Abstract
Background
Near-death experiences (NDE) occur with imminent death and in situations of stress and danger but are poorly understood. Evidence suggests that NDE are associated with rapid eye movement (REM) sleep intrusion, a feature of narcolepsy. Previous studies further found REM abnormalities and an increased frequency of dream-enacting behavior in migraine patients, as well as an association between migraine with aura and narcolepsy. We therefore investigated if NDE are more common in people with migraine aura.
Methods
We recruited 1,037 laypeople from 35 countries and five continents, without any filters except for English language and age ≥18 years, via a crowdsourcing platform. Reports were validated using the Greyson NDE Scale.
Results
Eighty-one of 1,037 participants had NDE (7.8%; CI [6.3–9.7%]). There were no significant associations between NDE and age (p > 0.6, t-test independent samples) or gender (p > 0.9, Chi-square test). The only significant association was between NDE and migraine aura: 48 (6.1%) of 783 subjects without migraine aura and 33 (13.0%) of 254 subjects with migraine aura had NDE (p < 0.001, odds ratio (OR) = 2.29). In multiple logistic regression analysis, migraine aura remained significant after adjustment for age (p < 0.001, OR = 2.31), gender (p < 0.001, OR = 2.33), or both (p < 0.001, OR = 2.33).
Conclusions
In our sample, migraine aura was a predictor of NDE. This indirectly supports the association between NDE and REM intrusion and might have implications for the understanding of NDE, because a variant of spreading depolarization (SD), terminal SD, occurs in humans at the end of life, while a short-lasting variant of SD is considered the pathophysiological correlate of migraine aura.
Keywords: Cardiac arrest, Coma, Consciousness, Intensive care, Migraine, Migraine aura, Near-death experience, Out-of-body experience, Rapid eye movement sleep, Spreading depolarization
Introduction
Near-death experiences (NDE) include emotional, self-related, spiritual and mystical perceptions and feelings, occurring in situations close to death or in other situations of imminent physical or emotional danger (Greyson, 1983; Parnia et al., 2014). Common themes of NDE comprise, but are not restricted to, out-of-body experiences, visual and auditory hallucinations, distortion of time perception, and increased speed of thoughts (Greyson, 1983). See Peinkhofer, Dreier & Kondziella (2019) for a recent overview on NDE.
The neuronal mechanisms of NDE are poorly understood. Nelson and colleagues previously proposed the concept that rapid eye movement (REM) sleep intrusion and REM related out-of-body experiences could occur at the time of a life-threatening event and might explain many elements of NDE (Nelson et al., 2006; Nelson, Mattingly & Schmitt, 2007). REM sleep is defined by rapid and random saccadic eye movements, loss of muscle tone, vivid dreaming, and cortical activation as revealed by desynchronization of the scalp electroencephalography (EEG). REM state features can intrude into wakefulness, both in healthy individuals and patients with narcolepsy. This may cause visual and auditory hallucinations at sleep onset (hypnagogic) or upon awakening (hypnopompic) and muscle atonia with sleep paralysis and cataplexy (Scammell, 2015). According to the hypothesis of Nelson and colleagues, danger provokes the arousal of neural pathways that, when stimulated, are known to generate REM-associated responses. This was interpreted as a “diathesis-stress model” (Nelson et al., 2006; Long & Holden, 2007). In this model, an unusually sensitive arousal system (i.e., the diathesis), as evidenced by the experience of REM intrusion, would predispose people to NDE in situations of stress and danger. To test their hypothesis, Nelson et al. (2006) conducted a survey comparing a group of individuals with self-reported NDE and an age- and sex-matched control group. The results suggested that episodes of REM intrusion are more common in individuals with NDE.
The study by Nelson et al. has been criticized (Long & Holden, 2007), however, which recently inspired us to carry out a follow-up study in a different setting to address some of the criticism (Kondziella, Dreier & Olsen, 2019). For example, Long & Holden (2007) pointed out that 40% of the people with NDE in the Nelson study denied ever having experienced an episode of REM intrusion, suggesting that there may be a link between the two phenomena, but not a 1:1 relationship. In our crowdsourced survey, 106 of 1,034 participants reported NDE according to a Greyson NDE scale (GNDES) score ≥7, and 50 (47%) of these individuals fulfilled the criteria of REM intrusion according to almost the identical questionnaire that Nelson and colleagues had used (Kondziella, Dreier & Olsen, 2019). In contrast, only 17% of individuals without NDE reported REM intrusions. Based on multivariate regression analysis, we found that REM intrusion is a predictor of NDE (Kondziella, Dreier & Olsen, 2019). Thus, we confirmed the results of Nelson and colleagues, but also the limitation that this is not a 1:1 relationship.
A more central point of criticism was related to the control group in Nelson and colleagues’ study which consisted mainly of medical personnel, a likely selection bias (Long & Holden, 2007). We countered this in our survey with a crowdsourced approach in which the control group originated from the same population as the NDE group (i.e., unprimed laypeople) (Kondziella, Dreier & Olsen, 2019). Our survey was announced under the headline “Survey on NDE and (related experiences),” but we did not provide further information about the content of the study. Participants were informed that their monetary reward was fixed, regardless of whether they would report having had an NDE or not. Then, we asked the participants to complete a questionnaire comprising demographic information, followed by the questions about REM intrusion. Subsequently, participants were asked if they ever had experienced an NDE. If not, the survey ended there; if yes, participants were asked in detail about this experience and information about all 16 GNDES items was collected (Kondziella, Dreier & Olsen, 2019). In this way, we think that we were able to dispel the previous criticism regarding the control group.
Long & Holden (2007) also explained how the questionnaire for REM intrusion could be misinterpreted by people with NDE, possibly leading to an overestimation of the association between REM intrusion and NDE. It is indeed difficult to address this problem with a questionnaire containing only closed questions. Therefore, we also gave our participants the opportunity to describe their experiences in their own words (Kondziella, Dreier & Olsen, 2019).
Another approach to address this problem is to investigate if comorbidities of REM intrusion, which might be easier to detect with a questionnaire, are associated with NDE too. In this context, it is interesting that REM sleep abnormalities have been linked to migraine. Thus, recurrent vivid dreams are associated with migraine attacks (Lippman, 1954); migraine attacks often occur during REM sleep (Levitan, 1984); hallucinations are not infrequent in people with migraine (Lippman, 1951, 1953; Daniel & Donnet, 2011); migraine patients exhibit increased REM sleep and prolonged REM sleep latencies (Drake et al., 1990); and they show a significantly increased frequency of dream-enacting behavior (Suzuki et al., 2013). In addition, several studies found an association between migraine and narcolepsy, a disorder involving REM intrusion (Dahmen et al., 1999, 2003; Longstreth et al., 2007; Suzuki et al., 2015; Yang et al., 2017). For example, Yang et al. (2017) found a consistently higher risk of developing narcolepsy in children with migraine compared to those without, and this risk was particularly high in children with migraine with aura.
On this basis, we hypothesized that, analogous to an unusually sensitive arousal system underlying REM intrusion, an increased susceptibility of the brain to spreading depolarization (SD), the assumed pathophysiological correlate of migraine aura (Fig. 1A), could predispose people to NDE. To test this hypothesis, we recruited a large global sample of laypersons and investigated if the lifetime occurrence of migraine aura is more common in people with NDE.
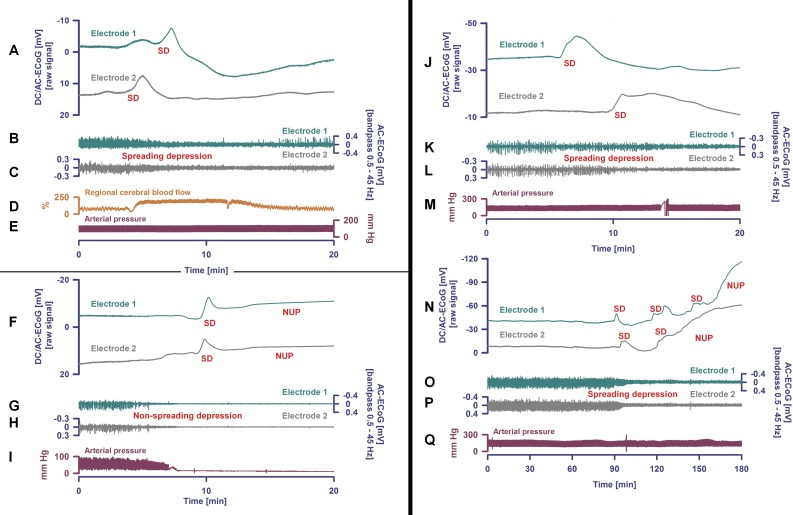
Figure 1. Spreading depolarization occurs both in migraine aura and in the dying human brain.
Examples from three patients with spreading depolarizations. Patient 1 (A–E). Spreading depolarization (SD) is observed as a large negative direct current (DC) shift propagating between different electrodes (A) (subdural full-band DC/alternate current (AC)-electrocorticography (ECoG) between 0 and 45 Hz, electrode separation: one cm) (Dreier et al., 2017). This short-lasting SD was recorded in a patient with aneurysmal subarachnoid hemorrhage (aSAH) in a metabolically largely intact and sufficiently perfused neocortex region. Based on measurements of regional cerebral blood flow (rCBF) using intracarotid 133Xe and positron emission tomography, blood-oxygen-level dependent (BOLD) imaging with functional magnetic resonance imaging (MRI) and magnetoencephalography (MEG), it is assumed that the SD underlying a migraine aura should be largely similar (Dreier & Reiffurth, 2015). The patient’s perception of a migraine aura is presumably triggered by the SD-induced spreading depression of spontaneous activity (Dreier & Reiffurth, 2015), which is shown here in (B) and (C) as a transient reduction in amplitudes propagating between electrodes (frequency band: 0.5–45 Hz). It should be noted, however, that a patient can only perceive a migraine aura if this spreading depression propagates through an eloquent region of the brain (Dreier & Reiffurth, 2015). SD is characterized by the almost complete collapse of ion gradients across cell membranes, causing water influx and an almost complete loss of Gibbs’ free energy contained in the ion gradients (Dreier et al., 2013). Recovery from SD requires activation of adenosine triphosphate (ATP)-dependent membrane pumps, in particular Na, K-ATPases. Therefore, tissue ATP declines by ca. 50% during SD not only in energy-deprived but also in well-nourished tissue (Dreier & Reiffurth, 2015). Consequently, rCBF significantly increases (D) in normal tissue to meet the enhanced energy demand and to clear the tissue of metabolites (measurement of rCBF using an optoelectrode and laser-Doppler flowmetry). The regional hyperemia is variably followed by a mild rCBF decrease (oligemia) during which the vascular reactivity is disturbed. The short initial hypoperfusion is an abnormality here that indicates mild impairment of the neurovascular coupling in the context of aSAH (Dreier & Reiffurth, 2015). The arterial blood pressure (E) measured in the radial artery was stable during the SD. Patient 2 (F–I). The second patient died from hepatorenal failure several days after aSAH (Dreier et al., 2018). The circulatory arrest is evidenced by the drop in arterial blood pressure (I). About 35 seconds after the circulatory arrest, the AC-ECoG in traces (G) and (H) begin to show the non-spreading depression of spontaneous activity. Phase 2 lasts 95 s at electrode 2 (H). Thereafter, the terminal SD occurs and spreads further from electrode 2 to electrode 1 (electrode separation: one cm) (F). Terminal SD consists of the initial SD component and the late negative ultraslow potential (NUP). It remains speculative if NDE can occur in ECoG phases 1, 2 or 3. According to current knowledge, however, the occurrence of NDE in phases 2 or 3 cannot be ruled out. As explained in the main text, ECoG and scalp electroencephalography (EEG) show a flat line in phase 2, but experiments in animals and brain slices with sophisticated electrophysiological techniques including patch-clamping have shown that the synaptic terminals remain highly active in this phase and that the neurons are polarized (Müller & Somjen, 2000; Fleidervish et al., 2001; Allen, Rossi & Attwell, 2004; Revah et al., 2016). Therefore, we cannot exclude with certainty that patients may experience a perception at this stage. The terminal depolarization takes place in phase 3. It cannot be excluded either that this may be associated with bright light phenomena or tunnel vision similar to what occurs during a migraine aura. Brain cells die only gradually in phase 4 which is characterized by the NUP (F). Patient 3 (J–Q). After onset of the terminal cluster of SDs shown in (J) and (N), this patient with aSAH was found to have lost brainstem reflexes with fixed dilated pupils, indicating the development of brain death (Dreier et al., 2019). The cluster starts here at electrode 1 and propagates to electrode 2 (J and N). The first SD occurs in electrically active tissue and therefore causes spreading depression of the spontaneous ECoG activity (K and L). In contrast to Patient 1 (B and C), however, activity depression then persists (O and P). After the first SD, a second SD occurs, which transforms into a NUP (N). In contrast to Patient 2 (F), further SDs are superimposed on the NUP, but their amplitudes become smaller and smaller (N). Like in patient 1 (E), the arterial blood pressure (Q) remains stable during the SDs and the NUP. The patient was terminally extubated 20 h later and shortly thereafter a circulatory arrest developed without further SD (Dreier et al., 2019). Data from Patient 2 (Dreier et al., 2018) and Patient 3 (Dreier et al., 2019) are presented here in abbreviated form to illustrate the pivotal aspects of brain death at the tissue level. The patients were enrolled at the Charité—Universitätsmedizin Berlin in research protocols of invasive neuromonitoring approved by the local ethics committee and written informed consent was obtained from the patients’ legally authorized representative, as described previously (Dreier et al., 2018, 2019).
Materials and Methods
Study design
Our objective was to investigate whether people with a history of migraine aura are more likely to have NDE, and vice versa, than people without migraine aura. We used an online platform, Prolific Academic (https://prolific.ac/), to recruit an international sample of laypeople. Like Amazon’s Mechanical Turk, Prolific Academic is a crowdsourcing online platform to recruit human subjects that can be used for research purposes (Kondziella, Dreier & Olsen, 2019; Kondziella, Cheung & Dutta, 2019) and that compares favorably in terms of data quality, including honesty and diversity of participants (Peer et al., 2017). Participants were recruited without any filters except for English language and age ≥18 years, and we excluded participants who had been enrolled in our previous study on NDE and REM intrusion (Kondziella, Dreier & Olsen, 2019). The study was announced under the headline “Survey on NDE and headache” using the following text: “We wish to explore the frequency with which NDE occur in the public. This should take no more than 1.5 min on average (a little bit longer, if you have had such an experience, and a little bit less, if you haven’t). You will be paid 0.20$ after completing the survey. Please note that we might use your anonymous answers when writing a paper.”
From all participants, we collected information about age, gender, place of residence and employment status (data provided automatically by Prolific Academic); if they had frequent headaches; if yes, if these headaches could last longer than 4 h and were associated with visual or non-visual aura (Kaiser et al., 2019); if participants ever had an NDE; if yes, if this experience occurred in a truly life-threatening situation or in a situation that just felt so; if the experience was neutral, pleasant or unpleasant; and all participants with an NDE were asked to provide information about all 16 items of the GNDES, the most widely used standardized tool to identify, confirm and characterize NDE in research (Greyson, 1983). Like in our previous study (Kondziella, Dreier & Olsen, 2019), NDE was defined by a GNDES score ≥7. We evaluated all reports of NDE, irrespective of whether they occurred in truly life-threatening situations or in situations that just felt so. Participants with an NDE (and those who claimed an NDE but scored six or less points on the GNDES) were also given the opportunity to describe this in their own words (optional). We did not inquire about REM sleep intrusion, which we assessed in our previous study (Kondziella, Dreier & Olsen, 2019). Table 1 provides details.
Table 1. Questionnaire on headaches, migraine aura and near-death experiences.
Questions about headache (adapted from Kaiser et al. (2019))
|
Questions about near-death experiences
|
GNDES (zero to two points for each answer; based on Greyson (1983).
|
Note:
In contrast to the Greyson Near-Death Experience Scale (GNDES), we also questioned about unpleasant experiences.
Statistics
Using a very high population size (300,000,000), a confidence level of 95% and a margin of error of 5%, we estimated the required sample size to be 384 participants. However, since previous studies have estimated the frequency with which NDE occur in the public to be 5–10%, including our own on NDE and REM intrusion (Kondziella, Dreier & Olsen, 2019), we decided to enroll approximately 1,000 participants to identify an estimated number of 100 individuals with an NDE.
In univariate analysis, associations between potential predictors (age, gender, migraine aura) for NDE were examined using Chi-square test and t-test for independent samples. Additionally, we used multivariable logistic regression to analyze the association between migraine aura and NDE adjusted for age and gender. The level of significance was 0.05 (two-sided) for all statistical tests. Statistical analysis was performed with SPSS 23.0 (IBM, Armonk, NY, USA).
Ethics
Participants gave consent for publication of their anonymous data. Participation was voluntary, anonymous and restricted to those aged 18 years or older. Participants received a monetary reimbursement after completing the survey, in accordance with the Prolific Academic’s ethical rewards principle (≥$6.50/h). The Ethics Committee of the Capital Region of Denmark waives approval for online surveys (Section 14 (1) of the Committee Act. 2; http://www.nvk.dk/english).
Data availability statement
The de-identified raw data are provided in the Supplemental Files.
Results
We recruited 1,037 laypeople from 35 countries and five continents (mean age: 31 years, standard deviation: 11.1 years, median age: 28 years, interquartile range (IQR): 23–36 years; 76% fully or part-time employed or in training), most of which were residing in Europe and North America (Fig. S1). Six participants identified themselves as transgender, 531 (52%) as female, and 500 (48%) as male.
Near-death experiences: frequency and phenomenology
A total of 286 participants (28% (95% CI [25–30%])) claimed an NDE. The most frequent symptoms were abnormal time perception (faster or slower than normal; reported by 257 participants; 90%); extraordinary speed of thoughts (n = 169; 59%); exceptional vivid senses (n = 165; 58%); and feeling separated from one’s body, including out-of-body experiences (n = 113; 40%). Participants perceived the situation in which they made their experience slightly more often as truly life-threatening (n = 165; 58%) than not (n = 121; 42%).
However, only 81 of 286 individuals who claimed an NDE reached the threshold of ≥7 points on the GNDES (28% (95% CI [23–34%])). Hence, confirmed NDE were reported by 81 of 1,037 participants (8% (95% CI [6.3–9.7%])) (Figs. 2 and 3). Confirmed NDE were perceived much more often as pleasant (n = 29; 49%) than experiences that did not qualify as NDE according to the GNDES (n = 21; 13%; p < 0.0001; Chi-square test; neutral experiences excluded). Table 2 provides selected written reports from participants with an NDE of ≥7 GNDES points and Table 3 from participants with <7 GNDES points.
Figure 2. Schematic overview of the study design.
Of 61.707 eligible lay people registered with Prolific Academic (https://prolific.ac/; accessed on February 4, 2019), we enrolled 1,037 participants; 81 (7.8% (95% CI [6.3–9.7%])) of whom reported a near-death experience that fulfilled established criteria (Greyson Near-Death Experience Scale score of 7 or higher). n, number of participants; NDE, near-death experience.
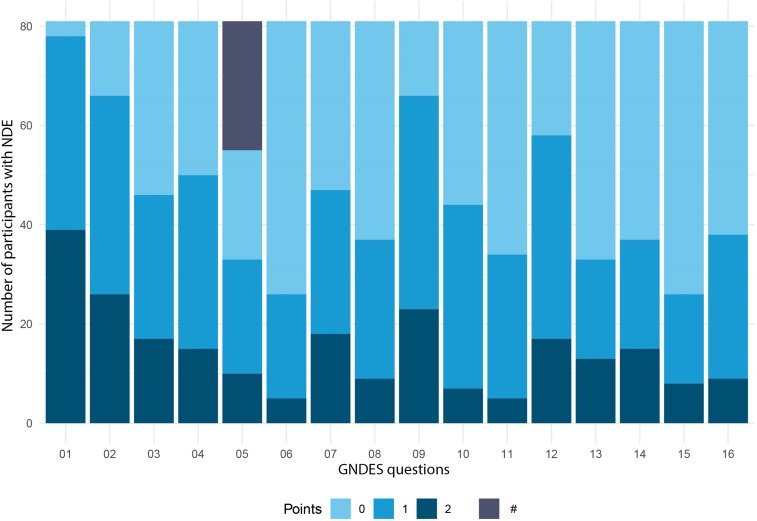
Figure 3. Experiences of participants with NDE.
This figure illustrates the experiences of 81 participants with NDE confirmed by the Greyson Near-Death Experience Scale (GNDES), that is, those with seven points or more on the GNDES. See Table 1 for GNDES questions 1–16. Each question is given zero to two points (0 = “no”; 1 and 2 = “yes,” weighted according to the intensity of the feature experienced). #—unpleasant experience (question 5); in contrast to the GNDES, we also inquired about unpleasant experiences.
Table 2. Selected reports from participants with an experience that reached the threshold of ≥7 points on the Greyson Near-Death Experience Scale (GNDES) and qualified as an NDE.
The last two comments describe experiences during ingestion of ketamine (which has been suggested as the chemical most likely to cause drug-induced near-death experiences (Martial et al., 2019)) and REM sleep disturbance (which has been identified in another recent study as a likely mechanism of near-death experiences (Kondziella, Dreier & Olsen, 2019)). Comments are edited for clarity and spelling.
|
Table 3. Selected reports from participants with an experience below the threshold of ≥7 points on Greyson Near-Death Experience Scale (GNDES) and that did not qualify as an NDE.
Comments are edited for clarity and spelling.
|
Headache and migraine aura
Seven-hundred-twenty of 1,037 individuals (69%) answered “yes” to the following question about a primary headache disorder: “Do you get headaches that are NOT caused by a head injury, hangover, or an illness such as the cold or the flu?” The male-to-female ratio of people who responded “yes” to this question was 1:1.3. Two-hundred-fifty-four of 1,037 individuals (24%) fulfilled criteria (Kaiser et al., 2019) of having experienced a migraine aura at any point during their lifetime. Individuals could have different types of migraine aura. Two-hundred-thirty of 254 (91%) individuals reported having had visual auras, 60 (24%) somato-sensory auras, 49 (19%) motor auras and 21 (8%) aphasic/dysarthric auras. Hundred-seventy-four of 531 women (33%) had a migraine aura and 77 of 500 men (15%). This difference was statistically significant (p < 0.001, Chi-square test; male-to-female ratio: 1:2.2). People with migraine aura were slightly older than people without migraine aura (median age: 30 (IQR: 24–38) years vs median age: 28 (IQR: 22–36) years, p = 0.005, Mann–Whitney Rank Sum Test).
Near-death experiences and evidence of migraine aura
There were no significant associations between confirmed NDE and age (p > 0.6, t-test independent samples) or gender (p > 0.9, Chi-square test). The only significant association was between confirmed NDE and migraine aura: 48 (6.1%) of 783 subjects without migraine aura and 33 (13.0%) of 254 subjects with migraine aura had experienced an NDE (p < 0.001, odds ratio (OR) = 2.29). In multiple logistic regression analysis with age, gender and the interaction of age and gender, none of these potential predictors was significant. However, migraine aura remained significant after adjustment for age (p < 0.001, OR = 2.31), gender (p < 0.001, OR = 2.33), and both age and gender (p < 0.001, OR = 2.33).
Discussion
The prevalence of NDE
The prevalence of individuals with an NDE is estimated at about 4–8% in the general population (Gallup & Proctor, 1982; Knoblauch, Schmied & Schnettler, 2001; Perera, Padmasekara & Belanti, 2005; Facco & Agrillo, 2012; Chandradasa et al., 2018). In our survey, it was 8%. We found a prevalence of 10% using the same criteria in our previous crowdsourcing online survey on NDE and REM intrusion (Kondziella, Dreier & Olsen, 2019), indicating that this prevalence is quite robust. Unlike most previous reports in which NDE were almost always associated with peace and well-being (Thonnard et al., 2013; Charland-Verville et al., 2014; Martial et al., 2017, 2018; Cassol et al., 2018), we confirmed recent findings that many people find their NDE unpleasant (Charland-Verville et al., 2015; Kondziella, Dreier & Olsen, 2019; Cassol et al., 2019). However, experiences with the cut-off score of ≥7 GNDES points were reported more often as pleasant (49%) than experiences with a lower score (13%).
Migraine aura is a predictor of NDE
Migraine aura was a predictor of NDE in our sample. This association was very stable. Regardless of whether either no adjustment, an adjustment for age, for sex or for both was performed, the ORs for migraine aura only varied between 2.29 and 2.33. However, a potential limitation of our study is the announcement of the internet query in which we stated that we would investigate for NDE and headache. This might have attracted more people with NDE and headache. The overall prevalence for all types of primary headache, including tension-type headache, was 69% in our survey. Tension-type headache is the most common form of headache (Jensen, 2018). Its aggregate prevalence in the general population across different studies was 38% (Jensen, 2018). Yet, in a population-based study in Denmark, a much higher lifetime prevalence of 78% was found (Lyngberg et al., 2005; Jensen, 2018). The high prevalence of primary headaches in our survey is hence within the realm of possibility but raises the question if we have attracted a disproportionate number of people with headache. This could include people with migraine with aura. The observation that 24% of the participants in our survey met criteria for a migraine aura, while population-based studies have estimated this prevalence at only 4% in the general population, renders this indeed likely (Russel et al., 1995). The young average age, typical of an Internet-based study, could have contributed to over-representation of migraineurs with aura. The way we phrased our headache questions could be another reason, as we did not intend to validate a migraine diagnosis according to established criteria (Kaiser et al., 2019). Instead, we used a more inclusive approach to identify people with a high likelihood of having migraine aura because we were not interested in migraine per se but rather in migraine aura as a possible predictor for an NDE (Kaiser et al., 2019). Since population-based studies suggest that spontaneous migraine aura is four times less common in people without typical migraine headache than in people with typical migraine headache (Russel et al., 1995), it is unlikely that the over-representation of people with migraine aura in our survey resulted from the fact that we also included people with migraine aura without typical migraine headache. However, we did not ask whether the aura symptoms lasted at least 5 min (It should be noted that the threshold of >5 min to classify as migraine aura is arbitrary). Accordingly, in humans it has been shown that SD, the pathophysiological correlate of migraine aura, may occur in spatially very limited fields and that the propagation speed in the cortical tissue ranges between ~2 and nine mm/min (Woitzik et al., 2013). On one hand, this could have contributed to the discrepancy between our data and population-based migraine studies. On the other hand, the male-to-female ratio in individuals with migraine aura was 1:2.2 in our survey, which is well in line with the results of population-based studies and supports that we indeed detected variants of migraine aura (Russel et al., 1995). In contrast, the male-to-female ratio of a primary headache disorder, be it tension-type headache, migraine or a rarer headache, was 1:1.3 overall. This ratio is again well in line with the assumption that the vast majority of primary headache sufferers in our survey had episodic tension-type headache (Jensen, 2018).
The recurrent burden of headache may have increased motivation to participate in our survey, although this remains entirely speculative. The important question, however, is whether the combination of NDE and migraine aura disproportionately increased the motivation of affected people to join our study. Mathematically, we deal with three random factors: migraine aura (yes/no), NDE (yes/no), and participation (yes/no). The twofold dependencies between participation and migraine aura or NDE appear unproblematic. In contrast, a threefold dependency between participation, migraine aura and NDE could have produced a spurious association. However, we consider this unlikely because, for instance, the entire survey was finished during such a short time frame (i.e., within 3 h after posting the survey online) that word-of-mouth communication of the survey’s topic seems very unlikely.
Internet-based surveys and more traditional mail-based questionnaires or laboratory-based studies each have their advantages and disadvantages (Kaiser et al., 2019). We suggest that a combination of the different approaches is more meaningful than using just one method (Kondziella, Dreier & Olsen, 2019). On one side, complex clinical and ethical concepts cannot be fully captured by an online survey (Woods et al., 2015; Peer et al., 2017). For instance, we did not inquire about precipitating factors/contexts in which participants had experienced their NDE (although we did so in our previous study on NDE and REM intrusion (Kondziella, Dreier & Olsen, 2019)). Future non-internet-based studies will therefore be necessary to verify that NDE and migraine aura are indeed associated. On the other side, the anonymous character of a crowdsourcing online survey decreases the influence of psychological bias (Woods et al., 2015; Peer et al., 2017), because there is no incentive to satisfy the investigator by exaggerating or inventing memories. There was no monetary incentive in our survey either, since we instructed participants that their reimbursement would be the same regardless of whether they reported an NDE or headache or not. In addition, we recruited a much larger sample than would have been feasible during a conventional survey. Although participants from Europe and North America made up the largest share, ours was indeed a global sample with people from 35 countries and five continents.
NDE and the neurobiology of dying
In the largest prospective multi-center observational trial on AWAreness during Resuscitation (AWARE), 46% of 140 survivors reported memories following their cardiac arrest with seven major cognitive themes (Parnia et al., 2014). Nine percent of the survivors met the criteria for an NDE according to the GNDES. Two percent described awareness with explicit memories of “seeing” or “hearing” real events related to their resuscitation. Importantly, one patient had a verifiable period of conscious awareness during which time cerebral function was not expected (Parnia et al., 2014). Although speculative, it seems likely that there is a neurobiological basis for this observation (Nelson et al., 2006; Parnia et al., 2014; Martial et al., 2019; Peinkhofer, Dreier & Kondziella, 2019). The pathophysiological events that occur during the process of dying are of obvious interest in this regard (Vrselja et al., 2019). The transition from life to death is thus characterized by four major events: loss of circulation, loss of respiration, loss of spontaneous electrocorticography (ECoG) activity, and a terminal SD without repolarization. These four events occur always, but not necessarily in the same order (Dreier et al., 2018, 2019; Carlson et al., 2018). In the most common scenario, arrest of systemic circulation, respiration and ECoG activity develops more or less simultaneously, while terminal SD follows the complete arrest of ECoG activity with a latency of 13–266 s (Dreier et al., 2018). Along this sequence, the invasively recorded direct current (DC)/alternate (AC)-ECoG activity can be roughly divided into four different phases which are illustrated with an original recording from a previous study (Dreier et al., 2018) in Fig. 1B: In phase 1, spontaneous ECoG activity is still measurable; phase 2 is characterized by a complete loss of ECoG activity starting simultaneously in different cortical regions and layers, which is referred to as non-spreading depression of spontaneous activity (Dreier, 2011); in phase 3, the terminal SD starts but, from a phenomenologically point of view, is initially similar to SD spreading in healthy grey brain matter (Fig. 1A) (Dreier & Reiffurth, 2015; Hartings et al., 2017a); and finally, in phase 4 a negative ultraslow potential signals the second phase of terminal SD (Oliveira-Ferreira et al., 2010; Hartings et al., 2017b; Dreier et al., 2018, 2019; Lückl et al., 2018; Carlson et al., 2018).
The pertinent question arising from the AWARE study is whether phase 2 and (the transition to) phase 3 are compatible with a conscious perception by the patient—and hence, might contribute to the pathophysiological mechanisms of an NDE. On closer examination of the experimental data, it is interesting that the non-spreading depression of spontaneous ECoG activity in phase 2 does not result from a loss of synaptic activity, but on the contrary from vesicular release of various transmitters, including GABA and glutamate, leading to an incoherent, massive increase in miniature excitatory and inhibitory postsynaptic potentials that replace the normal postsynaptic potentials (Fleidervish et al., 2001; Allen, Rossi & Attwell, 2004; Revah et al., 2016). This probably leads to gradual depletion of the releasable pool of vesicles in the synaptic terminals, and thereby significantly distorts neuronal interactions (Fleidervish et al., 2001; Revah et al., 2016) (Not only are the miniature potentials small, but the abnormal neuronal desynchronization also prevents these potentials from summing-up, which precludes their measurement using comparatively insensitive methods such as subdural and intracortical ECoG or the even cruder scalp EEG). Initially, neurons are hyperpolarized (Tanaka et al., 1997; Müller & Somjen, 2000). Over time, intracellular calcium and extracellular potassium concentrations gradually increase, while extracellular pH decreases (Kraig, Ferreira-Filho & Nicholson, 1983; Mutch & Hansen, 1984; Nedergaard & Hansen, 1993; Erdemli, Xu & Krnjevic, 1998; Müller & Somjen, 2000; Dreier et al., 2002). Eventually, hyperpolarization turns into neuronal depolarization. When the adenosine triphosphate (ATP) stores are exhausted, ATP-dependent membrane pumps such as the Na, K-ATPase become unable to replenish the leaking ions. Consequently, SD erupts at one or more sites of the cortical tissue and spreads into the environment as a giant wave of depolarization. It is important to understand that this terminal SD marks the onset of the toxic cellular changes that ultimately lead to death, but it is not a marker of death per se, since the SD is reversible—to a certain point—with restoration of the circulation (Hossmann & Sato, 1970; Heiss & Rosner, 1983; Memezawa, Smith & Siesjö, 1992; Ayad, Verity & Rubinstein, 1994; Shen et al., 2005; Pignataro, Simon & Boison, 2007; Nozari et al., 2010; Lückl et al., 2018). Thus, in contrast to what happens during coma or sedation, when the brain dies, it undergoes a massive and unstoppable depolarization process (and hence, a very last state of “activation”) (Dreier, 2011).
Returning to the association between NDE and REM intrusion, it would be interesting to know if also a link exists between miniature excitatory/inhibitory postsynaptic potentials and REM sleep. Information is scarce, but there is indeed evidence that these potentials occur in the healthy brain and are involved in the sleep-wake cycle and both REM and non-REM sleep (Yang & Brown, 2014; Christensen et al., 2014; Sangare et al., 2016). Yet, the connection between these potentials in healthy people, on one hand, and disordered neuronal processing, including NDE, on the other hand, has never been properly investigated.
Another unsolved question is if terminal SD could produce bright light phenomena and tunnel vision similar to what happens during a migraine aura, when SD spread through healthy cortical tissue. In this context, it is particularly thought-provoking that terminal SD is not always the final event, but data from so far three patients indicate that terminal SD can sometimes indeed precede circulatory arrest and initiate a spreading depression of spontaneous activity like that in migraineurs with aura (Fig. 1C) (Dreier et al., 2018, 2019; Carlson et al., 2018). In contrast to migraine aura, activity then remains depressed at the time of cardiac death.
It is important to bear in mind that virtually all humans (and all animals, including insects (Spong, Dreier & Robertson, 2017)) undergo terminal SD at the end of their life, whereas only a minority of people have a migraine aura during their lifetime. Hence, although terminal SD may play a role in the development of NDE, migraine aura during lifetime is probably not required for having an NDE with a bright light at the end of life. However, people with a propensity for migraine aura may be more likely to experience terminal SD while the brain is still electrically active (Fig. 1C). Thus, if terminal SD facilitates NDE, this would suggest that the event of a terminal SD can still be perceived and remembered.
To substantiate or dismiss these speculations, it would be necessary to fully understand how the changing polarization states of approximately 20 billion neurons in the neocortex (Mortensen et al., 2014) create the conscious awareness of an individual, an area of intense but unsolved research (Owen et al., 2006; Giacino et al., 2014; Kondziella et al., 2016; Paulson et al., 2017; Demertzi et al., 2019). This seems important because of the increasing practice of organ donation after cardio-circulatory death (DCD). In countries where DCD is practiced, physicians have reached consensus that death should occur somewhere between a few seconds and 10 min after loss of circulatory function (Boucek et al., 2008; Stiegler et al., 2012; Dhanani et al., 2012; Van Veen et al., 2018). Thus, a survey on postmortem organ donation in the framework of the CENTER-TBI study recently revealed that as many as 10 out of 64 centers (16%) in Europe and Israel immediately begin organ retrieval from the donor after a “flat line electrocardiogram” is detected on the monitor (Van Veen et al., 2018). Critical voices have been raised, however (Rady & Verheijde, 2016; Youngner & Hyun, 2019). Due to the above-mentioned uncertainties in our understanding of the dying process, we think it is indeed prudent to consider if organ removal should first be permitted when the neurons in the donor’s brain no longer exhibit synaptic transmission and alterations of their polarization state. In other words, organ harvesting should perhaps be postponed until the donor’s entire brain has unmistakably reached the negative ultraslow potential phase of terminal SD. It follows that a better understanding of NDE may be relevant to protect the interests of potential organ donors in the context of DCD.
Conclusions and Future Directions
In a large global sample of unprimed laypeople, migraine aura was significantly associated with NDE, even after multivariate adjustment. The connection between migraine aura, REM intrusion and NDE is complex. For instance, the brainstem plays an important role in REM intrusion, and dream-like hallucinations such as those in REM sleep are known from people with lesions near the meso-pontine paramedian reticular formation and the midbrain cerebral peduncles (i.e., peduncular hallucinations) (Galetta & Prasad, 2017), suggesting that dysfunction of the REM-inhibiting serotonergic dorsal raphe nuclei and the noradrenergic locus coeruleus facilitates REM intrusion (Hobson, McCarley & Wyzinski, 1975; Manford & Andermann, 1998; Kayama & Koyama, 2003; De Lecea, Carter & Adamantidis, 2012). A large body of evidence further indicates that the brainstem also plays an important role in the pathogenesis of migraine (Akerman, Holland & Goadsby, 2011). Moreover, REM sleep abnormalities have been described in migraineurs; and several reports have substantiated the notion that migraine, in particular migraine with aura, is associated with narcolepsy (Lippman, 1951; Levitan, 1984; Drake et al., 1990; Dahmen et al., 1999, 2003; Longstreth et al., 2007; Suzuki et al., 2013, 2015; Yang et al., 2017) and hallucinations (Lippman, 1951, 1953; Daniel & Donnet, 2011). Hence, although a propensity for REM intrusion is neither necessary, nor sufficient, for having NDE (Britton & Bootzin, 2004; Lopez et al., 2006; Long & Holden, 2007), we and others have suggested that REM intrusion is a predictor of NDE (Nelson et al., 2006; Kondziella, Dreier & Olsen, 2019). In the present study we found that migraine aura is also a predictor of NDE. The relationship between NDE and migraine aura raises many novel questions which deserve further investigations. In the broadest sense, excitation/inhibition imbalance across different brain structures is likely to play a role (Van den Maagdenberg et al., 2004; Tottene et al., 2009; Ambrosini et al., 2016). However, migraine aura also has an important vascular component that is particularly interesting for the study of NDE and the dying brain and further increases the complexity of these phenomena and their interactions (Van den Maagdenberg et al., 2004; Tottene et al., 2009; Dreier & Reiffurth, 2015).
Supplemental Information
Using an online crowdsourcing platform, we recruited 1.037 lay people from 35 countries on five continents, the majority from Europe and North America.
Funding Statement
The authors received funding from RH Forskningspulje; R143-A6132-B3632, Savværksejer Jeppe Juhl og Hustru Ovita Juhls Mindelegat; 27062019, Deutsche Forschungsgemeinschaft (DFG); DFG DR 323/5-1, DFG DR 323/10-1 and FP7; 602150. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Daniel Kondziella conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Markus Harboe Olsen analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Coline L. Lemale analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Jens P. Dreier analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The Ethics Committee of the Capital Region of Denmark waives approval for online surveys (Section 14 (1) of the Committee Act. 2).
Data Availability
The following information was supplied regarding data availability:
All anonymized raw study data are available in a Supplemental File.
References
- Akerman, Holland & Goadsby (2011).Akerman S, Holland PR, Goadsby PJ. Diencephalic and brainstem mechanisms in migraine. Nature Reviews Neuroscience. 2011;12(10):570–584. doi: 10.1038/nrn3057. [DOI] [PubMed] [Google Scholar]
- Allen, Rossi & Attwell (2004).Allen NJ, Rossi DJ, Attwell D. Sequential release of GABA by exocytosis and reversed uptake leads to neuronal swelling in simulated ischemia of hippocampal slices. Journal of Neuroscience. 2004;24(15):3837–3849. doi: 10.1523/JNEUROSCI.5539-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini et al. (2016).Ambrosini A, Iezzi E, Perrotta A, Kisialiou A, Nardella A, Berardelli A, Pierelli F, Schoenen J. Correlation between habituation of visual-evoked potentials and magnetophosphene thresholds in migraine: a case-control study. Cephalalgia. 2016;36(3):258–264. doi: 10.1177/0333102415590241. [DOI] [PubMed] [Google Scholar]
- Ayad, Verity & Rubinstein (1994).Ayad M, Verity MA, Rubinstein EH. Lidocaine delays cortical ischemic depolarization: relationship to electrophysiologic recovery and neuropathology. Journal of Neurosurgical Anesthesiology. 1994;6(2):98–110. doi: 10.1097/00008506-199404000-00005. [DOI] [PubMed] [Google Scholar]
- Boucek et al. (2008).Boucek MM, Mashburn C, Dunn SM, Frizell R, Edwards L, Pietra B, Campbell D, Denver Children’s Pediatric Heart Transplant Team Pediatric heart transplantation after declaration of cardiocirculatory death. New England Journal of Medicine. 2008;359(7):709–714. doi: 10.1056/NEJMoa0800660. [DOI] [PubMed] [Google Scholar]
- Britton & Bootzin (2004).Britton WB, Bootzin RR. Near-death experiences and the temporal lobe. Psychological Science. 2004;15(4):254–258. doi: 10.1111/j.0956-7976.2004.00661.x. [DOI] [PubMed] [Google Scholar]
- Carlson et al. (2018).Carlson AP, Shuttleworth CW, Major S, Lemale CL, Dreier JP, Hartings JA. Terminal spreading depolarizations causing electrocortical silencing prior to clinical brain death: case report. Journal of Neurosurgery. 2018 doi: 10.3171/2018.7.JNS181478. Epub ahead of print 1 December 2018. [DOI] [PubMed] [Google Scholar]
- Cassol et al. (2019).Cassol H, Martial C, Annen J, Martens G, Charland-Verville V, Majerus S, Laureys S. A systematic analysis of distressing near-death experience accounts. Memory. 2019;27(8):1122–1129. doi: 10.1080/09658211.2019.1626438. [DOI] [PubMed] [Google Scholar]
- Cassol et al. (2018).Cassol H, Pétré B, Degrange S, Martial C, Charland-Verville V, Lallier F, Bragard I, Guillaume M, Laureys S. Qualitative thematic analysis of the phenomenology of near-death experiences. PLOS ONE. 2018;13(2):e0193001. doi: 10.1371/journal.pone.0193001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandradasa et al. (2018).Chandradasa M, Wijesinghe C, Kuruppuarachchi KALA, Perera M. Near-death experiences in a multi-religious hospital population in Sri Lanka. Journal of Religion and Health. 2018;57(5):1599–1605. doi: 10.1007/s10943-017-0442-9. [DOI] [PubMed] [Google Scholar]
- Charland-Verville et al. (2014).Charland-Verville V, Jourdan J-P, Thonnard M, Ledoux D, Donneau A-F, Quertemont E, Laureys S. Near-death experiences in non-life-threatening events and coma of different etiologies. Frontiers in Human Neuroscience. 2014;8(490):203. doi: 10.3389/fnhum.2014.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charland-Verville et al. (2015).Charland-Verville V, Lugo Z, Jourdan J-P, Donneau A-F, Laureys S. Near-death experiences in patients with locked-in syndrome: not always a blissful journey. Consciousness and Cognition. 2015;34:28–32. doi: 10.1016/j.concog.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Christensen et al. (2014).Christensen MH, Ishibashi M, Nielsen ML, Leonard CS, Kohlmeier KA. Age-related changes in nicotine response of cholinergic and non-cholinergic laterodorsal tegmental neurons: implications for the heightened adolescent susceptibility to nicotine addiction. Neuropharmacology. 2014;85:263–283. doi: 10.1016/j.neuropharm.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmen et al. (2003).Dahmen N, Kasten M, Wieczorek S, Gencik M, Epplen JT, Ullrich B. Increased frequency of migraine in narcoleptic patients: a confirmatory study. Cephalalgia. 2003;23(1):14–19. doi: 10.1046/j.1468-2982.2003.00343.x. [DOI] [PubMed] [Google Scholar]
- Dahmen et al. (1999).Dahmen N, Querings K, Grün B, Bierbrauer J. Increased frequency of migraine in narcoleptic patients. Neurology. 1999;52(6):1291–1293. doi: 10.1212/WNL.52.6.1291. [DOI] [PubMed] [Google Scholar]
- Daniel & Donnet (2011).Daniel C, Donnet A. Migrainous complex hallucinations in a 17-year-old adolescent. Headache: The Journal of Head and Face Pain. 2011;51(6):999–1001. doi: 10.1111/j.1526-4610.2010.01823.x. [DOI] [PubMed] [Google Scholar]
- De Lecea, Carter & Adamantidis (2012).De Lecea L, Carter ME, Adamantidis A. Shining light on wakefulness and arousal. Biological Psychiatry. 2012;71(12):1046–1052. doi: 10.1016/j.biopsych.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demertzi et al. (2019).Demertzi A, Tagliazucchi E, Dehaene S, Deco G, Barttfeld P, Raimondo F, Martial C, Fernández-Espejo D, Rohaut B, Voss HU, Schiff ND, Owen AM, Laureys S, Naccache L, Sitt JD. Human consciousness is supported by dynamic complex patterns of brain signal coordination. Science Advances. 2019;5(2):eaat7603. doi: 10.1126/sciadv.aat7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanani et al. (2012).Dhanani S, Hornby L, Ward R, Shemie S. Variability in the determination of death after cardiac arrest. Journal of Intensive Care Medicine. 2012;27(4):238–252. doi: 10.1177/0885066610396993. [DOI] [PubMed] [Google Scholar]
- Drake et al. (1990).Drake ME, Pakalnis A, Andrews JM, Bogner JE. Nocturnal sleep recording with cassette EEG in chronic headaches. Headache: The Journal of Head and Face Pain. 1990;30(9):600–603. doi: 10.1111/j.1526-4610.1990.hed3009600.x. [DOI] [PubMed] [Google Scholar]
- Dreier (2011).Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nature Medicine. 2011;17(4):439–447. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- Dreier et al. (2002).Dreier JP, Kleeberg J, Petzold G, Priller J, Windmüller O, Orzechowski H-D, Lindauer U, Heinemann U, Einhäupl KM, Dirnagl U. Endothelin-1 potently induces Leão’s cortical spreading depression in vivo in the rat: a model for an endothelial trigger of migrainous aura? Brain. 2002;125(1):102–112. doi: 10.1093/brain/awf007. [DOI] [PubMed] [Google Scholar]
- Dreier et al. (2013).Dreier JP, Isele T, Reiffurth C, Offenhauser N, Kirov SA, Dahlem MA, Herreras O. Is spreading depolarization characterized by an abrupt, massive release of gibbs free energy from the human brain cortex? Neuroscientist. 2013;19(1):25–42. doi: 10.1177/1073858412453340. Epub ahead of print 24 July 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier et al. (2017).Dreier JP, Fabricius M, Ayata C, Sakowitz OW, Shuttleworth CW, Dohmen C, Graf R, Vajkoczy P, Helbok R, Suzuki M, Schiefecker AJ, Major S, Winkler MK, Kang EJ, Milakara D, Oliveira-Ferreira AI, Reiffurth C, Revankar GS, Sugimoto K, Dengler NF, Hecht N, Foreman B, Feyen B, Kondziella D, Friberg CK, Piilgaard H, Rosenthal ES, Westover MB, Maslarova A, Santos E, Hertle D, Sánchez-Porras R, Jewell SL, Balança B, Platz J, Hinzman JM, Lückl J, Schoknecht K, Schöll M, Drenckhahn C, Feuerstein D, Eriksen N, Horst V, Bretz JS, Jahnke P, Scheel M, Bohner G, Rostrup E, Pakkenberg B, Heinemann U, Claassen J, Carlson AP, Kowoll CM, Lublinsky S, Chassidim Y, Shelef I, Friedman A, Brinker G, Reiner M, Kirov SA, Andrew RD, Farkas E, Güresir E, Vatter H, Chung LS, Brennan KC, Lieutaud T, Marinesco S, Maas AI, Sahuquillo J, Dahlem MA, Richter F, Herreras O, Boutelle MG, Okonkwo DO, Bullock MR, Witte OW, Martus P, van den Maagdenberg AM, Ferrari MD, Dijkhuizen RM, Shutter LA, Andaluz N, Schulte AP, MacVicar B, Watanabe T, Woitzik J, Lauritzen M, Strong AJ, Hartings JA. Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: review and recommendations of the COSBID research group. Journal of Cerebral Blood Flow & Metabolism. 2017;37(5):1595–1625. doi: 10.1177/0271678X16654496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier et al. (2018).Dreier JP, Major S, Foreman B, Winkler MKL, Kang E-J, Milakara D, Lemale CL, DiNapoli V, Hinzman JM, Woitzik J, Andaluz N, Carlson A, Hartings JA. Terminal spreading depolarization and electrical silence in death of human cerebral cortex. Annals of Neurology. 2018;83(2):295–310. doi: 10.1002/ana.25147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier et al. (2019).Dreier JP, Major S, Lemale CL, Kola V, Reiffurth C, Schoknecht K, Hecht N, Hartings JA, Woitzik J. Correlates of spreading depolarization, spreading depression, and negative ultraslow potential in epidural versus subdural electrocorticography. Frontiers in Neuroscience. 2019;13:373. doi: 10.3389/fnins.2019.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier & Reiffurth (2015).Dreier JP, Reiffurth C. The stroke-migraine depolarization continuum. Neuron. 2015;86(4):902–922. doi: 10.1016/j.neuron.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Erdemli, Xu & Krnjevic (1998).Erdemli G, Xu YZ, Krnjevic K. Potassium conductance causing hyperpolarization of CA1 hippocampal neurons during hypoxia. Journal of Neurophysiology. 1998;80(5):2378–2390. doi: 10.1152/jn.1998.80.5.2378. [DOI] [PubMed] [Google Scholar]
- Facco & Agrillo (2012).Facco E, Agrillo C. Near-death experiences between science and prejudice. Frontiers in Human Neuroscience. 2012;6:209. doi: 10.3389/fnhum.2012.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleidervish et al. (2001).Fleidervish IA, Gebhardt C, Astman N, Gutnick MJ, Heinemann U. Enhanced spontaneous transmitter release is the earliest consequence of neocortical hypoxia that can explain the disruption of normal circuit function. Journal of Neuroscience. 2001;21(13):4600–4608. doi: 10.1523/JNEUROSCI.21-13-04600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galetta & Prasad (2017).Galetta KM, Prasad S. Historical trends in the diagnosis of peduncular hallucinosis. Journal of Neuro-Ophthalmology. 2017;38:1. doi: 10.1097/WNO.0000000000000599. [DOI] [PubMed] [Google Scholar]
- Gallup & Proctor (1982).Gallup G, Proctor W. Adventures in immortality: a look beyond the threshold of death. New York: McGraw-Hill; 1982. [Google Scholar]
- Giacino et al. (2014).Giacino JT, Fins JJ, Laureys S, Schiff ND. Disorders of consciousness after acquired brain injury: the state of the science. Nature Reviews Neurology. 2014;10(2):99–114. doi: 10.1038/nrneurol.2013.279. [DOI] [PubMed] [Google Scholar]
- Greyson (1983).Greyson B. The near-death experience scale. Construction, reliability, and validity. Journal of Nervous and Mental Disease. 1983;171(6):369–375. doi: 10.1097/00005053-198306000-00007. [DOI] [PubMed] [Google Scholar]
- Hartings et al. (2017a).Hartings JA, Shuttleworth CW, Kirov SA, Ayata C, Hinzman JM, Foreman B, Andrew RD, Boutelle MG, Brennan KC, Carlson AP, Dahlem MA, Drenckhahn C, Dohmen C, Fabricius M, Farkas E, Feuerstein D, Graf R, Helbok R, Lauritzen M, Major S, Oliveira-Ferreira AI, Richter F, Rosenthal ES, Sakowitz OW, Sánchez-Porras R, Santos E, Schöll M, Strong AJ, Urbach A, Westover MB, Winkler MK, Witte OW, Woitzik J, Dreier JP. The continuum of spreading depolarizations in acute cortical lesion development: examining Leão’s legacy. Journal of Cerebral Blood Flow & Metabolism. 2017a;37(5):1571–1594. doi: 10.1177/0271678X16654495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartings et al. (2017b).Hartings JA, York J, Carroll CP, Hinzman JM, Mahoney E, Krueger B, Winkler MKL, Major S, Horst V, Jahnke P, Woitzik J, Kola V, Du Y, Hagen M, Jiang J, Dreier JP. Subarachnoid blood acutely induces spreading depolarizations and early cortical infarction. Brain. 2017b;140(10):2673–2690. doi: 10.1093/brain/awx214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss & Rosner (1983).Heiss WD, Rosner G. Functional recovery of cortical neurons as related to degree and duration of ischemia. Annals of Neurology. 1983;14(3):294–301. doi: 10.1002/ana.410140307. [DOI] [PubMed] [Google Scholar]
- Hobson, McCarley & Wyzinski (1975).Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189(4196):55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- Hossmann & Sato (1970).Hossmann K-A, Sato K. Recovery of neuronal function after prolonged cerebral ischemia. Science. 1970;168(3929):375–376. doi: 10.1126/science.168.3929.375. [DOI] [PubMed] [Google Scholar]
- Jensen (2018).Jensen RH. Tension-type headache - the normal and most prevalent headache. Headache: The Journal of Head and Face Pain. 2018;58(2):339–345. doi: 10.1111/head.13067. [DOI] [PubMed] [Google Scholar]
- Kaiser et al. (2019).Kaiser EA, Igdalova A, Aguirre GK, Cucchiara B. A web-based, branching logic questionnaire for the automated classification of migraine. Cephalalgia. 2019;39(10):1257–1266. doi: 10.1177/0333102419847749. [DOI] [PubMed] [Google Scholar]
- Kayama & Koyama (2003).Kayama Y, Koyama Y. Control of sleep and wakefulness by brainstem monoaminergic and cholinergic neurons. Acta Neurochirurgica Supplement. 2003;87:3–6. doi: 10.1007/978-3-7091-6081-7_1. [DOI] [PubMed] [Google Scholar]
- Knoblauch, Schmied & Schnettler (2001).Knoblauch H, Schmied I, Schnettler B. Different kinds of near-death experience: a report on a survey of near-death experiences in Germany. Journal of Near-Death Studies. 2001;20(1):15–29. doi: 10.1023/A:1011112727078. [DOI] [Google Scholar]
- Kondziella, Cheung & Dutta (2019).Kondziella D, Cheung MC, Dutta A. Public perception of the vegetative state/unresponsive wakefulness syndrome: a crowdsourced study. PeerJ. 2019;7(6031):e6575. doi: 10.7717/peerj.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondziella, Dreier & Olsen (2019).Kondziella D, Dreier JP, Olsen MH. Prevalence of near-death experiences in people with and without REM sleep intrusion. PeerJ. 2019;7(2):e7585. doi: 10.7717/peerj.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondziella et al. (2016).Kondziella D, Friberg CK, Frokjaer VG, Fabricius M, Møller K. Preserved consciousness in vegetative and minimal conscious states: systematic review and meta-analysis. Journal of Neurology, Neurosurgery & Psychiatry. 2016;87(5):485–492. doi: 10.1136/jnnp-2015-310958. [DOI] [PubMed] [Google Scholar]
- Kraig, Ferreira-Filho & Nicholson (1983).Kraig RP, Ferreira-Filho CR, Nicholson C. Alkaline and acid transients in cerebellar microenvironment. Journal of Neurophysiology. 1983;49(3):831–850. doi: 10.1152/jn.1983.49.3.831. [DOI] [PubMed] [Google Scholar]
- Levitan (1984).Levitan H. Dreams which culminate in migraine headaches. Psychotherapy and Psychosomatics. 1984;41(4):161–166. doi: 10.1159/000287805. [DOI] [PubMed] [Google Scholar]
- Lippman (1951).Lippman C. Hallucinations in migraine. American Journal of Psychiatry. 1951;107(11):856–858. doi: 10.1176/ajp.107.11.856. [DOI] [PubMed] [Google Scholar]
- Lippman (1953).Lippman C. Hallucinations of physical duality in migraine. Journal of Nervous and Mental Disease. 1953;117(4):345–350. doi: 10.1097/00005053-195304000-00008. [DOI] [PubMed] [Google Scholar]
- Lippman (1954).Lippman C. Recurrent dreams in migraine: an aid to diagnosis. Journal of Nervous and Mental Disease. 1954;120(3):273–276. doi: 10.1097/00005053-195409000-00014. [DOI] [PubMed] [Google Scholar]
- Long & Holden (2007).Long J, Holden J. Does the arousal system contribute to near-death and out-of-body experiences? A summary and response. Journal of Near-Death Studies. 2007;25(3):135–169. doi: 10.17514/jnds-2007-25-3-p135-169. [DOI] [Google Scholar]
- Longstreth et al. (2007).Longstreth WT, Jr, Koepsell TD, Ton TG, Hendrickson AF, Van Belle G. The epidemiology of narcolepsy. Sleep. 2007;30(1):13–26. doi: 10.1093/sleep/30.1.13. [DOI] [PubMed] [Google Scholar]
- Lopez et al. (2006).Lopez U, Forster A, Annoni J-M, Habre W, Iselin-Chaves IA. Near-death experience in a boy undergoing uneventful elective surgery under general anesthesia. Pediatric Anesthesia. 2006;16(1):85–88. doi: 10.1111/j.1460-9592.2005.01607.x. [DOI] [PubMed] [Google Scholar]
- Lückl et al. (2018).Lückl J, Lemale CL, Kola V, Horst V, Khojasteh U, Oliveira-Ferreira AI, Major S, Winkler MKL, Kang E-J, Schoknecht K, Martus P, Hartings JA, Woitzik J, Dreier JP. The negative ultraslow potential, electrophysiological correlate of infarction in the human cortex. Brain. 2018;141(6):1734–1752. doi: 10.1093/brain/awy102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyngberg et al. (2005).Lyngberg AC, Rasmussen BK, Jørgensen T, Jensen R. Has the prevalence of migraine and tension-type headache changed over a 12-year period? A Danish population survey. European Journal of Epidemiology. 2005;20(3):243–249. doi: 10.1007/s10654-004-6519-2. [DOI] [PubMed] [Google Scholar]
- Manford & Andermann (1998).Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(Pt 10):1819–1840. doi: 10.1093/brain/121.10.1819. [DOI] [PubMed] [Google Scholar]
- Martial et al. (2017).Martial C, Cassol H, Antonopoulos G, Charlier T, Heros J, Donneau A-F, Charland-Verville V, Laureys S. Temporality of features in near-death experience narratives. Frontiers in Human Neuroscience. 2017;11:311. doi: 10.3389/fnhum.2017.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martial et al. (2018).Martial C, Cassol H, Charland-Verville V, Merckelbach H, Laureys S. Fantasy proneness correlates with the intensity of near-death experience. Frontiers in Psychiatry. 2018;9:190. doi: 10.3389/fpsyt.2018.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martial et al. (2019).Martial C, Cassol HH, Charland-verville V, Pallavicini C, Sanz C, Zamberlan FF, Martínez R, Martinez Vivo R, Erowid F, Erowid E, Laureys S, Greyson B, Tagliazucchi E. Neurochemical models of near-death experiences: a large-scale study based on the semantic similarity of written reports. Consciousness and Cognition. 2019;69:52–69. doi: 10.1016/j.concog.2019.01.011. [DOI] [PubMed] [Google Scholar]
- Memezawa, Smith & Siesjö (1992).Memezawa H, Smith ML, Siesjö BK. Penumbral tissues salvaged by reperfusion following middle cerebral artery occlusion in rats. Stroke. 1992;23(4):552–559. doi: 10.1161/01.STR.23.4.552. [DOI] [PubMed] [Google Scholar]
- Mortensen et al. (2014).Mortensen HS, Pakkenberg B, Dam M, Dietz R, Sonne C, Mikkelsen B, Eriksen N. Quantitative relationships in delphinid neocortex. Frontiers in Neuroanatomy. 2014;8(46):132. doi: 10.3389/fnana.2014.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller & Somjen (2000).Müller M, Somjen GG. Na+ and K+ concentrations, extra- and intracellular voltages, and the effect of TTX in hypoxic rat hippocampal slices. Journal of Neurophysiology. 2000;83(2):735–745. doi: 10.1152/jn.2000.83.2.735. [DOI] [PubMed] [Google Scholar]
- Mutch & Hansen (1984).Mutch WAC, Hansen AJ. Extracellular pH changes during spreading depression and cerebral ischemia: mechanisms of brain pH regulation. Journal of Cerebral Blood Flow & Metabolism. 1984;4(1):17–27. doi: 10.1038/jcbfm.1984.3. [DOI] [PubMed] [Google Scholar]
- Nedergaard & Hansen (1993).Nedergaard M, Hansen AJ. Characterization of cortical depolarizations evoked in focal cerebral ischemia. Journal of Cerebral Blood Flow & Metabolism. 1993;13(4):568–574. doi: 10.1038/jcbfm.1993.74. [DOI] [PubMed] [Google Scholar]
- Nelson et al. (2006).Nelson KR, Mattingly M, Lee SA, Schmitt FA. Does the arousal system contribute to near death experience? Neurology. 2006;66(7):1003–1009. doi: 10.1212/01.wnl.0000204296.15607.37. [DOI] [PubMed] [Google Scholar]
- Nelson, Mattingly & Schmitt (2007).Nelson KR, Mattingly M, Schmitt FA. Out-of-body experience and arousal. Neurology. 2007;68(10):794–795. doi: 10.1212/01.wnl.0000256784.85952.6f. [DOI] [PubMed] [Google Scholar]
- Nozari et al. (2010).Nozari A, Dilekoz E, Sukhotinsky I, Stein T, Eikermann-Haerter K, Liu C, Wang Y, Frosch MP, Waeber C, Ayata C, Moskowitz MA. Microemboli may link spreading depression, migraine aura, and patent foramen ovale. Annals of Neurology. 2010;67(2):221–229. doi: 10.1002/ana.21871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Ferreira et al. (2010).Oliveira-Ferreira AI, Milakara D, Alam M, Jorks D, Major S, Hartings JA, Lückl J, Martus P, Graf R, Dohmen C, Bohner G, Woitzik J, Dreier JP, COSBID study group Experimental and preliminary clinical evidence of an ischemic zone with prolonged negative DC shifts surrounded by a normally perfused tissue belt with persistent electrocorticographic depression. Journal of Cerebral Blood Flow & Metabolism. 2010;30(8):1504–1519. doi: 10.1038/jcbfm.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen et al. (2006).Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science. 2006;313(5792):1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- Parnia et al. (2014).Parnia S, Spearpoint K, De Vos G, Fenwick P, Goldberg D, Yang J, Zhu J, Baker K, Killingback H, McLean P, Wood M, Zafari AM, Dickert N, Beisteiner R, Sterz F, Berger M, Warlow C, Bullock S, Lovett S, McPara RMS, Marti-Navarette S, Cushing P, Wills P, Harris K, Sutton J, Walmsley A, Deakin CD, Little P, Farber M, Greyson B, Schoenfeld ER. AWARE—AWAreness during REsuscitation—a prospective study. Resuscitation. 2014;85(12):1799–1805. doi: 10.1016/j.resuscitation.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Paulson et al. (2017).Paulson S, Berlin HA, Ginot E, Makari G. Delving within: the new science of the unconscious. Annals of the New York Academy of Sciences. 2017;1406(1):12–27. doi: 10.1111/nyas.13390. [DOI] [PubMed] [Google Scholar]
- Peer et al. (2017).Peer E, Brandimarte L, Samat S, Acquisti A. Beyond the Turk: alternative platforms for crowdsourcing behavioral research. Journal of Experimental Social Psychology. 2017;70:153–163. doi: 10.1016/j.jesp.2017.01.006. [DOI] [Google Scholar]
- Peinkhofer, Dreier & Kondziella (2019).Peinkhofer C, Dreier JP, Kondziella D. Semiology and mechanisms of near-death experiences. Current Neurology and Neuroscience Reports. 2019;19(9) doi: 10.1007/s11910-019-0983-2. [DOI] [PubMed] [Google Scholar]
- Perera, Padmasekara & Belanti (2005).Perera M, Padmasekara G, Belanti J. Prevalence of near-death experiences in Australia. Journal of Near-Death Studies. 2005;24:109–116. [Google Scholar]
- Pignataro, Simon & Boison (2007).Pignataro G, Simon RP, Boison D. Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. Journal of Cerebral Blood Flow & Metabolism. 2007;27(1):1–5. doi: 10.1038/sj.jcbfm.9600334. [DOI] [PubMed] [Google Scholar]
- Rady & Verheijde (2016).Rady MY, Verheijde JL. Neuroscience and awareness in the dying human brain: Implications for organ donation practices. Journal of Critical Care. 2016;34:121–123. doi: 10.1016/j.jcrc.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Revah et al. (2016).Revah O, Lasser-Katz E, Fleidervish IA, Gutnick MJ. The earliest neuronal responses to hypoxia in the neocortical circuit are glutamate-dependent. Neurobiology of Disease. 2016;95:158–167. doi: 10.1016/j.nbd.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Russel et al. (1995).Russel M, Rasmussen B, Thorvaldsen P, Olesen J. Prevalence and sex-ratio of the subtypes of migraine. International Journal of Epidemiology. 1995;24(3):612–618. doi: 10.1093/ije/24.3.612. [DOI] [PubMed] [Google Scholar]
- Sangare et al. (2016).Sangare A, Dubourget R, Geoffroy H, Gallopin T, Rancillac A. Serotonin differentially modulates excitatory and inhibitory synaptic inputs to putative sleep-promoting neurons of the ventrolateral preoptic nucleus. Neuropharmacology. 2016;109:29–40. doi: 10.1016/j.neuropharm.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Scammell (2015).Scammell TE. Narcolepsy. New England Journal of Medicine. 2015;373(27):2654–2662. doi: 10.1056/NEJMra1500587. [DOI] [PubMed] [Google Scholar]
- Shen et al. (2005).Shen Q, Ren H, Cheng H, Fisher M, Duong TQ. Functional, perfusion and diffusion MRI of acute focal ischemic brain injury. Journal of Cerebral Blood Flow & Metabolism. 2005;25(10):1265–1279. doi: 10.1038/sj.jcbfm.9600132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spong, Dreier & Robertson (2017).Spong KE, Dreier JP, Robertson RM. A new direction for spreading depolarization: investigation in the fly brain. Channels. 2017;11(2):97–98. doi: 10.1080/19336950.2016.1239898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler et al. (2012).Stiegler P, Sereinigg M, Puntschart A, Seifert-Held T, Zmugg G, Wiederstein-Grasser I, Marte W, Meinitzer A, Stojakovic T, Zink M, Stadlbauer V, Tscheliessnigg K. A 10 min “no-touch” time - is it enough in DCD? A DCD Animal Study. Transplant International. 2012;25(4):481–492. doi: 10.1111/j.1432-2277.2012.01437.x. [DOI] [PubMed] [Google Scholar]
- Suzuki et al. (2015).Suzuki K, Miyamoto M, Miyamoto T, Inoue Y, Matsui K, Nishida S, Hayashida K, Usui A, Ueki Y, Nakamura M, Murata M, Numao A, Watanabe Y, Suzuki S, Hirata K. The prevalence and characteristics of primary headache and dream-enacting behaviour in Japanese patients with narcolepsy or idiopathic hypersomnia: a multi-centre cross-sectional study. PLOS ONE. 2015;10(9):e0139229. doi: 10.1371/journal.pone.0139229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki et al. (2013).Suzuki K, Miyamoto T, Miyamoto M, Suzuki S, Watanabe Y, Takashima R, Hirata K. Dream-enacting behaviour is associated with impaired sleep and severe headache-related disability in migraine patients. Cephalalgia. 2013;33(10):868–878. doi: 10.1177/0333102413477742. [DOI] [PubMed] [Google Scholar]
- Tanaka et al. (1997).Tanaka E, Yamamoto S, Kudo Y, Mihara S, Higashi H. Mechanisms underlying the rapid depolarization produced by deprivation of oxygen and glucose in rat hippocampal CA1 neurons in vitro. Journal of Neurophysiology. 1997;78(2):891–902. doi: 10.1152/jn.1997.78.2.891. [DOI] [PubMed] [Google Scholar]
- Thonnard et al. (2013).Thonnard M, Charland-Verville V, Brédart S, Dehon H, Ledoux D, Laureys S, Vanhaudenhuyse A. Characteristics of near-death experiences memories as compared to real and imagined events memories. PLOS ONE. 2013;8(3):e57620. doi: 10.1371/journal.pone.0057620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottene et al. (2009).Tottene A, Conti R, Fabbro A, Vecchia D, Shapovalova M, Santello M, Van den Maagdenberg AMJM, Ferrari MD, Pietrobon D. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Ca(v)2.1 knockin migraine mice. Neuron. 2009;61(5):762–773. doi: 10.1016/j.neuron.2009.01.027. [DOI] [PubMed] [Google Scholar]
- Van den Maagdenberg et al. (2004).Van den Maagdenberg AMJM, Pietrobon D, Pizzorusso T, Kaja S, Broos LAM, Cesetti T, Van de Ven RCG, Tottene A, Van der Kaa J, Plomp JJ, Frants RR, Ferrari MD. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41(5):701–710. doi: 10.1016/S0896-6273(04)00085-6. [DOI] [PubMed] [Google Scholar]
- Van Veen et al. (2018).Van Veen E, Van der Jagt M, Cnossen MC, Maas AIR, De Beaufort ID, Menon DK, Citerio G, Stocchetti N, Rietdijk WJR, Van Dijck JTJM, Kompanje EJO. Brain death and postmortem organ donation: report of a questionnaire from the CENTER-TBI study. Critical Care. 2018;22(1):306. doi: 10.1186/s13054-018-2241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrselja et al. (2019).Vrselja Z, Daniele SG, Silbereis J, Talpo F, Morozov YM, Sousa AMM, Tanaka BS, Skarica M, Pletikos M, Kaur N, Zhuang ZW, Liu Z, Alkawadri R, Sinusas AJ, Latham SR, Waxman SG, Sestan N. Restoration of brain circulation and cellular functions hours post-mortem. Nature. 2019;568(7752):336–343. doi: 10.1038/s41586-019-1099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woitzik et al. (2013).Woitzik J, Hecht N, Pinczolits A, Sandow N, Major S, Winkler MKL, Weber-Carstens S, Dohmen C, Graf R, Strong AJ, Dreier JP, Vajkoczy P, COSBID study group Propagation of cortical spreading depolarization in the human cortex after malignant stroke. Neurology. 2013;80(12):1095–1102. doi: 10.1212/WNL.0b013e3182886932. [DOI] [PubMed] [Google Scholar]
- Woods et al. (2015).Woods AT, Velasco C, Levitan CA, Wan X, Spence C. Conducting perception research over the internet: a tutorial review. PeerJ. 2015;3:e1058. doi: 10.7717/peerj.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang & Brown (2014).Yang C, Brown RE. The cholinergic agonist carbachol increases the frequency of spontaneous GABAergic synaptic currents in dorsal raphe serotonergic neurons in the mouse. Neuroscience. 2014;258:62–73. doi: 10.1016/j.neuroscience.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2017).Yang C-P, Hsieh M-L, Chiang J-H, Chang H-Y, Hsieh VC-R. Migraine and risk of narcolepsy in children: a nationwide longitudinal study. PLOS ONE. 2017;12(12):e0189231. doi: 10.1371/journal.pone.0189231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner & Hyun (2019).Youngner S, Hyun I. Pig experiment challenges assumptions around brain damage in people. Nature. 2019;568(7752):302–304. doi: 10.1038/d41586-019-01169-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Using an online crowdsourcing platform, we recruited 1.037 lay people from 35 countries on five continents, the majority from Europe and North America.
Data Availability Statement
The de-identified raw data are provided in the Supplemental Files.
The following information was supplied regarding data availability:
All anonymized raw study data are available in a Supplemental File.