur metabolite with sFd-35 exhibiting the highest tunable range. In the case of sFd-35, a low level of complementation was observed when cells were exposed to aTc in the absence of IPTG. This finding is thought to arise as a consequence of leaky expression of the C-terminal Fd fragment.
To investigate whether sFd ET can be regulated post-translationally, we created vectors for expressing the three most active split proteins as fusions to the FKBP12 and the FKBP-rapamycin binding (FRB) domain of mTOR (Fig. 2a), proteins whose interaction is stabilized by rapamycin32, and we assessed their complementation of E. coli EW11 growth ±rapamycin. These sFds were expressed using TetR- and LacI-repressible promoters (Supplementary Figure 4a), and complementation was measured using inducer concentrations that yielded maximal growth when sFds were fused to SYNZIP peptides. In the absence of rapamycin, none of the sFds complemented growth. Addition of rapamycin enhanced the growth of cells expressing sFd-35 (Fig. 2b), but not sFd-65 and sFd-72 (Supplementary Figure 5). With sFd-35, growth was dose dependent and could be tuned to a similar maximal density as cells expressing Ml-Fd.
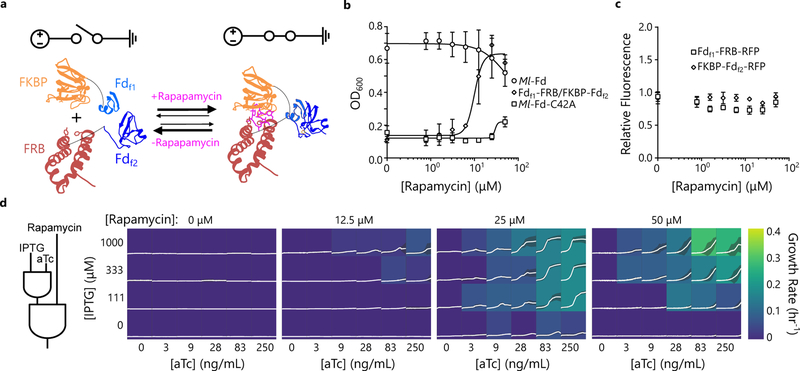
Figure 2. Post-translational control over Fd electron transfer in cells.
(a) sFd-35 fragments Fdf1 and Fdf2 (blue) were expressed as fusions to FKBP (orange) and FRB (red), respectively. (b) Effect of rapamycin concentration on E. coli EW11 growth in the presence of aTc (150 ng/mL) and IPTG (500 μM) concentrations that yielded strong complementation with SZ17/SZ18 fusions. Complementation by Fdf1-FRB/FKBP-Fdf2 (k1/2 = 9.95 μM rapamycin) is compared with Ml-Fd and a C42A mutant of Ml-Fd that lacks a cysteine required for coordinating iron. Growth of cells expressing Fdf1-FRB/FKBP-Fdf2 is enhanced significantly above the C42A mutant when ≥25 μM rapamycin is added (p-values [25 μM-1×10–4, 50 μM-6×10–5] using a two-tailed, independent t-test). Symbols and error bars represent the mean and s.d., respectively (n=3 biologically independent samples). (c) The effect of rapamycin on the relative fluorescence of cells expressing Fdf1-FKBP-RFP and FRB-Fdf2-RFP. Upon addition of rapamycin in ethanol, there was no significant increase in fluorescence for either fragment compared to ethanol alone (p-value > 0.01 using a two-tailed, independent t-test). Symbols and error bars represent the mean and s.d., respectively (n=3 biologically independent samples). All fluorescent reporters were amended to the C-terminus of proteins to maintain the context of the ribosomal binding sites and translation initiation43 (d) Fdf1-FRB and FKBP-Fdf2 can function as a three-input AND gate that uses aTc, IPTG, and rapamycin to control electron flow. Each box shows cell density from cultures with the growth rate (h–1) indicated by the color. The white lines and shaded region within each box represent the cell density mean and s.d., respectively (n=3 biologically independent samples).
To evaluate whether rapamycin affected sFd-35 expression, we examined rapamycin effects on protein accumulation using fragments fused to red fluorescent protein (RFP). With each sFd fragment, this analysis revealed similar RFP levels in the presence and absence of rapamycin (Fig. 2c). These findings provide evidence that ET is controlled post-translationally by rapamycin stabilization of the sFd fragment complex. Complementation measurements performed using different combinations of aTc, IPTG, and rapamycin revealed that sFd-35 can be used as a three-input AND gate to control ET for sulfide production and growth (Fig. 2d).
A ferredoxin switch created using domain insertion.
Allosteric protein switches have been engineered by fusing protein fragments to the termini of domains that undergo chemical-dependent conformational changes25,26. To test whether this domain-insertion strategy can be used to diversify the chemical regulation of Ml-Fd, we inserted the ligand-binding domain (LBD) of the human estrogen receptor (ER) after residue 35 to create sFd-35-ER (Fig. 3a). This LBD was chosen because it undergoes a conformational change upon 4-hydroxytamoxifen (4-HT) binding that alters the proximity of the protein termini33. In addition, we recently found that insertion of the ER-LBD into an amino acyl tRNA synthetase yields protein switches with 4-HT dependent activities25.
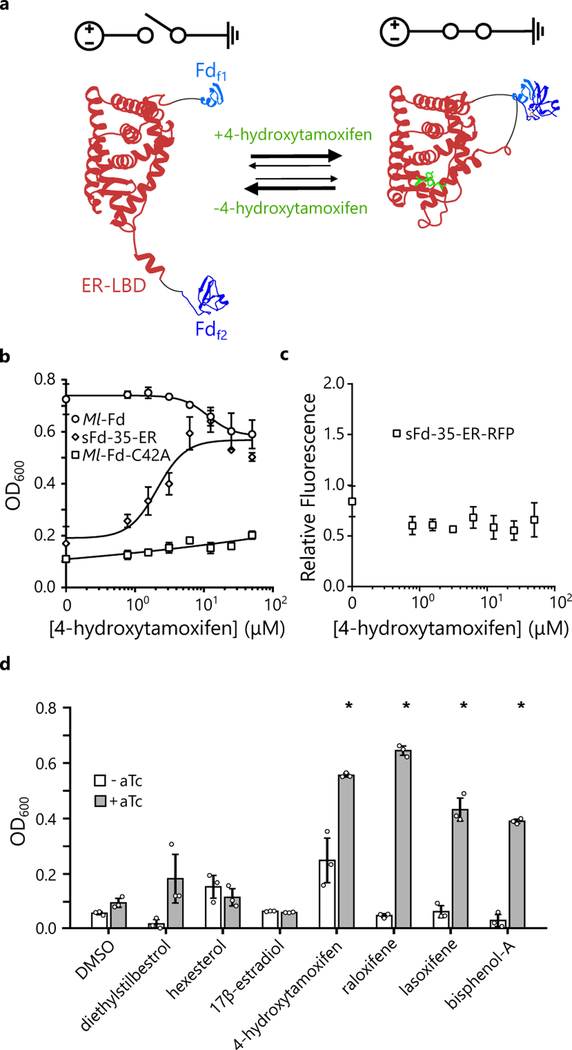
Figure 3. Using domain insertion to create a Fd switch.
(a) The ER-LBD was inserted after residue 35 in Ml-Fd to create sFd-35-ER. (b) Effect of 4-HT concentration on the complementation of E. coli EW11 by sFd-35-ER (k1/2 = 2.11 μM 4-HT), Ml-Fd, and Ml-Fd-C42A. Growth of sFd-35-ER is enhanced significantly above the C42A mutant in the presence of ≥6.25 μM 4-HT (p-values [6.25 μM-6×10–3, 12.5 μM-4×10–4, 25 μM-1×10–4, 50 μM-2×10–5] using a two-tailed, independent t-test). Symbols and error bars represent the mean and s.d., respectively (n=3 biologically independent samples). (c) The effect of 4-HT on the relative fluorescence of cells expressing sFd-35-ER-RFP. Upon addition of 4-HT in ethanol, there was no significant increase in fluorescence compared to ethanol alone (p-value > 0.01 using a two-tailed, independent t-test). Symbols and error bars represent the mean and s.d., respectively (n=3 biologically independent samples). All fluorescent reporters were amended to the C-terminus of proteins to maintain the context of the ribosomal binding sites and translation initiation43. (d) Effect of estrogen receptor modulators (50 μM) on complementation of E. coli EW11 by sFd-35-ER with 0 ng/mL aTc (white bars) or 200 ng/mL aTc (gray bars). The agonists (diethylstilbesterol, hexesterol, and 17β-estradiol) do not significantly enhance growth of EW11 cells compared to DMSO alone (p-value > 0.01 using a two-tailed, independent t-test), while the antagonists (4-hydroxytamoxifen, raloxifene, and lasoxifene) as well as bisphenol-A significantly enhance growth compared to DMSO alone. * represents significant growth (p-values [4-hydroxytamoxifen-1×10−4, raloxifene-5×10−6, lasoxifene-3×10−3, bisphenol-A-2×10−4] using a two-tailed, independent t-test). Bars and error bars represent the average and s.d., respectively (n=3 biologically independent samples).
We evaluated the activity of sFd-35-ER by expressing this protein using an aTc-inducible promoter (Supplementary Figure 4b). In medium containing aTc, sFd-35-ER did not significantly enhance E. coli EW11 growth following a 48 hour incubation (Fig. 3b). In contrast, complementation was enhanced to Ml-Fd levels when cells were grown in the presence of aTc and 4-HT. We also expressed this protein as a fusion to RFP (sFd-35-ER-RFP) to evaluate how 4-HT affects protein expression. This analysis revealed similar expression across a range of 4-HT concentrations (Fig. 3c), providing evidence that the increased complementation arises from 4-HT effects on sFd-35-ER ET rather than increases in expression.
The ER-LBD binds two classes of estrogen receptor modulators, agonists and antagonists, which elicit distinct conformations (Supplementary Figure 6) and tissue-specific responses in vivo33,34. To evaluate how these different modulators affect sFd-35-ER ET, we evaluated sFd-35-ER growth complementation in the presence of agonists, antagonists, and an endocrine disruptor (Fig. 3d). This analysis revealed that sFd-35-ER ET is chemically selective and activated by the ER antagonists (4-HT, raloxifene, lasoxifene) and endocrine disruptor (bisphenol-A). However, this switch is not activated by ER agonists (17β-estradiol, diethylstilbestrol, hexestrol).
In vitro characterization of the ferredoxin switch.
To understand how Ml-Fd Fe-S cluster binding and ET are affected by domain insertion, sFd-35-ER was overexpressed in E. coli and purified under aerobic conditions. Recombinant sFd-35-ER was brown in color throughout the purification protocol (Supplementary Figure 7) and presented absorbance (Fig. 4a) and visible circular dichroism (CD) spectra (Fig. 4b) that were consistent with the presence of a 2Fe-2S metallocluster and similar to Ml-Fd and other plant-type Fds35,36.
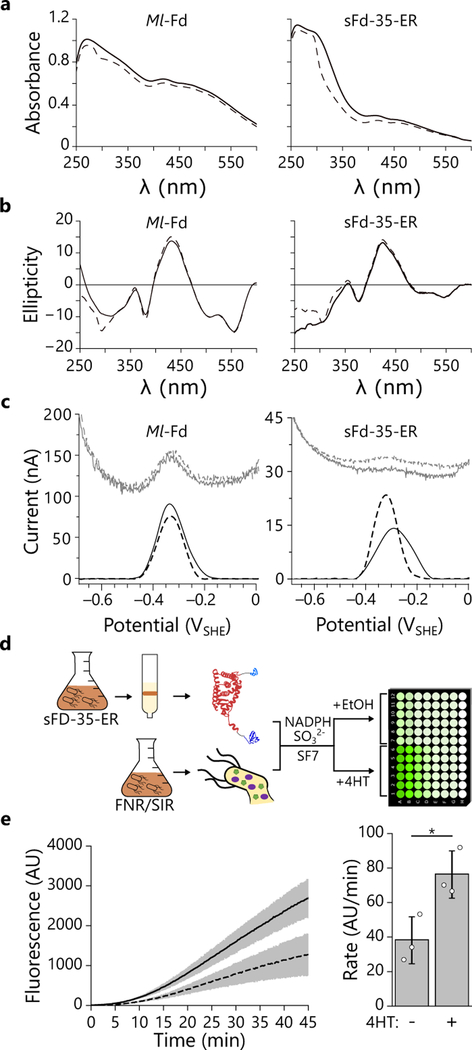
Figure 4. Using purified sFd-35-ER to control metabolite production in cell lysates.
(a) The absorbance and (b) circular dichroism spectra of purified sFd-35-ER and Ml-Fd (150 μM each) in the presence (solid line) or absence (dashed line) of 4-HT display features characteristic of 2Fe-2S proteins36. (c) The midpoint potentials of Ml-Fd and sFd-35-ER in and presence (solid line) or absence (dashed line) of 4-HT. Raw square wave voltammetry data are plotted in gray, baseline-subtracted data are plotted in black. Experiments depicted in a-c were performed twice using two independent protein preparations yielded the same results. (d) Lysates generated from E. coli EW11 constitutively expressing FNR and SIR were mixed with purified sFd-35-ER (50 nM), NADPH (40 μM), SO32− (10 mM), and SF7 (2 μM). Following mixing, 4-HT in ethanol (line) or an equivalent amount of ethanol (dashed line) were added to reactions, and fluorescence was monitored at 37°C. (e) The sFd-35-ER switch displayed a significant increase in sulfide production rate upon 4-HT addition (p = 0.027, using a two-tailed, independent t-test) relative to the ethanol carrier. The black lines and bars represent the mean while the gray shaded region and error bars represent the s.d. (n = 3 independent experiments).
To investigate if domain insertion affects the reduction potential of Ml-Fd, we characterized Ml-Fd and sFd-35-ER using protein film voltammetry37. In the absence of 4-HT, we found that the midpoint potentials of both Ml-Fd and sFd-35-ER (Fig. 4c) were −336 and −320 mV, respectively. These measurements indicate that sFd-35-ER retains a potential that is adequate for coupling NADPH oxidation by FNR (FAD E° = −337 mV)38 to sulfite reduction by SIR ([4Fe-4S] E° = −415 mV, siroheme = −285 mV)39. In the presence of 4-HT, Ml-Fd presented a similar midpoint potential (−330 mV) as in the absence of ligand, while sFd-35-ER yielded a +27 mV shift in potential (−293 mV). These findings provide evidence that domain insertion can be used to create a Fd whose redox potential is tuned by chemical binding, and they suggest that this design approach could be applied to proteins with alternative 2Fe-2S cluster ligands to create switches capable of regulating ET over a range of potentials11,40.
To directly demonstrate that purified sFd-35-ER functions as a switch in vitro, we examined whether this protein could support sulfide production in lysates derived from E. coli EW11 expressing only FNR and SIR (Fig. 4d). We monitored SIR activity in desalted cell lysates using sulfidefluor-7 (SF7)41, a fluorescent probe for hydrogen sulfide. Cell extracts derived from E. coli EW11 expressing FNR and SIR presented sulfide production rates that depended upon NADPH concentration (Supplementary Figure 8). To minimize this background signal, we added a low level of NADPH (40 μM) that did not yield large sulfide production rates over the time course of our measurements. With this in vitro assay, the rate of sulfide production is proportional to the concentration of Ml-Fd added to cell lysates (Supplementary Figure 9). The sulfide production rate was not enhanced by adding 4-HT alone, demonstrating that this chemical does not support ET to SIR in the absence of a Fd. Addition of purified sFd-35-ER to cell lysates containing FNR and SIR increased the sulfide production rate (Fig. 4e), indicating that this protein can mediate ET to SIR in the absence of 4-HT. However, in the presence of 4-HT, the sulfide production rate was significantly enhanced. Taken together, these measurements provide direct evidence that 4-HT regulates sulfide production by altering the conformation of sFd-35-ER and the efficiency with which it transfers electrons from FNR to SIR.
DISCUSSION
Chemical-dependent protein electron carriers represent a new class of synthetic switches that will enable precision control over electron flow in cells. Herein, we show that these electrical switches can be used to control ET from FNR to SIR. These electron donor and acceptor proteins were used as partner proteins because they allowed for the rapid assessment of Fd-mediated ET using growth complementation of a nutritional requirement. Previous studies have shown that Fds can support ET in diverse non-native pathways, such as hydrogen13,19,20, propane7, and antibiotic synthesis6. These observations suggest that our chemical-dependent Fds could be used to regulate diverse metabolic outputs by expressing them in parallel with FNR and alternative electron acceptors that are also dependent on 2Fe-2S Fds, such as aldehyde decarbonylase7, cytochrome P450s22, and glutamine oxoglutarate aminotransferase42.
A thermostable Fd was used a starting point for switch design to maximize the likelihood of discovering Fd variants capable of ET. Among the different backbone fission sites tested, cleavage after residue 35 yielded the most robust fragments for different design strategies. With this sFd, ET was enhanced by fusion to SYNZIP-17/18 or fusion to FKBP/FRB; the other sFds only displayed function when fused to the SYNZIP peptides. Additionally, sFd-35 fragments could be fused to the end of the ER LBD to create a chemical-dependent Fd. These findings suggest that structurally-related fragments in other 2Fe-2S Fds could be used to create similar protein switches. By applying this design strategy to Fds with distinct partner specificities from Ml-Fd, Fds could be created that extend the diversity of metabolic outputs that can be regulated using ER antagonists at the level of ET.
Fd switches having 2Fe-2S clusters should be useful for regulating ET across the range of midpoint potentials where native Fds function (−455 to −262 mV)11. To create switches that support ET at more negative potentials, our design approach could be applied to clostridial-type Fds which possess multiple 4Fe-4S clusters11,40. Modern clostridial Fds are thought to have arisen from duplication of smaller peptides that required association to function8. These smaller peptides have been synthesized in vitro and shown to coordinate iron-sulfur clusters in vitro43, suggesting that they may be capable of supporting ET from FNR to SIR when expressed in cells. Additionally, our design approach could be applied to families of protein electron carriers that support ET at more positive midpoint potentials, such as cytochromes, thioredoxins, rieske-type Fds, and cupredoxins11. The major challenge with applying this design strategy to other protein electron carriers will be the development of high throughput assays that can be used to rapidly mine combinatorial libraries of engineered proteins for variants that display chemical-dependent ET.
Electrical protein switches will have a wide range of applications in bioelectronics, metabolic engineering, and synthetic biology. They can be used in place of native oxidoreductases to regulate the flow of electrons derived from oxidative metabolism and photosynthesis in response to specific metabolic or environmental cues. They can also be used to dynamically regulate electron flow between central metabolism and non-native metabolic pathways6,7,13,19,20. Furthermore, electrical protein switches can be used to couple the exquisite sensing capabilities of proteins to electrical communication between cells and conductive materials outside of cells1. To date, electron conduits have been described whose extracellular ET can be tuned using transcriptional regulation2–4. By coupling exoelectrogen current production to electrical protein switches, post-translational control over cellular current production may be possible that does not require slow transcriptional and translational processes. In the future, the ligand specificity of these switches can be diversified by fusing Fd fragments to different ligand-binding domains and by mutating the ligand-binding site of our prototype switches.
ONLINE METHODS
Materials.
Rapamycin was from TCI America, isopropyl β-D-1-thiogalactopyranoside (IPTG) was from RPI, and all other chemicals were purchased from Sigma-Aldrich.
Vector design.
Supplementary Table 1 lists all of the plasmids. These were constructed using Golden Gate DNA assembly43 of PCR products amplified using Phusion DNA polymerase (Thermo-Fisher). The genes encoding Zea mays FNR, Zea mays SIR, and Spinacia oleracea Fd were a gift from Pam Silver (Harvard University). The genes encoding Mastigocladus laminosus, Zea mays, Chlamydomonas reinhardtii, and cyanomyophage PSSM-2 Fd were synthesized as G-blocks by Integrated DNA Technologies. The genes for Ml-Fd and sFd-35-ER were cloned into pET-28b to create expression vectors for protein purification. All vectors were sequence verified.
Calculations.
A multiple sequence alignment (MSA) of 60 plant-type Fd sequences was generated using MUSCLE5. Positional amino acid sequence divergence was calculated as the number of unique amino acids observed at each Ml-Fd native site. Any sites containing a gap in one or more Fd sequences was given a sequence divergence value of 20. The sequence divergence value for each Fd native site was calculated as the average for a sliding window of three sites. The UniProt numbers for each Fd are provided in Supplementary Figure 1. The FASTA formatted MSA is included as Supplementary MSA.fasta and described in Supplementary Note 1.
Growth assay.
For all growth experiments, E. coli EW11 were freshly transformed with two plasmids using electroporation, one encoding the e-donor and acceptor pair (FNR and SIR) and the other encoding either a native Fd, a C42A mutant of Ml-Fd that is unable to coordinate a 2Fe-2S cluster, or an engineered Fd. Starter cultures were inoculated using single colonies obtained by selecting for colonies containing both plasmids on LB-agar plates. These starter cultures were grown in deep-well 96-well plates for 18 hours at 37°C in 1 mL of a non-selective modified M9 medium (M9c), which contained sodium phosphate, dibasic (6.8 g/L), sodium phosphate, monobasic (3 g/L), sodium chloride (0.5 g/L), 2% glucose, ammonium chloride (1 g/L), calcium chloride (0.1 mM), magnesium sulfate (10 mM), ferric citrate (24 mg/L), p-aminobenzoic acid (2 mg/L), inositol (20 mg/L), adenine (5 mg/L), uracil (20 mg/L), tryptophan (40 mg/L), tyrosine (1.2 mg/L), and the remaining 18 amino acids (80 mg/L each). For complementation analysis, starter cultures that had been grown to stationary phase in M9c were diluted ~1:100 using a 96-well replicator pin into 100 μL of a selective modified M9 medium (M9sa), which is identical to M9c but lacks cysteine and methionine. Cells were grown in the presence of the indicated amount of inducers (aTc, IPTG, rapamycin, or 4-HT) in an Infinite m1000 Pro plate reader (Tecan) at 37°C with shaking at 250 rpm at an amplitude of 1.5 mm in double-orbital mode. Optical density (OD) measurements were taken every 10 minutes. To select for the Fd and e-donor/acceptor plasmids, all growth steps included chloramphenicol and streptomycin at 34 μg/mL and 100 μg/mL, respectively.
Fluorescence spectroscopy.
Whole cell RFP measurements were done as with the growth assay except starter cultures were diluted ~1:100 using a 96-well replicator pin into M9c medium (100 μL) and grown in an incubator at 37°C with shaking at 250 rpm. After 24 hours of growth, OD and fluorescence (λex = 560 nm, λem = 650 nm) were measured using an Infinite m1000 Pro plate reader (Tecan). Fluorescence was normalized to OD and scaled relative to the condition without chemical inducer.
Protein purification.
E. coli Rosetta transformed with pET28b containing the Ml-Fd or sFd-35-ER genes were grown at 37°C in LB medium containing 50 μg/mL kanamycin to mid-log phase, induced using 50 μM IPTG, and grown overnight at 30°C while shaking at 250 rpm. Cells harvested by centrifugation (4000g) were resuspended in 20 mL of lysis buffer (per L of culture), which contained 10 mM Tris pH 8, 5 mM dithiothreitol (DTT), 10 mg/L DNase I, and 0.5 mg/mL lysozyme. After freezing overnight at −80°C, cells were thawed and mixed with a cOmplete™ Mini, EDTA-Free protease inhibitor tablet (Sigma-Aldrich) at a ratio of one tablet per 400 mL of total cell lysate. Cell lysates were loaded onto a DE52 anion exchange column (Whatman) that had been equilibrated with TED buffer (25mM Tris pH 8, 1 mM EDTA, and 1 mM DTT), the column was washed with TED containing 200 mM NaCl, and Fe-S proteins were eluted using TED containing 300 mM NaCl. The brown eluent was diluted with TED to bring NaCl below 100 mM and loaded onto HiTrap Q XL column (GE Healthcare) that had been equilibrated with TED using an AKTA Start FPLC system (GE Healthcare). This column was washed using TED buffer, a linear gradient was run from 0 to 375 mM NaCl TED, and an isocratic 500 mM NaCl TED solution was used to elute the Fe-S proteins. SDS-page was performed using NuPage 12% Bis-Tris Gels (Invitrogen) and the PageRuler Unstained Broad Range Protein Ladder (Thermo Scientific). Samples were concentrated 20-fold using an Amicon Ultra 10K MWCO spin column. Concentrated samples were flash frozen with liquid nitrogen.
Spectroscopy.
Purified Ml-Fd and sFd-35-ER were dialyzed into TED buffer (25 mM Tris pH 8, 1 mM EDTA, 1 mM DTT). Each protein was incubated with 4-HT (100 μM) or the carrier DMSO used to dissolve 4-HT (1% of final volume) for 30 min. A J-815 spectropolarimeter (Jasco) was used to measure the ellipticity of samples from 700 nm to 300 nm. UV/Vis absorbance of samples was read using a Cary 50 UV/Vis Spectrophotometer (Varian) from 600 nm to 250 nm. Measurements used quartz cuvettes with a 1 cm path length. Experiments performed twice using two distinct protein preparations yielded the same result.
Cell lysates.
Electrocompetent E. coli EW11 were transformed with the plasmid (pSAC01) that constitutively expresses Zea mays FNR and SIR. Cells were grown to stationary phase at 37°C and 250 rpm in 2xYTPG medium (16 g/L tryptone, 10 g/L yeast extract, 5 g/L NaCl, 3 g/L KH2PO4, 7 g/L K2HPO4, and 18 g/L glucose) containing 100 μg/mL streptomycin. Cells from overnight cultures were diluted 1:100 in fresh 2xYTPG medium containing streptomycin, grown for 2 hours at 37°C while shaking at 250 rpm until mid-log phase, and then shifted to 30°C and grown for an additional 4 hours. Cell cultures were harvested by centrifugation (4000g), cells were resuspended in equal volume of S30 buffer (10 mM Tris-acetate, 14 mM magnesium acetate, 60 mM potassium acetate pH 8.2), cells were washed twice with S30 buffer, and the pellets were weighed and flash frozen with liquid nitrogen. Pellets were thawed, resuspended in 2 mL of S30 buffer per gram of cell pellet, and sonicated using a Q500 Sonicator (Qsonica) with the probe at 20 kHz and 40% maximal amplitude until samples had been exposed to ~0.5 Joules of sonication energy per μL of cell slurry36. To minimize sample overheating, sonication proceeded for 25 seconds followed by a 15 second rest period between sonication pulses. To remove native reduced cofactors like NADH and NADPH, lysates were applied to a Zeba Spin Desalting Column 7K MWCO (Thermo Scientific) that had been equilibrated with S30 buffer. Protein content in lysates was quantified using Bio-Rad Protein Assay Dye Reagent Concentrate (Bio-Rad), using bovine serum albumin (NEB) as a standard.
Sulfide production assay.
To visualize sulfide production, cells lysates were diluted 2-fold into S30 buffer to 4.8 mg/mL of total protein. Desalted lysates were diluted 1.25-fold with 5x LSR buffer, which contained 50 mM sodium sulfite, 200 μM β-Nicotinamide adenine dinucleotide 2’-phosphate reduced tetrasodium salt (NADPH), 10 μM Sulfidefluor 7 AM (Tocris)26, 670 mM potassium acetate, 50 mM ammonium acetate, 40 mM magnesium acetate, and 50 mM potassium phosphate, dibasic pH 7.2. To analyze background sulfide production from lysates alone, additional NADPH was added to the lysate/LSR mixture to achieve higher concentrations. To analyze Fd-dependent sulfide production, purified Ml-Fd or sFd-35-ER was added at the indicated concentrations to the lysate diluent S30 buffer. To limit disulfide bond formation in the ER-LBD sFd-35-ER, which has been observed in past studies42, this protein was first reduced with freshly made 1 mM DTT in S30 buffer for 20 minutes on ice and then desalted immediately before addition to the assay using a Zeba Spin Desalting Column 7K MWCO. Following NADPH or protein addition, reactions were arrayed in a Corning 96 well plate at room temperature (Costar #3603), transferred to an Infinite m1000 Pro plate reader (Tecan), and heated to 37°C and shaken at 250 rpm with fluorescence readings (λex = 495 nm, λem = 520 nm) every 15 seconds. When analyzing chemical-dependent sulfide production, 12.5 μM 4-hdyroxytamoxifen (4HT) or blank ethanol was injected into the plate following two minutes of incubation and the lysate diluent S30 buffer volume was reduced accordingly.
Midpoint potentials.
Electrochemical experiments were carried out anaerobically in a MBraun Labmaster glovebox using a PGSTAT12 potentiostat. A three-electrode configuration was used in a water-jacketed glass cell. A platinum wire was used as the counter electrode and a standard calomel electrode was used as the reference electrode. Reported potentials are relative to the standard hydrogen electrode. Baseline measurements were collected using an edge-plane graphite (EPG) electrode that was modified with a 100mM neomycin trisulfate solution, rinsed, and placed into a glass cell containing a 23.5 ºC mixed buffer solution (5mM acetate/MES/MOPS/TAPS/CHES/CAPS) pH 7.0, with 100 mM NaCl. A 5 μL aliquot of 720μM Ml-Fd or 5mM sFd-35-ER was applied directly to the electrode surface with or without 1 μL of 50mM 4-HT, the protein was allowed to reduce in size for approximately one minute at room temperature before being placed into the buffer cell solution. Square wave voltammograms were collected at 23.5 ºC with a frequency of 10Hz and electrochemical signals were analyzed using QSoas. Experiments performed twice using two distinct protein preparations yielded the same result.
Statistical analysis.
Growth assays and whole cell fluorescence data are reported as the mean and standard deviation of biologically independent samples (n=3). The sulfide production data are reported as the mean and standard deviation of independent experiments (n=3). All reported p-values were obtained using two-tailed, independent t-tests. Sample sizes were in accordance with community standards.
Supplementary Material
ACKNOWLEDGEMENTS
E. coli EW11 and the genes encoding Zea mays FNR, Zea mays SIR, and Spinacia oleracea Fd were a gift from P. Silver (Harvard University). Cellular assay development was supported by DOE grant DE-SC0014462 (G.N.B. and J.J.S.), split Fd efforts were supported by NASA grant NNX15AL28G (J.J.S. and G.N.B), domain insertion efforts were supported by ONR grant N00014–17-1–2639 (J.J.S.), and electrochemistry was supported by DOE grant DE-SC0012598 (S.J.E.). J.T.A. was supported by NSF GRFP and DOE SGCSR fellowships.
Footnotes
COMPETING INTERESTS
J.J.S., J.T.A., G.N.B, and I.J.C. have submitted a patent application (No 62/583,770) covering the use of fragmented Fds as chemical-dependent electron carriers, entitled “Regulating electron flow using fragmented proteins.”
REFERENCES
- 1.Jensen HM et al. Engineering of a synthetic electron conduit in living cells. Proceedings of the National Academy of Sciences of the United States of America 107, 19213–19218 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Webster DP et al. An arsenic-specific biosensor with genetically engineered Shewanella oneidensis in a bioelectrochemical system. Biosensors & Bioelectronics 62, 320–324 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Golitsch F, Bücking C. & Gescher J. Proof of principle for an engineered microbial biosensor based on Shewanella oneidensis outer membrane protein complexes. Biosensors & Bioelectronics 47, 285–291 (2013). [DOI] [PubMed] [Google Scholar]
- 4.West EA, Jain A. & Gralnick JA Engineering a native inducible expression system in Shewanella oneidensis to control extracellular electron transfer. ACS Synthetic Biology 6, 1627–1634 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Kracke F, Vassilev I. & Krömer JO Microbial electron transport and energy conservation – the foundation for optimizing bioelectrochemical systems. Frontiers in Microbiology 6, 575 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shomar H. et al. Metabolic engineering of a carbapenem antibiotic synthesis pathway in Escherichia coli. Nature Chemical Biology (2018). doi: 10.1038/s41589-018-0084-6 [DOI] [PubMed] [Google Scholar]
- 7.Kallio P, Pásztor A, Thiel K, Akhtar KM & Jones PR An engineered pathway for the biosynthesis of renewable propane. Nature Communications 5, 4731 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eck RV & Dayhoff MO Evolution of the Structure of Ferredoxin Based on Living Relics of Primitive Amino Acid Sequences. Science 152, 363–366 (1966). [DOI] [PubMed] [Google Scholar]
- 9.Harel A, Bromberg Y, Falkowski PG & Bhattacharya D. Evolutionary history of redox metal-binding domains across the tree of life. Proceedings of the National Academy of Sciences 111, 7042–7047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sousa FL et al. Early bioenergetic evolution. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 368, 20130088 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkinson JT, Campbell I, Bennett GN & Silberg JJ Cellular Assays for Ferredoxins: A Strategy for Understanding Electron Flow through Protein Carriers That Link Metabolic Pathways. Biochemistry 55, 7047–7064 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Hall D, Cammack R. & Rao K. Role for ferredoxins in the origin of life and biological evolution. Nature 233, 136–8 (1971). [DOI] [PubMed] [Google Scholar]
- 13.Barstow B. et al. A synthetic system links FeFe-hydrogenases to essential E. coli sulfur metabolism. Journal of Biological Engineering 5, 7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Xie X, Yang M, Dixon R. & Wang Y-P Modular electron-transport chains from eukaryotic organelles function to support nitrogenase activity. Proceedings of the National Academy of Sciences of the United States of America 114, E2460–E2465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlesier J, Rohde M, Gerhardt S. & Einsle O. A Conformational Switch Triggers Nitrogenase Protection from Oxygen Damage by Shethna Protein II (FeSII). Journal of the American Chemical Society 138, 239–247 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Milton RD et al. The In Vivo Potential-Regulated Protective Protein of Nitrogenase in Azotobacter vinelandii Supports Aerobic Bioelectrochemical Dinitrogen Reduction In Vitro. Journal of the American Chemical Society 139, 9044–9052 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Angeleri M, Zorina A, Aro E-M & Battchikova N. Interplay of SpkG kinase and the Slr0151 protein in the phosphorylation of ferredoxin 5 in Synechocystis sp. strain PCC 6803. FEBS letters 592, 411–421 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Moscatiello R. et al. Identification of ferredoxin II as a major calcium binding protein in the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. BMC microbiology 15, 16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rumpel S. et al. Enhancing hydrogen production of microalgae by redirecting electrons from photosystem I to hydrogenase. Energy Environ. Sci. 7, 3296–3301 (2014). [Google Scholar]
- 20.Eilenberg H. et al. The dual effect of a ferredoxin-hydrogenase fusion protein in vivo: successful divergence of the photosynthetic electron flux towards hydrogen production and elevated oxygen tolerance. Biotechnology for Biofuels 9, 182 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yacoby I. et al. Photosynthetic electron partitioning between [FeFe]-hydrogenase and ferredoxin:NADP+-oxidoreductase (FNR) enzymes in vitro. Proceedings of the National Academy of Sciences of the United States of America 108, 9396–9401 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellor S. et al. Fusion of Ferredoxin and Cytochrome P450 Enables Direct Light-Driven Biosynthesis. ACS chemical biology 11, 1862–1869 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein V. & Alexandrov K. Synthetic protein switches: design principles and applications. Trends in Biotechnology 33, 101–110 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Pelletier J, Campbell-Valois F. & Michnick S. Oligomerization domain-directed reassembly of active dihydrofolate reductase from rationally designed fragments. Proceedings of the National Academy of Sciences of the United States of America 95, 12141–12146 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas EE, Pandey N, Knudsen S, Ball ZT & Silberg JJ Programming Post-Translational Control over the Metabolic Labeling of Cellular Proteins with a Noncanonical Amino Acid. ACS synthetic biology 6, 1572–1583 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guntas G, Mansell TJ, Kim J. & Ostermeier M. Directed evolution of protein switches and their application to the creation of ligand-binding proteins. Proceedings of the National Academy of Sciences of the United States of America 102, 11224–11229 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fish A, Danieli T, Ohad I, Nechushtai R. & Livnah O. Structural basis for the thermostability of ferredoxin from the cyanobacterium Mastigocladus laminosus. Journal of Molecular Biology 350, 599–608 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Segall-Shapiro TH et al. Mesophilic and hyperthermophilic adenylate kinases differ in their tolerance to random fragmentation. Journal of Molecular Biology 406, 135–148 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Nakayama M, Toyota H, Kurisu G. & Hase T. Structural and mutational studies of an electron transfer complex of maize sulfite reductase and ferredoxin. Journal of Biochemistry 160, 101–109 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Kurisu G. et al. Structure of the electron transfer complex between ferredoxin and ferredoxin-NADP(+) reductase. Nature Structural Biology 8, 117–121 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Reinke AW, Grant RA & Keating AE A synthetic coiled-coil interactome provides heterospecific modules for molecular engineering. Journal of the American Chemical Society 132, 6025–6031 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michnick S, Rosen M, Wandless T, Karplus M. & Schreiber S. Solution structure of FKBP. Science (New York, N.Y.) 252, 836–839 (1991). [DOI] [PubMed] [Google Scholar]
- 33.Shiau A. et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95, 927–937 (1998). [DOI] [PubMed] [Google Scholar]
- 34.Paige L. et al. Estrogen receptor (ER) modulators each induce distinct conformational changes in ER alpha and ER beta. Proceedings of the National Academy of Sciences of the United States of America 96, 3999–4004 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stephens P. et al. Circular dichroism and magnetic circular dichroism of iron-sulfur proteins. Biochemistry 17, 4770–8 (1978). [DOI] [PubMed] [Google Scholar]
- 36.Ta D. & Vickery L. Cloning, sequencing, and overexpression of a [2Fe-2S] ferredoxin gene from Escherichia coli. The Journal of Biological Chemistry 267, 11120–11125 [PubMed] [Google Scholar]
- 37.Bak DW, Zuris JA, Paddock ML, Jennings PA & Elliott SJ Redox Characterization of the FeS Protein MitoNEET and Impact of Thiazolidinedione Drug Binding. Biochemistry 48, 10193–10195 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aliverti A. et al. Biochemical and crystallographic characterization of ferredoxin-NADP(+) reductase from nonphotosynthetic tissues. Biochemistry 40, 14501–14508 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Hirasawa M, Nakayama M, Hase T. & Knaff DB Oxidation-reduction properties of maize ferredoxin: sulfite oxidoreductase. Biochimica Et Biophysica Acta 1608, 140–148 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Bak DW & Elliott SJ Alternative FeS cluster ligands: tuning redox potentials and chemistry. Current Opinion in Chemical Biology 19, 50–58 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Lin VS, Lippert AR & Chang CJ Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production. Proceedings of the National Academy of Sciences of the United States of America 110, 7131–7135 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navarro F, Martín-Figueroa E, Candau P. & Florencio F. Ferredoxin-dependent iron-sulfur flavoprotein glutamate synthase (GlsF) from the Cyanobacterium synechocystis sp. PCC 6803: expression and assembly in Escherichia coli. Archives of biochemistry and biophysics 379, 267–76 (2000). [DOI] [PubMed] [Google Scholar]
- 43.Mulholland SE, Gibney BR, Rabanal F. & Dutton LP Characterization of the Fundamental Protein Ligand Requirements of [4Fe-4S]2+/+Clusters with Sixteen Amino Acid Maquettes. Journal of the American Chemical Society 120, 10296–10302(1998). [Google Scholar]
- 44.Salis HM, Mirsky EA & Voigt CA Automated design of synthetic ribosome binding sites to control protein expression. Nature Biotechnology 27, 946–950 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
METHODS-ONLY REFERENCES
- 45.Engler C, Kandzia R. & Marillonnet S. A One Pot, One Step, Precision Cloning Method with High Throughput Capability. PLoS ONE 3, e3647 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.