Abstract
Background
Because less-invasive techniques can obviate the need for brain biopsy in the diagnosis of primary central nervous system lymphoma (PCNSL), it is common practice to wait for a thorough initial work-up, which may delay treatment. We conducted a systematic review and reviewed our own series of patients to define the role of LP and early brain biopsy in the diagnosis of PCNSL.
Methods
Our study was divided into 2 main sections: 1) systematic review assessing the sensitivity of cerebrospinal fluid (CSF) analysis on the diagnosis of PCNSL, and 2) a retrospective, single-center patient series assessing the diagnostic accuracy and safety of early biopsy in immunocompetent PCNSL patients treated at our institution from 2012 to 2018.
Results
Our systematic review identified 1481 patients with PCNSL. A preoperative LP obviated surgery in 7.4% of cases. Brain biopsy was the preferred method of diagnosis in 95% of patients followed by CSF (3.1%). In our institutional series, brain biopsy was diagnostic in 92.3% of cases (24/26) with 2 cases that required a second procedure for diagnosis. Perioperative morbidity was noted in 7.6% of cases (n = 2) due to hemorrhages after stereotactic brain biopsy that improved at follow-up.
Conclusions
The diagnostic yield of CSF analyses for PCNSL in immunocompetent patients remains exceedingly low. Our institutional series demonstrates that early biopsy for PCNSL is safe and accurate, and may avert protracted work-ups. We conclude that performing an early brain biopsy in a suspected case of PCNSL is a valid, safe option to minimize diagnostic delay.
Keywords: brain biopsy, lumbar puncture, primary central nervous system lymphoma
Primary central nervous system lymphoma (PCNSL) is a rare subtype of extranodal non-Hodgkin lymphoma that can involve the brain, eyes, meninges, or spinal cord without evidence of systemic disease, accounting for approximately 3% to 4% of all CNS tumors. The annual incidence of PCNSL is around 7 cases per 1 000 000 people in the United States, with a male predominance occurring mostly in the sixth decade of life.1,2 Epidemiological studies have demonstrated a rise in the incidence of PCNSL over the last 40 years with higher overall rates in immunocompetent patients age 65 years or older.3,4 Initial clinical symptoms can vary depending on lesion location and size; however, most patients present with focal deficits (70%), neuropsychiatric symptoms (43%), increased intracranial pressure (33%), or seizures (14%), rather than systemic “B” signs (fever, night sweats, and unintentional weight loss).5–7 When suspecting PCNSL, contrast MR is the diagnostic modality of choice; PCNSL is typically iso-hypointense on T1-weighted imaging and iso-hypointense to gray matter on T2-weighted imaging, with a strong homogeneous pattern of enhancement in 85% of patients because of its hypercellularity. However, radiographic imaging patterns are suggestive but not diagnostic of PCNSL, and definitive diagnosis must be achieved by histopathological confirmation by stereotactic (SBB) or open brain biopsy. Brain biopsy (open or SBB) is the preferred surgical procedure; although a positive cerebrospinal fluid (CSF) or vitreous biopsy for lymphoma can obviate the need for a surgical procedure.8,9
These adjuvant studies are typically recommended in the majority of cases; however, they may not be diagnostic and may delay prompt treatment. Here, we conducted a systematic review of the literature to characterize the diagnostic sensitivity of LP for the diagnosis of PCNSL. We also supplemented this discussion with a review of our patient series that advocates for an early biopsy, prior or simultaneously to the time of the LP. Our overarching goal is to characterize and elucidate the management paradigms to define the role of early brain biopsy in the diagnosis of PCNSL.
Materials and Methods
Our study was divided into 2 main sections: 1) systematic review assessing the sensitivity of CSF analysis on the diagnosis of PCNSL, and 2) a prospective, single-center patient series assessing the diagnostic accuracy and safety of early biopsy for patients with PCNSL.
Study Selection
A systematic literature search was performed following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines between the years 1985 and 2018 using the PubMed database for all articles containing the terms primary central nervous system lymphoma, and CSF or cerebrospinal fluid or study or trial: (((“primary”[All Fields] AND “central nervous system”[All Fields] AND “lymphoma”[MeSH Terms])) AND ((((trial) OR study) OR CSF) OR cerebrospinal fluid)).10 The objective was to screen for articles containing series of patients with PCNSL and CSF assessment for lymphoma or leptomeningeal disease (LD), at any stage of the disease (diagnosis or follow-up). Inclusion criteria were as follows: 1) series with more than 20 patients with CSF assessment for lymphoma or LD, 2) diagnosis of PCNSL, and 3) HIV-negative patients.
The article search was limited to English with humans as the only study participants. All articles were specified as retrospective or prospective patient studies, clinical trials, randomized clinical trials, or post hoc analyses of clinical trials. Reviews, editorials, commentaries, and case reports were excluded.
Data Analysis
All articles included were reviewed for data available on number of patients in the study, number of patients with CSF screening for lymphoma or LD, method used to diagnose PCNSL, initial staging work-up, CSF screening method, number of patients with positive CSF at any stage of the disease, and steroid use before CSF or biopsy screening. The type of study and general characteristics of the population included were also mentioned.
The second part of our study included a retrospective, single-center patient series assessing the diagnostic accuracy and safety of early biopsy for patients with PCNSL. We conducted a retrospective review of all cases with histological confirmation of PCNSL in immunocompetent patients treated at our institution from 2012 to 2018. Patients were excluded if they had a prior diagnosis of lymphoma, history of immunodeficiency, and systemic disease as demonstrated by positive body imaging. All relevant demographic, epidemiologic, and clinical variables were collected.
Results
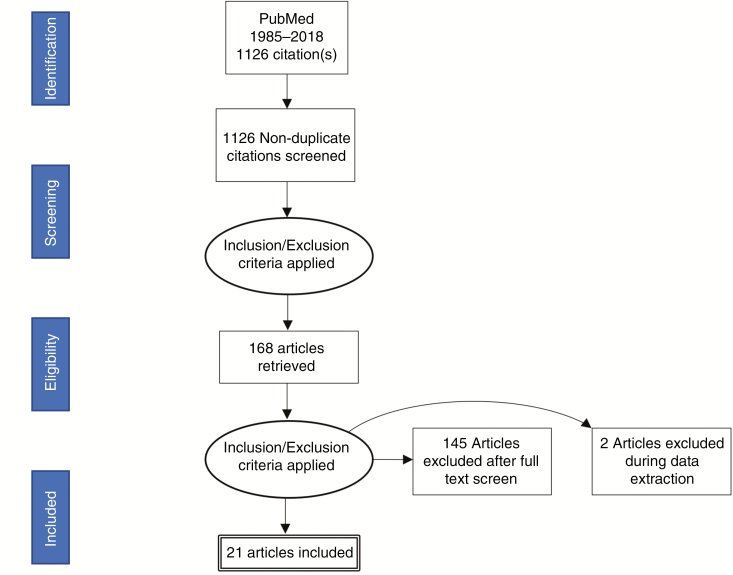
After applying the inclusion criteria, 23 studies were included for data analysis, 2 of which were excluded because of inadequate data description (Fig. 1). A final number of 21 studies with a total of 1481 patients with PCNSL were included in our analysis: retrospective series (11), followed by prospective clinical trials (6), prospective descriptive studies (2), a randomized clinical trial (1), and post hoc analysis of a randomized clinical trial study (1) (Table 1). A PRISMA flow sheet of the articles screened can be found in Fig. 1. Two articles were included with a minor percentage (2% to 4%) of patients who were diagnosed by radiological/clinical findings rather than histopathologic report of tumor tissue.
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Guidelines and How They Were Used for Our Assessment of the Literature.
Table 1.
Results of the Systematic Review: CSF Analysis in PCNSL
| Author and Year | No. of Pts with CSF Screening | Population | Diagnostic Method | Type of Study | Initial Staging Work-Up | CSF Screening Method | CTC | Positive CSF | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Balmaceda et al 19958 | 86 | Consecutive PCNSL | BB: 78,1% Vitrectomy: 5.2% CSF: 14.5% Radiologic and clinical features: 2.1% |
PS | CT scan Bone marrow biopsy Ophthalmological exam HIV test |
Cytology | Yes: 65.1% No: 15.1% NA: 19.7% |
26% |
| 2 | Schlegel et al 200111 | 20 | Consecutive PCNSL | BB:100% | PCT | CT scan Bone marrow biopsy Ophthalmological exam HIV test |
Cytology | 0% | |
| 3 | DeAngelis et al 200212 | 81 | PCNSL, including brain, eye, and meninges | Stereotactic or open biopsy, vitrectomy or CSF | PS | CT scan Bone marrow biopsy Ophthalmological exam HIV test |
Cytology | 21% | |
| 4 | Ferreri et al 200213 | 241 | PCNSL | BB: 95% CSF cytology: 4% Vitrectomy: 0.27% Autopsy: 0.27% |
RS | CT scan Bone marrow biopsy Ophthalmological exam HIV test |
Cytology | 16% | |
| 5 | Pels et al 200314 | 58 | PCNSL, including brain, eyes, and meninges | BB: 100% | PCT | CT scan Bone marrow biopsy Ophthalmological exam HIV test |
Cytology | 12% | |
| 6 | Jahnke et al 200615 | 25 | Low-grade PCNSL | BB: 98% CSF cytology: 2.5% |
RS | CT scan Bone marrow biopsy Ophthalmological exam HIV test |
Cytology | 16% | |
| 7 | Shenkier et al 200516 | 32 | PCNSL (T-cell) | BB: 95.5% CSF cytology: 2.2% Autopsy: 2.2% |
RS | Ophthalmological exam HIV test |
Cytology | 19% | |
| 8 | Quek et al 200617 | 23 | PCNSL | BB: 100% | RS | CT Scan Bone marrow biopsy Ophthalmological exam HIV test |
Cytology | 21.7% | |
| 9 | Kiewe et al 200818 | 34 | Consecutive PCNSL | BB: 90.2% Vitrectomy: 2.7% CSF cytology: 2.7% Radiologic and clinical features: 4.1% |
RS | CT scan Bone marrow biopsy Ophthalmological exam (only in patients with ocular symptoms) HIV test |
Cytology | 18% | |
| 10 | Agarwal et al 200919 | 20 | Consecutive PCNSL | Stereotactic brain biopsy: 20 (100%) | RS | CT scan Bone marrow biopsy Ophthalmological exam HIV test |
Cytology | 0% | |
| 11 | Korfel et al 201220 | 361 for CSF Cytology 152 for PCR |
PCNSL | NA | PHCT | NA | CSF cytology IgH PCR (only in B-cell lymphoma) |
12,2% in CSF cytology 10.5% in IgH PCR |
|
| 12 | Pasricha et al 201121 | 48 | Consecutive PCNSL | BB: 100% | RS | Bone marrow biopsy Lymphadenopathy and organomegaly screening |
Cytology | 2.08% | |
| 13 | Kim et al 201422 | 32 | PCNSL, including brain, eyes, cranial nerves, meninges, and spinal cord. | NA | RS | CT scan PET scan Bone marrow biopsy Ophthalmologic exam HIV test |
Cytology | 28.1% | |
| 14 | Rubenstein et al 201323 | 41 | PCNSL, including brain, and spine | NA | PCT | CT scan Bone marrow biopsy Ophthalmological exam HIV test |
Cytology | 24% | |
| 15 | Xie et al 201524 | 54 | >60 years with PCNSL | BB: 98% Slit-lamp examination of the eye: 7.4% Vitrectomy: 5.6% CSF cytology: 3.7% CSF flow cytometry: 1.9% |
RS | NA | Cytology | 5.6% | |
| 16 | Ferreri et al 201425 | 29 | PCNSL, including brain, eyes, and meninges | BB: 97.6% Vitrectomy: 2.4% |
PCT | HIV | Cytology | 10.3% | |
| 17 | Wang et al 201426 | 41 | PCNSL, including brain, eyes, and meninges | NA | PCT | HIV test | Cytology | 4.8% | |
| 18 | Pulczynski et al 201527 | 44 | PCNSL | BB: 100% | PCT | CT scan Bone marrow biopsy Ophthalmologic exam HIV test US of the testes inage >60 years |
Cytology flow cytometry | Cytology: 18% Flow cytometry: 6.6% |
|
| 19 | Omuro et al 201528 | 78 | Age >60 years with PCNSL | NA | RCT | NA | Cytology | 29.4% | |
| 20 | Patel et al 201529 | 76 | Consecutive PCNSL Brain only, not eyes or meninges |
Stereotactic or open biopsy: 100% | RS | CT scan Bone marrow biopsy Ophthalmologic exam HIV test |
Cytology | Yes: 18.4% | 2.6% |
| 21 | Houillier et al 201730 | 57 | Age >60 years with PCNSL | NA | RS | NA | Cytology | 21% |
Abbreviations: BB, brain biopsy; CSF, cerebrospinal fluid; CTC, corticosteroids; PCNSL, primary central nervous system lymphoma; PCR, polymerase chain reaction; PCT, prospective clinical trial; PHCT, post hoc analysis of a randomized clinical trial; PS, prospective descriptive study; RCT, randomized controlled trial; RS, retrospective series; US, ultrasound.
We found 35 patients in 6 studies8,15,17,18,20,26 in which surgery was obviated because of positive CSF for lymphoma, with a total of 472 patients screened (7.4%, n = 35/472). CSF analysis during all stages of disease (diagnosis or staging) was positive in 14.9% of cases (222/1481). In 2 studies, different methods were used in addition to standard cytology (flow cytometry in 1 and polymerase chain reaction [PCR] analysis of immunoglobulin heavy chain in another). Brain biopsy was the preferred method of diagnosis in 95% of patients (n = 1029) followed by CSF (3.1%, n = 35)) and vitreous sampling (1%, n = 16). The initial assessment in most studies included a combination of HIV test, bone marrow biopsy, CT of the chest, abdomen, and pelvis, and ophthalmologic exam with slit lamp. The use of steroids at the time of diagnosis was described in only 2 studies and varied from 18.4% to 65% of cases.
Retrospective Series
From 2012 to 2018, 26 patients were newly diagnosed with PCNSL at our 2 major teaching hospitals (Table 2). All cases were confirmed by brain biopsy and were histologically confirmed by the pathology department. The surgical procedures were frameless SBB in 16 patients (61.5%), craniotomy for tumor resection in 8 cases (30.8%), and endoscopy-guided biopsy in 2 cases (7.7%). The most common regions affected were the frontal lobes (13), followed by temporal lobes (6), parietal (3), thalamic (2), occipital (1), and brainstem (1). Owing to our institutional bias for early biopsy, only 7 patients (26.9%) were screened prior to surgery with LP for CSF analysis for cytology and flow cytometry. CSF analysis was negative for all cases. Initial brain biopsy was diagnostic in 92.3% of cases (24/26), with 2 cases that required a second procedure for diagnosis. In both cases, the patients were on preoperative steroids, and the pathology reports were not conclusive. A second procedure (SBB) was performed in each case after the patients were completely off steroids, permitting a definitive diagnosis of PCNSL. Although 42.3% (n = 11) of patients were on steroids preoperatively, a brain biopsy was still diagnostic in the majority of these patients (n = 9, 81.8%). Perioperative morbidity was noted in 7.6% of cases (n = 2) due to hemorrhages after SBB that improved at follow-up. The first patient developed mild dysarthria in the first 12 hours of the procedure that resolved entirely in 2 weeks with conservative treatment. The second patient had an event 24 hours after the surgery, becoming lethargic and showing a new motor deficit (hemiparesis) that slightly improved at last follow-up. No perioperative mortality was noted in our series.
Table 2.
PCNSL Treated Between 2012 and 2018
| Patient | Age | Procedure | Localization | Anesthesia | Preoperative Work-Up | Preoperative LP Result | Preoperative Steroids | Postoperative Complications |
|---|---|---|---|---|---|---|---|---|
| 1 | 74 | Craniotomy | Brainstem | General | Preoperative CT-CAP | Yes, negative | Yes | No |
| 2 | 80 | SBB | Frontal | General | No | No | No | No |
| 3 | 67 | SBB | Frontal | General | No | No | No | No |
| 4 | 86 | SBB | Frontal | MAC sedation | No | No | No | No |
| 5 | 72 | Craniotomy | Frontal | General | No | No | Yes | No |
| 6 | 88 | SBB | Frontal | General | No | No | Yes | No |
| 7 | 80 | Craniotomy | Frontal | Awake | No | No | No | No |
| 8 | 71 | SBB | Frontal | General | No | No | No | No |
| 9 | 79 | SBB | Frontal | General | No | Yes, negative | No | Second biopsy |
| 10 | 79 | Endoscopy | Frontal | General | No | No | Yes | No |
| 11 | 66 | SBB | Frontal | General | No | Yes, negative | No | No |
| 12 | 84 | SBB | Frontal | General | Preoperative CT-CAP | No | No | Mild dysarthria |
| 13 | 84 | SBB | Frontal | General | No | No | Yes | Small hemorrhage |
| 14 | 68 | SBB | Frontal | General | Preoperative CT-CAP | Yes, negative | Yes | No |
| 15 | 60 | SBB | Parietal | General | No | No | No | No |
| 16 | 64 | SBB | Parietal | General | Preoperative CT-CAP | No | Yes | No |
| 17 | 62 | SBB | Parietal | General | Preoperative CT-CAP | No | Yes | No |
| 18 | 80 | Craniotomy | Occipital | General | No | No | No | No |
| 19 | 69 | Craniotomy | Temporal | General | No | Yes, negative | No | Second biopsy |
| 20 | 36 | Craniotomy | Temporal | General | No | No | Yes | No |
| 21 | 60 | Craniotomy | Temporal | Awake | No | No | No | No |
| 22 | 60 | SBB | Temporal | General | Preoperative CT-CAP | Yes, negative | Yes | No |
| 23 | 52 | SBB | Parietal | General | No | No | No | No |
| 24 | 45 | Craniotomy | Parietal | General | No | No | Yes | No |
| 25 | 45 | SBB | Thalamic | General | No | Yes, negative | No | No |
| 26 | 80 | Endoscopy | Thalamic | General | No | No | No | No |
Abbreviations: CT-CAP, CT scan chest, abdomen and pelvis; MAC, monitored anesthesia care; PCNSL, primary central nervous system lymphoma; SBB, stereotactic frameless brain biopsy.
Discussion
Appropriate management of PCNSL comprises an adequate use of diagnostic and therapeutic tools. Traditionally, less-invasive modalities (LP, vitreous sample, steroid trial) have been suggested to preclude brain biopsies for the diagnosis of PCNSL. However, the utility of these diagnostic procedures was previously undefined. Our study remains the first systematic review that helps define the diagnostic yield of CSF studies for PCNSL.
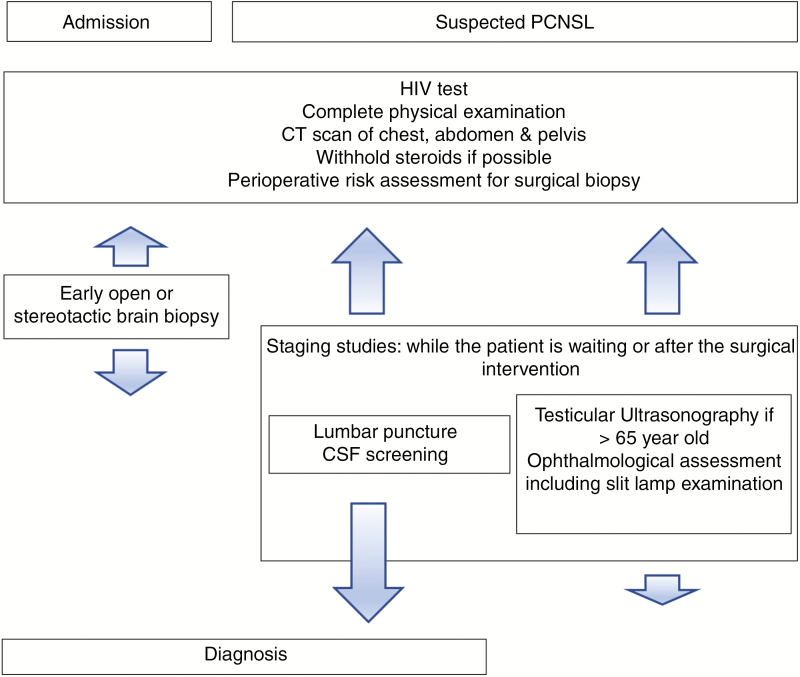
If there is suspicion of primary CNS lymphoma, initial work-up typically includes at least 1 HIV blood test, an LP (if there are no signs of possible contraindications), and ophthalmological assessment including slit-lamp examination. Also, systemic staging should include testicular ultrasonography (mostly in older patients) and either PET/CT or CT scan of the chest, abdomen, and pelvis.9,31 Cytomorphology of the CSF is the most common technique for diagnosing leptomeningeal spread, but cellular immunophenotyping by flow cytometry or PCR analysis of immunoglobulin heavy and light chain genes may help when the cytological examination is negative.32,33 It is important to acknowledge that many earlier studies on PCNSL predated the adoption of sophisticated molecular and cytometric tests.
In 1995, Balmaceda et al8 proposed a diagnostic algorithm for patients with possible PCNSL that suggested that when a CT/MRI is suspicious for PCNSL, you should withhold corticosteroids and perform a slit-lamp exam and LP, postponing brain biopsy. In this series, they were able to diagnose only 14.5% of the patients by CSF analysis, avoiding the need to perform a brain biopsy in those cases. They also stated that most of the samples were obtained after corticosteroids were started, with a possible underestimation of the real value of performing an LP before a brain biopsy. Although a reasonable and less-invasive approach, available data from recent studies suggest that only a minor fraction of patients can be diagnosed by CSF analysis, ranging from 0% to 4% of the total cases (Table 1).
However, positive CSF analysis during any stage of PCNSL (diagnosis or follow-up screening) tends to be more common, with an average of 14.9% (0% to 28%) of all patients with PCNSL. Although these numbers seem to show a higher sensitivity, we must understand that multiple LPs are performed in some centers as part of the follow-up of these patients at a more advanced disease, leading to a higher percentage of positive results. Most articles suggesting a less-invasive and more staged approach support recommendations for CSF analysis in the postdiagnostic stage of the PCNSL. This discrepancy (7.1% at diagnosis and 14.9% at follow-up) helps define the role of early biopsy for PCNSL.
Early brain biopsy has also been recommended in the literature because of the low rates of complications, high diagnostic rates, and to prevent the diagnostic delay that patients with PCNSL present.34–38 In our series, definitive diagnosis was achieved in the first surgical intervention (regardless of preoperative LP) in 92.3% of the cases, similar to other publications.39–42 Additionally, brain biopsies in our modern series remained relatively safe with low permanent morbidity (3.8%, hemiparesis in 1 patient). Overall, these data help advocate for early biopsies in immunocompetent patients with suspected PCNSL regardless of CSF analyses to help prevent diagnostic delays and start earlier treatment.
As previously reported, diagnostic delays in patients with PCNSL can occur frequently. In a study published by Cerqua and colleagues,35 the authors compared time spans from clinical onset to final diagnosis in 28 patients with glioblastoma multiforme and 28 patients with PCNSL. They found that mean time span from first neuroimaging examination to final histologic diagnosis was much longer in PCNSL patients (41.7 vs 16.2 days, P = .008). Other studies also confirm considerable delays in PCNSL diagnoses, ranging up to 124 days in certain patient populations.37 In many hospitals, part of the delay includes lengthy cytology or flow cytometry analysis that may take up to 1-2 weeks in some hospitals. As a result, early, safe diagnostic options may be offered to patients to mitigate these diagnostic delays and reduce costs associated with extended hospitalizations. In our series, the diagnostic yield of CSF screening (n = 7/26) was 0%, and the time to diagnosis from first consultation was considerably higher in patients who underwent preoperative/diagnostic LP compared with those in the early biopsy strategy (21 vs. 11 days, respectively).
Some critics suggest that LPs should always be performed prior to brain biopsy because the benefits of noninvasive diagnosis outweigh the undue risks of brain biopsies. Previous literature suggests that brain biopsies may have a nontrivial risk of procedural complications including hemorrhage, nondiagnosis, and infection. However, many of these studies (premillennial) predate the invention of neuronavigation and intraoperative imaging (CT/MRI) that have intrinsically advanced the accuracy of SBBs. Nevertheless, LP has some utility in cases in which there is some equivocality on the diagnosis, especially in immunocompromised patients or patients with multifocal/LD. Lastly, we maintain that early brain biopsies do not preclude LPs if they are needed but should be offered simultaneously under anesthesia for appropriate staging at the time of diagnosis.
As has been reported, the use of steroids preoperatively can compromise the efficacy of brain biopsy and LPs in cases of PCNSL.43,44 As such, the classic rationale recommends withholding steroid treatment for at least 14 days prior to a brain biopsy. Our data suggest that the diagnostic yield of brain biopsies is only slightly affected (~80%) when patients remain on steroids prior to surgery. This is comparable with other retrospective studies45 that found no difference in the rate of definitive diagnosis in the first biopsy in patients on or off corticosteroids (88% vs 87%). However, the length of steroid treatment before the biopsy may remain important; another study by Manoj et al45 reported a high incidence of false-negative results in the first biopsy when the treatment was longer than 1 week, compared with less than 1 week with no steroid treatment at all (44% vs 5.8% vs 0%, respectively). Therefore, withholding initial treatment or tapering corticosteroids is recommended until histologic confirmation has been obtained if the patient can tolerate the steroid wean. In those patients in whom steroid treatment is mandatory, performing an early biopsy may still remain effective (Fig. 2).
Fig. 2.
Management Paradigm for Primary Central Nervous System Lymphoma. CSF indicates cerebrospinal fluid.
Establishing a minimally invasive approach in the diagnosis of brain tumors has always been a goal. In recent years, the detection of circulating tumor DNA in serum, plasma, or CSF has become a field of major interest in neuro-oncology. The molecular analysis of these fragments of tumor DNA or microRNA can identify genetic hallmark mutations of PCNSL, being remarkably useful in the diagnosis of these types of tumors.46–48 However, further studies are needed for these promising minimally invasive techniques to be included in the standard diagnostic paradigm.
Our study provides an updated framework that advocates for an earlier surgical biopsy when a PCNSL is suspected. However, in our systematic review we could find data only from retrospective studies that were not designed to assess the sensitivity of CSF screening, and many of them were multicenter studies with heterogeneous or incomplete data (ie, use of corticosteroids). In the future, we expect to see prospective studies about the efficacy of new minimally invasive techniques such as molecular analysis in the diagnosis of PCNSL.
Conclusions
The diagnostic yield of CSF analyses for PCNSL in immunocompetent patients remains exceedingly low (7.4%), although follow-up CSF screening may be positive in up to 14.9% of cases. Our institutional series demonstrates that early biopsy for PCNSL is safe and accurate, and may avert lengthy extensive work-ups. We conclude that performing an early brain biopsy in a suspected case of PCNSL is a valid option to minimize diagnostic delay, with a high rate of definitive diagnosis, and a low rate of complications.
Funding
None.
Conflict of interest statement. None declared.
References
- 1. Schabet M. Epidemiology of primary CNS lymphoma. J Neurooncol. 1999;43(3):199–201. [DOI] [PubMed] [Google Scholar]
- 2. Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ.. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105(9):1414–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mrugala MM, Rubenstein JL, Ponzoni M, Batchelor TT.. Insights into the biology of primary central nervous system lymphoma. Curr Oncol Rep. 2009;11(1):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shiels MS, Pfeiffer RM, Besson C, et al. . Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br J Haematol. 2016;174(3):417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bataille B, Delwail V, Menet E, et al. . Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg. 2000;92(2):261–266. [DOI] [PubMed] [Google Scholar]
- 6. Batchelor T, Loeffler JS. Primary CNS lymphoma. J Clin Oncol. 2006;24(8):1281–1288. [DOI] [PubMed] [Google Scholar]
- 7. Giannini C, Dogan A, Salomão DR. CNS lymphoma: a practical diagnostic approach. J Neuropathol Exp Neurol. 2014;73(6):478–494. [DOI] [PubMed] [Google Scholar]
- 8. Balmaceda C, Gaynor JJ, Sun M, Gluck JT, DeAngelis LM.. Leptomeningeal tumor in primary central nervous system lymphoma: recognition, significance, and implications. Ann Neurol. 1995;38(2):202–209. [DOI] [PubMed] [Google Scholar]
- 9. Hoang-Xuan K, Bessell E, Bromberg J, et al. . Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: guidelines from the European Association for Neuro-Oncology. Lancet Oncol. 2015;16(7):e322–e332. [DOI] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, Altman DG,. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. [Erratum in Int J Surg. 2010;8(8):658.] [DOI] [PubMed] [Google Scholar]
- 11. Schlegel U, Pels H, Glasmacher A, et al. . Combined systemic and intraventricular chemotherapy in primary CNS lymphoma: a pilot study. J Neurol Neurosurg Psychiatry. 2001;71(1):118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ;. Radiation Therapy Oncology Group Study 93-10 Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol. 2002;20(24):4643–4648. [DOI] [PubMed] [Google Scholar]
- 13. Ferreri AJ, Reni M, Pasini F, et al. . A multicenter study of treatment of primary CNS lymphoma. Neurology. 2002;58(10):1513–1520. [DOI] [PubMed] [Google Scholar]
- 14. Pels H, Schmidt-Wolf IG, Glasmacher A, et al. . Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol. 2003;21(24):4489–4495. [DOI] [PubMed] [Google Scholar]
- 15. Jahnke K, Korfel A, O’Neill BP, et al. . International study on low-grade primary central nervous system lymphoma. Ann Neurol. 2006;59(5):755–762. [DOI] [PubMed] [Google Scholar]
- 16. Shenkier TN, Blay JY, O’Neill BP, et al. . Primary CNS lymphoma of T-cell origin: a descriptive analysis from the international primary CNS lymphoma collaborative group. J Clin Oncol. 2005;23(10):2233–2239. [DOI] [PubMed] [Google Scholar]
- 17. Quek R, Ty A, Lim ST, et al. . Primary central nervous system lymphoma in an Asian population: a 15-year experience. Onkologie. 2006;29(10):455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kiewe P, Fischer L, Martus P, Thiel E, Korfel A.. Primary central nervous system lymphoma: monocenter, long-term, intent-to-treat analysis. Cancer. 2008;112(8):1812–1820. [DOI] [PubMed] [Google Scholar]
- 19. Agarwal PA, Menon S, Smruti BK, Singhal BS.. Primary central nervous system lymphoma: a profile of 26 cases from Western India. Neurol India. 2009;57(6):756–763. [DOI] [PubMed] [Google Scholar]
- 20. Korfel A, Weller M, Martus P, et al. . Prognostic impact of meningeal dissemination in primary CNS lymphoma (PCNSL): experience from the G-PCNSL-SG1 trial. Ann Oncol. 2012;23(9):2374–2380. [DOI] [PubMed] [Google Scholar]
- 21. Pasricha S, Gupta A, Gawande J, Trivedi P, Patel D.. Primary central nervous system lymphoma: a study of clinicopathological features and trend in western India. Indian J Cancer. 2011;48(2):199–203. [DOI] [PubMed] [Google Scholar]
- 22. Kim YR, Kim SH, Chang JH, et al. . Early response to high-dose methotrexate, vincristine, and procarbazine chemotherapy-adapted strategy for primary CNS lymphoma: no consolidation therapy for patients achieving early complete response. Ann Hematol. 2014;93(2):211–219. [DOI] [PubMed] [Google Scholar]
- 23. Rubenstein JL, Hsi ED, Johnson JL, et al. . Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol. 2013;31(25):3061–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xie H, Ahluwalia MS, Peereboom DM. The Cleveland Clinic experience with primary central nervous system lymphoma. Am J Clin Oncol. 2015;38(2):140–146. [DOI] [PubMed] [Google Scholar]
- 25. Ferreri AJ, Ciceri F, Brandes AA, et al. . MATILDE chemotherapy regimen for primary CNS lymphoma: results at a median follow-up of 12 years. Neurology. 2014;82(15):1370–1373. [DOI] [PubMed] [Google Scholar]
- 26. Wang XX, Huang HQ, Bai B, et al. . Clinical outcomes of patients with newly diagnosed primary central nervous system lymphoma are comparable on treatment with high-dose methotrexate plus temozolomide and with high-dose methotrexate plus cytarabine: a single-institution experience. Leuk Lymphoma. 2014;55(11):2497–2501. [DOI] [PubMed] [Google Scholar]
- 27. Pulczynski EJ, Kuittinen O, Erlanson M, et al. . Successful change of treatment strategy in elderly patients with primary central nervous system lymphoma by de-escalating induction and introducing temozolomide maintenance: results from a phase II study by the Nordic Lymphoma Group. Haematologica. 2015;100(4):534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Omuro A, Chinot O, Taillandier L, et al. . Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: an intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol. 2015;2(6):e251–e259. [DOI] [PubMed] [Google Scholar]
- 29. Patel B, Chacko G, Nair S, et al. . Clinicopathological correlates of primary central nervous system lymphoma: experience from a tertiary care center in South India. Neurol India. 2015;63(1):77–82. [DOI] [PubMed] [Google Scholar]
- 30. Houillier C, Ghesquières H, Chabrot C, et al. . Rituximab, methotrexate, procarbazine, vincristine and intensified cytarabine consolidation for primary central nervous system lymphoma (PCNSL) in the elderly: a LOC network study. J Neurooncol. 2017;133(2):315–320. [DOI] [PubMed] [Google Scholar]
- 31. Han CH, Batchelor TT. Diagnosis and management of primary central nervous system lymphoma. Cancer. 2017;123(22):4314–4324. [DOI] [PubMed] [Google Scholar]
- 32. Weller M. Glucocorticoid treatment of primary CNS lymphoma. J Neurooncol. 1999;43(3):237–239. [DOI] [PubMed] [Google Scholar]
- 33. Chiavazza C, Pellerino A, Ferrio F, Cistaro A, Soffietti R, Rudà R.. Primary CNS lymphomas: challenges in diagnosis and monitoring. Biomed Res Int. 2018;2018:3606970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baraniskin A, Deckert M, Schulte-Altedorneburg G, Schlegel U, Schroers R.. Current strategies in the diagnosis of diffuse large B-cell lymphoma of the central nervous system. Br J Haematol. 2012;156(4):421–432. [DOI] [PubMed] [Google Scholar]
- 35. Cerqua R, Balestrini S, Perozzi C, et al. . Diagnostic delay and prognosis in primary central nervous system lymphoma compared with glioblastoma multiforme. Neurol Sci. 2016;37(1):23–29. [DOI] [PubMed] [Google Scholar]
- 36. Deckert M, Brunn A, Montesinos-Rongen M, Terreni MR, Ponzoni M.. Primary lymphoma of the central nervous system—a diagnostic challenge. Hematol Oncol. 2014;32(2):57–67. [DOI] [PubMed] [Google Scholar]
- 37. Haldorsen IS, Espeland A, Larsen JL, Mella O.. Diagnostic delay in primary central nervous system lymphoma. Acta Oncol. 2005;44(7):728–734. [DOI] [PubMed] [Google Scholar]
- 38. Chen CC, Hsu PW, Erich Wu TW, et al. . Stereotactic brain biopsy: single center retrospective analysis of complications. Clin Neurol Neurosurg. 2009;111(10):835–839. [DOI] [PubMed] [Google Scholar]
- 39. Dammers R, Haitsma IK, Schouten JW, Kros JM, Avezaat CJ, Vincent AJ.. Safety and efficacy of frameless and frame-based intracranial biopsy techniques. Acta Neurochir (Wien). 2008;150(1):23–29. [DOI] [PubMed] [Google Scholar]
- 40. Baraniskin A, Kuhnhenn J, Schlegel U, et al. . Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood. 2011;117(11):3140–3146. [DOI] [PubMed] [Google Scholar]
- 41. Grossman R, Sadetzki S, Spiegelmann R, Ram Z.. Haemorrhagic complications and the incidence of asymptomatic bleeding associated with stereotactic brain biopsies. Acta Neurochir (Wien). 2005;147(6):627–631; discussion 631. [DOI] [PubMed] [Google Scholar]
- 42. Kongkham PN, Knifed E, Tamber MS, Bernstein M.. Complications in 622 cases of frame-based stereotactic biopsy, a decreasing procedure. Can J Neurol Sci. 2008;35(1):79–84. [DOI] [PubMed] [Google Scholar]
- 43. Binnahil M, Au K, Lu JQ, Wheatley BM, Sankar T.. The influence of corticosteroids on diagnostic accuracy of biopsy for primary central nervous system lymphoma. Can J Neurol Sci. 2016;43(5):721–725. [DOI] [PubMed] [Google Scholar]
- 44. Porter AB, Giannini C, Kaufmann T, et al. . Primary central nervous system lymphoma can be histologically diagnosed after previous corticosteroid use: a pilot study to determine whether corticosteroids prevent the diagnosis of primary central nervous system lymphoma. Ann Neurol. 2008;63(5):662–667. [DOI] [PubMed] [Google Scholar]
- 45. Manoj N, Arivazhagan A, Mahadevan A, et al. . Central nervous system lymphoma: patterns of incidence in Indian population and effect of steroids on stereotactic biopsy yield. Neurol India. 2014;62(1):19–25. [DOI] [PubMed] [Google Scholar]
- 46. Hattori K, Sakata-Yanagimoto M, Suehara Y, et al. . Clinical significance of disease-specific MYD88 mutations in circulating DNA in primary central nervous system lymphoma. Cancer Sci. 2018;109(1):225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fontanilles M, Marguet F, Bohers É, et al. . Non-invasive detection of somatic mutations using next-generation sequencing in primary central nervous system lymphoma. Oncotarget. 2017;8(29):48157–48168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Y, Springer S, Zhang M, et al. . Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci U S A. 2015;112(31):9704–9709. [DOI] [PMC free article] [PubMed] [Google Scholar]